- 1Key Laboratory of Agri-Food Safety of Anhui Province, School of Resources and Environment, Anhui Agricultural University, Hefei, China
- 2State Key Laboratory Breeding Base of Green Pesticide and Agricultural Bioengineering, Key Laboratory of Green Pesticide and Agricultural Bio-engineering, Ministry of Education, Guizhou University, Guiyang, China
Structural optimization of natural products has become one of the most effective ways to develop novel pesticides. In this study, 30 novel pesticide derivatives containing a linear bisamide were synthesized. Then, their insecticidal activities against P. xylostella were evaluated. Results indicate that different bisamide substitutes show different larvicidal structure–activity relationships. At the same time, 2-trifluoroethyl is the most efficient substituent. The bioactivity results showed that most of the desired compounds exhibited better insecticidal activity against P. xylostella than piperine. Among them, compound D28 resulted in 90% mortality at 1 mg/ml concentration. This study provides a novel protocol for the discovery of new insecticides. The molecular docking results indicated that compound D28 could act on γ-aminobutyric acid receptors.
1 Introduction
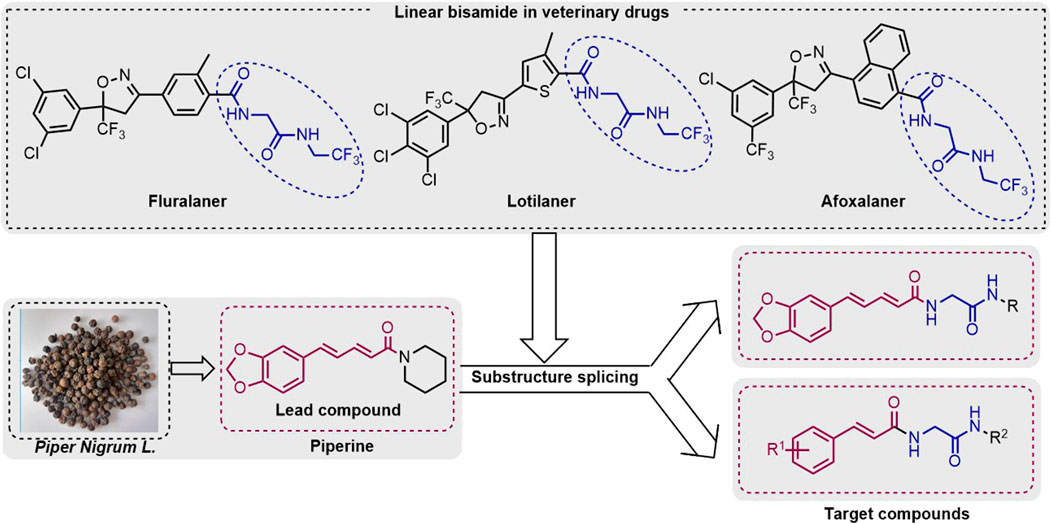
Plutella xylostella, also known as “dangling silkworm” or “diamondback moth,” is a pest commonly found on vegetables, causing damage by larvae feeding on the leaves of cruciferous vegetables. In recent years, the damage of Plutella xylostella has become increasingly serious, with significant adverse effects on the yield and quality of cruciferous vegetables (Wang et al., 2011; Zhao et al., 2022). The primary commercial agents used to control Plutella xylostella are traditional insecticides such as emamectin. However, long-term use of these insecticides results in moderate to high resistance (Lima Neto et al., 2021; Shen et al., 2017). Consequently, developing novel insecticides is an important endeavor (He et al., 2016; Liu et al., 2020; Xia et al., 2022). During the past few decades, natural product structural optimization has been a promising way to develop high-efficiency pesticides (Swain, 1977; Sun et al., 2013). In agricultural pest prevention, natural products have unique advantages (Tang et al., 2012; Roman, 2016; Liu et al., 2018), such as 1) it is relatively safe for higher mammals and natural enemies of pests; 2) it is environmentally friendly (Tong et al., 2018); 3) it has a new insecticidal mode of action (Gaur and Bao, 2021); and 4) it reduces pesticide resistance (Chen et al., 2017). For example, Neemaceae showed broad-spectrum insecticidal activity against many plant pests. Euonymus has been successfully commercialized to control rice pests, stored grain pests, and tree pests (Wu et al., 2001; Tang et al., 2004).
Piperine, as a cinnamon amide alkaloid (Parmar et al., 1997), shows a broad range of bioactivities such as antiobesity (Sunila and Kuttan, 2004), antiparasitic (Ribeiro et al., 2004.; Franklim et al., 2013), and lipid-lowering effects (Kimura et al., 2006; Park et al., 2012). In addition, piperine and its derivatives exhibit effective insecticidal properties against various agricultural pests. For example, Barbosa et al. reported a series of piperine derivatives by modifying the piperidine ring of piperine, which showed effective insecticidal activity against Ascia monuste orseis (Paula et al., 2000). Ribero et al. (2004) designed a series of piperine derivatives with effective insecticidal activity against Trypanosoma cruzi. Xu and Yang, (2017) found that piperine derivatives show stomach toxicity activity against agricultural pests with effects comparable to the commercial botanical pesticide toosendanin (CN107892685A). Han et al. (2021) designed a series of compounds that combined the benzo[d][1,3]dioxole moiety of piperine, which showed effective insecticidal activity against Ostrinia furnacalis.
Bisamide compounds show effective biological activities for agricultural pest control. However, the use of linear bisamides in the development of insecticides is still in its infancy. In contrast, linear bisamides are significant structural motifs in some veterinary drugs (for example, fluralaner, lotilaner, and afoxolaner) (Figure 1). Due to the novel action mechanism and viable activity of linear bisamides and the piperine skeleton, this study designed and synthesized a variety of piperine bisamide derivatives. The target compounds’ insecticidal activities against P. xylostella were systematically investigated.
2 Materials and methods
2.1 Chemical part
2.1.1 General information
Most of the chemicals were purchased from Aladdin, energy-chemical, TCI, or Alfa Aesar. NMR spectra were recorded on Bruker Avance 600 spectrometers. Chemical shifts (ppm) were given relative to the solvent. Melting points of the target molecules were determined using a Shanghai Yice WRX-4 melting point meter. High-resolution mass spectrometry data were determined on a Thermo Scientific (UHPLC-Q-Orbitrap).
2.1.2 Preparation of compound A
A solution of 20% KOH–EtOH (44 ml) was added to 5.7 g (20.0 mmol) of piperine at room temperature. The mixture was stirred at 80 C for 20 h. Then, 10% hydrochloric acid was added to adjust the pH of the mixture to 3. The mixture was filtered under reduced pressure, washed with water, and recrystallized in ethanol to obtain intermediate A in 81% yield (3.5 g).
(2E,4E)-5-(Benzo[d][1,3]dioxol-5-yl)penta-2,4-dienoic (A): brown solid, 3.5g, yield 81%, mp: 163.4–166.5°C, 1H NMR (600 MHz, DMSO-d6) δ = 7.32–7.23 (m, 1H, Ar-H), 7.19 (s, 1H, Ar-H), 7.03–6.83 (m, 4H, -CH = CH-), 6.01 (s, 2H, -CH2), 5.90 (d, J = 15.1 Hz, 1H, Ar-H). 13C NMR (151 MHz, DMSO-d6) δ = 168.02, 148.52, 148.40, 144.97, 140.15, 130.96, 125.27, 123.42, 121.58, 108.91, 106.20, 101.77. HRMS (ESI) calcd for C12H11O4 [M + H]+: 219.06519, found 219.06514.
2.1.3 General procedure for preparing compound C
A mixture of intermediate A (10.0 mmol, 2.18 g), glycine methyl ester hydrochloride (9.0 mmol, 1.13 g), HOBt (10.0 mmol, 1.35 g), DIEPA (10.0 mmol, 1.29 g), and 20 ml of DCM was stirred at 0°C for 15 min. Then, EDCI (10 mmol, 1.92 g) was added. The reaction was stirred at 25°C for 18 h. After the reaction was completed, the solvent was removed under reduced pressure, and the crude product was purified by column chromatography to obtain intermediate B. Then, a mixture of intermediate B (10.0 mmol, 2.88 g) and KOH (20.0 mmol, 1.12 g) was added in 30 ml of H2O/CH3OH/THF(v:v:v = 1:1:1) and stirred at room temperature for 12 h. Then, 10% of HCl solution was added until the solid no longer formed, and the crude product was purified by column chromatography to obtain intermediate C.
((2E,4E)-5-(Benzo[d][1,3]dioxol-5-yl)penta-2,4-dienoyl)glycine (C): brown solid, 2.56 g, yield 93%, mp: 214.5–216.7°C, 1H NMR (600 MHz, DMSO-d6) δ = 8.35 (s, 1H, -NH), 7.26–7.09 (m, 2H, Ar-H), 6.96 (d, J = 7.8 Hz, 1H, Ar-H), 6.92–6.65 (m, 3H, -CH = CH-), 6.13 (d, J = 15.0 Hz, 1H, -CH = CH-), 5.99 (s, 2H, -CH2), 3.82 (d, J = 5.5 Hz, 2H, -CH2). 13C NMR (151 MHz, DMSO-d6) δ = 171.72, 166.21, 148.33, 148.19, 140.49, 138.70, 131.18, 125.53, 124.22, 123.06, 108.88, 106.13, 101.67, 41.21. HRMS (ESI) calcd for C14H14O5N [M + H]+: 276.08665, found 276.08572.
2.1.4 Preparation of target compounds D1–D28
A mixture of intermediate C (1.0 mmol, 275 mg), amine (1.2 mmol), HOBt (1.2 mol, 162.14 mg), DIEPA (1.2 mol, 155.1 mg), and 10 ml of DCM was stirred at 0°C for 15 min. Then, EDCI (1.2 mol, 230.0 mg) was added, and the reaction mixture was stirred at 25°C for 18 h. After the reaction was completed, the solvent was removed under reduced pressure to give the crude product, and the target compounds were obtained via recrystallization using ethyl acetate and petroleum ether as the solvent.
(2E,4E)-5-(Benzo[d][1,3]dioxol-5-yl)-N-(2-oxo-2-(Phenylamino)ethyl)penta-2,4-dienamide (D1): brown solid, 81.2 mg, yield 23.2%, mp: >250.0°C, 1H NMR (600 MHz, DMSO-d6) δ = 9.99 (s, 1H, NH), 8.39 (t, J = 5.8 Hz, 1H, -NH), 7.57 (d, J = 7.8 Hz, 2H, Ar-H), 7.28 (t, J = 7.9 Hz, 2H, Ar-H), 7.24 (d, J = 1.1 Hz, 1H, Ar-H), 7.19–7.15 (m, 1H, Ar-H), 7.02 (t, J = 7.4 Hz, 1H, Ar-H), 6.98–6.83 (m, 4H, Ar-H and -CH = CH-), 6.19 (d, J = 15.0 Hz, 1H, -CH = CH-), 6.02 (s, 2H, -CH2), 3.96 (d, J = 5.9 Hz, 2H, -CH2). 13C NMR (151 MHz, DMSO-d6) δ = 168.24, 166.12, 148.37, 148.19, 140.24, 139.33, 138.57, 131.28, 129.14, 125.66, 124.56, 123.66, 123.06, 119.61, 108.86, 106.15, 101.68, 43.33. HRMS (ESI) calcd for C20H19O4N2 [M + H]+: 351.13393, found 351.13257.
(2E,4E)-5-(Benzo[d][1,3]dioxol-5-yl)-N-(2-oxo-2-(o-tolylamino)ethyl)penta-2,4-dienamide (D2): red solid, 88.0 mg, yield 24.1%, mp: >250.0°C, 1H NMR (600 MHz, DMSO-d6) δ = 9.32 (s, 1H, -NH), 8.43 (s, 1H, -NH), 7.40 (d, J = 7.4 Hz, 1H, Ar-H), 7.25–7.11 (m, 4H, Ar-H), 7.05 (d, J = 6.9 Hz, 1H, Ar-H), 6.98–6.84 (m, 4H, Ar-H and -CH = CH-), 6.19 (d, J = 15.0 Hz, 1H, -CH = CH-), 6.01 (s, 2H, -CH2), 4.00 (d, J = 4.5 Hz, 2H, -CH2), 2.17 (s, 3H, -CH3). 13C NMR (151 MHz, DMSO-d6) δ = 168.32, 166.25, 148.37, 148.20, 140.32, 138.63, 136.51, 131.83, 131.26, 130.71, 126.39, 125.64, 125.53, 125.00, 124.50, 123.07, 108.86, 106.15, 101.68, 43.24, 18.13. HRMS (ESI) calcd for C21H21O4N2 [M + H]+: 365.14958, found 365.14801.
(2E,4E)-5-(Benzo[d][1,3]dioxol-5-yl)-N-(2-oxo-2-(m-tolylamino)ethyl)penta-2,4-dienamide (D3): red solid, 148.1 mg, yield 40.6%, mp: 225.6–226.1°C, 1H NMR (600 MHz, DMSO-d6) δ = 9.90 (s, 1H, -NH), 8.37 (s, 1H, -NH), 7.42–7.32 (m, 2H, Ar-H), 7.24 (s, 1H, Ar-H), 7.18–7.14 (m, 2H, Ar-H), 6.97 (d, J = 7.9 Hz, 1H, Ar-H), 6.95–6.82 (m, 4H, Ar-H and -CH = CH-), 6.18 (d, J = 15.0 Hz, 1H, -CH = CH-), 6.01 (s, 2H, -CH2), 3.94 (d, J = 5.1 Hz, 2H, -CH2), 2.24 (s, 3H, -CH3). 13C NMR (151 MHz, DMSO-d6) δ = 168.17, 166.13, 148.36, 148.19, 140.26, 139.23, 138.60, 138.32, 131.26, 128.99, 125.64, 124.53, 124.38, 123.09, 120.13, 116.80, 108.87, 106.12, 101.68, 43.32, 21.60. HRMS (ESI) calcd for C21H21O4N2 [M + H]+: 365.14958, found 365.14841.
(2E,4E)-5-(Benzo[d][1,3]dioxol-5-yl)-N-(2-oxo-2-(p-tolylamino)ethyl)penta-2,4-dienamide (D4): red solid, 116 mg, yield 31.5%, mp: 178.8–180.7°C, 1H NMR (600 MHz, DMSO-d6) δ = 9.90 (s, 1H, -NH), 8.37 (t, J = 5.5 Hz, 1H, -NH), 7.44 (d, J = 8.2 Hz, 2H, Ar-H), 7.24 (s, 1H, Ar-H), 7.16 (m, 1H, Ar-H), 7.08 (d, J = 8.1 Hz, 2H, Ar-H), 6.99–6.81 (m, 4H, Ar-H and -CH = CH-), 6.18 (d, J = 15.0 Hz, 1H, -CH = CH-), 6.02 (s, 2H, -CH2), 3.94 (d, J = 5.7 Hz, 2H, -CH2), 2.22 (s, 3H, -CH3). 13C NMR (151 MHz, DMSO-d6) δ = 167.98, 166.09, 148.37, 148.19, 140.21, 138.55, 136.81, 132.58, 131.28, 129.51, 125.66, 124.59, 123.06, 119.64, 108.86, 106.15, 101.68, 43.28, 20.84. HRMS (ESI) calcd for C21H21O4N2 [M + H]+: 365.14958, found 365.14871.
(2E,4E)-5-(Benzo[d][1,3]dioxol-5-yl)-N-(2-((4-(tert-butyl)phenyl)amino)-2-oxoethyl)penta-2,4-dienamide (D5): red solid, 198.3 mg, yield 48.7%, mp: 124.4–125.1°C, 1H NMR (600 MHz, DMSO-d6) δ = 9.92 (s, 1H, NH), 8.38 (t, J = 5.3 Hz, 1H, -NH), 7.47 (d, J = 8.3, 2H, Ar-H), 7.29 (d, J = 8.4 Hz, 2H, Ar-H), 7.23 (s, 1H, Ar-H), 7.17 (m, 1H, Ar-H), 7.00–6.84 (m, 4H, Ar-H and -CH = CH-), 6.19 (d, J = 15.0 Hz, 1H, -CH = CH-), 6.01 (s, 2H, -CH = CH-), 3.95 (d, J = 5.6 Hz, 2H, -CH2), 1.22 (s, 9H, -CH3). 13C NMR (151 MHz, DMSO-d6) δ = 168.01, 166.13, 148.36, 148.19, 146.06, 140.24, 138.57, 136.71, 131.27, 125.72, 124.57, 123.05, 119.45, 114.17, 108.86, 106.15, 101.67, 43.24, 34.41, 31.62. HRMS (ESI) calcd for C24H27O4N2 [M + H]+: 407.19653, found 407.19501.
(2E,4E)-5-(Benzo[d][1,3]dioxol-5-yl)-N-(2-((3,5-dimethylphenyl)amino)-2-oxoethyl)penta-2,4-dienamide (D6): yellow solid, 73.4 mg, yield 19.4%, mp: >250.0°C 1H NMR (600 MHz, DMSO-d6) δ = 9.81 (s, 1H, -NH), 8.36 (t, J = 5.5 Hz, 1H, -NH), 7.24 (s, 1H, Ar-H), 7.21–7.12 (m, 3H, Ar-H), 6.98–6.83 (m, 4H, Ar-H and -CH = CH-), 6.66 (s, 1H, Ar-H), 6.18 (d, J = 15.0 Hz, 1H, -CH = CH-), 6.01 (s, 2H, -CH2), 3.92 (d, J = 5.7 Hz, 2H, -CH2), 2.20 (s, 6H, -CH3).13C NMR (151 MHz, DMSO-d6) δ = 168.08, 166.13, 148.37, 148.19, 140.22, 139.15, 138.57, 138.09, 131.27, 125.65, 125.22, 124.56, 123.06, 117.43, 108.86, 106.14, 101.68, 43.37, 21.51. HRMS (ESI) calcd for C22H23O4N2 [M + H]+: 379.16523, found 379.16409.
(2E,4E)-5-(Benzo[d][1,3]dioxol-5-yl)-N-(2-((3-ethynylphenyl)amino)-2-oxoethyl)penta-2,4-dienamide (D7): red solid, 59.8 mg, yield 15.9%, mp: 203.5–205.5°C, 1H NMR (600 MHz, DMSO-d6) δ = 10.10 (s, 1H, -NH), 8.40 (t, J = 5.5 Hz, 1H, -NH), 7.76 (s, 1H, Ar-H), 7.55 (d, J = 8.2 Hz, 1H, Ar-H), 7.30 (t, J = 7.9 Hz, 1H, Ar-H), 7.24 (s, 1H, Ar-H), 7.19–7.12 (m, 2H, Ar-H), 6.99–6.96 (m, 1H, Ar-H), 6.94–6.90 (m, 1H, -CH = CH-), 6.89–6.86 (m, 2H, -CH = CH-), 6.18 (d, J = 15.0 Hz, 1H, -CH = CH-), 6.02 (s, 2H, -CH2), 4.13 (s, 1H, CH≡), 3.96 (d, J = 5.8 Hz, 2H, -CH2). 13C NMR (151 MHz, DMSO-d6) δ = 168.58, 166.15, 148.37, 148.20, 140.28, 139.54, 138.61, 131.28, 129.63, 126.92, 125.65, 124.50, 123.08, 122.47, 122.44, 120.19, 108.86, 106.15, 101.68, 83.79, 80.90, 43.37. HRMS (ESI) calcd for C22H19O4N2 [M + H]+: 375.13393, found 375.13269.
(2E,4E)-5-(Benzo[d][1,3]dioxol-5-yl)-N-(2-oxo-2-((3,4,5-trimethoxyphenyl)amino)ethyl) penta-2,4-dienamide (D8): red solid, 183.3 mg, yield 41.6%, mp: 189.3–192.9°C, 1H NMR (600 MHz, DMSO-d6) δ = 9.92 (s, 1H, -NH), 8.36 (t, J = 5.7 Hz, 1H, -NH), 7.24 (s, 1H, Ar-H), 7.18–7.14 (m, 1H, Ar-H), 6.99–6.94 (m, 3H, Ar-H), 6.93–6.84 (m, 3H, -CH = CH-), 6.18 (d, J = 15.0 Hz, 1H, -CH = CH-), 6.01 (s, 2H, -CH2), 3.92 (d, J = 5.8 Hz, 2H, -CH2), 3.70 (s, 6H, -CH3), 3.59 (s, 3H, -CH3). 13C NMR (151 MHz, DMSO-d6) δ = 168.08, 166.14, 153.15, 148.36, 148.19, 140.28, 138.61, 135.43, 131.25, 125.62, 124.50, 123.09, 108.87, 106.13, 101.68, 97.52, 60.54, 56.17, 43.32. HRMS (ESI) calcd for C23H25O7N2 [M + H]+: 441.16563, found 441.16418.
(2E,4E)-5-(Benzo[d][1,3]dioxol-5-yl)-N-(2-((2-fluorophenyl)amino)-2-oxoethyl)penta-2,4-dien-amide (D9): white solid, 122.4 mg, yield 33%, mp: 185.5–188.9°C, 1H NMR (600 MHz, DMSO-d6) δ = 9.77 (s, 1H, -NH), 8.41 (t, J = 5.7 Hz, 1H, -NH), 7.85 (d, J = 6.6 Hz, 1H, Ar-H), 7.32–7.06 (m, 5H, Ar-H), 6.99–6.82 (m, 4H, Ar-H and -CH = CH-), 6.18 (d, J = 15.0 Hz, 1H, -CH = CH-), 6.02 (s, 2H, -CH2), 4.03 (d, J = 5.8 Hz, 2H, -CH2). 13C NMR (151 MHz, DMSO-d6) δ = 168.74, 166.19, 154.01 (d, 1JC-F = 246.1 Hz), 148.37, 148.21, 140.36, 138.64, 131.27, 126.36 (d, 2JC-F = 12.1 Hz), 125.64, 124.78, 124.76, 124.44, 123.08, 115.95, 115.82, 108.86, 106.15, 101.68, 43.16.19F NMR (564 MHz, DMSO) δ = -124.94. HRMS (ESI) calcd for C20H18O4N2F [M + H]+: 369.12451, found 369.12305.
(2E,4E)-5-(Benzo[d][1,3]dioxol-5-yl)-N-(2-((3-fluorophenyl)amino)-2-oxoethyl)penta-2,4-dien-amide (D10): red solid, 67.1 mg, yield 18.2%, mp: 226.0–227.5°C, 1H NMR (600 MHz, DMSO-d6) δ = 10.22 (s, 1H, -NH), 8.41 (s, 1H, -NH), 7.56 (d, J = 11.6 Hz, 1H, Ar-H), 7.30 (dt, J = 17.2, 7.9 Hz, 2H, Ar-H), 7.24 (s, 1H, Ar-H), 7.18–7.14 (m, 1H, Ar-H), 6.97–6.83 (m, 5H, Ar-H and -CH = CH-), 6.18 (d, J = 15.0 Hz, 1H, -CH = CH-), 6.01 (s, 2H, -CH2), 3.96 (d, J = 5.6 Hz, 2H, -CH2). 13C NMR (151 MHz, DMSO-d6) δ = 168.66, 166.19, 162.57 (d, 1JC-F = 241.6 Hz), 148.37, 148.20, 141.04 (d, 2JC-F = 12.1 Hz), 140.34, 138.64, 131.26, 130.8 (d, 3JC-F = 9.1 Hz), 125.63, 124.43, 123.08, 115.31, 110.12 (d, 2JC-F = 21.2 Hz), 108.87, 106.37 (d, 2JC-F = 27.2 Hz), 106.15, 101.68, 43.36.19F NMR (564 MHz, DMSO) δ = -112.05. HRMS (ESI) calcd for C20H18O4N2F [M + H]+: 369.12451, found 369.12344.
(2E,4E)-5-(Benzo[d][1,3]dioxol-5-yl)-N-(2-((4-fluorophenyl)amino)-2-oxoethyl)penta-2,4-dien- amide (D11): white solid, 120.0 mg, yield 32.6%. mp: 251.8–253.6°C 1H NMR (600 MHz, DMSO-d6) δ = 10.05 (s, 1H, -NH), 8.40 (t, J = 5.8 Hz, 1H, -NH), 7.59–7.57 (m, 2H, Ar-H), 7.24 (d, J = 1.2 Hz, 1H, Ar-H), 7.14 (dt, J = 17.7, 9.8 Hz, 3H, Ar-H), 6.98–6.84 (m, 4H, Ar-H and -CH = CH-), 6.18 (d, J = 15.0 Hz, 1H, -CH = CH-), 6.02 (s, 2H, -CH2), 3.94 (d, J = 5.8 Hz, 2H, -CH2). 13C NMR (151 MHz, DMSO-d6) δ = 168.19, 166.11, 159.43 (d, 1JC-F = 240.1 Hz), 148.28 (d, 2JC-F = 27.3 Hz), 140.25, 138.59, 135.71, 131.27, 125.65, 124.53, 123.07, 121.38, 115.70 (d, 2JC-F = 22.7 Hz), 109.99, 108.86, 106.14, 101.68, 43.25.19F NMR (564 MHz, DMSO) δ = -119.48. HRMS (ESI) calcd for C20H18O4N2F [M + H]+: 369.12451, found 369.12338.
(2E,4E)-5-(Benzo[d][1,3]dioxol-5-yl)-N-(2-((3-chlorophenyl)amino)-2-oxoethyl)penta-2,4-dien-amide (D12): yellow solid, 82.5 mg, yield 21.5% mp: 223.8–225.7°C, 1H NMR (600 MHz, DMSO-d6) δ = 10.19 (s, 1H, -NH), 8.41 (t, J = 5.7 Hz, 1H, -NH), 7.77 (s, 1H, Ar-H), 7.43 (d, J = 8.2 Hz, 1H, Ar-H), 7.31 (t, J = 8.1 Hz, 1H, Ar-H), 7.24 (s, 1H, Ar-H), 7.18–7.14 (m, 1H, Ar-H), 7.08 (d, J = 8.0 Hz, 1H, Ar-H), 6.98–6.83 (m, 4H, Ar-H and -CH = CH-), 6.18 (d, J = 15.1 Hz, 1H, -CH = CH-), 6.02 (s, 2H, -CH2), 3.95 (d, J = 5.8 Hz, 2H, -CH2). 13C NMR (151 MHz, CDCl3) δ = 173.45, 170.92, 153.12, 152.95, 145.52, 145.07, 143.39, 138.25, 136.02, 135.62, 130.39, 129.19, 128.14, 127.84, 123.80, 122.72, 113.62, 110.90, 106.43, 48.12. HRMS (ESI) calcd for C20H18O4N2Cl [M + H]+: 385.09496, found 385.09415.
(2E,4E)-N-(2-((3-Acetylphenyl)amino)-2-oxoethyl)-5-(benzo[d][1,3]dioxol-5-yl)penta-2,4-dien-amide (D13): brown solid, 137 mg, yield 35.0%, mp: >250.0°C, 1H NMR (600 MHz, DMSO-d6) δ = 10.20 (s, 1H, -NH), 8.40 (s, 1H, -NH), 8.15 (s, 1H, Ar-H), 7.83 (d, J = 7.6 Hz, 1H, Ar-H), 7.64 (d, J = 7.4 Hz, 1H, Ar-H), 7.45 (t, J = 7.8 Hz, 1H, Ar-H), 7.24 (s, 1H, Ar-H), 6.97 (d, J = 7.4 Hz, 2H, Ar-H), 6.90–6.83 (m, 3H, Ar-H and -CH = CH-), 6.19 (d, J = 15.0 Hz, 1H, -CH = CH-), 6.02 (s, 2H, -CH2), 3.98 (d, J = 5.3 Hz, 2H, -CH2), 2.53 (s, 3H, -CH3).13C NMR (151 MHz, DMSO-d6) δ = 198.01, 168.62, 166.16, 148.37, 148.20, 140.29, 139.70, 138.62, 137.82, 131.27, 129.60, 125.64, 124.50, 124.09, 123.72, 123.08, 118.84, 108.86, 106.15, 101.68, 43.37, 27.11. HRMS (ESI) calcd for C22H21O5N2 [M + H]+: 393.14450, found 393.14301.
(2E,4E)-5-(Benzo[d][1,3]dioxol-5-yl)-N-(2-((4-(methylthio)phenyl)amino)-2-oxoethyl)penta-2,4-dienamide (D14): brown solid, 54.5 mg, yield 13.7%, mp: 208.9–211.3°C, 1H NMR (600 MHz, DMSO-d6) δ = 10.01 (s, 1H, -NH), 8.39 (t, J = 5.7 Hz, 1H, -NH), 7.53 (d, J = 8.6 Hz, 2H, Ar-H), 7.23 (s, 1H, Ar-H), 7.22–7.18 (m, 2H, Ar-H), 7.17–7.10 (m, 1H, Ar-H), 6.99–6.95 (m, 1H, Ar-H), 6.89 (m, 3H, -CH = CH-), 6.18 (d, J = 15.0 Hz, 1H, -CH = CH-), 6.01 (s, 2H, -CH2), 3.95 (d, J = 5.8 Hz, 2H, -CH2), 2.41 (s, 3H, -CH3). 13C NMR (151 MHz, DMSO-d6) δ = 168.16, 166.14, 148.37, 148.19, 140.26, 138.58, 136.87, 132.24, 131.27, 127.67, 125.64, 124.54, 123.05, 120.33, 108.86, 106.15, 101.68, 43.31, 16.05. HRMS (ESI) calcd for C21H21O4N2S [M + H]+: 397.12165, found 397.12054.
(2E,4E)-5-(benzo[d][1,3]dioxol-5-yl)-N-(2-((3-(benzyloxy)phenyl)amino)-2-oxoethyl)penta-2,4-dienamide (D15): red solid, 99.2 mg, yield 21.7%, mp: 191.0–192.0°C, 1H NMR (600 MHz, DMSO) δ = 9.97 (s, 1H, -NH), 8.37 (t, J = 5.5 Hz, 1H, -NH), 7.41 (d, J = 7.6 Hz, 2H, Ar-H), 7.36 (t, J = 6.8 Hz, 3H, Ar-H), 7.30 (t, J = 6.8 Hz, 1H, Ar-H), 7.24 (s, 1H, Ar-H), 7.17 (dt, J = 15.2, 9.2 Hz, 2H, Ar-H), 7.10 (d, J = 7.9 Hz, 1H, Ar-H), 6.97 (d, J = 8.1 Hz, 1H, Ar-H), 6.95–6.78 (m, 4H, Ar-H and -CH = CH-), 6.69 (d, J = 8.0 Hz, 1H, -CH = CH-), 6.18 (d, J = 15.0 Hz, 1H, Ar-H), 6.02 (s, 2H, -CH2), 5.04 (s, 2H, -CH2), 3.95 (d, J = 5.8 Hz, 2H, -CH2). 13C NMR (151 MHz, DMSO-d6) δ = 168.31, 166.10, 159.06, 148.37, 148.19, 140.53, 140.25, 138.59, 137.49, 131.28, 129.97, 128.84, 128.21, 128.01, 125.65, 124.53, 123.09, 112.15, 109.97, 108.87, 106.38, 106.13, 101.68, 69.60, 43.34. HRMS (ESI) calcd for C27H25O5N2 [M + H]+: 457.17580, found 457.17459.
(2E,4E)-5-(Benzo[d][1,3]dioxol-5-yl)-N-(2-((4-(benzyloxy)phenyl)amino)-2-oxoethyl)penta-2,4-dienamide (D16): yellow solid, 97.6 mg, yield 21.3%, mp: 231.4–233.5°C, 1H NMR (600 MHz, DMSO) δ = 9.86 (s, 1H, -NH), 8.37 (t, J = 5.6 Hz, 1H, -NH), 7.47 (d, J = 8.9 Hz, 2H, Ar-H), 7.41 (d, J = 7.4 Hz, 2H, Ar-H), 7.35 (dt, J = 7.1, 5.0 Hz, 3H, Ar-H), 7.29 (t, J = 7.1 Hz, 1H, Ar-H), 7.24 (s, 1H, Ar-H), 6.97 (d, J = 7.7 Hz, 1H, Ar-H), 6.95–6.85 (m, 5H, Ar-H and -CH = CH-), 6.18 (d, J = 15.0 Hz, 1H, -CH = CH-), 6.01 (s, 2H, -CH = CH-), 5.03 (s, 2H, -CH2), 3.92 (d, J = 5.8 Hz, 2H, -CH2). 13C NMR (151 MHz, DMSO-d6) δ = 167.76, 166.11, 154.75, 148.36, 148.19, 140.21, 138.55, 137.63,132.69, 131.27, 128.81, 128.06, 125.65, 124.60, 123.05, 121.16, 116.24, 115.35, 108.87, 106.15, 101.68, 69.89, 43.21. HRMS (ESI) calcd for C27H25O5N2 [M + H]+: 457.17580, found 457.17438.
(2E,4E)-5-(Benzo[d][1,3]dioxol-5-yl)-N-(2-((4-((2,6-difluorobenzyl)oxy)phenyl)amino)-2-oxoethyl)penta-2,4-dienamide (D17): yellow solid, 89.7 mg, yield 18.2%, mp: 203.9–206.5°C, 1H NMR (600 MHz, DMSO-d6) δ = 9.89 (s, 1H, -NH), 8.38 (s, 1H, -NH), 7.49 (d, J = 8.9 Hz, 3H, Ar-H), 7.24 (s, 1H, Ar-H), 7.17–7.13 (m, 3H, Ar-H), 6.97 (t, J = 7.9 Hz, 3H, Ar-H), 6.92–6.84 (m, 3H, -CH = CH-), 6.19 (d, J = 15.0 Hz, 1H, -CH = CH-), 6.02 (s, 2H, -CH2), 5.04 (s, 2H, -CH2), 3.94 (d, J = 5.7 Hz, 2H, -CH2). 13C NMR (151 MHz, DMSO-d6) δ = 167.83, 166.08, 162.49, 160.84, 154.46, 148.28 (d, 2JC-F = 28.3 Hz), 140.21, 138.56, 133.17, 132.05, 131.28, 125.66, 124.59, 123.09, 121.10, 115.92 (d, 1JC-F = 199.3 Hz), 115.42, 112.18 (dd, J = 21.2, 4.5 Hz), 108.86, 106.13, 101.68, 58.38, 43.21.19F NMR (564 MHz, DMSO) δ = -115.17. HRMS (ESI) calcd for C27H23O5N2F2 [M + H]+: 493.15695, found 493.15533.
(2E,4E)-5-(Benzo[d][1,3]dioxol-5-yl)-N-(2-oxo-2-((4-((4-(trifluoromethyl)benzyl)oxy)phenyl)-amino) ethyl) penta-2,4-dienamide (D18): brown solid, 118.5 mg, yield 22.5%, mp: 200.6–202.7°C, 1H NMR (600 MHz, DMSO-d6) δ = 9.90 (s, 1H, -NH), 8.39 (s, 1H, -NH), 7.72 (d, J = 7.4 Hz, 2H, Ar-H), 7.63 (d, J = 7.5 Hz, 2H, Ar-H), 7.50–7.46 (m, 2H, Ar-H), 7.24 (s, 1H, Ar-H), 7.19–7.13 (m, 1H, Ar-H), 6.96 (t, J = 8.7 Hz, 3H, Ar-H), 6.91–6.83 (m, 3H, -CH = CH-), 6.19 (d, J = 15.0 Hz, 1H, -CH = CH-), 6.02 (s, 2H, -CH2), 5.16 (s, 2H, -CH2), 3.94 (d, J = 5.6 Hz, 2H, -CH2). 13C NMR (151 MHz, DMSO-d6) δ = 167.81, 166.09, 154.39, 148.37, 148.19, 142.54, 140.21, 138.56, 132.96, 131.27, 128.38, 125.68 (t, 4JC-F = 4.53 Hz), 124.59, 123.08, 121.15, 115.37, 108.85, 106.12, 101.68, 68.96, 43.21.19F NMR (564 MHz, DMSO) δ = -60.97. HRMS (ESI) calcd for C28H24O5N2F3 [M + H]+: 525.16318, found 525.16119.
(2E,4E)-5-(Benzo[d][1,3]dioxol-5-yl)-N-(2-oxo-2-((4-(pyridin-2-ylmethoxy)phenyl)amino)ethyl)-penta-2,4-dienamide (D19): brown solid, 95.2 mg, yield 20.8%, mp: 211.2–214.5°C 1H NMR (600 MHz, DMSO-d6) δ = 9.86 (s, 1H, -NH), 8.54 (d, J = 4.1 Hz, 1H, -NH), 8.36 (t, J = 5.5 Hz, 1H, Ar-H), 7.80 (td, J = 7.7, 1.7 Hz, 1H, Ar-H), 7.48 (d, J = 8.9 Hz, 3H, Ar-H), 7.33–7.29 (m, 1H, Ar-H), 7.24 (d, J = 1.2 Hz, 1H, Ar-H), 7.18–7.14 (m, 1H, Ar-H), 7.00–6.79 (m, 7H, Ar-H and -CH = CH-), 6.18 (d, J = 15.0 Hz, 1H, -CH = CH-), 6.02 (s, 2H, -CH2), 5.11 (s, 2H, -CH2), 3.93 (d, J = 5.8 Hz, 2H, -CH2). 13C NMR (151 MHz, DMSO-d6) δ = 167.79, 166.07, 157.25, 154.51, 149.49, 148.37, 148.18, 140.20, 138.56, 137.34, 132.88, 131.28, 125.66, 124.60, 123.32, 123.08, 122.05, 121.17, 115.30, 108.86, 106.13, 101.68, 70.95, 43.20. HRMS (ESI) calcd for C26H24O5N3 [M + H]+: 458.17105, found 458.16992.
(2E,4E)-5-(Benzo[d][1,3]dioxol-5-yl)-N-(2-((3-(benzyloxy)phenyl)amino)-2-oxoethyl)penta-2,4-dienamide (D20): brown solid, 110.4 mg, yield 24.8%, mp: 182.9–185.0°C 1H NMR (600 MHz, DMSO-d6) δ = 10.02 (s, 1H, -NH), 8.39 (t, J = 5.6 Hz, 1H, -NH), 7.58 (d, J = 8.8 Hz, 2H, Ar-H), 7.33 (t, J = 7.9 Hz, 2H, Ar-H), 7.24 (s, 1H, Ar-H), 7.18–7.14 (m, 1H, Ar-H), 7.07 (t, J = 7.3 Hz, 1H, Ar-H), 7.02–6.82 (m, 8H, Ar-H and -CH = CH-), 6.19 (d, J = 15.0 Hz, 1H, -CH = CH-), 6.02 (s, 2H, -CH2), 3.95 (d, J = 5.7 Hz, 2H, -CH2). 13C NMR (151 MHz, DMSO-d6) δ = 168.08, 166.12, 157.78, 152.22, 148.37, 148.19, 140.24, 138.58, 135.24, 131.28, 130.36, 125.66, 124.56, 123.40, 123.06, 121.31, 119.85, 118.32, 108.86, 106.15, 101.68, 43.28. HRMS (ESI) calcd for C26H23O5N2 [M + H]+: 443.16015, found 443.15891.
(2E,4E)-5-(Benzo[d][1,3]dioxol-5-yl)-N-(2-(benzylamino)-2-oxoethyl)penta-2,4-dienamide (D21): red solid, 62.2 mg, yield 17.1%, mp: 173.6–175.8°C, 1H NMR (600 MHz, DMSO-d6) δ = 8.38 (t, J = 5.3 Hz, 1H, -NH), 8.33 (t, J = 5.6 Hz, 1H, -NH), 7.29 (t, J = 7.4 Hz, 2H, Ar-H), 7.26–7.07 (m, 5H, Ar-H), 6.97 (d, J = 7.9 Hz, 1H, Ar-H), 6.94–6.82 (m, 3H, -CH = CH-), 6.15 (d, J = 15.0 Hz, 1H, -CH = CH-), 6.01 (s, 2H, -CH2), 4.27 (d, J = 5.8 Hz, 2H, -CH2), 3.81 (d, J = 5.7 Hz, 2H, -CH2). 13C NMR (151 MHz, DMSO-d6) δ = 169.42, 166.04, 148.37, 148.18, 140.10, 139.80, 138.50, 131.28, 128.64, 127.63, 127.15, 125.66, 124.73, 123.04, 108.86, 106.13, 101.68, 42.72, 42.52. HRMS (ESI) calcd for C21H21O4N2 [M + H]+: 365.14958, found 365.14828.
(2E,4E)-5-(Benzo[d][1,3]dioxol-5-yl)-N-(2-((2-chlorobenzyl)amino)-2-oxoethyl)penta-2,4-dienamide (D22): red solid, 182 mg, yield 45.6%, mp: 180.1–183.0°C, 1H NMR (600 MHz, DMSO-d6) δ = 8.40 (s, 1H, -NH), 8.36 (t, J = 5.4 Hz, 1H, -NH), 7.40 (d, J = 7.6 Hz, 1H, Ar-H), 7.35–7.21 (m, 4H, Ar-H), 7.18–7.14 (m, 1H, Ar-H), 6.97 (d, J = 8.0 Hz, 1H, Ar-H), 6.95–6.79 (m, 3H, -CH = CH-), 6.15 (d, J = 15.0 Hz, 1H, -CH = CH-), 6.01 (s, 2H, -CH2), 4.32 (d, J = 5.7 Hz, 2H, -CH2), 3.84 (d, J = 5.8 Hz, 2H, -CH2). 13C NMR (151 MHz, DMSO-d6) δ = 169.72, 166.10, 148.37, 148.18, 140.14, 138.54, 136.66, 132.36, 131.27, 129.46, 129.18, 128.98, 127.53, 125.65, 124.67, 123.08, 108.86, 106.12, 101.68, 42.72, 40.43. HRMS (ESI) calcd for C21H20O4N2Cl [M + H]+: 399.11061, found 399.10913.
(2E,4E)-5-(Benzo[d][1,3]dioxol-5-yl)-N-(2-oxo-2-(phenethylamino)ethyl)penta-2,4-dienamide (D23):white solid, 147.5 mg, yield 38.9%, mp: 166.5–168.4°C, 1H NMR (600 MHz, DMSO-d6) δ = 8.26 (t, J = 5.4 Hz, 1H, -NH), 7.93 (s, 1H, -NH), 7.27–7.23 (m, 3H, Ar-H), 7.21–7.09 (m, 4H, Ar-H), 7.00–6.82 (m, 4H, Ar-H and -CH = CH-), 6.15 (d, J = 15.0 Hz, 1H, -CH = CH-), 6.01 (s, 2H, -CH2), 3.73 (d, J = 5.8 Hz, 2H, -CH2), 3.29–3.25 (m, 2H, -CH2), 2.69 (t, J = 7.3 Hz, 2H, -CH2). 13C NMR (151 MHz, DMSO-d6) δ = 169.23, 165.96, 148.37, 148.18, 140.11, 139.83, 138.51, 131.28, 129.03, 128.75, 126.50, 125.65, 124.69, 123.07, 108.86, 106.12, 101.68, 42.69, 40.66, 35.62. HRMS (ESI) calcd for C22H23O4N2 [M + H]+: 379.16523, found 379.16385.
(2E,4E)-5-(Benzo[d][1,3]dioxol-5-yl)-N-(2-oxo-2-((3-phenylpropyl)amino)ethyl)penta-2,4-dienamide (D24): white solid, 161.9 mg, yield 44.6%, mp: 166.5–168.4°C, 1H NMR (600 MHz, DMSO-d6) δ = 8.29 (t, J = 5.5 Hz, 1H, -NH), 7.90 (d, J = 4.9 Hz, 1H, -NH), 7.30–7.22 (m, 3H, Ar-H), 7.20–7.09 (m, 4H, Ar-H), 6.98–6.94 (m, 1H, Ar-H), 6.94–6.77 (m, 3H, -CH = CH-), 6.16 (d, J = 15.0 Hz, 1H, -CH = CH-), 6.01 (s, 2H, -CH2), 3.74 (d, J = 5.8 Hz, 2H, -CH2), 3.08–3.04 (m, 2H, -CH2), 2.57–2.51 (m, 2H, -CH2), 1.73–1.63 (m, 2H, -CH2). 13C NMR (151 MHz, DMSO-d6) δ = 169.20, 165.95, 148.36, 148.17, 142.15, 140.05, 138.47, 131.28, 128.70, 128.68, 126.12, 125.66, 124.75, 123.05, 108.86, 106.12, 101.68, 42.71, 38.60, 32.89, 31.30. HRMS (ESI) calcd for C23H25O4N2 [M + H]+: 393.18088, found 393.17938.
(2E,4E)-5-(Benzo[d][1,3]dioxol-5-yl)-N-(2-(naphthalen-2-ylamino)-2-oxoethyl)penta-2,4-dienamide (D25): brown solid, 93.3 mg, yield 23.31%, mp: 141.9–144.2°C 1H NMR (600 MHz, DMSO-d6) δ = 10.01 (s, 1H, -NH), 8.50 (s, 1H, -NH), 8.07 (d, J = 6.8 Hz, 1H, Ar-H), 7.91 (d, J = 6.7 Hz, 1H, Ar-H), 7.75 (d, J = 7.5 Hz, 1H, Ar-H), 7.58–7.46 (m, 3H, Ar-H), 7.24 (s, 1H, Ar-H), 6.92 (dt, J = 37.5, 12.0 Hz, 6H, Ar-H and -CH = CH-), 6.23 (d, J = 14.8 Hz, 1H, -CH = CH-), 6.01 (s, 2H, -CH2), 4.15 (s, 2H, -CH2). 13C NMR (151 MHz, DMSO-d6) δ = 169.14, 166.35, 148.37, 148.20, 140.32, 138.62, 134.15, 133.80, 131.27, 128.52, 128.21, 126.46, 125.98, 125.66, 124.59, 124.05, 123.17, 123.07, 115.91, 108.87, 107.96, 106.15, 101.69, 43.37. HRMS (ESI) calcd for C24H21O4N2 [M + H]+: 401.14958, found 401.14816.
(2E,4E)-5-(Benzo[d][1,3]dioxol-5-yl)-N-(2-(benzo[d][1,3]dioxol-5-ylamino)-2-oxoethyl)penta-2,4-dienamide (D26): black solid, 110.4 mg, yield 27.9%, mp: 198.5–201.9°C 1H NMR (600 MHz, DMSO-d6) δ = 9.91 (s, 1H, -NH), 8.37 (s, 1H, -NH), 7.28–7.21 (m, 2H, Ar-H), 7.18–7.14 (m, 1H, Ar-H), 6.99–6.91 (m, 3H, Ar-H), 6.90–6.86 (m, 2H, -CH = CH-), 6.83 (t, J = 5.8 Hz, 1H, -CH = CH-), 6.18 (d, J = 15.1 Hz, 1H, -CH = CH-), 6.02 (s, 2H, -CH2), 5.95 (s, 2H, -CH2), 3.92 (d, J = 5.7 Hz, 2H, -CH2). 13C NMR (151 MHz, DMSO-d6) δ = 167.88, 166.08, 148.37, 148.19, 147.47, 143.33, 140.22, 138.56, 133.74, 131.28, 125.65, 124.57, 123.06, 112.44, 108.86, 108.44, 106.14, 101.82, 101.68, 101.37, 43.24. HRMS (ESI) calcd for C21H19O6N2 [M + H]+: 395.12376, found 395.12210.
(2E,4E)-5-(Benzo[d][1,3]dioxol-5-yl)-N-(2-((1-methyl-1H-indol-5-yl)amino)-2-oxoethyl)penta-2,4-dienamide (D27): yellow solid, 142.8 mg, yield 35.4%, mp: 218.4–221.1°C, 1H NMR (600 MHz, DMSO-d6) δ = 9.81 (s, 1H, -NH), 8.38 (t, J = 5.6 Hz, 1H, -NH), 7.84 (s, 1H, Ar-H), 7.33 (d, J = 8.8 Hz, 1H, Ar-H), 7.28–7.22 (m, 2H, Ar-H), 7.19–7.15 (m, 1H, Ar-H), 7.00–6.82 (m, 4H, Ar-H and -CH = CH-), 6.34 (d, J = 2.8 Hz, 1H, -CH = CH-), 6.20 (d, J = 15.0 Hz, 1H, -CH = CH-), 6.02 (s, 2H, -CH2), 3.97 (d, J = 5.8 Hz, 2H, -CH2), 3.73 (s, 3H, -CH3).13C NMR (151 MHz, DMSO-d6) δ = 167.62, 166.07, 148.37, 148.18, 140.15, 138.52, 133.78, 131.47, 131.30, 130.56, 128.26, 125.69, 124.70, 123.06, 115.24, 111.42, 109.87, 108.86, 106.14, 101.68, 100.65, 43.33, 32.92. HRMS (ESI) calcd for C23H22O4N3 [M + H]+: 404.16048, found 404.15900.
(2E,4E)-5-(Benzo[d][1,3]dioxol-5-yl)-N-(2-oxo-2-((2,2,2-trifluoroethyl)amino)ethyl)penta-2,4-dienamide (D28): yellow solid, 70.5 mg, yield 19.7%, mp: 154.2–156.1°C 1H NMR (600 MHz, DMSO-d6) δ = 8.52 (t, J = 5.9 Hz, 1H, -NH), 8.32 (t, J = 5.6 Hz, 1H, -NH), 7.23 (s, 1H, Ar-H), 7.17–7.13 (m, 1H, Ar-H), 6.97 (d, J = 7.9 Hz, 1H, Ar-H), 6.91–6.87 (m, 3H, -CH = CH-), 6.14 (d, J = 15.0 Hz, 1H, -CH = CH-), 6.01 (s, 2H, -CH2), 3.90–3.86 (m, 2H, -CH2), 3.83 (d, J = 5.8 Hz, 2H, -CH2). 13C NMR (151 MHz, DMSO-d6) δ = 170.38, 166.05, 148.37, 148.19, 140.25, 138.59, 131.26, 125.62, 124.52, 123.07, 108.86, 106.13, 101.68, 42.33.19F NMR (564 MHz, DMSO) δ = -70.71. HRMS (ESI) calcd for C16H16O4N2F3 [M + H]+: 357.10567, found 357.10431.
2.1.5 Preparation of intermediates F1 and F2
A mixture of intermediate E (10.0 mmol), glycine methyl ester hydrochloride (9.0 mmol, 1.13 g), HOBt (10.0 mmol, 1.35 g), DIEPA (10.0 mmol, 1.29 g), and 20 ml of DCM was stirred at 0°C for 15 min. Then, EDCI (10 mol, 1.92 g) was added, and the mixture was stirred at 25°C for 18 h. After the reaction was completed, the solvent was removed under reduced pressure. Then, a mixture of KOH (20.0 mmol, 1.12 g) and 30 ml of H2O/CH3OH/THF (v:v:v = 1:1:1) was added to the crude products, and the reaction mixture was stirred at room temperature for 12 h. Then, 10% of HCl solution was added until a solid precipitate formed. The solvent was removed under reduced pressure, and the crude product was purified by column chromatography to obtain intermediates F1 and F2.
Cinnamoylglycine (F1): white solid, 1.75 g, yield 85%, mp: 198.4–201.5°C, 1H NMR (600 MHz, DMSO-d6) δ = 8.42 (s, 1H, -NH), 7.55 (d, J = 7.4 Hz, 2H, Ar-H), 7.47–7.28 (m, 4H, Ar-H and -CH = CH-), 6.71 (d, J = 15.8 Hz, 1H, -CH = CH-), 3.87 (d, J = 5.1 Hz, 2H, -CH2). 13C NMR (151 MHz, DMSO-d6) δ = 171.64, 165.77, 139.63, 135.25, 129.95, 129.35, 127.99, 122.17, 41.30. HRMS (ESI) calcd for C11H12O3N [M + H]+: 206.08117, found 206.08066.
(E)-(3-(3,4-Difluorophenyl)acryloyl)glycine (F2): white solid, 1.98 g, yield 82%, mp: 210.0–213.1°C, 1H NMR (600 MHz, DMSO-d6) δ = 8.38 (s, 1H, -NH), 7.72–7.62 (m, 1H, Ar-H), 7.49–7.27 (m, 3H, Ar-H and -CH = CH-), 6.68 (d, J = 15.6 Hz, 1H, -CH = CH-), 3.87 (d, J = 3.0 Hz, 2H, -CH2). 13C NMR (151 MHz, DMSO-d6) δ = 171.58, 165.39, 151.07 (dd, J = 46.8, 12.1 Hz), 149.47 (dd, J = 45.3, 12.1 Hz), 137.52, 133.21 (dd, J = 6.0, 4.5 Hz), 125.19 (dd, J = 7.6, 4.5 Hz), 123.52, 118.42 (d, 3JC-F = 18.1 Hz), 116.69 (d, 3JC-F = 16.6 Hz), 41.31.19F NMR (564 MHz, DMSO) δ = -136.97, -138.11. HRMS (ESI) calcd for C11H10O3NF2 [M + H]+: 242.06233, found 242.06166.
2.1.6 Preparation of target compounds J1 and J2
A mixture of intermediate F (1.0 mmol), amine (1.2 mmol), HOBt (1.2 mol, 162.14 mg), DIEPA (1.2 mol, 155.1 mg), and 10 ml of DCM was stirred at 0°C for 15 min. Then, EDCI (1.2 mol, 230.0 mg) was added, and the reaction mixture was stirred at 25°C for 18 h. After the reaction was completed, the solvent was removed under reduced pressure to give the crude product. Then, the target compounds J1 and J2 were obtained via recrystallization using ethyl acetate and petroleum ether as the solvent.
N-(2-oxo-2-((4-Phenoxyphenyl)amino)ethyl) cinn-amamide (J1): white solid, 58.2 mg, yield 15.6%, mp: 213.4–217.5°C, 1H NMR (600 MHz, DMSO-d6) δ = 10.07 (s, 1H, -NH), 8.45 (t, J = 5.5 Hz, 1H, -NH), 7.59–7.55 (m, 4H, Ar-H), 7.47–7.30 (m, 6H, Ar-H), 7.07 (t, J = 7.4 Hz, 1H, Ar-H), 6.97–6.93 (m, 4H, Ar-H), 6.76 (d, J = 15.8 Hz, 1H, -CH = CH-), 4.01 (d, J = 5.8 Hz, 2H, -CH2). 13C NMR (151 MHz, DMSO-d6) δ = 167.96, 165.88, 157.76, 152.24, 139.54, 135.28, 135.19, 130.37, 129.95, 129.37, 128.00, 123.42, 122.31, 121.34, 119.87, 118.32, 43.31. HRMS (ESI) calcd for C23H21O3N2 [M + H]+: 373.15467, found 373.15344.
(E)-N-(2-((4-(benzyloxy)phenyl)amino)-2-oxo-ethyl)-3-(3,4-difluorophenyl)acrylamide (J2): white solid, 49.5 mg, yield 11.7%, mp: 246.2–248.3°C, 1H NMR (600 MHz, DMSO-d6) δ = 9.90 (s, 1H, -NH), 8.39 (t, J = 5.6 Hz, 1H, Ar-H), 7.69–7.65 (m, 1H, Ar-H), 7.51–7.39 (m, 7H, Ar-H), 7.35 (t, J = 7.5 Hz, 2H, Ar-H), 7.29 (t, J = 7.2 Hz, 1H, Ar-H), 6.94 (d, J = 8.9 Hz, 2H, Ar-H), 6.75 (d, J = 15.8 Hz, 1H, -CH = CH-), 5.03 (s, 2H, -CH2), 3.98 (d, J = 5.6 Hz, 2H, -CH2). 13C NMR (151 MHz, DMSO-d6) δ = 167.53, 165.45, 154.77, 151.12 (dd, J = 43.8, 12.1 Hz), 149.48 (dd, J = 40.8, 13.6 Hz), 137.48 (d, 2JC-F = 39.3 Hz), 133.29 (dd, J = 6.0, 3.0 Hz), 132.64, 128.81, 128.32 (d, 1JC-F = 117.8 Hz), 128.18, 128.06, 125.15 (dd, J = 6.0, 3.0 Hz), 123.77, 121.16, 118.45 (d, 2JC-F = 18.1 Hz), 116.68 (d, 2JC-F = 18.1 Hz), 115.35, 69.87, 43.25.19F NMR (564 MHz, DMSO) δ = -137.01, -138.10.
2.2 Bioactivity assay against P. xylostella
The biological activity was evaluated using the leaf dipping method. The stock solution of insecticides was diluted using an aqueous solution of 0.05% Triton X-80. Cabbage leaf discs were dipped in solutions with the insecticide concentration (0.2 mg/ml) for 15 s and allowed to dry for 2 h. Control discs were treated with a 0.05% Triton X-80 solution. All the dipped leaf discs were dried at room temperature before being placed in Petri dishes (10 cm in diameter). Each set of concentrations was replicated three times. Next, 10 s-instar larvae were transferred to each Petri dish. The dishes were then stored in an incubator at 25 ± 2°C, 70 ± 20% RH (relative humidity) and kept under a 14:10 h light/dark photoperiod. Larvae mortality was recorded at 48 h.
2.3 Docking
A bioinformatics analysis for the molecular docking of D28 with GABAA receptor was conducted according to the method used by Sun et al. (2021). A homology model of GABAA was constructed using the online server SWISS-MODEL (https://swissmodel.expasy.org/). Molecular docking of D28 with GABAA receptor was performed using Autodock software (version 4.2) (Bajaj et al., 1996). The energetically minimized three-dimensional structure of AITC was constructed using Chem 3D ultra 2010. The pdb files of D28 and GABAA receptor were set as the ligand and the receptor, respectively, followed by sequenced procedures using Autogrid and Autodock. Docking results with minimized reaction energy were selected, and binding sites were analyzed with PyMOL software (Seeliger and de Groot, 2010).
3 Results and discussion
3.1 Chemistry
The synthetic routes of compounds D1–D28, J1, and J2 are outlined in Figure 2. The pathway started from the reaction of piperine and NaOH in EtOH under 85°C for 12 h, which produced intermediate A. Then, intermediate A reacted with methyl glycinate to produce intermediate B in the presence of HOBt, DIPEA, and EDCI using CH2Cl2 as the solvent. Next, intermediate B reacted with KOH in H2O/CH3OH/THF (v:v:v = 1:1:1) to produce intermediate C. The target compounds D1–D28 were obtained via a condensation reaction between intermediate C and different amines. The compounds J1 and J2 were prepared using E1 and E2 as the starting materials that was followed by two condensation reactions to form the target compounds J1 and J2. All desired products were confirmed by 1H-NMR, 13C-NMR, 19F-NMR, and HRMS.
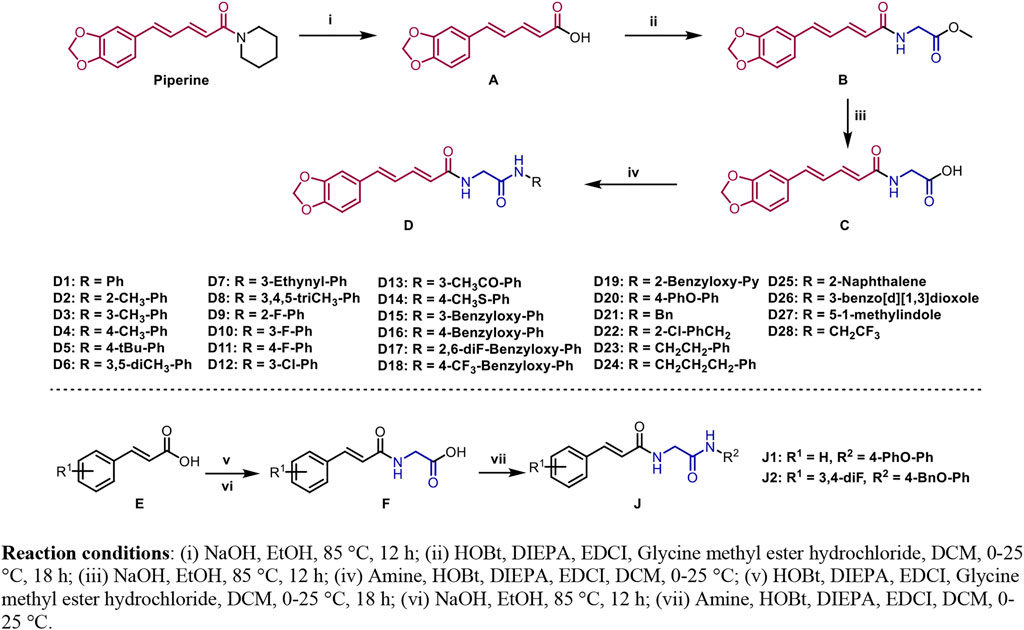
FIGURE 2. Synthesis route of the target compounds. Reaction conditions: (i) NaOH and EtOH, 85°C, 12 h; (ii) HOBt, DIEPA, EDCI, glycine methyl ester hydrochloride, and DCM, 0–25°C, 18 h; (iii) NaOH and EtOH, 85°C, 12 h; (iv) amine, HOBt, DIEPA, EDCI, and DCM, 0–25°C; (v) HOBt, DIEPA, EDCI, glycine methyl ester hydrochloride, and DCM, 0–25°C, 18 h; (vi) NaOH and EtOH, 85°C, 12 h; (vii) amine, HOBt, DIEPA, EDCI, and DCM, 0–25°C.
3.2 Insecticidal biological activity
The results of the insecticidal activity against P. xylostella are shown in Table 1. These compounds showed better insecticidal activities against P. xylostella than piperine, although most of the target compounds had low insecticidal activities with a mortality rate of less than 20% at a concentration of 0.2 mg/ml. Compounds D12, D23, and D28 showed the highest activity among these compounds, with a mortality rate of 26.7, 26.7, and 43.3% at 48h, respectively. To further compare the difference in insecticidal activities of compound D28 and piperine, the mortality rates of these two compounds were tested at 1 mg/ml (Table 2), and compound D28 showed 90% mortality. From the structure–activity relationship, R showed significant effects on the insecticidal activities of the target compounds. When R was trifluoroethyl, compound D28 showed the best insecticidal activities, followed by arenethyl. When chlorine was substituted at the 3-position of the benzene ring, compound D12 showed higher insecticidal activity. When the substituent R was CH3, compounds with CH3 at the meta-position showed higher activity, for instance, D3 (3-CH3) > D2 (2-CH3) ≈ D4 (4-CH3). Meanwhile, when R was benzyloxy-substituted phenyl, it seemed to have higher activity than alkyl-substituted phenyl. For instance, D18 (4-CF3-Benzyloxy-Ph) > D8 (3,4,5-triCH3-Ph) > D21 (Bn).
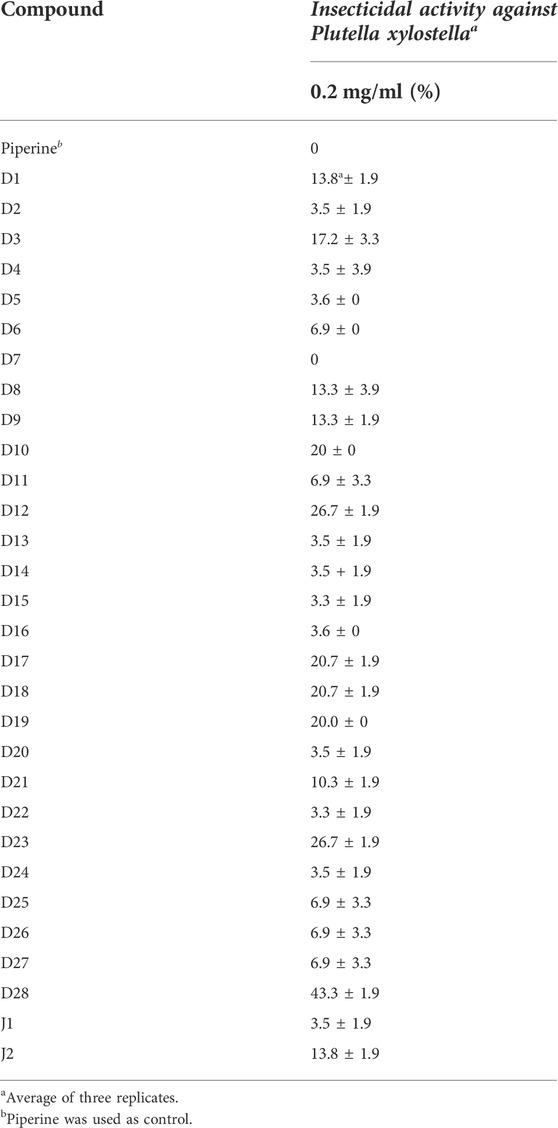
TABLE 1. Insecticidal activity of piperine and target compounds against P. xylostella on larvae (mortality (%) ± SD) (48 h).
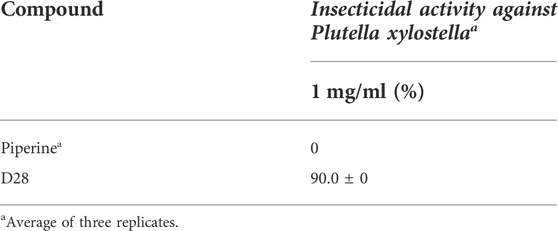
TABLE 2. Insecticidal activity of piperine and D28 against P. xylostella at 1 mg/ml on larvae (mortality (%) ± SD) (48 h).
3.3 Molecular docking of compound D28 with GABAA receptor
To identify the binding affinity of D28 and GABAA receptor, we determined the molecular docking of D28 and GABAA receptor using Autodock software (Figure 3). Figure 3A and b indicate that D28 binds at ARG180, GLN246, and LEU182 within the GABAA receptor by hydrogen bonds, of which distances were 1.927, 2.026, and 2.058 Å, respectively. The molecular docking results indicated that compound D28 could act on the GABAA receptor.
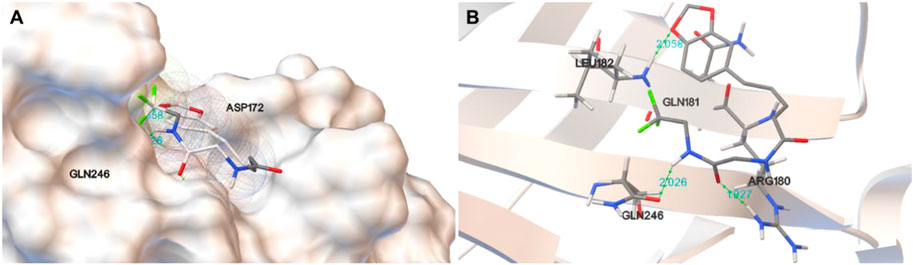
FIGURE 3. Molecular docking results of D28 with GABAA receptor analyzed using PyMOL software. (A) Protein surface view for docking of D28 with GABAA receptor with the interaction of a hydrogen bond; (B) picture of the docking of D28 with GABAA receptor, with binding sites at ARG180, GLN246, and LEU182.
4 Conclusion
In summary, 30 novel piperine derivatives containing linear bisamide were designed and synthesized. The structures of these compounds were confirmed via 1H-NMR, 13C-NMR, and HRMS. The insecticidal activities of these compounds were evaluated, and all of them have better insecticidal activities against P. xylostella than piperine. In addition, compound D28 displayed good insecticidal activity. The insecticidal mechanism of compound D28 was studied using molecular docking, and the results indicated that compound 34 may act on GABAA receptors. These findings indicated that these piperine derivatives have the potential to be a promising lead compound for further study.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
Author contributions
YL designed the experiments. CZ and QT performed the experiments and analyzed the data. YL wrote the manuscript.
Funding
This study was funded by the Natural Science Foundation of Anhui Province (1908085MC71), National Key Research and Development Program of China (2021YFD1700104), Key R&D Projects of Anhui Province (202104a06020008), and Postgraduate Scientific Research Projects of Universities in Anhui Province (YJS20210197).
Acknowledgments
The authors are thankful for the helpful discussion from Chao Zhang (AHAU).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fchem.2022.973630/full#supplementary-material
References
Bajaj, C. L., Pascucci, V., and Schikore, D. R. (1996). “Fast isocontouring for improved Interactivity,” in Proceedings of 1996 Symposium on Volume Visualization, San Francisco, CA, USA, 29-29 Oct. 1996, 39–46.
Chen, Y., Wu, J., Wang, A., Qi, Z., Jiang, T., Chen, C., et al. (2017). Discovery of N-(5-((5-Chloro-4-((2-(isopropylsulfonyl)phenyl)amino)pyrimidin-2-yl)amino)-4-methoxy-2-(4-methyl-1, 4 -diazepan-1-yl)phenyl)acrylamide (CHMFL-ALK/EGFR-050) as a potent ALK/EGFR dual kinase inhibitor capable of overcoming a variety of ALK/EGFR associated drug resistant mutants in NSCLC. Eur. J. Med. Chem. 139, 674–697. doi:10.1016/j.ejmech.2017.08.035
Franklim, T. N., Freire-de-Lima, L., Diniz, J. D. N. S., Previato, J. O., Castro, R. N., Mendonça-Previato, L., et al. (2013). Design, synthesis and trypanocidal evaluation of novel 1, 2, 4-Triazoles-3-thiones derived from natural piperine. Molecules 18, 6366–6382. doi:10.3390/molecules18066366
Gaur, R., and Bao, G.-H. (2021). Chemistry and pharmacology of natural catechins from camellia sinensis as anti-MRSA agents. Curr. Top. Med. Chem. 21, 1519–1537. doi:10.2174/1568026621666210524100632
Han, Q., Wu, N., Li, H.-L., Zhang, J.-Y., Li, X., Deng, M.-F., et al. (2021). A piperine-based scaffold as a novel starting point to develop inhibitors against the potent molecular target OfChtI. J. Agric. Food Chem. 69, 7534–7544. doi:10.1021/-acs.jafc.0c08119
He, F., Li, B., Ai, G., Kange, A. M., Zhao, Y., Zhang, X., et al. (2016). Transcriptomics analysis of the Chinese pear pathotype of Alternaria alternata gives insights into novel mechanisms of HSAF antifungal activities. Int. J. Mol. Sci. 19, 1841. doi:10.3390/ijms19071841
Kimura, K., Yamaoka, M., and Kamisaka, Y. (2006). Inhibition of lipid accumulation and lipid body formation in oleaginous yeast by effective components in spices, carvacrol, eugenol, thymol, and piperine. J. Agric. Food. Chem. 54, 3528–3534. doi:10.1021/jf0531149
Lima Neto, J. E., da Solidade Ribeiro, L. M., and de Siqueira, H. Á. A. (2021). Inheritance and fitness of Plutella xylostella (Lepidoptera: Plutellidae) resistance to chlorfenapyr. J. Econ. Entomol. 114, 875–884. doi:10.1093/jee/toaa299
Liu, H., Ren, Z.-L., Wang, W., Gong, J.-X., Chu, M.-J., Ma, Q.-W., et al. (2018). Novel coumarin-pyrazole carboxamide derivatives as potential topoisomerase II inhibitors: Design, synthesis and antibacterial activity. Eur. J. Med. Chem. 157, 81–87. doi:10.1016/j.ejmech.2018.07.059
Liu, Z., Li, Q., and Song, B. (2020). Recent research progress in and perspectives of mesoionic insecticides: Nicotinic acetylcholine receptor inhibitors. J. Agric. Food Chem. 68, 11039–11053. doi:10.1021/acs.jafc.0c02376
Park, U. H., Jeong, H. S., Jo, E. Y., Park, T., Yoon, S. K., Kim, E.-J., et al. (2012). Piperine, a component of black pepper, inhibits adipogenesis by antagonizing PPARγ activity in 3T3-L1 cells. J. Agric. Food Chem. 60, 3853–3860. doi:10.1021/jf204514a
Parmar, V. S., Jain, S. C., Bisht, K. S., Jain, R., Taneja, P., Jha, A., et al. (1997). Phytochemistry of the genus piper. Phytochemistry 46, 597–673. doi:10.1016/S0031-9422(97)00328-2
Paula, V. F. d., Barbosa, L. C. d. A., Demuner, A. J., Piló-Veloso, D., and Picanço, M. C. (2000). Synthesis and insecticidal activity of new amide derivatives of piperine. Pest Manag. Sci. 56, 168–174. doi:10.1002/(sici)1526-4998(200002)56:2<168:aid-ps110>3.0.co;2-h
Ribeiro, T. S., Freire-de-Lima, Le., Previato, J. O., Previato, L. M., Heise, N., Freire de Lima, M. E., et al. (2004). Toxic effects of natural piperine and its derivatives on epimastigotes and amastigotes of trypanosoma cruzi. Bioorg. Med. Chem. Lett. 14, 3555–3558. doi:10.1016/j.bmcl.2004.04.019
Roman, P. (2016). History, presence and perspective of using plant extracts as commercial botanical insecticides and farm products for protection against insects – A review. Plant Prot. Sci. 52, 229–241. doi:10.17221/31/2016-PPS
Seeliger, D., and de Groot, B. L. (2010). Ligand docking and binding site analysis with PyMOL and autodock/vina. J. Comput. Aided. Mol. Des. 24, 417–422. doi:10.1007/s10822-010-9352-6
Shen, J., Li, D., Zhang, S., Zhu, X., Wan, H., Li, J., et al. (2017). Fitness and inheritance of metaflumizone resistance in Plutella xylostella. Pestic. Biochem. Physiol. 139, 53–59. doi:10.1016/j.pestbp.2017.04.010
Sun, J., Lv, X.-H., Qiu, H.-Y., Wang, Y.-T., Du, Q.-R., Li, D.-D., et al. (2013). Synthesis, biological evaluation and molecular docking studies of pyrazole derivatives coupling with a thiourea moiety as novel CDKs inhibitors. Eur. J. Med. Chem. 68, 1–9. doi:10.1016/j.ejmech.2013.07.003
Sun, Y., Jiang, Y., Wu, H., Xu, N., Ma, Z., Zhang, C., et al. (2021). Function of four mitochondrial genes in fumigation lethal mechanisms of allyl isothiocyanate against Sitophilus zeamais adults. Pestic. Biochem. Physiol. 179, 104947. doi:10.1016/j.pestbp.2021.104947
Sunila, E. S., and Kuttan, G. (2004). Immunomodulatory and antitumor activity of piper longum linn and piperine. J. Ethnopharmacol. 90, 339–346. doi:10.1016/j.jep.2003.10.016
Swain, T. (1977). Secondary compounds as protective agents. Annu. Rev. Plant Physiol. 28, 479–501. doi:10.1146/annurev.pp.28.060177.002403
Tang, J.-F., Lv, X.-H., Wang, X.-L., Sun, J., Zhang, Y.-B., Yang, Y.-S., et al. (2012). Design, synthesis, biological evaluation and molecular modeling of novel 1, 3, 4-oxadiazole derivatives based on vanillic acid as potential immunosuppressive agents. Bioorg. Med. Chem. 20, 4226–4236. doi:10.1016/j.bmc.2012.05.055
Tang, M., Wang, Z., and Shi, Y. (2004). Involvement of cytochrome c release and caspase activation in toosendanin-induced PC12 cell apoptosis. Toxicology 201, 31–38. doi:10.1016/j.tox.2004.03.023
Tong, Z., Duan, J., Wu, Y., Liu, Q., He, Q., Shi, Y., et al. (2018). Evaluation of highly detectable pesticides sprayed in Brassica napus L.: Degradation behavior and risk assessment for honeybees. Molecules 23, 2482. doi:10.3390/molecules23102482
Wang, H., Yang, Z., Fan, Z., Wu, Q., Zhang, Y., Mi, N., et al. (2011). Synthesis and insecticidal activity of N-tert-Butyl-N, N′-diacylhydrazines containing 1, 2, 3-thiadiazoles. J. Agric. Food Chem. 59, 628–634. doi:10.1021/jf104004q
Wu, W.-J., Wang, M.-A., Zhu, J.-B., Zhou, W., Hu, Z., Ji, Z., et al. (2001). Five new insecticidal sesquiterpenoids from Celastrus angulatus. J. Nat. Prod. 64, 364–367. doi:10.1021/np0004193
Xia, D., Liu, H., Cheng, X., Maraswami, M., Chen, Y., Lv, X., et al. (2022). Recent developments of coumarin-based hybrids in drug discovery. Curr. Top. Med. Chem. 22, 269–283. doi:10.2174/1568026622666220105105450
Xu, H., and Yang, R. (2017). Piperine derivatives, preparation method and application of preparation of botanical insecticides. Xi’an, Shanxi Province: Northwest A&F University. CN 107892685 A.
Keywords: piperine derivatives, insecticidal activity, linear bisamide, Plutella xylostella, Lepidoptera
Citation: Zhang C, Tian Q and Li Y (2022) Design, synthesis, and insecticidal activity evaluation of piperine derivatives. Front. Chem. 10:973630. doi: 10.3389/fchem.2022.973630
Received: 20 June 2022; Accepted: 01 July 2022;
Published: 26 July 2022.
Edited by:
Pei Li, Kaili University, ChinaReviewed by:
Fengxiang Zhu, Shanxi University, ChinaZhengjun Liu, Anshun University, China
Zechao Wang, Zhengzhou University, China
Copyright © 2022 Zhang, Tian and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yahui Li, WWFodWkuTGlAYWhhdS5lZHUuY24=
†These authors have contributed equally to this work
 Chiying Zhang1†
Chiying Zhang1† Yahui Li
Yahui Li