- 1Institute of Biochemistry, University of Greifswald, Greifswald, Germany
- 2Institute for Materials Discovery, University College London, London, United Kingdom
- 3Department of Chemistry, Institute for Advanced Studies in Basic Sciences (IASBS), Zanjan, Iran
Editorial on the Research Topic
Advances in Inorganic Materials for Supercapacitors and Batteries
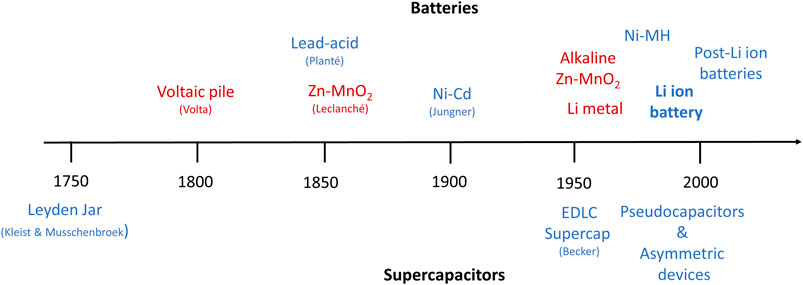
Batteries and supercapacitors have become important elements of everyday life, thanks to the extensive effort contributed to their development. It is interesting to look back at the historical development of batteries and supercapacitors during the last two centuries since the invention of the first battery—called voltaic pile—by Alessandro Volta in 1800 and the first patent on electrochemical double layer capacitors by H. I. Becker in 1957 (Figure 1). A survey of this timeline reveals that 1) progress happens rather incrementally over a long time with few paradigm changes such as lithium ion batteries, and 2) while early developments may be attributed to specific individuals, recent advances are the result of extensive collaborative work of many researchers from different disciplines. Indeed, such schematic illustrations only list the outcome technologies, and the implicit influence of many material scientists, electrochemists, and physicists who discovered new materials, developed double layer theories, and introduced new concepts such as ion insertion/deinsertion cannot be overlooked.
It often happens that improving the performance of an energy storage system becomes increasingly difficult as the system approaches the practically-attainable power/energy density which is usually 50%–60% of the theoretical limit. This along with the insufficient cycling stability especially in the case of batteries constitute two of the most important challenges in today’s energy storage research. Therefore, a marked further development of batteries and supercapacitors definitely requires both fundamental and applied research covering various aspects such as the underlying electrochemical/electronic processes, structure-performance relationships, new storage mechanisms, as well as material discovery and optimization. Accordingly, in the last 2 decades several new research directions have been followed that may be all together called “post-lithium ion batteries.” The most important ones are probably solid state batteries, flow batteries, lithium metal batteries (Li/O2 and Li/S), as well as sodium ion and dual-ion insertion batteries.
The present Research Topic aims at reflecting on the recent advances in batteries and supercapacitors based on inorganic materials. The electrode materials based on inorganics possess tunable properties such as various metal oxidation states, diverse crystal phases, high amenability to defects/vacancies, synergetic intermetallic effects, and so on; hence, there is a large playground for improving their electrochemical performances. A good example of how introducing vacancy (or defect) through metal ion doping can significantly influence the electrochemical performance is presented here by Le Calvez et al. They have doped the perovskite structure AgNbO3 partially by La3+ and observed a substantial enhancement on its capacity for lithium ion insertion/deinsertion. Interestingly, the defective perovskite Ag1-3xLaxNbO3 shows no detectable phase transition during lithium insertion/deinsertion and remains a solid solution. Akintola et al., on the other hand, have modified an anionic MOF with lithium and sodium ions through ionic exchange and investigated them as anodes for lithium and sodium ion batteries. The as-treated MOF electrode exhibits an improved capacity and stability. Norouzi et al. report on the preparation of an electrode based on MoO3/carbon by a sol-gel/hydrothermal process and show that the capacitance of the electrode in aqueous ZnCl2 electrolyte improves due to a carbonization process. Finally, Esteve-Adell et al. have studied the lithium ion storage capacity of graphene nanoplatelets with different surface area as anodes for lithium-ion batteries and have concluded that the introduction of defect/disorder into the graphene structure through porosification is important for enhancing capacity.
Last but not least, as Research Topic Editors, we would like to thank all authors, peer-reviewers, and the Frontiers’ team for their valuable contributions to this Research Topic, and hope the readers will appreciate the reported findings as much as we did.
Author Contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Keywords: batteries, supercapacitors, inorganic materials, metal oxides, ion insertion
Citation: Malaie K, Dall’Agnese Y and Kazemi SH (2022) Editorial: Advances in Inorganic Materials for Supercapacitors and Batteries. Front. Chem. 10:955942. doi: 10.3389/fchem.2022.955942
Received: 29 May 2022; Accepted: 31 May 2022;
Published: 15 June 2022.
Edited and reviewed by:
Xifei Li, Xi’an University of Technology, ChinaCopyright © 2022 Malaie, Dall’Agnese and Kazemi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Keyvan Malaie, a2V5dmFuLm1hbGFpZUB1bmktZ3JlaWZzd2FsZC5kZQ==; Yohan Dall’Agnese, eS5kYWxsYWduZXNlQHVjbC5hYy51aw==; Sayed Habib Kazemi, aGFiaWJrYXplbWlAaWFzYnMuYWMuaXI=
 Keyvan Malaie
Keyvan Malaie Yohan Dall’Agnese
Yohan Dall’Agnese Sayed Habib Kazemi
Sayed Habib Kazemi