- 1State Key Laboratory Breeding Base of Green Pesticide and Agricultural Bioengineering, Key Laboratory of Green Pesticide and Agricultural Bioengineering, Ministry of Education, Guizhou University, Guiyang, China
- 2Tea Research Institute, Guizhou Academy of Agricultural Sciences, Guiyang, China
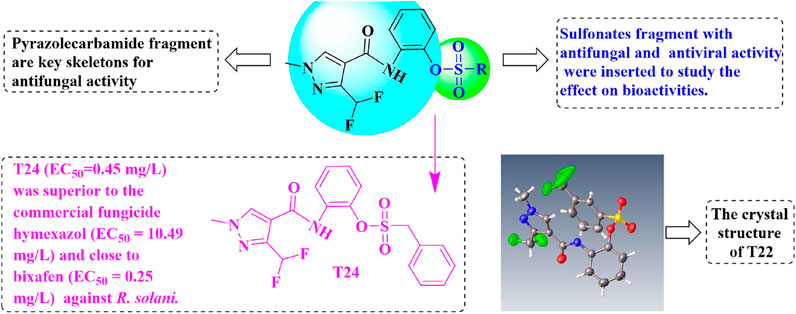
Novel pyrazolecarbamide derivatives bearing a sulfonate fragment were synthesized to identify potential antifungal and antiviral agents. All the structures of the key intermediates and target compounds were confirmed by nuclear magnetic resonance (NMR) and high-resolution mass spectrometry (HRMS). The single-crystal X-ray diffraction of the compound T22 showed that pyrazole carbamide is a sulfonate. The in vitro antifungal activities of the target compounds against Colletotrichum camelliae, Pestalotiopsis theae, Gibberella zeae, and Rhizoctonia solani were evaluated at 50 μg/ml. Among the four pathogens, the target compounds exhibited the highest antifungal activity against Rhizoctonia solani. The compound T24 (EC50 = 0.45 mg/L) had higher antifungal activity than the commercial fungicide hymexazol (EC50 = 10.49 mg/L) against R. solani, almost similar to bixafen (EC50 = 0.25 mg/L). Additionally, the target compounds exhibited protective effects in vivo against TMV. Thus, this study reveals that pyrazolecarbamide derivatives bearing a sulfonate fragment exhibit potential antifungal and antiviral activities.
Introduction
Phytopathogenic microorganisms, such as Rhizoctonia solani, Gibberella zeae, Pestalotiopsis theae, Colletotrichum camelliae, and tobacco mosaic virus (TMV) reduce the yield and quality of food and cash crops (Fisher et al., 2012). Chemical pesticides are still the most commonly used control measure for these diseases; however, the associated pesticide resistance and environmental hazards (Wei et al., 2020) impede their usage. Therefore, there is an urgent need to develop novel eco-friendly antifungal and antiviral agents agent with low toxicity and high efficiency.
Pyrazole and its derivatives have received considerable attention because of their diverse agrochemical and pharmaceutical applications. Most pyrazole derivatives exhibit a broad spectrum of biological activities, including antifungal (Kanungo and Joshi, 2014; Mu et al., 2016; Yan et al., 2018), insecticidal (Wu et al., 2012; Jiang et al., 2020), antibacterial (El Shehry et al., 2018; Wang et al., 2021), and other antimicrobial activities (Kasiotis et al., 2014; Saleh et al., 2020). Especially, pyrazole carboxamide derivatives, such as penthiopyrad, furametpyr, penflufen, isopyrazam, and bixafen, which could inhibit the succinate dehydrogenase, have been developed and commercialized as fungicides (Si et al., 2019).
Sulfonates are also widely applied in agrochemical and medical industries because of their insecticidal (Sun et al., 2013; Wang et al., 2015), antifungal (Kang et al., 2019; Zhou et al., 2022), and antibacteria (Su et al., 2021) Moreover, the heterocyclic compounds containing aryl sulfonate moiety exhibit excellent antiviral activities (Zeng et al., 2010; Huang et al., 2015; Hadházi et al., 2017).
Therefore, we designed and synthesized a series of novel pyrazolecarbamide derivatives bearing a sulfonate moiety based on the active splicing principle and used the mycelial growth rate and half-leaf blight spot methods to evaluate their antifungal and antiviral activities.
Materials and Methods
Chemistry
The 1H and 13C NMR spectra were recorded in CDCl3 using 400 and 101 MHz spectrophotometers (Bruker BioSpin GmbH, Rheinstetten, Germany), respectively, while high-resolution mass spectrometry (HRMS) was performed using Thermo Scientific Q Exactive (Thermo Fisher Scientific, Massachusetts, America). The X-ray crystallographic data were collected and processed on a D8 Quest X-ray diffractometer (Bruker BioSpin GmbH, Rheinstetten, German). All solvents were dried using the standard methods and distilled before use.
3-(Difluoromethyl)-1-Methyl-1H-Pyrazole-4-Carboxylic Acid (4)
As shown in Scheme 1, the key intermediate 4 was synthesized using a previously published three-step procedure (Wang et al., 2020). White powder, yield 46%. m.p 201.1-201.9°C.1H NMR (400 MHz, CDCl3) δ 7.98 (s, 1H), 7.12 (t, J = 54.3 Hz, 1H), 4.02 (s, 3H). HRMS (ESI): calculated for C6H6F2N2O2 [M + Na]+: 199.02950, found: 199.02896.
2-(Difluoromethyl)-N-(2-Hydroxyphenyl)-1-Methyl-1H-Pyrazole-4-Carboxamide (6)
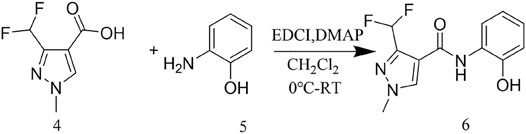
A mixture of 1-Ethyl-3-(3-dimethyllaminopropyl)carbodiimide hydrochloride (EDCI, 120 mmol), Intermediate 4 (100 mmol) and o-aminophenol (100 mmol), and dimethylaminopyridine (DMAP, 10 mmol) were dissolved in CH2Cl2 (500 ml) at −10°C for 1 h. Thereafter, the mixture was stirred at room temperature for 8 h, and the key intermediate 6 was purified using column chromatography. Light yellow solid, yield 62%. m.p. 181.1-182.3°C .1H NMR (400 MHz, CDCl3) δ 8.99 (s, 1H), 8.37 (s, 1H), 8.08 (s, 1H), 7.17 (td, J = 7.7, 1.6 Hz, 1H), 7.05 (ddd, J = 7.8, 6.0, 1.5 Hz, 2H), 6.91 (dd, J = 7.4,1.5 Hz, 1H), 6.88 (t, J = 54.1Hz, 1H), 3.97 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 160.74, 149.12(t, J = 26.5 Hz), 136.77, 127.62, 125.42, 122.63, 120.51, 120.10, 115.5, 112.18, 110.40 (t, J = 235.3 Hz), 39.71. HRMS (ESI): calculated for C12H11F2N3O2 [M + Na]+: 290.07170, found: 290.07126.
General Procedure for the Preparation of the Target Compounds (T1-27)
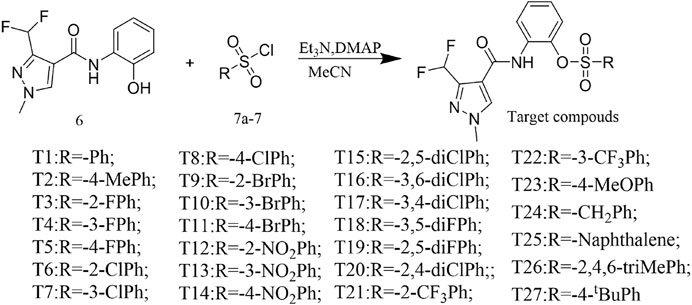
Catalytic DMAP, arylsulfonyl chloride (1.1 mmol), and Et3N (2 mmol) were added to a stirred CH3CN (20 ml) solution of the key intermediate 6 (1 mmol), and the reaction was monitored at room temperature using TLC. Thereafter, the solvent was removed by rotary evaporation, and 10 ml of water was added to the residue, followed by extraction of the aqueous layer three times (30 ml × 3) using ethyl acetate. The organic layers were then combined and dried using anhydrous Na2SO4 and later concentrated under reduced pressure to form a crude product, purified using flash chromatography to obtain the target product.
2-(3-(Difluoromethyl)-1-Methyl-1H-Pyrazole-4-Carboxamido)Phenyl Benzenesulfonate (T1)
Gray powder, yield 72%. m.p. 138.3-139.6°C .1H NMR (400 MHz, CDCl3) δ 8.33 (s, 1H), 8.27 (dd, J = 8.3, 1.6 Hz, 1H), 7.92 (s, 1H), 7.87–7.80 (m, 2H), 7.70–7.61 (m, 1H), 7.52–7.45 (m, 2H), 7.26 (dd, J = 15.7, 1.5 Hz, 1H), 7.08 (t, J = 54.1 Hz, 1H), 7.04–6.97 (m, 1H), 6.90 (dd, J = 8.2, 1.5 Hz, 1H), 4.00 (s, 3H).13C NMR (101 MHz, CDCl3) δ 159.33, 144.86(t, J = 26.5 Hz), 139.41, 134.99, 134.50, 133.41, 131.01, 129.44(×2), 128.65(×2), 128.01, 124.78, 123.27, 122.71, 116.68, 110.50 (t, J = 235.3 Hz), 39.92. HRMS (ESI): calculated for C18H15F2N3O4S[M + Na]+: 430.06490, found: 430.06531.
2-(3-(Difluoromethyl)-1-Methyl-1H-Pyrazole-4-Carboxamido)Phenyl 4-Methylbenzenesulfonate (T2)
Light yellow power, yield 79%. m.p. 126.2-126.9°C .1H NMR (400 MHz, CDCl3) δ 8.35 (s, 1H), 8.28 (dd, J = 8.3, 1.6 Hz, 1H), 7.92 (s, 1H), 7.76–7.64 (m, 2H), 7.31–7.21 (m, 4H), 7.09 (t, J = 54.1 Hz, 1H), 7.00 (td, J = 7.9, 1.6 Hz, 1H), 6.88 (dd, J = 8.2, 1.5 Hz, 1H), 4.00 (s, 3H), 2.42 (s, 3H).13C NMR (101 MHz, CDCl3) δ 159.35, 146.34, 145.05(t, J = 29.3 Hz), 139.50, 133.19, 131.54, 131.13, 130.07(×2), 128.72(×2), 127.94, 124.73, 123.20, 122.78, 116.82, 110.44(t, J = 235.8 Hz), 39.92, 21.87. HRMS (ESI): calculated for C19H17F2N3O4S [M + Na]+: 444.08055, found: 444.08109.
2-(3-(Difluoromethyl)-1-Methyl-1H-Pyrazole-4-Carboxamido)Phenyl 2-Fluorobenzenesulfonate (T3)
White powder, yield 78%. m.p. 123.9-124.5°C.1H NMR (400 MHz, CDCl3) δ 8.40 (s, 1H), 8.33 (dd, J = 8.3, 1.6 Hz, 1H), 7.96 (s, 1H), 7.89 (ddd, J = 8.3, 6.9, 1.8 Hz, 1H), 7.76–7.66 (m, 1H), 7.30 (qd, J = 7.7, 1.3 Hz, 3H), 7.26–7.19 (m, 2H), 7.12 (t, J = 54.0 Hz, 1H), 7.15 (dd, J = 8.2, 1.5 Hz, 1H), 7.10-7.02 (m, 1H), 4.02 (s, 3H).13C NMR (101 MHz, CDCl3) δ 160.92, 159.53, 158.34, 138.73, 137.67, 137.58, 132.71, 131.57, 131.09, 128.31, 125.00, 124.96, 124.88, 123.24, 122.66, 117.81, 117.60, 116.77, 112.53, 110.19, 39.97. HRMS (ESI): calculated for C18H14F3N3O4S [M + Na]+: 448.05548, found: 448.05454.
2-(3-(Difluoromethyl)-1-Methyl-1H-Pyrazole-4-Carboxamido)Phenyl 3-Fluorobenzenesulfonate (T4)
Gray powder, yield 76%. m.p. 119.3-120.9°C.1H NMR (400 MHz, CDCl3) δ 8.31–8.19 (m, 2H), 7.92 (s, 1H), 7.63–7.53 (m, 2H), 7.47 (td, J = 8.1, 5.2 Hz, 1H), 7.33 (tdd, J = 8.3, 2.5, 1.0 Hz, 1H), 7.30–7.27 (m, 1H), 7.05 (ddd, J = 8.7, 7.2, 1.5 Hz, 1H),7.02 (t, J = 54.1 Hz, 1H), 7.00 (dd, J = 8.2, 1.7 Hz, 1H), 3.99 (s, 3H).13C NMR (101 MHz, CDCl3) δ 163.61, 161.09, 159.26, 144.67, 144.40, 144.13, 139.44, 136.61, 136.53, 133.98, 131.37, 131.30, 130.85, 128.18, 124.96, 124.58, 124.54, 123.55, 122.56, 122.35, 122.14, 116.55, 116.08, 115.83, 113.16, 110.82, 108.49, 39.86. HRMS (ESI): calculated for C18H14F3N3O4S [M + Na]+:448.05548, found: 448.05454.
2-(3-(Difluoromethyl)-1-Methyl-1H-Pyrazole-4-Carboxamido)Phenyl 4-Fluorobenzenesulfonate (T5)
Light yellow powder, yield 69%. m.p. 165.2-165.9°C.1H NMR (400 MHz, CDCl3) δ 8.32–8.21 (m, 2H), 7.93 (s, 1H), 7.88–7.80 (m, 2H), 7.33–7.26 (m, 1H), 7.18–7.07 (m, 2H), 7.05 (ddd, J = 8.9, 7.4, 1.6 Hz, 1H),7.00 (t, J = 54.0 Hz, 1H), 6.98 (dd, J = 8.3, 1.6 Hz, 1H), 4.00 (s, 3H).13C NMR (101 MHz, CDCl3) δ 166.37(d, J = 259.6 Hz), 159.22, 144.15(t, J = 26.2 Hz), 144.18, 139.45, 134.24, 131.69, 131.59, 130.89, 130.69, 128.13, 124.92, 123.48, 122.78, 116.96, 116.73, 116.65, 110.90(t, J = 235.8 Hz), 105.41, 39.90. HRMS (ESI): calculated for C18H14F3N3O4S [M + Na]+:448.05548, found: 448.05454.
2-(3-(Difluoromethyl)-1-Methyl-1H-Pyrazole-4-Carboxamido)Phenyl 2-Chlorobenzenesulfonate (T6)
Gray powder, yield 70%. m.p. 116.3-117.2°C.1H NMR (400 MHz, CDCl3) δ 8.48 (s, 1H), 8.39–8.30 (m, 1H), 8.03 (dd, J = 8.0, 1.5 Hz, 1H), 7.97 (s, 1H), 7.67–7.57 (m, 2H), 7.44 (ddd, J = 8.0, 7.1, 1.6 Hz, 1H), 7.29 (ddd, J = 8.6, 5.6, 3.4 Hz, 1H), 7.11 (d, J = 54.1 Hz, 1H), 7.06–6.99 (m, 2H), 4.01 (s, 3H).13C NMR (101 MHz, CDCl3) δ 159.59, 145.46(t, J = 26.5 Hz), 139.00, 135.92, 133.48, 133.15, 132.92, 132.54, 132.50, 131.21, 128.26, 127.52, 124.89, 123.48, 122.61, 116.70, 110.23 (t, J = 236.3 Hz), 39.96. HRMS (ESI): calculated for C18H14ClF2N3O4S [M + Na]+: 464.02593, found: 464.02521.
2-(3-(Difluoromethyl)-1-Methyl-1H-Pyrazole-4-Carboxamido)Phenyl 3-Chlorobenzenesulfonate (T7)
Light yellow powder, yield 73%. m.p. 110.0-111.9°C.1H NMR (400 MHz, CDCl3) δ 8.24 (dd, J = 8.2, 1.4 Hz, 1H), 8.19 (s, 1H), 7.91 (s, 1H), 7.86 (t, J = 1.9 Hz, 1H), 7.63 (dt, J = 7.9, 1.4 Hz, 1H), 7.58 (ddd, J = 8.1, 2.1, 1.0 Hz, 1H), 7.41 (t, J = 8.0 Hz, 1H), 7.29 (ddd, J = 8.5, 6.6, 2.4 Hz, 1H), 7.11–7.02 (m, 2H), 7.01 (d, J = 54.1 Hz, 1H), 3.99 (s, 3H).13C NMR (101 MHz, CDCl3) δ 159.16, 144.30(t, J = 27.3 Hz), 139.40, 136.43, 135.79, 134.99, 134.11, 130.81, 130.70, 128.47, 128.22, 126.77, 124.98, 123.52, 122.72, 116.53, 110.90(t, J = 235.3 Hz), 39.88. HRMS (ESI): calculated for C18H14ClF2N3O4S [M + Na]+: 464.02593, found: 464.02521.
2-(3-(Difluoromethyl)-1-Methyl-1H-Pyrazole-4-Carboxamido)Phenyl 4-Chlorobenzenesulfonate (T8)
Light yellow powder, yield 79%. m.p. 185.6-185.9°C.1H NMR (400 MHz, CDCl3) δ 8.24 (dd, J = 8.2, 1.5 Hz, 2H), 8.22 (s, 1H), 7.93 (s, 1H), 7.77–7.69 (m, 2H), 7.44–7.36 (m, 2H), 7.29 (ddd, J = 8.6, 7.2, 1.8 Hz, 1H), 7.07 (td, J = 7.7, 1.5 Hz, 2H), 7.02 (dd, J = 8.2, 1.8 Hz, 1H), 6.98 (t, J = 54.1 Hz, 1H), 3.99 (s, 3H).13C NMR (101 MHz, CDCl3) δ 159.15, 143.97(t, J = 26.7 Hz), 141.62, 139.54, 134.48, 133.37, 130.80, 130.04(×2), 129.75(×2), 128.15, 125.00, 123.59, 122.89, 116.64, 111.03(t, J = 234.7 Hz), 39.88. HRMS (ESI): calculated for C18H14ClF2N3O4S [M + Na]+: 464.02593, found: 464.02521.
2-(3-(Difluoromethyl)-1-Methyl-1H-Pyrazole-4-Carboxamido)Phenyl 2-Bromobenzenesulfonate (T9)
Gray powder, yield 69%. m.p. 133.9-134.2°C.1H NMR (400 MHz, CDCl3) δ 8.48 (s, 1H), 8.36–8.28 (m, 1H), 8.04 (dd, J = 7.8, 1.9 Hz, 1H), 7.98 (s, 1H), 7.81 (dd, J = 7.8, 1.4 Hz, 1H), 7.53 (td, J = 7.6, 1.9 Hz, 1H), 7.48 (td, J = 7.7, 1.4 Hz, 1H), 7.32–7.25 (m, 1H), 7.00 (t, J = 54.0 Hz, 1H), 7.04–6.98 (m, 2H), 4.00 (s, 3H).13C NMR (101 MHz, CDCl3) δ 159.59, 145.42(t, J = 25.8 Hz), 139.06, 136.05, 135.79, 134.98, 132.99, 132.79, 131.24, 128.23, 128.07, 124.89, 123.52, 122.66, 121.38, 116.82, 110.22(t, J = 235.6 Hz), 39.94. HRMS (ESI): calculated for C18H14BrF2N3O4S [M + Na]+: 507.97542, found: 507.97227.
2-(3-(Difluoromethyl)-1-Methyl-1H-Pyrazole-4-Carboxamido)Phenyl 3-Bromobenzenesulfonate (T10)
Light yellow powder, yield 80%. m.p. 128.4-128.5°C.1H NMR (400 MHz, CDCl3) δ 8.30–8.21 (m, 1H), 8.18 (s, 1H), 8.01 (t, J = 1.9 Hz, 1H), 7.91 (s, 1H), 7.73 (ddd, J = 8.1, 1.9, 1.0 Hz, 1H), 7.66 (ddd, J = 7.9, 1.8, 1.0 Hz, 1H), 7.33 (t, J = 8.0 Hz, 1H), 7.29 (td, J = 6.1, 3.3 Hz, 1H), 7.11–7.03 (m, 2H), 7.01 (t, J = 54.1 Hz, 1H), 3.99 (s, 3H).13C NMR (101 MHz, CDCl3) δ 159.13, 144.26 (t, J = 27.3 Hz), 139.35, 137.90, 136.47, 134.16, 131.24, 130.87, 130.75, 128.23, 127.18, 125.00, 123.50, 123.38, 122.78, 116.47, 110.91(t, J = 235.3 Hz), 39.91.HRMS (ESI): calculated for C18H14BrF2N3O4S [M + Na]+: 507.97542, found: 507.97227.
2-(3-(Difluoromethyl)-1-Methyl-1H-Pyrazole-4-Carboxamido)Phenyl 4-Bromobenzenesulfonate (T11)
Light yellow powder, yield 79%. m.p. 175.8-176.4°C.1H NMR (400 MHz, CDCl3) δ 8.23 (dd, J = 8.2, 1.5 Hz, 1H), 8.20 (s, 1H), 7.94 (s, 1H), 7.68–7.61 (m, 2H), 7.60–7.50 (m, 2H), 7.29 (ddd, J = 8.5, 7.0, 1.9 Hz, 1H), 7.07 (ddd, J = 8.6, 7.1, 1.5 Hz, 1H), 7.03 (dd, J = 8.2, 1.9 Hz, 1H), 6.97 (t, J = 54.1 Hz, 1H), 4.00 (s, 3H).13C NMR (101 MHz, CDCl3) δ 159.12, 143.91(t, J = 28.8 Hz), 139.54, 134.57, 133.94, 132.73(×2), 130.77, 130.26, 130.02(×2), 128.15, 125.02, 123.60, 122.92, 116.60, 111.06(t, J = 235.02 Hz), 39.89.HRMS (ESI): calculated for C18H14BrF2N3O4S [M + Na]+: 507.97542, found: 507.97227.
2-(3-(Difluoromethyl)-1-Methyl-1H-Pyrazole-4-Carboxamido)Phenyl 2-Nitrobenzenesulfonate (T12)
Light yellow powder, yield 83%. m.p. 146.0-147.8°C.1H NMR (400 MHz, CDCl3) δ 8.31 (s, 1H), 8.26 (dd, J = 8.3, 1.6 Hz, 1H), 7.92 (dd, J = 7.9, 1.4 Hz, 1H), 7.90 (d, J = 1.2 Hz, 1H), 7.81 (td, J = 7.8, 1.4 Hz, 1H), 7.69 (td, J = 7.8, 1.3 Hz, 1H), 7.66 (dd, J = 7.9, 1.3 Hz, 1H), 7.38 (dd, J = 8.3, 1.5 Hz, 1H), 7.34–7.28 (m, 1H), 7.13 (ddd, J = 8.3, 7.4, 1.6 Hz, 1H),7.11 (t, J = 54.1 Hz, 1H), 4.00 (s, 3H).13C NMR (101 MHz, CDCl3) δ 159.55, 148.37, 146.02(t, J = 25.3 Hz), 138.50, 136.28, 132.83, 132.35(×2), 130.76, 128.57, 128.06, 125.10, 124.90, 123.43, 123.06, 116.02(t, J = 2.7 Hz), 109.75(t, J = 236.8 Hz), 39.91. HRMS (ESI): calculated for C18H14F2N4O6S [M + Na]+: 475.04998, found: 475.04948.
2-(3-(Difluoromethyl)-1-Methyl-1H-Pyrazole-3-Carboxamido)Phenyl 4-Nitrobenzenesulfonate (T13)
Gray powder, yield 79%. m.p. 160.4-160.9°C.1H NMR (400 MHz, CDCl3) δ 8.70 (t, J = 2.0 Hz, 1H), 8.42 (ddd, J = 8.2, 2.2, 1.1 Hz, 1H), 8.14 (dd, J = 8.2, 1.6 Hz, 1H), 8.11 (d, J = 4.6 Hz, 1H), 7.99 (dt, J = 8.0, 1.3 Hz, 1H), 7.88 (s, 1H), 7.66 (t, J = 8.1 Hz, 1H), 7.32 (ddd, J = 8.3, 7.5, 1.5 Hz, 1H), 7.26 (dd, J = 8.3, 1.5 Hz, 1H), 7.14 (ddd, J = 8.5, 7.4, 1.6 Hz, 1H), 6.92 (t, J = 54.1 Hz, 1H), 3.98 (s, 3H).13C NMR (101 MHz, CDCl3) δ 158.87, 148.24, 143.29(t, J = 27.7 Hz),, 139.43, 136.92, 135.14, 134.01, 130.78, 130.38, 129.07, 128.44, 125.34, 123.96, 123.77, 122.79, 116.11, 111.46(t, J = 234.2 Hz), 39.83.HRMS (ESI): calculated for C18H14F2N4O6S [M + Na]+: 475.04998, found:475.04948.
2-(3-(Difluoromethyl)-1-Methyl-1H-Pyrazole-4-Carboxamido)Phenyl 4-Nitrobenzenesulfonate (T14)
Light yellow powder, yield 80%. m.p. 198.9-199.6°C.1H NMR (400 MHz, CDCl3) δ 8.24–8.17 (m, 2H), 8.15 (dd, J = 8.3, 1.6 Hz, 1H), 8.07 (s, 1H), 7.99–7.94 (m, 2H), 7.86 (s, 1H), 7.33 (td, J = 7.8, 1.6 Hz, 1H), 7.21 (dd, J = 8.3, 1.6 Hz, 1H), 7.14 (ddd, J = 8.5, 7.3, 1.6 Hz, 1H), 6.88 (t, J = 54.1 Hz, 1H), 3.98 (s, 3H).13C NMR (101 MHz, CDCl3) δ 158.82, 151.19, 144.20(t, J = 26.5 Hz), 140.75, 139.69, 135.60, 130.30, 130.00(×2), 128.42, 125.40, 124.41(×2), 124.16, 123.00, 116.31, 111.61(t, J = 234.7 Hz), 39.81. HRMS (ESI): calculated for C18H14F2N4O6S [M + Na]+: 475.04998, found:475.04948.
2-(3-(Difluoromethyl)-1-Methyl-1H-Pyrazole-4-Carboxamido)Phenyl 2,5-Dichlorobenzenesulfonate (T15)
Light yellow powder, yield 82%. m.p. 155.6-157.3°C.1H NMR (400 MHz, CDCl3) δ 8.40 (s, 1H), 8.35–8.27 (m, 1H), 8.01 (d, J = 2.4 Hz, 1H), 7.95 (s, 1H), 7.57 (dd, J = 8.6, 2.4 Hz, 1H), 7.52 (d, J = 8.5 Hz, 1H), 7.31 (ddd, J = 8.5, 5.4, 3.5 Hz, 1H), 7.10–7.03 (m, 2H), 7.06 (t, J = 54.1 Hz, 1H), 3.99 (s, 3H).13C NMR (101 MHz, CDCl3) δ 159.52, 145.01(t, J = 26.2 Hz), 138.96, 135.74, 134.44, 133.74, 133.54, 133.36, 132.06, 131.70, 131.01, 128.43, 125.11, 123.77, 122.47, 116.62, 110.43(t, J = 235.3 Hz), 39.93. HRMS (ESI): calculated for C18H13Cl2F2N3O4S [M + Na]+: 497.98696, found: 497.98602.
2-(3-(Difluoromethyl)-1-Methyl-1H-Pyrazole-4-Carboxamido)Phenyl 3,5-Dichlorobenzenesulfonate (T16)
Gray powder, yield 78%. m.p. 128.9-129.5°C.1H NMR (400 MHz, CDCl3) δ 8.27–8.22 (m, 1H), 8.15 (s, 1H), 7.94 (s, 1H), 7.68 (d, J = 1.9 Hz, 2H), 7.56 (t, J = 1.9 Hz, 1H), 7.33 (ddd, J = 8.5, 5.7, 3.3 Hz, 1H), 7.15–7.12 (m, 2H), 7.06 (t, J = 54.0 Hz, 1H), 3.99 (s, 3H).13C NMR (101 MHz, CDCl3) δ 159.03, 143.78(t, J = 25.6 Hz), 139.33, 137.56, 136.50, 134.78, 134.76, 130.61, 128.44, 126.84(×2), 125.19, 123.82, 122.63, 116.40, 111.22(t, J = 234.9 Hz), 76.84, 39.89. HRMS (ESI): calculated for C18H13Cl2F2N3O4S [M + Na]+: 497.98696, found: 497.98602.
2-(3-(Difluoromethyl)-1-Methyl-1H-Pyrazole-4-Carboxamido)Phenyl 3,4-Dichlorobenzenesulfonate (T17)
Gray powder, yield 79%. m.p. 173.4-174.4°C.1H NMR (400 MHz, CDCl3) δ 8.18 (dd, J = 8.2, 1.5 Hz, 1H), 8.11 (d, J = 4.1 Hz, 1H), 7.92 (d, J = 2.4 Hz, 2H), 7.56–7.43 (m, 2H), 7.32 (ddd, J = 8.5, 7.0, 1.9 Hz, 1H), 7.20–7.09 (m, 2H), 6.93 (t, J = 54.1 Hz, 1H), 3.99 (s, 3H).13C NMR (101 MHz, CDCl3) δ 158.92, 143.41(t, J = 28.3 Hz), 139.81, 139.59, 135.04, 134.65, 134.32, 131.42, 130.50, 130.29, 128.31, 127.52, 125.24, 123.89, 123.05, 116.33, 111.39(t, J = 234.8 Hz), 39.84.HRMS (ESI): calculated for C18H13Cl2F2N3O4S [M + Na]+: 497.98696, found: 497.98602.
2-(3-(Difluoromethyl)-1-Methyl-1H-Pyrazole-4-Carboxamido)Phenyl 3,5-Difluorobenzenesulfonate (T18)
Gray powder, yield 86%. m.p. 143.1-144.0°C.1H NMR (400 MHz, CDCl3) δ 8.33–8.12 (m, 2H), 7.94 (s, 1H), 7.45–7.22 (m, 3H), 7.11–7.03 (m, 3H),7.00 (t, J = 54.0 Hz, 1H), 3.98 (s, 3H).13C NMR (101 MHz, CDCl3) δ 164.11, 164.00, 161.57, 161.45, 159.20, 144.23, 143.95, 143.68, 139.45, 137.91, 137.82, 137.73, 134.57, 130.68, 128.36, 125.15, 123.86, 122.38, 116.42, 113.44, 112.50, 112.41, 112.30, 112.21, 111.11, 110.83, 110.58, 110.33(t, J = 235.4 Hz), 39.84. HRMS (ESI): calculated for C18H13F4N3O4S [M + Na]+:466.04606, found: 466.04663.
2-(3-(Difluoromethyl)-1-Methyl-1H-Pyrazole-4-Carboxamido)Phenyl 2,5-Difluorobenzenesulfonate (T19)
Light yellow powder, yield 80%. m.p. 141.4-141.6°C.1H NMR (400 MHz, CDCl3) δ 8.33 (s, 1H), 8.29 (dd, J = 8.2, 1.6 Hz, 1H), 7.95 (s, 1H), 7.58 (ddd, J = 7.0, 5.2, 3.2 Hz, 1H), 7.43–7.32 (m, 1H), 7.33–7.27 (m, 1H), 7.25–7.20 (m, 1H), 7.18 (dd, J = 8.1, 1.7 Hz, 2H), 7.09 (t, J = 54.1 Hz, 1H), 7.08 (dd, J = 15.6, 1.6 Hz, 2H), 3.99 (s, 3H).13C NMR (101 MHz, CDCl3) δ 159.50, 159.06, 156.94, 156.55, 154.39, 145.44, 145.18, 144.92, 138.78, 133.15, 130.87, 128.43, 125.06, 124.42, 124.34, 124.27, 124.19, 124.10, 124.04, 123.55, 122.51, 119.42, 119.34, 119.18, 119.10, 118.33, 118.06, 116.57, 112.73, 110.39, 108.05, 39.91. HRMS (ESI): calculated for C18H13F4N3O4S [M + Na]+: 466.04606, found: 466.04663.
2-(3-(Difluoromethyl)-1-Methyl-1H-Pyrazole-4-Carboxamido)Phenyl 2,4-Difluorobenzenesulfonate (T20)
light yellow powder, yield 79%. m.p. 148.7-149.6°C.1H NMR (400 MHz, CDCl3) δ 8.36 (s, 1H), 8.30 (dd, J = 8.3, 1.6 Hz, 1H), 7.95 (s, 1H), 7.90 (ddd, J = 8.9, 7.8, 5.9 Hz, 1H), 7.30 (ddd, J = 8.5, 7.4, 1.6 Hz, 1H), 7.15 (dd, J = 8.3, 1.6 Hz, 1H), 7.10–7.05 (m, 1H),7.08 (t, J = 54.1 Hz, 1H), 7.04–6.95 (m, 2H), 4.00 (s, 3H).13C NMR (101 MHz, CDCl3) δ 159.46, 145.53, 138.87, 135.14, 133.40, 133.01, 132.81, 132.69, 130.97, 129.09, 129.00, 128.94, 128.76, 128.36, 124.93, 123.41, 123.01, 121.07, 116.49, 112.41, 110.07, 107.73, 39.90. HRMS (ESI): calculated for C18H13F4N3O4S [M + Na]+: 466.04606, found: 466.04663.
2-(3-(Difluoromethyl)-1-Methyl-1H-Pyrazole-4-Carboxamido)Phenyl 2-(Trifluoromethyl)Benzenesulfonate (T21)
Gray powder, yield 69%. m.p. 120.6-121.2°C.1H NMR (400 MHz, CDCl3) δ 8.36 (s, 1H), 8.30 (dd, J = 8.3, 1.6 Hz, 1H), 7.95 (s, 1H), 7.90 (ddd, J = 8.9, 7.8, 5.9 Hz, 1H), 7.30 (ddd, J = 8.5, 7.4, 1.6 Hz, 1H), 7.15 (dd, J = 8.3, 1.6 Hz, 1H), 7.10–7.05 (m, 2H),7.09 (t, J = 54.1 Hz, 1H), 7.04–6.95 (m, 2H), 4.00 (s, 3H).13C NMR (101 MHz, CDCl3) δ 168.62, 166.13, 166.02, 162.04, 161.91, 159.48, 159.31, 145.37, 145.11, 144.85, 138.85, 133.57, 133.46, 133.26, 130.97, 128.36, 125.00, 123.51, 122.57, 119.62, 119.49, 116.67, 112.78, 112.75, 112.56, 112.53, 110.44, 108.10, 106.65, 106.41, 106.39, 106.15, 39.92.HRMS (ESI): calculated for C19H14F5N3O4S [M + Na]+: 498.05229, found: 498.05078.
2-(3-(Difluoromethyl)-1-Methyl-1H-Pyrazole-3-Carboxamido)Phenyl 3-(Trifluoromethyl)Benzenesulfonate (T22)
Light yellow powder, yield 73%. m.p. 147.2-148.3°C.1H NMR (400 MHz, CDCl3) δ 8.27–8.15 (m, 2H), 8.12 (s, 1H), 7.92 (d, J = 7.8 Hz, 1H), 7.91 (s, 1H), 7.87 (d, J = 7.9 Hz, 1H), 7.62 (t, J = 7.9 Hz, 1H), 7.29 (ddd, J = 8.6, 5.8, 3.1 Hz, 1H), 7.13–7.03 (m, 2H), 6.96 (t, J = 54.1 Hz, 1H), 3.97 (s, 3H).13C NMR (101 MHz, CDCl3) δ 159.09, 144.17, 143.89, 143.62, 139.32, 136.04, 134.52, 132.64, 132.30, 131.96, 131.82, 131.63, 131.46, 131.43, 131.39, 131.36, 130.75, 130.30, 128.28, 125.69, 125.65, 125.62, 125.58, 125.02, 124.22, 123.59, 122.64, 121.50, 116.36, 113.43, 111.11, 108.78, 39.79.HRMS (ESI): calculated for C19H14F5N3O4S [M + Na]+: 498.05229, found: 498.05078.
2-(3-(Difluoromethyl)-1-Methyl-1H-Pyrazole-4-Carboxamido)Phenyl 4-Methoxybenzenesulfonate (T23)
Gray powder, yield 83%. m.p. 130.0-131.1°C.1H NMR (400 MHz, CDCl3) δ 8.35 (s, 1H), 8.28 (dd, J = 8.2, 1.6 Hz, 1H), 7.91 (s, 1H), 7.78–7.70 (m, 2H), 7.30–7.23 (m, 1H), 7.09 (t, J = 54.0 Hz, 1H), 7.01 (td, J = 7.8, 1.6 Hz, 1H), 6.93-6.86 (m, 3H), 4.00 (s, 3H), 3.86 (s, 3H).13C NMR (101 MHz, CDCl3) δ 164.70, 159.36, 144.97(t, J = 25.8 Hz), 139.56, 133.27, 131.15, 131.02(×2), 127.90, 125.68, 124.72, 123.18, 122.93, 116.83, 114.64(×2), 110.48(t, J = 235.4 Hz), 55.93, 39.90.HRMS (ESI): calculated for C19H17F2N3O5S [M + Na]+:460.07547, found: 460.07503.
2-(3-(Difluoromethyl)-1-Methyl-1H-Pyrazole-4-Carboxamido)Phenyl Phenylmethanesulfonate (T24)
Light yellow powder, yield 83%. m.p. 123.4-124.2°C.1H NMR (400 MHz, CDCl3) δ 8.35 (dd, J = 8.3, 1.6 Hz, 1H), 8.27 (s, 1H), 7.68 (s, 1H), 7.51–7.44 (m, 2H), 7.40 (dd, J = 5.0, 2.0 Hz, 3H), 7.31 (td, J = 7.9, 1.5 Hz, 1H), 7.20 (m, 1H),7.15 (t, J = 54.0 Hz, 1H), 7.09 (td, J = 7.8, 1.6 Hz, 1H), 7.02–6.97 (m, 1H), 4.65 (s, 2H), 3.97 (s, 3H).13C NMR (101 MHz, CDCl3) δ 159.54, 145.26(t, J = 25.8 Hz),138.25-132.95, 131.24, 131.09(×2), 129.67, 129.24, 128.23(×2), 126.88, 125.03, 123.19, 122.90,115.83,110.39 (t, J = 235.4 Hz), 57.29, 39.92. HRMS (ESI): calculated for C19H17F2N3O4S [M + Na]+:444.08055, found: 448.07975.
2-(3-(Difluoromethyl)-1-Methyl-1H-Pyrazole-4-Carboxamido)Phenyl Naphthalene-2-Sulfonate (T25)
Light yellow powder, yield 80%. m.p. 158.7-159.5°C.1H NMR (400 MHz, CDCl3) δ 8.43 (d, J = 1.9 Hz, 1H), 8.30–8.17 (m, 2H), 7.94–7.81 (m, 3H), 7.71 (ddd, J = 13.8, 8.5, 1.6 Hz, 2H), 7.65–7.58 (m, 2H), 7.31–7.21 (m, 1H), 7.04–6.99 (m, 2H), 6.98 (t, J = 54.1 Hz, 1H), 3.90 (s, 3H).13C NMR (101 MHz, CDCl3) δ 159.11, 144.39(t, J = 25.7 Hz), 139.66, 135.70, 133.52, 131.91, 131.81, 130.94, 130.72, 129.89, 129.82, 129.55, 128.15, 128.01, 128.00, 124.87, 123.32, 123.04, 122.76, 116.48, 110.66 (t, J = 234.8 Hz, 1H), 39.76. HRMS (ESI): calculated for C22H17F2N3O4S [M + Na]+:480.08055, found: 448.08005.
2-(3-(Difluoromethyl)-1-Methyl-1H-Pyrazole-4-Carboxamido)Phenyl 2,4,6-Trimethylbenzenesulfonate (T26)
White powder, yield 81%. m.p. 155.0-155.4°C.1H NMR (400 MHz, CDCl3) δ 8.56 (s, 1H), 8.35 (dd, J = 8.3, 1.6 Hz, 1H), 7.95 (s, 1H), 7.28–7.23 (m, 1H), 7.15 (t, J = 54.1 Hz, 1H), 7.01 (s, 2H), 6.91 (td, J = 7.8, 1.6 Hz, 1H), 6.52 (dd, J = 8.2, 1.5 Hz, 1H), 4.01 (s, 3H), 2.55 (s, 6H), 2.35 (s, 3H).13C NMR (101 MHz, CDCl3) δ 159.50, 145.40 (t, J = 25.3 Hz), 144.77, 140.77(×2), 139.25, 132.64, 132.05(×2), 131.52, 129.59, 127.78, 124.57, 123.25, 121.97, 116.80, 110.09 (t, J = 234.8Hz, 1H), 39.85, 22.85(×2), 21.23.HRMS (ESI): calculated for C21H21F2N3O4S [M + Na]+:472.11185, found: 472.11150.
2-(3-(Difluoromethyl)-1-Methyl-1H-Pyrazole-4-Carboxamido)Phenyl 4-(Tert-butyl)Benzenesulfonate (T27)
Light yellow powder, yield 82%. m.p.149.7-150.5°C.1H NMR (400 MHz, CDCl3) δ 8.41 (s, 1H), 8.31 (dd, J = 8.3, 1.6 Hz, 1H), 7.94 (s, 1H), 7.81–7.73 (m, 2H), 7.56–7.46 (m, 2H), 7.26 (td, J = 7.8, 1.5 Hz, 1H),7.11 (t, J = 54.1 Hz, 1H), 7.00 (td, J = 7.9, 1.6 Hz, 1H), 6.89 (dd, J = 8.2, 1.5 Hz, 1H), 4.00 (s, 3H), 1.32 (s, 9H).13C NMR (101 MHz, CDCl3) δ 159.33, 159.29, 145.04(t, J = 25.2 Hz), 139.43, 133.31, 131.48, 131.22, 128.57(×2), 127.93, 126.46(×2), 124.66, 123.12, 122.75, 116.81, 110.45 (t, J = 236.3 Hz), 39.91, 35.54, 31.05(×3). HRMS (ESI): calculated for C22H23F2N3O4S [M + Na]+: 486.12750, found: 486.12686.
In Vitro Biological Evaluation
In Vitro Antifungal Assay
The test strains were Colletotrichum camelliae (C.camelliae), Pestalotiopsis theae (P. theae) provided by Guizhou Tea Research Institute, and Gibberella zeae (G. zeae), Rhizoctonia solani (R. solani) provided by Guizhou Institute of Plant Protection. In this study, the in vitro antifungal activity of the target compounds T1-27 against four plant pathogens was screened by the mycelial growth rate method (Zhang et al., 2019). The tested compounds were dissolved in DMSO to prepare a 10 mg/ml stock solution before mixing with PDA. The PDA containing compounds at a concentration of 50 mg/L were then poured into sterilized Petri dishes for primary screening. Data Processing System (DPS, V9.50) was used for statistical analysis of test data, and Duncan’s new multiple range method was used to test the significance of differences. The EC50 values and 95% confidence limits were calculated after testing the inhibition rates, based on the above method. The inhibition rate of the potent compounds was further tested and the corresponding EC50 values were calculated by using DPS. This test method is provided in the Supporting information.
In Vivo Antiviral Activities Assay
The in vivo antiviral activities of target compounds T1-27 against TMV were tested by the half leaf blight spot method previously reported in the literature(Chen et al., 2021; Xie et al., 2018). TMV was propagated in Nicotiana tabacum cv. K326 by the Gooding method. Antiviral activities of the target compounds against TMV in vivo were at 500 mg/L. The commercial antiviral agents Ningnanmycin and Chitosan oligosaccharides were severed as the positive controls. Data is processed in the same way as that of antifungal activity.
Results and Discussion
Chemistry
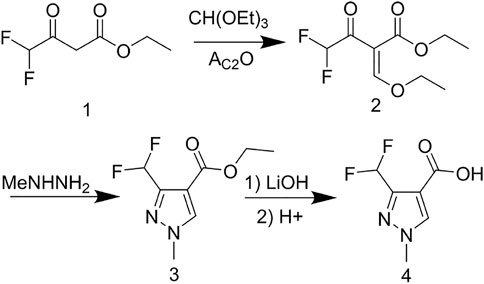
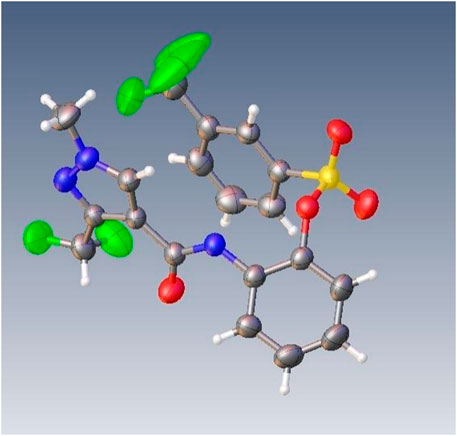
The reaction between the starting material, ethyl 4,4-difluoro-3-oxobutanoate 1) and triethyl orthoformate in acetic anhydride at 140°C, yielded ethyl 2-(ethoxymethylene)-4,4-difluoro-3-oxobutanoate (compound 2) (Sun and Zhou, 2015). Compound 2 was then treated with methylhydrazine to yield compound 3, which was successively hydrolyzed with lithium hydroxide and hydrochloric acid to obtain a white solid of the key intermediate 3-(difluoromethyl)-1-methyl-1H-pyrazole-4-carboxylic acid (compound 4) (Scheme 1). Thereafter, compound 6, a light yellow solid, was formed by conjugating compound 4 with 2-aminophenol in CH2Cl2 using EDCI and DMAP (Scheme 2). Finally, different substituted moieties of arylsulfonyl chloride were reacted with compound 5 to yield the target compounds (Scheme 3). The structures of all key intermediates and target compounds were confirmed via 1H and 13C NMR and HRMS, and their spectra data are shown in the Supplementary Material. The single-crystal X-ray diffraction of compound T22 showed that the compound is a sulfonate and not a sulfonamide. Figure 1 shows the crystal structure of T22, whose deposition number is CCDC 2168151.
In Vitro Biological Evaluation
In Vitro Antifungal Assay
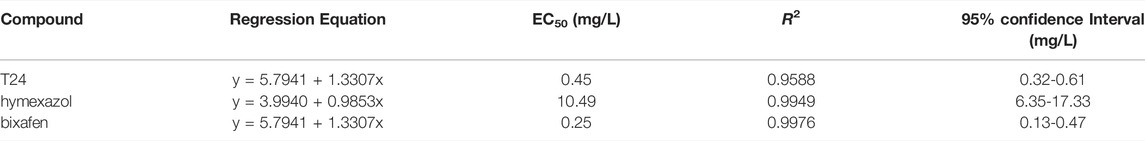
The preliminary in vitro antifungal activities of the 27 target compounds are presented in Tables 1, 2. Most of the target compounds exhibited some degree of antifungal activities against the four plant pathogens at 50 μg/ml (Table 1). Among the four plant pathogens, the target compounds, particularly T24, exhibited remarkable antifungal activity against R. solani. When R group was nitro group, the antifugal activity against R. solani was no more than 20%. It can be known from these data that the substituent on the benzene ring was a strong electron-withdrawing group, the antifungal activity was adversely affected. We also found that the activity of T24 against R. solani was much higher than that of T1 (Table 1). The only structural difference between these two compounds is the presence of an extra methylene group in T24, which is thought to enhance its antifungal activity. The compound T24 (EC50 = 0.45 mg/L) was superior to the commercial fungicide hymexazol (EC50 = 10.49 mg/L), but closer to bixafen (EC50 = 0.25 mg/L) in its activity against R. solani (Table 2).
In Vivo Antiviral Activities of Compounds T1-27
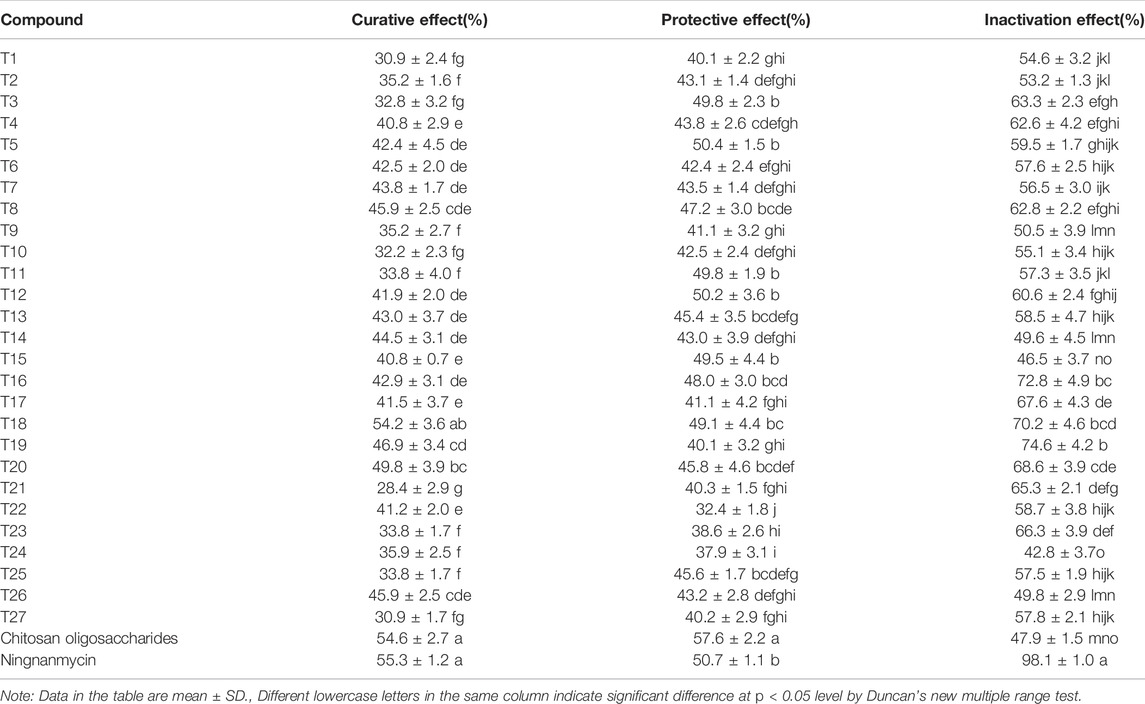
The phenylsulfonyl fragment has been reported to increase the antiviral activity (Hadházi et al., 2017), we synthesised novel sulfonate scaffold-containing pyrazolecarbamide and evaluated their antiviral activities.The curative, protective, and inactivation effects of the 27 target compounds against TMV were evaluated using the half leaf blight spot method (Liu et al., 2021; Zhang et al., 2021), and the commercial agents, Ningnanmycin and Chitosan oligosaccharide, served as positive controls. Compound T18 (54.2%) exhibited a close curative activity to ningnanmycin (55.3%) at 500 mg/ml. Additionally, most of the target compounds exhibited protective effects in vivo, and the protective effects of compounds T5 (50.4%) and T12 (50.2%) were similar to that of Ningnanmycin (50.7%). Although the target compounds had lower inactivation effects than ningnanmycin, most of them exhibited better inactivation activities than Chitosan oligosaccharides (Table 3).
Conclusion
In summary, 27 novel pyrazolecarbamide derivatives bearing a sulfonate fragment were synthesized and screened for their in vitro antifungal and in vivo antiviral activities against four plant pathogens (C. camelliae, P, theae, G. zeae, and R. solani). The structures of these compounds were identified using the single-crystal X-ray diffraction and spectral data obtained via 1H and 13C NMR and HRMS spectroscopy. The preliminary bioassay results showed that the target compounds exhibited certain inhibitory activities against the test fungi and TMV. Compound T24 exhibited excellent antifungal activities against R. solani compared to the commercial fungicide hymexazol, almost similar to bixafen. Moreover, the target compounds displayed protective effects in vivo against TMV. Thus, our research group is conducting further structural optimization of the target compounds for wide-scale field application.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
Z-WL and WY conceived and designed the paper. Z-WL and HL contributed to the synthesis, purification, characterization of all compounds. JY and CM performed the biological activity research. Z-WL wrote the manuscript. All authors have read and reviewed the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank the National Natural Science Foundation of China (no. 31860517) and the China Postdoctoral Science Foundation (2017M623069) for supporting this project. We deeply thank Dandan xie for his help in elucidating the single crystal structure.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fchem.2022.928842/full#supplementary-material
References
Chen, M., Su, S., Zhou, Q., Tang, X., Liu, T., Peng, F., et al. (2021). Antibacterial and Antiviral Activities and Action Mechanism of Flavonoid Derivatives with a Benzimidazole Moiety. J. Saudi Chem. Soc. 25, 101194. doi:10.1016/j.jscs.2020.101194
El Shehry, M. F., Ghorab, M. M., Abbas, S. Y., Fayed, E. A., Shedid, S. A., and Ammar, Y. A. (2018). Quinoline Derivatives Bearing Pyrazole Moiety: Synthesis and Biological Evaluation as Possible Antibacterial and Antifungal Agents. Eur. J. Med. Chem. 143, 1463–1473. doi:10.1016/j.ejmech.2017.10.046
Fisher, M. C., Henk, D. A., Briggs, C. J., Brownstein, J. S., Madoff, L. C., McCraw, S. L., et al. (2012). Emerging Fungal Threats to Animal, Plant and Ecosystem Health. Nature 484, 186–194. doi:10.1038/nature10947
Hadházi, Á., Pascolutti, M., Bailly, B., Dyason, J. C., Borbás, A., Thomson, R. J., et al. (2017). A Sialosyl Sulfonate as a Potent Inhibitor of Influenza Virus Replication. Org. Biomol. Chem. 15, 5249–5253. doi:10.1039/C7OB00947J
Huang, T.-J., Chuang, H., Liang, Y.-C., Lin, H.-H., Horng, J.-C., Kuo, Y.-C., et al. (2015). Design, Synthesis, and Bioevaluation of Paeonol Derivatives as Potential Anti-HBV Agents. Eur. J. Med. Chem. 90, 428–435. doi:10.1016/j.ejmech.2014.11.050
Jiang, X., Wei, X., Lin, F., Zhang, Z., Yao, G., Yang, S., et al. (2020). Substrate-Controlled [5+1] Annulation of 5-Amino-1H -phenylpyrazoles with Alkenes: Divergent Synthesis of Multisubstituted 4,5-Dihydropyrazolo[1,5-A ]quinazolines. Eur. J. Org. Chem. 2020, 3997–4003. doi:10.1002/ejoc.202000536
Kang, G.-Q., Duan, W.-G., Lin, G.-S., Yu, Y.-P., Wang, X.-Y., and Lu, S.-Z. (2019). Synthesis of Bioactive Compounds from 3-Carene (II): Synthesis, Antifungal Activity and 3D-QSAR Study of (Z)- and (E)-3-Caren-5-One Oxime Sulfonates. Molecules 24 (3), 477. doi:10.3390/molecules24030477
Kanungo, M., and Joshi, J. (2014). Impact of Pyraclostrobin (F-500) on Crop Plants. Plant Sci. Today. 1, 174–178. doi:10.14719/pst.2014.1.3.60
Kasiotis, K. M., Tzanetou, E. N., and Haroutounian, S. A. (2014). Pyrazoles as Potential Anti-angiogenesis Agents: A Contemporary Overview. Front. Chem. 2, 78. doi:10.3389/fchem.2014.00078
Liu, T., Peng, F., Cao, X., Liu, F., Wang, Q., Liu, L., et al. (2021). Design, Synthesis, Antibacterial Activity, Antiviral Activity, and Mechanism of Myricetin Derivatives Containing a Quinazolinone Moiety. ACS Omega 6, 30826–30833. doi:10.1021/acsomega.1c05256
Mu, J.-X., Shi, Y.-X., Yang, M.-Y., Sun, Z.-H., Liu, X.-H., Li, B.-J., et al. (2016). Design, Synthesis, DFT Study and Antifungal Activity of Pyrazolecarboxamide Derivatives. Molecules 21 (1), 68. doi:10.3390/molecules21010068
Saleh, N. M., El-Gazzar, M. G., Aly, H. M., and Othman, R. A. (2020). Novel Anticancer Fused Pyrazole Derivatives as EGFR and VEGFR-2 Dual TK Inhibitors. Front. Chem. 7, 917. doi:10.3389/fchem.2019.00917
Si, W.-J., Wang, X.-B., Chen, M., Wang, M.-Q., Lu, A.-M., and Yang, C.-L. (2019). Design, Synthesis, Antifungal Activity and 3D-QSAR Study of Novel Pyrazole Carboxamide and Niacinamide Derivatives Containing Benzimidazole Moiety. New J. Chem. 43, 3000–3010. doi:10.1039/C8NJ05150J
Su, S., Zhou, Q., Tang, X., Peng, F., Liu, T., Liu, L., et al. (2021). Design, Synthesis, and Antibacterial Activity of Novel Myricetin Derivatives Containing Sulfonate. Monatsh. Chem. 152, 345–356. doi:10.1007/s00706-021-02739-1
Sun, J., and Zhou, Y. (2015). Synthesis and Antifungal Activity of the Derivatives of Novel Pyrazole Carboxamide and Isoxazolol Pyrazole Carboxylate. Molecules 20, 4383–4394. doi:10.3390/molecules20034383
Sun, R., Wang, Z., Li, Y., Xiong, L., Liu, Y., and Wang, Q. (2013). Design, Synthesis, and Insecticidal Evaluation of New Benzoylureas Containing Amide and Sulfonate Groups Based on the Sulfonylurea Receptor Protein Binding Site for Diflubenzuron and Glibenclamide. J. Agric. Food Chem. 61, 517–522. doi:10.1021/jf304468b
Wang, R., Zhi, X., Li, J., and Xu, H. (2015). Synthesis of Novel Oxime Sulfonate Derivatives of 2′(2′,6′)-(Di)chloropicropodophyllotoxins as Insecticidal Agents. J. Agric. Food Chem. 63, 6668–6674. doi:10.1021/acs.jafc.5b02036
Wang, X., Wang, A., Qiu, L., Chen, M., Lu, A., Li, G., et al. (2020). Expedient Discovery for Novel Antifungal Leads Targeting Succinate Dehydrogenase: Pyrazole-4-Formylhydrazide Derivatives Bearing a Diphenyl Ether Fragment. J. Agric. Food Chem. 68, 14426–14437. doi:10.1021/acs.jafc.0c03736
Wang, X., Wang, X., Zhou, B., Long, J., and Li, P. (2021). Design, Synthesis, and Evaluation of New 4( 3 H )‐quinazolinone Derivatives Containing a Pyrazole Carboxamide Moiety. J. Heterocycl. Chem. 58, 2109–2116. doi:10.1002/jhet.4334
Wei, C., Zhao, L., Sun, Z., Hu, D., and Song, B. (2020). Discovery of Novel Indole Derivatives Containing Dithioacetal as Potential Antiviral Agents for Plants. Pestic. Biochem. Physiol. 166, 104568. doi:10.1016/j.pestbp.2020.104568
Wu, J., Song, B.-A., Hu, D.-Y., Yue, M., and Yang, S. (2012). Design, Synthesis and Insecticidal Activities of Novel Pyrazole Amides Containing Hydrazone Substructures. Pest. Manag. Sci. 68 (5), 801–810. doi:10.1002/ps.2329
Xie, D., Shi, J., Zhang, A., Lei, Z., Zu, G., Fu, Y., et al. (2018). Syntheses, Antiviral Activities and Induced Resistance Mechanisms of Novel Quinazoline Derivatives Containing a Dithioacetal Moiety. Bioorg. Chem. 80, 433–443. doi:10.1016/j.bioorg.2018.06.026
Yan, Z., Liu, A., Huang, M., Liu, M., Pei, H., Huang, L., et al. (2018). Design, Synthesis, DFT Study and Antifungal Activity of the Derivatives of Pyrazolecarboxamide Containing Thiazole or Oxazole Ring. Eur. J. Med. Chem. 149, 170–181. doi:10.1016/j.ejmech.2018.02.036
Zeng, X.-W., Huang, N., Xu, H., Yang, W.-B., Yang, L.-M., Qu, H., et al. (2010). Anti Human Immunodeficiency Virus Type 1 (HIV-1) Agents 4. Discovery of 5,5'-(p-Phenylenebisazo)-8-Hydroxyquinoline Sulfonates as New HIV-1 Inhibitors In Vitro. Chem. Pharm. Bull. 58, 976–979. doi:10.1248/cpb.58.976
Zhang, A., Yue, Y., Yang, J., Shi, J., Tao, K., Jin, H., et al. (2019). Design, Synthesis, and Antifungal Activities of Novel Aromatic Carboxamides Containing a Diphenylamine Scaffold. J. Agric. Food Chem. 67, 5008–5016. doi:10.1021/acs.jafc.9b00151
Zhang, J., He, F., Chen, J., Wang, Y., Yang, Y., Hu, D., et al. (2021). Purine Nucleoside Derivatives Containing a Sulfa Ethylamine Moiety: Design, Synthesis, Antiviral Activity, and Mechanism. J. Agric. Food Chem. 69, 5575–5582. doi:10.1021/acs.jafc.0c06612
Keywords: pyrazolecarbamide, sulfonate, antifungal activity, antiviral activity, synthesis
Citation: Lei Z-W, Yao J, Liu H, Ma C and Yang W (2022) Synthesis and Bioactivity of Novel Sulfonate Scaffold-Containing Pyrazolecarbamide Derivatives as Antifungal and Antiviral Agents. Front. Chem. 10:928842. doi: 10.3389/fchem.2022.928842
Received: 26 April 2022; Accepted: 13 May 2022;
Published: 22 June 2022.
Edited by:
Pei Li, Kaili University, ChinaReviewed by:
Hanxiang Wu, Institute of Plant Protection (IPP) (CAAS), ChinaGaopeng Song, South China Agricultural University, China
Copyright © 2022 Lei, Yao, Liu, Ma and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhi-Wei Lei, bGVpemhpd2VpODE2QDE2My5jb20=
 Zhi-Wei Lei
Zhi-Wei Lei Jianmei Yao2
Jianmei Yao2