
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
PERSPECTIVE article
Front. Chem., 14 July 2022
Sec. Catalytic Reactions and Chemistry
Volume 10 - 2022 | https://doi.org/10.3389/fchem.2022.959525
This article is part of the Research TopicCatalysis Rising Stars in ChinaView all 10 articles
Single-atom catalysis is a research Frontier and has attracted extensive interests in catalysis. Significant progresses have been carried out in the synthesis and characterization of metal single-atom catalysts (SACs). However, the stability and catalytic reactivity of metal SAC at elevated temperatures are not well documented because single atoms sinter at elevated temperatures. Therefore, the development of stable and reactive SAC at high temperatures remains a formidable challenge. In this perspective, we summarize recent efforts on the preparation of the thermally-stable SACs synthesized at elevated temperature via the reverse-Ostwald ripening mechanism, including the approaches of atom trapping and vapor-phase self-assembly. The reducibility of lattice oxygen, the loading upper limit and the location of the metal single atom are discussed, combining experiments with simulations. In addition, we demonstrate that the coordination structure of the metal single atom can be tailored to address the relationship of structure and performances of the metal SAC in reactions. We expect that this perspective can provide some insights to guide the study for the rational design of thermally-stable and active single atom catalysts, which are especially suitable for high-temperature reactions.
Catalysis has been in the era of the precise design and manipulation of catalyst structure in the atomic scale for developing highly efficient catalysts (Guan et al., 2021). Single-atom catalysis has attracted extensive attention since it was proposed by Zhang et al., in 2011 (Qiao et al., 2011) in that it can bridge the gap between homogeneous catalysis and heterogeneous catalysis. Because of the high atom efficiency, single-atom catalysts (SACs) have therefore attracted ever-increasing attention from numerous fields including material science, catalysis and electrochemistry. To date, much progress has been achieved on the studies of metal SACs in catalysis, especially on the synthesis and characterization of metal SACs (Li et al., 2018; Ji et al., 2020). Furthermore, metal SACs have been found to present superior reactivity (selectivity or activity) than nanoparticles in several catalytic reactions, such as hydrogenation (Yan et al., 2015) and oxidation (Zhang et al., 2022a). However, most of these reactions were carried out at relatively low reaction temperatures (<100°C) and catalytic reactions performed at temperatures of >200°C, such as dehydrogenation and reforming, are rarely reported since metal SACs are thermodynamically unstable and are prone to agglomerate at elevated temperatures due to Ostwald ripening (OR).
Metal SACs must keep stable during chemical reactions under industrial conditions, including at elevated temperatures (Xiong et al., 2021a). Especially, in the transition from the academic curiosity to an industrially relevant technology, the thermal stability of metal SAC became much more important (Datye and Guo, 2021). Previous work has indicated that metal SACs prepared by atom trapping (AT) can withstand the temperature up to 800°C in oxidizing conditions (Jones et al., 2016; Lang et al., 2019). However, many metal SACs are not stable under reaction atmospheres or at elevated temperatures. These SACs have been demonstrated to agglomerate to form nanoclusters due to the high free energy of SACs under reaction conditions (Yang et al., 2013). For example, atomically dispersed metal atoms can rapidly agglomerate to metal clusters or nanoparticles under catalytic reactions (CO oxidation, hydrogenation and dehydrogenation) (Liu et al., 2019). Moreover, the size of Pt particles formed from single atoms on a Pt/Al2O3 increases with the reaction temperature from 150 to 325°C, indicating that Pt species undergo dynamic structural transformation during reaction (Liu et al., 2019). Therefore, the development of metal SACs that are stable under reaction conditions is pivotal, which is prerequisite for future commercialization of these catalysts (Datye and Guo, 2021).
In this perspective, we discuss the progress on the preparation and activation of thermally-stable single-atom catalysts (TSSAC) in catalysis. We firstly introduce atom trapping (AT) and vapor-phase self-assembly (VPSA) to prepare thermally stable single atom catalysts, including on the supports of ceria (CeO2) and MgAl2O4. The ceria represents the supports with strong metal-support interaction, while the MgAl2O4 represents the supports with weak metal-support interaction. We also describe two approaches to tailor the coordination structure of the Pt single atoms on the thermally-stable Pt1/CeO2 catalyst. Finally, the atom-trapped Pt1/CeO2 SAC can be used to load a second metal atoms to generate two-dimensional metal oxides which shows superior catalytic reactivity than three-dimensional metal oxides. For the general and comprehensive understanding of SAC or TSSAC, such as support choices or carbon-supported SAC, the readers are referred to other important review articles (Liu et al., 2019; Xiong et al., 2021a; Singh et al., 2021).
Metal atoms in nanoparticles are mobile at high temperatures, leading to the sintering of the nanoparticles via the Ostwald Ripening mechanism. The mobile atom can be trapped by oxide supports such as PdO (Xiong et al., 2016), CeO2 (Jones et al., 2016; Wan et al., 2018) and Fe2O3 (Lang et al., 2019) or carbon materials (Wei et al., 2018) to form single-atom catalysts (Figures 1A,B). The trapping of the mobile metal atom by CeO2 and Fe2O3 is due to the strong metal atom-support interaction. In 2016, we first reported the evaporated migration of Pt species from Pt nanoparticles on alumina to ceria surface via a reverse Ostwald ripening mechanism (Jones et al., 2016). The Pt species was then trapped by the ceria and located on the ceria surface to form Pt1/CeO2 SAC. Further study found that the Pt1/CeO2 SAC can be formed by directly dispersing Pt salt precursors onto the ceria, followed by annealing the material at elevated temperatures in air. The locations and the maximum loading of the Pt single atom on the polyhedral ceria was further investigated (Kunwar et al., 2019). It is demonstrated that cerium oxide supported Pt single atoms at high metal loading up to 3 wt.% Pt. This Pt loading corresponds to the maximum density of Pt single atom on the ceria surface is ca. 1 Pt atom/nm2.
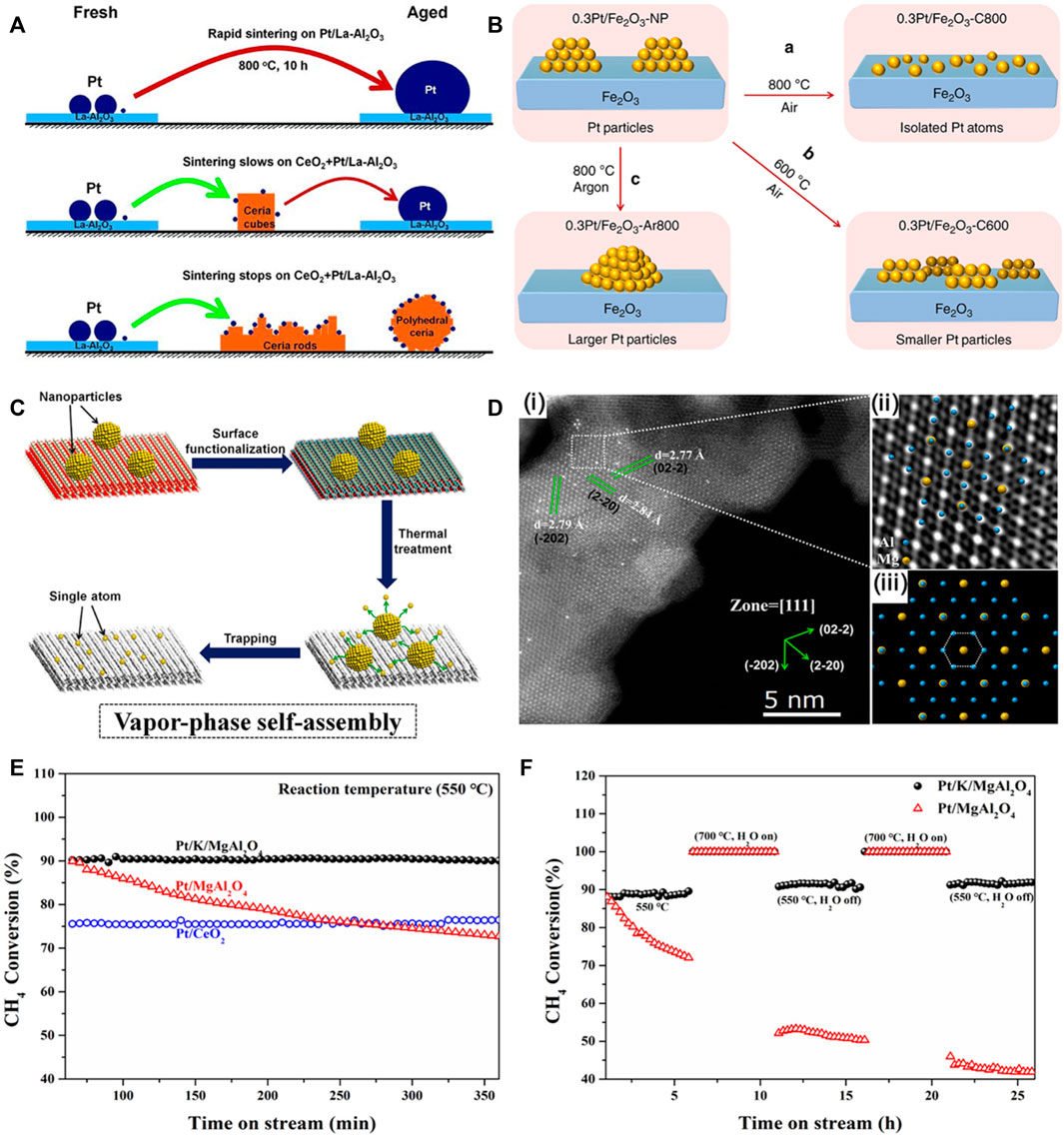
FIGURE 1. Atom trapping for the preparation of thermally-stable Pt single atom catalysts. (A) Illustration of Pt nanoparticle sintering and redispersion. Reproduced with permission (Jones et al., 2016). Copyright 2016, American Association for the Advancement of Science. (B) Illustration of the redispersion of Pt nanoparticle to Pt single atoms on Fe2O3. Reproduced with permission (Lang et al., 2019). Copyright 2019, Springer Nature. (C) Schematic illustration of the vapor-phase self-assembly processes. (D) AC-STEM image of Pt/K/MgAl2O4 SAC. (E) Catalytic reactivity and stability of Pt catalysts. (F) Reactivity and stability of Pt/K/MgAl2O4 SAC and Pt/MgAl2O4 nanocatalyst in methane oxidation. Reproduced with permission (Li et al., 2022). Elsevier.
The catalytic reactivity of the Pt1/CeO2 SAC prepared by the high temperature vapor phase synthesis (atom trapping, AT) was compared with Pt1/CeO2 SAC prepared by conventional wetness synthesis (strong electrostatic adsorption-SEA) with calcination at 350°C in air in CO oxidation (Pereira-Hernández et al., 2019). The AT sample led to ionic Pt being trapped on the CeO2 in a thermally stable form. The as-synthesized, both SACs are inactive for low- temperature (<150°C) CO oxidation. After treatment in CO at 275°C, both catalysts show enhanced reactivity. Despite similar Pt metal particle size, the AT catalyst is significantly more active, with onset of CO oxidation near room temperature. A combination of near-ambient pressure X-ray photoelectron spectroscopy (NAP-XPS) and CO temperature-programmed reduction (CO-TPR) showed that the high reactivity at low temperatures was related to the improved reducibility of lattice oxygen on the CeO2 support.
For the oxide supports with a weak metal-support interaction such as Al2O3 and MgAl2O4, experiment results showed that these oxide supports could not directly trap the mobile metal atom to form single-atom catalyst at high temperatures. Instead, the mobile metal atoms agglomerated to form large particles due to sintering at elevated temperatures (Xiong et al., 2016). Recently, an approach termed as “vapor-phase self-assembly” was reported to trap the mobile metal single atoms on the support having a weak metal-support interaction (Li et al., 2022). The new design principle, efficiently anchored Pt single atoms on a conventional support MgAl2O4 with the assistant of K ions at elevated temperature in air. A stable triangular K3O3 structure serves as sites for trapping isolated Pt species over MgAl2O4 (111), leading to a superior stability in methane oxidation. Such Pt single atoms trapped by the triangle motif are capable of withstanding exposure to high temperatures in oxidizing conditions. The Pt/K/MgAl2O4 SAC presented excellent thermal/hydrothermal stability and reactivity in methane oxidation in the presence of steam at elevated temperatures (Figures 1C–F), as compared to the Pt/MgAl2O4 nanocatalyst without K and the Pt1/CeO2 SAC.
The generalizability of the vapor-phase self-assembly mechanism on stabilizing metal single atoms was investigated on other alkali metal cations, metal centers and oxide supports. Alkali metal cations of Li+, Na+ and Cs+, metal centers (Ru, Ir and Au) and oxide supports (SiO2, NiAl2O4, CoAl2O4 and MgCr2O4) as well as density functional theory (DFT) simulations were used to explain the different effects of these structural parameters on stabilizing the metal single atoms during the VPSA process. Results show that alkali Na+ and Cs+ can stabilize the Pt single atoms on MgAl2O4 after the VPSA, whereas Li+ cannot trap Pt single atoms. DFT simulations revealed that for the Na+, K+ and Cs+, the atoms in the M-O motifs (M = Na, K and Cs) are in the same plane, while the atoms of Li-O motif in the Z direction are not in the same plane, leading to a weak interaction between Li and O on MgAl2O4 (111). As for other metal single atoms (Ru, Ir and Au), the K-modified MgAl2O4 can stabilize Ru and Ir atoms with VPSA, while it cannot stabilize Au atom. DFT calculations showed that after depositing M1, a stable triangle M1O3 structure is formed for Ru and Ir, similar to the case of Pt1 single atom. For oxide supports, γ-Al2O3 cannot trap Pt single atoms via VPSA because of the irregular surface revealed by DFT simulation, which cannot stabilize KO motif. The K/NiAl2O4 and K/CoAl2O4 cannot trap the Pt single atoms, whereas K/MgCr2O4 can partially trap the mobile Pt atoms at elevated temperatures. DFT simulations confirm the above observations.
Pt1/CeO2 SAC prepared by AT is thermally stable, while it is not as active as Pt/CeO2 nanocatalyst in CO oxidation (Gänzler et al., 2017; Pereira-Hernández et al., 2019). This is because the Pt single atom on the AT sample was strongly bound on the ceria. To improve the reactivity of the Pt1/CeO2 SAC, the coordination environment of the Pt single atom must be tailored. Two strategies were reported to modulate the coordination structure of the Pt single atom in the Pt1/CeO2 SAC. One is the addition of a second metal atom into the Pt1/CeO2 SAC (Xiong et al., 2017), such as Sn or Ga. The reactivity and stability of Pt single-atom species was investigated in the industrially important light alkane dehydrogenation reaction. The Pt1/CeO2 single-atom catalyst is active during propane dehydrogenation, but not selective for yielding propylene. DFT calculations show strong adsorption of the olefin produced, leading to C-C cleavage to produce CH4. In contrast, the addition of tin (Sn) into Pt1/CeO2 SAC allows the modified SAC to achieve high selectivity towards propylene because of facile desorption of the product. Furthermore, upon oxidation the Pt-Sn species readily revert to the atomically dispersed species on CeO2, making Pt-Sn/CeO2 a fully regenerable catalyst. Ga was also used to modify the coordination structure of the Pt1/CeO2 SAC in CO oxidation (Feng et al., 2018). Significantly, the stability of Pt single atoms anchored on the Ga site was enhanced compared with those on the bare ceria surface.
The other approach used to modify the coordination structure of Pt1/CeO2 SAC is to treat the Pt1/CeO2 SAC in a high-temperature steam (750°C) (Nie et al., 2017). The results demonstrated that the Pt1/CeO2 was activated via steam treatment (at 750°C) to simultaneously achieve the low-temperature CO oxidation activity while keeping Pt atomically dispersed after the high-temperature steam treatment. The treated Pt1/CeO2 SAC reached 100% CO conversion at 150°C, as compared to the inactive Pt1/CeO2 SAC at the same temperature in CO oxidation. A new type of active site is created on CeO2 in the vicinity of Pt2+, where Pt cation is coordinated with OlatticH to provide the improved reactivity in the oxidations of CO, NO and propane. These active sites are stable up to 800°C in oxidizing environments.
Metal single atoms in SAC can be used to engineer the properties of the support surface. Relying on the trapping of Pt single atoms on the CeO2 surface in thermally stable form, the nature of the deposited metal/metal oxide clusters can be modified (Xiong et al., 2021b). In particular, two-dimensional (2D) rafts of PtOx on the engineered catalyst support are formed by this approach (Figures 2A–C), as opposed to three-dimensional (3D) metal oxide nanoparticles on conventional supports. This 2D rafts of PtOx showed much higher reactivity than a Pt1/CeO2 SAC and the 3D Pt/CeO2 catalyst in CO oxidation (Xiong et al., 2021b). Adopting this approach for the synthesis of bimetallic catalysts via addition of Pd to the atom-trapped catalyst support (Pt@CeO2), the resulting Pd/Pt@CeO2 catalyst (Figures 2D–F) provides three times higher reaction rate and improved water tolerance than an impregnated PtPd/CeO2 catalyst during methane oxidation. The improved performance is attributed to the 2D morphology of the PdOx phase presented on the atom-trapped Pt@CeO2 support. The results showed that modifying the support by trapping single atoms provided an important addition to the toolkit of catalyst designers to engineer catalyst supports for controlling the nucleation and growth of metal and metal oxide clusters in heterogeneous catalysts.
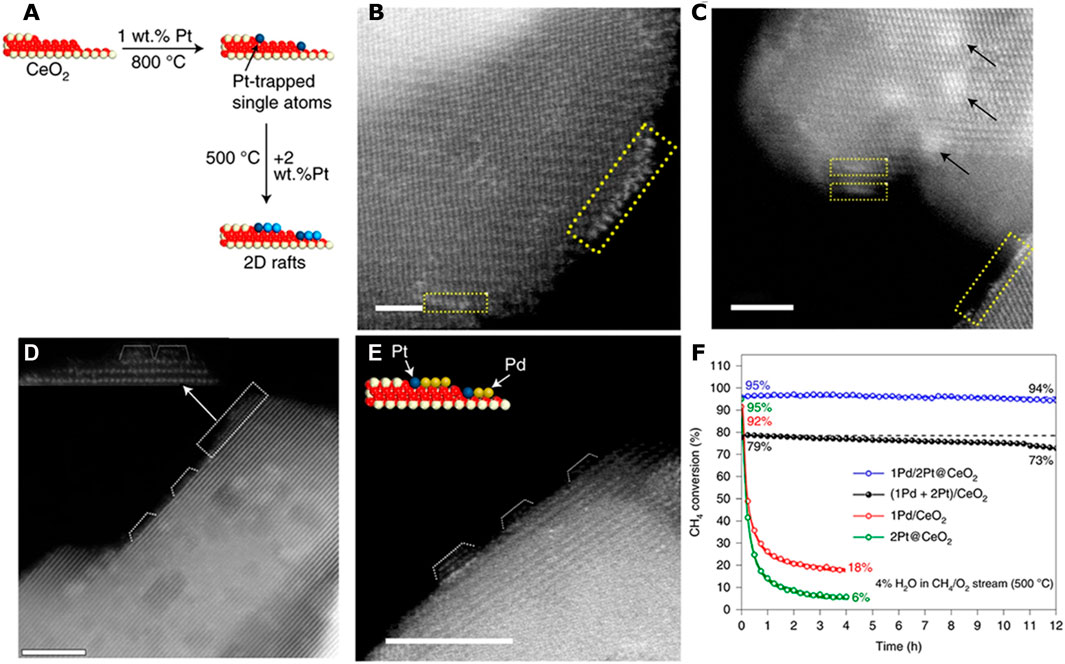
FIGURE 2. Engineering catalyst support via thermally-stable single-atom catalyst. (A) Schematic illustration showing the morphologies of Pt catalysts supported on ceria prepared by depositing Pt on a Pt-trapped ceria (Pt@CeO2) (B,C) AC-STEM images of the catalyst prepared by depositing 2 wt.% Pt on atom-trapped 1 wt.% Pt@CeO2. (D,E) AC-STEM image of Pd deposited on the catalyst shown in (E). (F) Comparison of catalyst stability for the as-synthesized 1Pd/2Pt@CeO2 and (1Pd + 2Pt)/CeO2 catalysts, reduced 2Pt@CeO2 and reduced 1Pd/CeO2 catalysts in CH4 oxidation at 500°C in 4% H2O. Reproduced with permission (Xiong et al., 2021b). Copyright 2021, Springer Nature.
To summarize, single-atom catalysts (SACs) are promising because of their maximum atom efficiency and unique property in catalysis. However, recent studies demonstrate that metal SACs tend to sinter to form clusters under reaction conditions, especially at elevated temperatures. Considering many catalytic reactions such as dehydrogenation, syngas chemistry and reforming, are carried out at temperatures of >200°C, it is therefore important to develop approaches to prepare thermally stable and active SACs for their future commercialization. In this perspective, the preparation and catalytic application of thermally-stable metal SACs are summarized, mainly on Pt-based SACs. Via the reverse-Ostwald ripening mechanism, approaches including atom trapping and vapor-phase self-assembly were applied to prepare stable and active Pt SACs. The locations of the Pt single atoms on the supports (CeO2 and MgAl2O4) were also corroborated using both experiments and simulations, and the properties of the Pt SACs were well demonstrated by advanced techniques such as XAS, LEIS, AC-STEM and CO-DRIFTS. In addition, the coordination environment of the metal SACs was tailored by different approaches such as adding the second metal or treating SAC in high-temperature steam. We also discuss the engineering of the catalyst support by atom-trapped single atoms, which modified the morphology of the deposited metal/metal oxide to achieve unique catalytic performances in catalysis.
Metal SAC used in practical catalytic reactions must be thermally stable under realistic conditions and therefore this area will unambiguously attract continuing attention in future. Focusing on thermally stable and active metal SACs, we would like to provide our insights on the future studies in this field. Firstly, metal SAC that is stable under oxidizing conditions may be not stable under reducing conditions, especially at elevated temperatures. Future work needs to perform to improve the catalyst stability under reducing conditions at elevated temperatures. Secondly, not all stable metal SACs are active or selective in catalysis. Therefore, the coordination structure of the metal single atom needs to be tailored to improve the activity/selectivity. Although there are a couple of strategies reported to adjust the coordination structure of metal center, a general approach applicable to other metal SACs is lacking. Thirdly, photocatalysis and electrocatalysis have attracted the ever-increasing interests in catalysis because these energies are renewable, and the use of these energies have the potent to go net zero. Since noble metal catalysts are widely used in these processes (Zhang et al., 2022b), photo-/electro- stability of metal single-atom catalysts suitable for both photocatalysis and electrocatalysis is therefore worthy investigating. Finally, current studies on metal SACs are mostly tested in several model reactions, including CO oxidation, semi-alkyne hydrogenation and the preferential oxidation of CO (PROX). The temperatures in these reactions are relatively low. Therefore, the stability of metal SACs must be examined in other conventional reactions such as syngas conversion and reforming, where a couple of recent studies have pioneered in this field recently (Akri et al., 2019; Tang et al., 2019).
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
SL wrote the first draft of the manuscript. JL participated in literature collection and collation. HX supervised the project, revised the manuscript and provided the financial supports. All authors agree to be accountable for the content of the work.
The work was financially supported by the National High-Level Talent Fund and the National Natural Science Foundation of China (Grant Nos 22072118 and 22121001).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer SL declared a past co-authorship with the author HX to the handling editor.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We also thank financial support from the State Key Laboratory of Physical Chemistry of Solid Surfaces of Xiamen University. Part fund was supported by Science and Technology Projects of Innovation Laboratory for Sciences and Technologies of Energy Materials of Fujian Province (IKKEM) (HRTP-[2022]-3).
Akri, M., Zhao, S., Li, X., Zang, K., Lee, A. F., Isaacs, M. A., et al. (2019). Atomically dispersed nickel as coke-resistant active sites for methane dry reforming. Nat. Commun. 10, 5181. doi:10.1038/s41467-019-12843-w
Datye, A. K., and Guo, H. (2021). Single atom catalysis poised to transition from an academic curiosity to an industrially relevant technology. Nat. Commun. 12, 895. doi:10.1038/s41467-021-21152-0
Feng, Y., Wan, Q., Xiong, H., Zhou, S., Chen, X., Pereira Hernandez, X. I., et al. (2018). Correlating DFT calculations with CO oxidation reactivity on Ga-doped Pt/CeO2 single-atom catalysts. J. Phys. Chem. C 122, 22460–22468. doi:10.1021/acs.jpcc.8b05815
Gänzler, A. M., Casapu, M., Vernoux, P., Loridant, S., Cadete Santos Aires, F. J., Epicier, T., et al. (2017). Angew. Chem. Int. Ed. 56, 13078–13082.
Guan, Q., Zhu, C., Lin, Y., Vovk, E. I., Zhou, X., Yang, Y., et al. (2021). Bimetallic monolayer catalyst breaks the activity-selectivity trade-off on metal particle size for efficient chemoselective hydrogenations. Nat. Catal. 4, 840–849. doi:10.1038/s41929-021-00679-x
Ji, S., Chen, Y., Wang, X., Zhang, Z., Wang, D., and Li, Y. (2020). Chemical synthesis of single atomic site catalysts. Chem. Rev. 120 (21), 11900–11955. doi:10.1021/acs.chemrev.9b00818
Jones, J., Xiong, H., Delariva, A. T., Peterson, E. J., Pham, H., Challa, S. R., et al. (2016). Thermally stable single-atom platinum-on-ceria catalysts via atom trapping. Science 353, 150–154. doi:10.1126/science.aaf8800
Kunwar, D., Zhou, S., DeLaRiva, A., Peterson, E. J., Xiong, H., Pereira-Hernández, X. I., et al. (2019). Stabilizing high metal loadings of thermally stable platinum single atoms on an industrial catalyst support. ACS Catal. 9, 3978–3990. doi:10.1021/acscatal.8b04885
Lang, R., Xi, W., Liu, J.-C., Cui, Y.-T., Li, T., Lee, A. F., et al. (2019). Non defect-stabilized thermally stable single-atom catalyst. Nat. Commun. 10, 234. doi:10.1038/s41467-018-08136-3
Li, H., Wan, Q., Du, C., Liu, Q., Qi, J., Ding, X., et al. (2022). Vapor-phase self-assembly for generating thermally stable single-atom catalysts. Chem 8, 731–748. doi:10.1016/j.chempr.2021.11.002
Li, Z., Wang, D., Wu, Y., and Li, Y. (2018). Recent advances in the precise control of isolated single-site catalysts by chemical methods. Natl. Sci. Rev. 5, 673–689. doi:10.1093/nsr/nwy056
Liu, L., Meira, D. M., Arenal, R., Concepcion, P., Puga, A. V., and Corma, A. (2019). Determination of the evolution of heterogeneous single metal atoms and nanoclusters under reaction conditions: Which are the working catalytic sites? ACS Catal. 9, 10626–10639. doi:10.1021/acscatal.9b04214
Nie, L., Mei, D., Xiong, H., Peng, B., Ren, Z., Hernandez, X. I. P., et al. (2017). Activation of surface lattice oxygen in single-atom Pt/CeO 2 for low-temperature CO oxidation. Science 358, 1419–1423. doi:10.1126/science.aao2109
Pereira-Hernández, X. I., DeLaRiva, A., Muravev, V., Kunwar, D., Xiong, H., Sudduth, B., et al. (2019). Nat. Commu. 10, 1358.
Qiao, B., Wang, A., Yang, X., Allard, L. F., Jiang, Z., Cui, Y., et al. (2011). Single-atom catalysis of CO oxidation using Pt1/FeOx. Nat. Chem. 3, 634–641. doi:10.1038/nchem.1095
Singh, B., Gawande, M. B., Kute, A. D., Varma, R. S., Fornasiero, P., McNeice, P., et al. (2021). Single-atom (Iron-Based) catalysts: Synthesis and applications. Chem. Rev. 121, 13620–13697. doi:10.1021/acs.chemrev.1c00158
Tang, Y., Wei, Y., Wang, Z., Zhang, S., Li, Y., Nguyen, L., et al. (2019). Synergy of single-atom Ni1 and Ru1 sites on CeO2 for dry reforming of CH4. J. Am. Chem. Soc. 141, 7283–7293. doi:10.1021/jacs.8b10910
Wan, Q., Wei, F., Wang, Y., Wang, F., Zhou, L., Lin, S., et al. (2018). Single atom detachment from Cu clusters, and diffusion and trapping on CeO2(111): Implications in Ostwald ripening and atomic redispersion. Nanoscale 10, 17893–17901. doi:10.1039/c8nr06232c
Wei, S., Li, A., Liu, J.-C., Li, Z., Chen, W., Gong, Y., et al. (2018). Direct observation of noble metal nanoparticles transforming to thermally stable single atoms. Nat. Nanotech 13, 856–861. doi:10.1038/s41565-018-0197-9
Xiong, H., Datye, A. K., and Wang, Y. (2021a). Thermally stable single‐atom heterogeneous catalysts. Adv. Mat. 33, 2004319. doi:10.1002/adma.202004319
Xiong, H., Kunwar, D., Jiang, D., García-Vargas, C. E., Li, H., Du, C., et al. (2021b). Engineering catalyst supports to stabilize PdOx two-dimensional rafts for water-tolerant methane oxidation. Nat. Catal. 4, 830–839. doi:10.1038/s41929-021-00680-4
Xiong, H., Lin, S., Goetze, J., Pletcher, P., Guo, H., Kovarik, L., et al. (2017). Thermally stable and regenerable platinum-tin clusters for propane dehydrogenation prepared by atom trapping on ceria. Angew. Chem. Int. Ed. 56, 8986–8991. doi:10.1002/anie.201701115
Xiong, H., Peterson, E., Qi, G., and Datye, A. K. (2016). Trapping mobile Pt species by PdO in diesel oxidation catalysts: Smaller is better. Catal. Today 272, 80–86. doi:10.1016/j.cattod.2016.01.022
Yan, H., Cheng, H., Yi, H., Lin, Y., Yao, T., Wang, C., et al. (2015). Single-atom Pd1/graphene catalyst achieved by atomic layer deposition: Remarkable performance in selective hydrogenation of 1,3-butadiene. J. Am. Chem. Soc. 137, 10484–10487. doi:10.1021/jacs.5b06485
Yang, X.-F., Wang, A., Qiao, B., Li, J., Liu, J., and Zhang, T. (2013). Single-atom catalysts: A new frontier in heterogeneous catalysis. Acc. Chem. Res. 46, 1740–1748. doi:10.1021/ar300361m
Zhang, L., Xue, L., Lin, B., Zhao, Q., Wan, S., Wang, Y., et al. (2022). Noble metal single-atom catalysts for the catalytic oxidation of volatile organic compounds. ChemSusChem 15, e202200357. doi:10.1002/cssc.202200357
Keywords: single-atom catalysts, thermally stable, atom trapping, metal-support interaction, vapor-phase self-assembly
Citation: Liu S, Li J and Xiong H (2022) Thermally-stable single-atom catalysts and beyond: A perspective. Front. Chem. 10:959525. doi: 10.3389/fchem.2022.959525
Received: 01 June 2022; Accepted: 27 June 2022;
Published: 14 July 2022.
Edited by:
Yuefeng Liu, Dalian Institute of Chemical Physics (CAS), ChinaReviewed by:
Sen Lin, Fuzhou University, ChinaCopyright © 2022 Liu, Li and Xiong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haifeng Xiong, aGFpZmVuZ3hpb25nQHhtdS5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.