- State Key Laboratory Breeding Base of Green Pesticide and Agricultural Bioengineering, Key Laboratory of Green Pesticide and Agricultural Bioengineering, Ministry of Education, Guizhou University, Guiyang, China
Oxathiapiprolin was developed with high antifungal activity and novel target protein and is used in the oomycetes control for crop protection. The structural modifications of oxathiapiprolin are summarized. The achievements and challenges in the structural modification of oxathiapiprolin are also discussed in this mini review. The outlook in this field is perspected according to our own opinion and understanding on the development of oxysterol binding protein inhibition fungicides.
Introduction
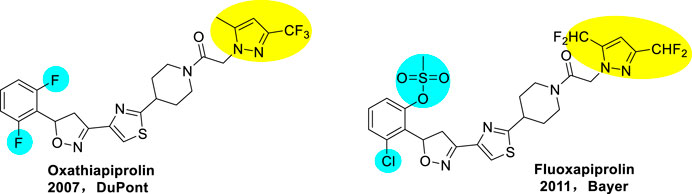
Oxathiapiprolin was developed as the first piperidinyl thiazole isoxazoline fungicide by Dupont in 2007 (Hanagan and Pasteris, 2009; Cristau et al., 2010; Pasteris, 2010), which was a new fungicide with novel chemical structure, containing pyrazole ring, thiazole ring, and isoxazoline ring (Figure 1). It is a class of oomycetes (Hardham, 2007; Kamoun et al., 2015) inhibitor, with the highest biological activity so far. It is also the most representative new pesticide in the 21st century and is expected to form a series of commercial products. Miao et al. (2016) demonstrated that the agent shows excellent biological activity against pathogens oomycetes. The EC50 and EC90 values of anti-oomycetes effect were 10–4 and 10–3 µg/ml, respectively, and there was no cross resistance with other oomycetes inhibitors. The agent was registered in China at the end of 2015 for the control of Phytophthora and downy mildew. In 2011, Bayer developed another piperidyl thiazole isoxazoline fungicide fluoxapiprolin (Tsuchiya et al., 2013) (Figure 1). These fungicides have great potential to control oomycetes for crop protection.
Due to the excellent biological activity and novel target proteins, many structural modifications of the oxathiapiprolin have been developed in recent years. Therefore, we consider that it is the right time to provide a systematic summary on the modification of this highly active fungicide. In this mini review, the structural modification and structure–activity relationship are discussed. A brief summary on the achievements and the challenges remained in the development of oomycetes fungicides is provided at the end of this review. An outlook into the future research direction within this field is also given based on our own opinion and knowledge on the trends of the development of oomycetes fungicides.
Structural modification of commercial oxathiapiprolin
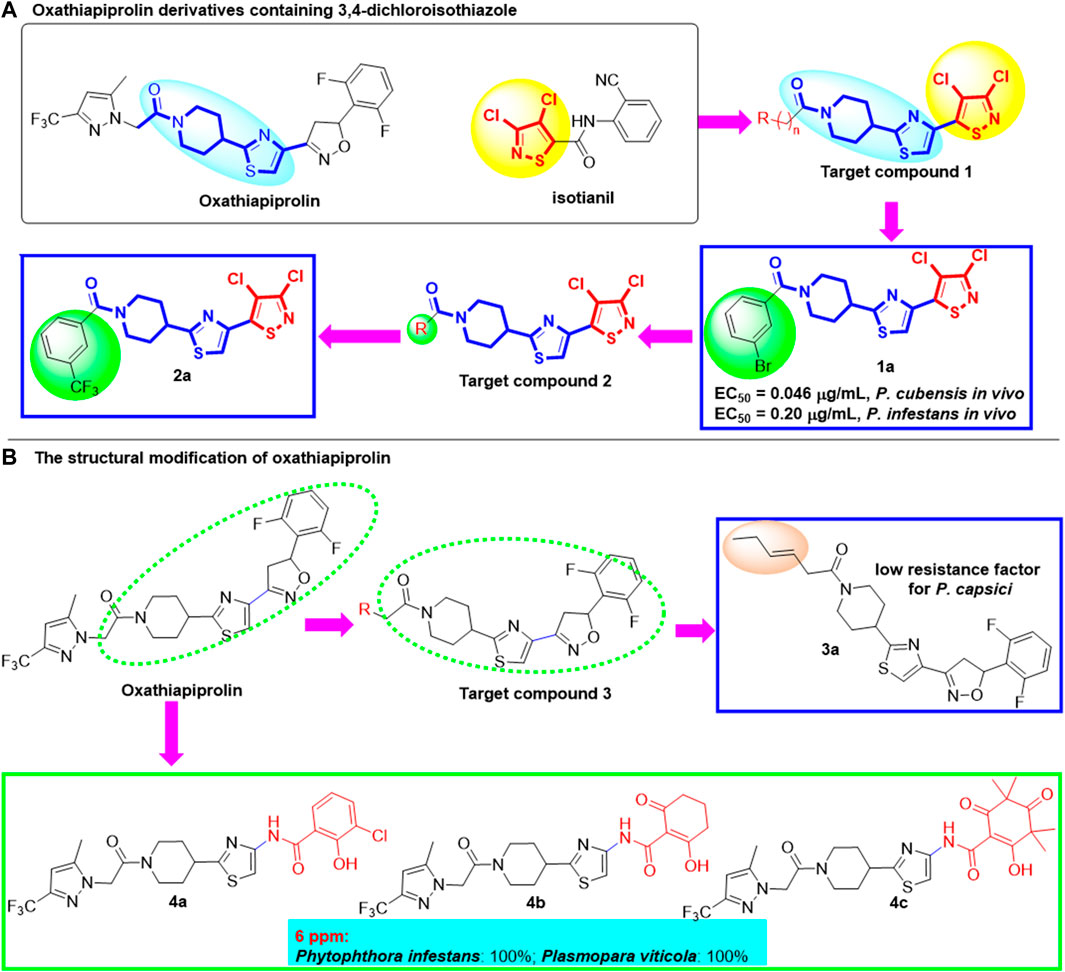
In 2018, in order to develop new fungicides with new mechanism of action and no resistance risk, Wu et al. (2018) designed and synthesized a series of novel target compounds based on commercial drugs oxathiapiprolin and isotianil and evaluated their antifungal and anti-oomycetes activities (Figure 2A). 3,4-dichloroisothiazole, which can induce the systemic acquired resistance (SAR), was introduced into the main scaffold of oxathiapiprolin. When n = 0 and R is m-Br phenyl group, compound 1a shows excellent biological activity against P. cubensis and P. infestans in vivo with the EC50 values of 0.046 μg/ml and 0.2 μg/ml, respectively. At the same time, the expression of defense genes npr1 and pr1 was increased after treated with 1a and infer that compound 1a could induce SAR to improve the anti-oomycete activity in vivo. Subsequently, Wu et al. (2019) explored the substituents on the phenyl group of compound 2 in 2019 (Figure 2A). When the m-CF3 group was installed on the benzene ring, the corresponding compound 2a exhibits better activity against P. cubensis and P. infestans in vivo at 1 μg/ml with the inhibitory rate of 100%, which is superior to that of oxathiapiprolin and isotianil. Although the field efficacy of compound 2a against P. cubensis was 74.91% at 2 g ai/667 m2 in field efficacy trials, it can be used as a novel fungicide lead compound.
In order to find new compounds with low resistance factor for P. capsici, structural modification was carried out in 2020 by modifying the pyrazole ring (Wang et al., 2020; Wang Y. et al., 2022; Li et al., 2022) of oxathiapiprolin (Li et al., 2020), with most of the oxathiapiprolin skeleton remained unchanged. Compound 3a possessed a low resistance factor for P. capsici and can be used as the lead compound for new fungicide development (Figure 2B). In 2015, Sulzer-Mosse et al. (2015) designed and synthesized a series of oxathiapiprolin derivatives and studied the structure–activity relationship. Compounds 4a, 4b, and 4c exhibited excellent biological effects against phytopathogens Phytophthora infestans and Plasmopara viticola, with the inhibitory rate of 100% at 6 μg/ml. Meanwhile, structure–activity relationship studies revealed that a phenolic or enolic hydroxy function installed on the β-position of a carboxamide is significant for their bioactivities.
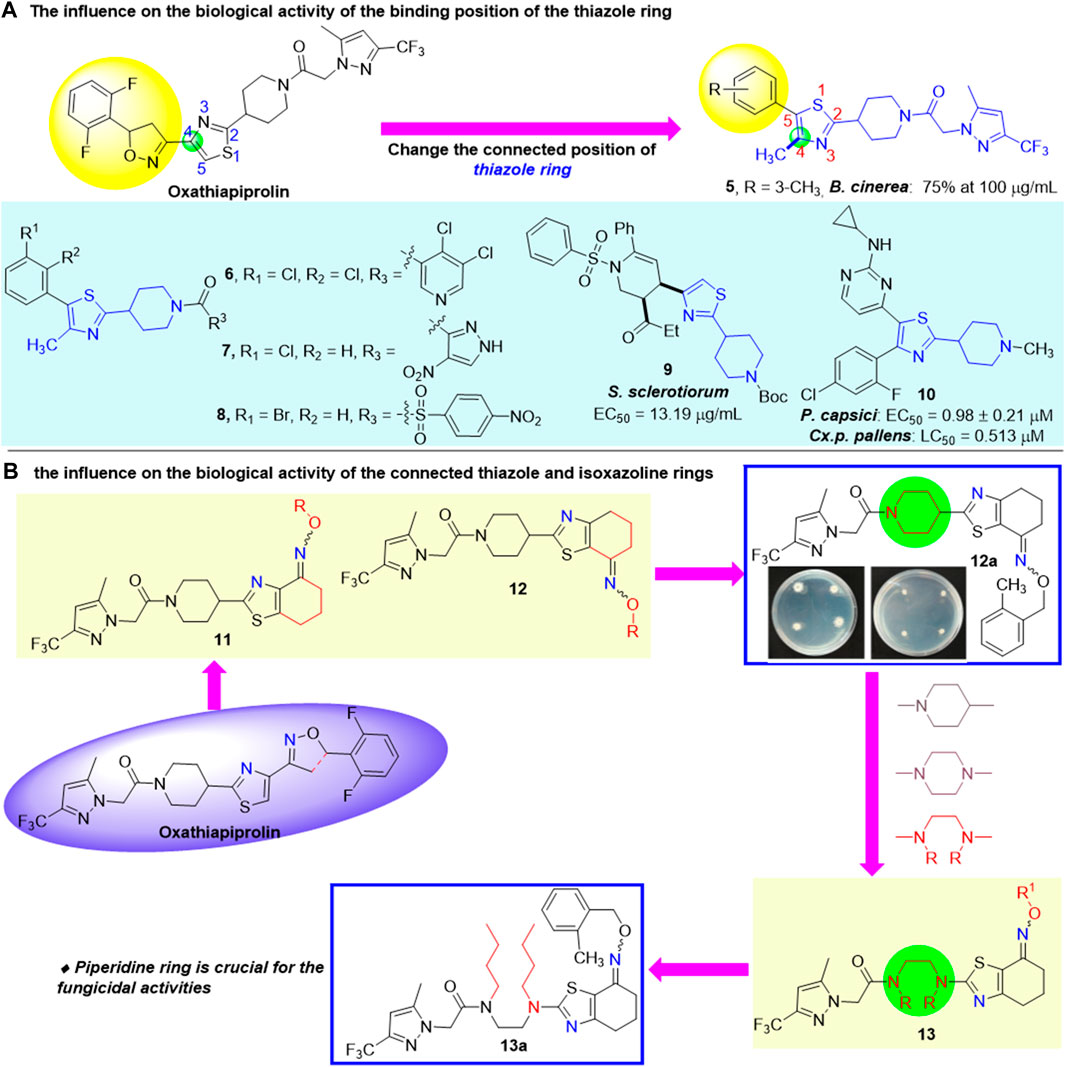
The binding position of the thiazole ring (Wang M. et al., 2022) was also explored and proved to have significant influence on the biological activity. In 2019, Ding et al. (2019b)designed and synthesized a number of oxathiapiprolin derivatives (Figure 3A). The preliminary fungicidal activity showed that the inhibition rate of 5 against B. cinerea was 75% at 100 μg/ml. Other compounds exhibited poor fungicidal activities, which demonstrated that the thiazole ring is critical for the activity of oxathiapiprolin. Subsequently, when the terminal group was replaced and the remaining thiazole and piperidine rings in the oxathiapiprolin molecule unchanged (e.g., compounds 6, 7, 8, and 9), their fungicidal activities have not been significantly improved, instead of certain insecticidal activities (Zhu et al., 2016; Ding et al., 2019a; Ding et al., 2019c; Ding et al., 2020). However, Choi et al. (2015) reported a novel compound 10 with high fungicidal and insecticidal activities through the modification of the thiazole ring in 2015, with the EC50 value of 0.98 μM against P. capsici and the LC50 value of 0.513 μM against Cx. p. pallens.
The connected thiazole and isoxazoline rings existed in the oxathiapiprolin molecule are heteroatom-rich fragments bearing two nitrogen atoms positioned either at the same or opposite conformations. In 2021, Bian et al. (2021) revealed that the position of the two nitrogen atoms has significant influence on the bioactivity through the biological activity screening in vitro of novel piperidyl thiazole derivatives, containing oxime ether and oxime ester derivatives (Figure 3B). The bioassay results showed that the target compounds possessed moderate to good fungicidal activities against P. capsici. Compound 12a exhibited the highest antifungal activity in vitro (EC50 = 0.0104 μg/ml), which was higher than dimethomorph (EC50 = 0.1148 μg/ml) and diacetylenyl amide (EC50 = 0.040 μg/ml). The activities of oxime ester compounds were lower than oxime ether compounds when the two nitrogen atoms are positioned on the opposite sides. Subsequently, based on the aforementioned results, a series of novel oxathiapiprolin derivatives containing oxime ether and oxime ester moieties were synthesized, in which the piperidine ring was opened (Tian et al., 2022) (Figure 3B). Their antifungicidal activities were evaluated and showed that the target compounds possessed moderate fungicidal activities against Phytophthora capsici, and the activities of these compounds were lower than that of oxathiapiprolin, suggesting that the piperidine ring was crucial for the fungicidal activities of these compounds.
Conclusion and outlook
Oxathiapiprolin targets at the protein of oxysterol binding protein (OSBP) to give high fungicidal activity and have been used as the effective pesticide to control oomycetes for crop protection. It can be found that the piperidine and thiazole rings are crucial for the biological activity. Some heterocycles can be introduced into the structure to induce systemic acquired resistance and thus improve the in vivo control effects to various pathogens. Although a few achievements have been made, challenges still remain. The structural modification of oxathiapiprolin faces considerable limitations. All the derivatives disclosed to date showed lower activity than the commercial fungicide oxathiapiprolin. The introductions of additional bioactive functional groups and chiral fragments are promising strategies in the search for novel oxathiapiprolin-derived structures with greater fungicidal activities and lower risks.
Author contributions
TL and JJ contributed equally to this work and drafted the manuscript. JS and JL participated in some manuscript writing and checking. ZJ conceptualized and directed the whole project. All of the authors contributed to scientific discussions.
Funding
We acknowledge funding supports from the National Natural Science Foundation of China (22061007, 22071036). Frontiers Science Center for Asymmetric Synthesis and Medicinal Molecules, Department of Education, Guizhou Province (Qianjiaohe KY number (2020)004). The 10 Talent Plan (Shicengci) of Guizhou Province ((2016)5649). Science and Technology Department of Guizhou Province ((2018)2802, (2019)1020). Program of Introducing Talents of Discipline to Universities of China (111 Program, D20023) at Guizhou University.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer XC declared a past co-authorship with the author ZJ to the handling editor.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Bian, Q., Zhao, R.-Q., Peng, X.-J., Gao, L.-J., Zhou, G.-N., Yu, S.-J., et al. (2021). Design, synthesis, and fungicidal activities of novel piperidyl thiazole derivatives containing oxime ether or oxime ester moieties. J. Agric. Food Chem. 69 (13), 3848–3858. doi:10.1021/acs.jafc.0c07581
Choi, W.-S., Nam, S.-W., Kim, I.-D., Kim, S.-H., Park, K.-H., Bae, I.-K., et al. (2015). Synthesis and pesticidal activities of 5-(2-Cyclopropylaminopyrimidin-4-yl)-4-(thiophenyl)thiazole derivatives. J. Chem. 241793, 1–6. doi:10.1155/2015/241793
Cristau, P., Rahn, N., Herrmann, S., Tsuchiya, T., Wachendorf-Neumann, U., Voerste, A., et al. (2010). Preparation of heterocyclic thiazoles as agrochemical fungicides. WO2010037479A1. Geneva: World Intellectual Property Organization.
Ding, C., Pan, Y., and Tan, C. (2020). Synthesis and biological activity of aryl thiazole piperidine amide compounds. Chin. J. Org. Chem. 40 (2), 528–535. doi:10.6023/cjoc201907034
Ding, C., Pan, Y., Yin, X., and Tan, C. (2019a). Synthesis and biological activity of thiazolidine piperidine nicotinamide compounds. Chin. J. Org. Chem. 39 (7), 2099–2105. doi:10.6023/cjoc201812027
Ding, C., Pan, Y., Yin, X., Tan, C., and Wang, X. (2019b). Synthesis and fungicidal activity of novel oxathiapiprolin derivatives. Chin. J. Org. Chem. 39 (7), 2062–2069. doi:10.6023/cjoc201901009
Ding, C., Pan, Y., Yin, X., Tan, C., and Zhang, G. (2019c). Synthesis and insecticidal activity of novel piperidine thiazole compounds. Chin. J. Org. Chem. 39 (3), 836–841. doi:10.6023/cjoc201809009
Hanagan, M. A., and Pasteris, R. J. (2009). Isoxazolyl-thiazole derivatives as fungicidal compounds and their preparation and use in controlling plant disease. WO 2009094407A2. Geneva: World Intellectual Property Organization.
Hardham, A. R. (2007). Cell biology of plant-oomycete interactions. Cell. Microbiol. 9 (1), 31–39. doi:10.1111/j.1462-5822.2006.00833.x
Kamoun, S., Furzer, O., Jones, J. D., Judelson, H. S., Ali, G. S., Dalio, R. J., et al. (2015). The top 10 oomycete pathogens in molecular plant pathology. Mol. Plant Pathol. 16 (4), 413–434. doi:10.1111/mpp.12190
Li, J.-L., Zhou, L.-M., Gao, M.-Q., Huang, Z.-Q., Liu, X.-L., Zhu, X.-L., et al. (2020). Design, synthesis, and fungicidal evaluation of novel oxysterol binding protein inhibitors for combatting resistance associated with oxathiapiprolin. Pestic. Biochem. Physiol. 169, 104673. doi:10.1016/j.pestbp.2020.104673
Li, L., Liu, T., Zhang, X., Hou, X., Dong, H., Li, X., et al. (2022). Catalyst-free and atom-economical 1, 3-dipolar cycloaddition of C, N-cyclic azomethine imines: facile synthesis of isoquinoline-fused spirocycles. Green Synth. Catal. 3 (1), 69–78. doi:10.1016/j.gresc.2021.11.005
Miao, J., Dong, X., Lin, D., Wang, Q., Liu, P., Chen, F., et al. (2016). Activity of the novel fungicide oxathiapiprolin against plant-pathogenic oomycetes. Pest Manag. Sci. 72 (8), 1572–1577. doi:10.1002/ps.4189
Pasteris, R. J. (2010). Fungicidal heterocyclic compounds and their preparation. WO2010065579A2. Geneva: World Intellectual Property Organization.
Sulzer-Mosse, S., Cederbaum, F., Lamberth, C., Berthon, G., Umarye, J., Grasso, V., et al. (2015). Synthesis and fungicidal activity of N-thiazol-4-yl-salicylamides, A new family of anti-oomycete compounds. Bioorg. Med. Chem. 23 (9), 2129–2138. doi:10.1016/j.bmc.2015.03.007
Tian, X., Peng, X., Zhao, T., Bian, Q., and Zhao, W. (2022). Design, synthesis, and fungicidal activities of novel ethylenediamine bridged thiazole derivatives containing oxime ether or oxime ester moieties. J. Heterocycl. Chem., 1–22. doi:10.1002/jhet.4484
Tsuchiya, T., Wasnaire, P., Hoffmann, S., Cristau, P., Seitz, T., Kluth, J., et al. (2013). Heteroarylpiperidine and -piperazine derivatives as fungicides and their preparation. WO2013098229A2. Geneva: World Intellectual Property Organization.
Wang, M., Meng, X., Cai, C., Wang, L., and Gong, H. (2022a). Synthesis of benzisothiazoles by a three-component reaction using elemental sulfur and ammonium as heteroatom components under transition metal-free conditions. Green Synth. Catal. 3 (2), 168–174. doi:10.1016/j.gresc.2022.03.005
Wang, S., Zhang, B., Chen, J., Zheng, Y., Feng, N., Ma, A., et al. (2020). Recent progress in synthesis of polysubstituted pyrazoles. Chin. J. Org. Chem. 40 (1), 15–27. doi:10.6023/cjoc201906007
Wang, Y., Wang, S., Liu, J., Lian, M., Chen, Y., Wang, K., et al. (2022b). Visible light-promoted enantioselective aerobic oxidation of pyrazolones by phase transfer catalysis. Green Synth. Catal. 3 (1), 102–109. doi:10.1016/j.gresc.2021.11.004
Wu, Q.-F., Zhao, B., Fan, Z.-J., Zhao, J.-B., Guo, X.-F., Yang, D.-Y., et al. (2018). Design, synthesis and fungicidal activity of isothiazole-thiazole derivatives. RSC Adv. 8 (69), 39593–39601. doi:10.1039/c8ra07619g
Wu, Q., Zhao, B., Fan, Z., Guo, X., Yang, D., Zhang, N., et al. (2019). Discovery of novel piperidinylthiazole derivatives as broad-spectrum fungicidal candidates. J. Agric. Food Chem. 67 (5), 1360–1370. doi:10.1021/acs.jafc.8b06054
Keywords: oxathiapiprolin, oomycetes, structural modification, fungicidal activity, OSBPI
Citation: Li T, Jin J, Song J, Lv J and Jin Z (2022) Advances in the green synthesis and agrichemical applications of oxathiapiprolin derivatives. Front. Chem. 10:987557. doi: 10.3389/fchem.2022.987557
Received: 06 July 2022; Accepted: 25 July 2022;
Published: 29 August 2022.
Edited by:
Huanzhen Ni, Eli Lilly, United StatesReviewed by:
Jing Mu, Shenzhen Hospital, Peking University, ChinaXingkuan Chen, Jinan University, China
Copyright © 2022 Li, Jin, Song, Lv and Jin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhichao Jin, emNqaW5AZ3p1LmVkdS5jbg==
 Tingting Li
Tingting Li Jiamiao Jin
Jiamiao Jin Zhichao Jin
Zhichao Jin