- Department of Chemistry and Chemical Engineering, Hunan Institute of Science and Technology, Yueyang, China
In recent years, unsupported MoS2-based catalysts have been reported as promising candidates in the hydrodeoxygenation (HDO) of bio-oil. However, preparing MoS2-based catalysts with both high activity and good stability for HDO reaction is still challenging and of great importance. Hence, this mini-review is focused on the recent development of unsupported MoS2-based HDO catalysts from the understanding of catalyst design. The three aspects including morphology and defect engineering, metal doping, and deactivation mechanism are highlighted in adjusting the HDO performance of MoS2-based catalysts. Finally, the key challenges and future perspectives about how to design efficient catalysts are also summarized in the conclusions.
Introduction
With the continuous consumption of fossil fuels, the energy problem has become more serious in modern society. Therefore, a lot of research studies in the energy field have focused on finding a new fuel that can replace traditional fossil fuels, which could be compatible with existing infrastructure, sustainable, and reduce CO2 emissions (Jiang et al., 2021). In recent years, bio-oil obtained from flash pyrolysis of biomass is considered a potential fuel in the future because of its wide range of raw materials and renewable (Qu et al., 2021).
Compared with fossil fuels, the oxygen content in bio-oil is very high (up to 40 wt.%). This component characteristic results in the disadvantages of low heating value, high viscosity and acidity, and poor thermal stability. Therefore, bio-oil must be upgraded through hydrodeoxygenation (HDO) to reduce the oxygen content before being used as an excellent fuel (Figure 1A). Thus, developing catalysts with excellent catalytic performance is crucial for the bio-oil HDO process. As the most commonly used catalysts for hydrodesulfurization (HDS) in industry, supported transition metal sulfides (CoMoS/Al2O3 or NiMoS/Al2O3) also have been frequently investigated in the HDO reaction due to their low cost and mature technology. However, the HDO activity of supported catalysts is not very ideal, which usually requires harsh reaction conditions of 300°C or above. Under high reaction temperature, the sulfide catalysts will undergo a fast deactivation due to sulfur loss without sulfur compensation from external sources during the reaction process (Wandas et al., 1996; Wang et al., 2015a; Liu et al., 2017). Recently, unsupported MoS2-based catalysts have been reported to show much better HDS and HDO activity than supported counterparts because of the high density of active sites, which is especially suitable for dealing with large molecules (Wang et al., 2009; Wang et al., 2014b). More importantly, the unsupported catalysts provide an ideal platform for studying the structure-performance relationship due to the absence of support. Over the past decade, a lot of studies have been carried out on how to improve the HDO activity, selectivity, and stability of unsupported MoS2-based catalysts.
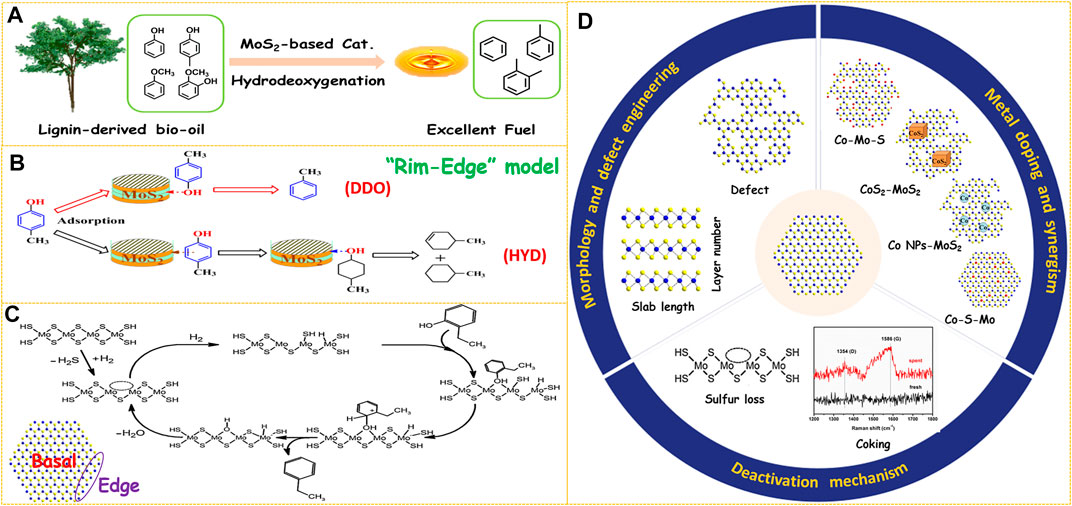
FIGURE 1. (A) Upgrading of lignin-derived bio-oil through HDO process. (B) Reaction pathways over MoS2-based catalysts. Reproduced with permission from (Wang et al., 2014d). Copyright (2014) American Chemical Society. (C) Proposed HDO mechanism of 2-ethylphenol on MoS2-based catalyst. Reproduced with permission from (Mortensen et al., 2011). Copyright (2011) Elsevier. (D) Three aspects to improve the HDO performance of MoS2-based catalysts.
Hence, in this mini-review, we firstly reviewed in detail how to study the structure-performance relationship through morphology and defect engineering of MoS2 catalysts. Then, the effect of metal doping on the active phase structure and HDO performance of the MoS2 catalyst are summarized. Moreover, the deactivation mechanism and how to inhibit the deactivation of MoS2-based catalysts in the HDO process is also introduced. Finally, we analyzed the challenges and future opportunities to design highly efficient unsupported MoS2-based HDO catalysts.
Morphology and Defect Engineering
MoS2 is a typical two-dimensional layered compound, which has two morphology parameters of slab length and layer number (Figure 1D). These two parameters could be visualized by high-resolution transmission electron microscopy (HRTEM) observation from the length and number of black fringes in the HRTEM images, respectively (Lai et al., 2016; Cao et al., 2021b). For unsupported MoS2-based catalysts, it is important to boost the number of active sites by increasing the Mo dispersion. Hence, shortening the slab length or decreasing the layer number of MoS2 can both improve its HDO activity. Due to the weak Van der Waals force between the adjacent layers, MoS2 can be easily exfoliated into few-layer or even single-layer nanosheets by some physical or chemical methods (Zhu et al., 2018; Cao et al., 2021c). A simple and effective hydrazine-assisted liquid exfoliation method was developed to exfoliate the commercial MoS2 to few-layer nanosheets (Liu et al., 2016). The HDO activity was remarkably enhanced for few-layer MoS2 nanosheets due to the exposure of more active surface sites. In addition, the layer number of bulk MoS2 was further decreased to monolayer by n-butyllithium exfoliation, and the conversion was extraordinarily improved from 25.8% of bulk MoS2 to 98.7% of single-layer MoS2 in the HDO of 4-methylphenol (Liu et al., 2017). This result demonstrates that preparing few-layer, especially monolayer MoS2 is an effective way to design highly efficient HDO catalysts. Various methods have been developed to synthesize unsupported MoS2 catalysts such as hydrothermal (Cao et al., 2021a; Zhang C. et al., 2021), ball milling (Wang C. et al., 2014), thermal decomposition (Yi et al., 2011), and solution method (Genuit et al., 2005). Among the abovementioned methods, the hydrothermal method is most widely used due to the advantages of simple operation and controllable morphology of the product. In the hydrothermal procedure, temperature, pH value, and surfactants showed a great effect on the morphology of the MoS2 product. For example, an acidic environment in the hydrothermal process was helpful to facilitate the nucleation and prepare smaller MoS2 particles, thus resulting in enhanced activity in the HDO of p-cresol (Wang et al., 2015b; Zhang C. et al., 2018). A higher initial synthesis temperature could promote fast nucleation and shorten the MoS2 slab length (Zhang et al., 2015). The layer number of MoS2 was adjusted successfully by adding different types of surfactants in the hydrothermal procedure and then the morphology-performance relation in the HDO of 4-methylphenol was studied (Wang et al., 2014d). There are two parallel reaction routes for the HDO of phenols over MoS2-based catalysts, which are defined as direct deoxygenation (DDO) and hydrogenation-dehydration (HYD). It was found that MoS2 catalyst with lower stacking showed a higher HYD selectivity, and the DDO route was favored by using MoS2 with higher stacking degree (Figure 1B). This phenomenon was well explained by the Rim-Edge model proposed in the HDS field (Daage and Chianelli, 1994). Therefore, the HDO selectivity could be precisely regulated by adjusting the layer number of MoS2 particles.
Generally, the coordinatively unsaturated sites (CUS) on the edge planes are considered active centers of MoS2 catalysts while the atoms on the basal planes are catalytically inert (Figure 1C) (Lauritsen et al., 2004). Therefore, creating defects on the basal planes is another important approach to increase the number of active sites (Figure 1D). Some strategies such as etching (Zhang W. et al., 2018; Wang et al., 2020), gas treatment (Li et al., 2019), or plasma bombardment (Tao et al., 2015) have been utilized to create defect sites on the basal planes. The location and type of defects can be directly observed by HRTEM, and the quantity information can be obtained by electron paramagnetic resonance (EPR) characterization (Wu et al., 2020). Recently, we have synthesized MoS2 nanosheets with rich defects through in situ etching by adding excess sulfur sources via a simple one-pot hydrothermal method (Zhang C. et al., 2021). The HDO activity was improved 1.7 folds through defect engineering within the basal planes. Zhang et al. reported a facile H2O2 etching strategy to tailor the concentration of sulfur vacancies by altering the H2O2/MoS2 molar ratio (Zhang Y. et al., 2021). The catalytic performance evaluation results revealed a linear relationship between the HDO activity and the degree of sulfur vacancies, further suggesting that sulfur vacancies acted as catalytic centers for the HDO reaction. The synthesis of amorphous MoS2 with low crystallinity is also one of the means to increase the number of defects. It was found that incorporating organic solvents or hydrazine in the hydrothermal method was conducive to obtaining amorphous MoS2 with a defect-rich structure. A bent and multilayered amorphous MoS2 was synthesized by utilizing (NH4)2MoS4 as a raw material and decalin as an organic solvent, and the HDO activity was enhanced by 31% compared with the crystalline MoS2(Yoosuk et al., 2012). Co-doped MoS2 nanoflowers with abundant defects by adding hydrazine to the hydrothermal system was prepared, which displayed an excellent p-cresol HDO performance (Song et al., 2018a). More importantly, the defects on the basal planes could provide additional anchor sites to accommodate promoter atoms. Recently, we have found that the content of the Co-Mo-S active phase was improved significantly by creating numerous defects on the basal planes of MoS2 support even impregnated with the same amount of Co promoters (Zhang C. et al., 2021). As a result, the Co-doped few-layer and defect-rich MoS2 achieved a 100% HDO conversion of p-cresol and 99.7% selectivity of toluene at 230°C. The increased surface sulfur defects produced by H2O2 etching were ideal platforms for stabilizing Co atoms to form the Co-Mo-S phase. The HDO activity of Co-MoS2 catalysts was increased 3.4 times by optimizing the etching conditions (Zhang Y. et al., 2021).
Metal Doping and Synergism Study
In general, the activity of unpromoted MoS2 is far from sufficient for catalyzing the HDO reaction effectively. Hence, it is essential to weaken the Mo-S bond through metal doping to reduce the formation temperature of sulfur vacancy. Co is the most commonly used metal to promote MoS2 because the DDO route is more favored for Co-doped catalysts which could minimize the H2 consumption. Based on the reported literature, there are mainly four different structures of catalytic centers for Co-promoted MoS2 catalysts (Figure 1D): (I) Co-Mo-S, (II) CoS2-MoS2, (III) Co-S-Mo (or called Co atom-MoS2), and (IV) Co NPs-MoS2 (NPs: nanoparticles). The four different catalytic active phases were obtained by tailored preparation methods, which resulted in a variety of synergistic mechanisms.
The Co-Mo-S model is the most accepted active phase structure in the field of HDS, in which Co atoms are preferentially located on the edge planes of MoS2. In our recent work, Co-doped nano-MoS2 catalysts with Co-Mo-S as an active phase were obtained by impregnating the as-prepared MoS2 with Co(CH3COO)2 followed by sulfidation in H2S/H2 atmosphere (Cao et al., 2021a). The temperature-programmed reduction (TPR) results displayed that the reduction peak was shifted from 285°C for MoS2 to 224°C for Co-doped nano-MoS2, which suggests a greatly decreased temperature for producing coordinative unsaturated sites (CUS). Also, it was found the Co content had a huge effect on the structure of the active phase and catalytic performance. If a small amount of Co are introduced, all Co atoms are preferentially located at the edge planes to form the Co-Mo-S phase. When the content of Co is further increased, the Co9S8 phase is gradually formed after the edge sites are fully occupied. However, too much Co content is not suggested because the larger Co9S8 would block some of the active sites. Also, the optimized catalyst with a Co/(Co + Mo) molar ratio of 0.3 had both Co-Mo-S and small Co9S8 particles, which embodies a synergistic effect between Co-Mo-S and Co9S8 phases. The HDO activity and toluene selectivity were improved dramatically from 16.9 to 65.0% of MoS2 to 98.7 and 98.9% of Co/MoS2-0.3 at 220°C. Another well-known model in the field of hydrorefining is the Remote-Control model, in which the independent CoxSy phase acts the role to provide spillover hydrogen. Wang et al. adopted a two-step hydrothermal method which obtained separated CoS2 and MoS2 phases in the resulted catalysts rather than the Co-Mo-S phase prepared by one-step hydrothermal method (Wang et al., 2016b). The CoS2/MoS2 showed an excellent HDO activity of 97.8% and a high toluene selectivity of 99.2% at 250°C. The unprecedented HDO performance was attributed to the large surface area and the synergism between CoS2 and MoS2. More interestingly, the CoS2 and MoS2 interface was in situ reconstructed into Co-Mo-S by H2 pre-reduction treatment, resulting in a large amount of surface Co-Mo-S active phase toward efficient HDO of p-cresol (Liu et al., 2020). In early electrochemical research, researchers found that sulfur vacancy has a strong adsorption capacity for Co complexes. On the basis of this discovery, Liu et al. prepared a single-layer MoS2 catalyst doped with isolated Co atoms (Co-SMoS2) by hydrothermal treatment of monolayer MoS2 with Co(thiourea)42+. The EXAFS and HAADF-STEM characterization results confirmed that single Co atoms were covalently bonded to the sulfur vacancies on the basal planes to form a new Co-S-Mo active phase (Liu et al., 2017). The theoretical calculation results showed that the proximal sites around Co-S-Mo were energetically favorable for the formation of sulfur vacancies, thus making Co-SMoS2 an extremely active and selective catalyst in the HDO reactions. Meanwhile, superior stability was also observed due to the low operating temperature at 180°C. An in situ interfacial reactions was adopted to prepare MoS2-x catalyst supported by Co nanoparticles (Co NPs-MoS2) based on the reducibility of defection-rich MoS2-x (Wu et al., 2020). The HRTEM and DFT results showed that Co NPs were energetically favored to adsorb on the defects to form a metal-vacancy interface. Also, the Co NPs promoted the formation of new sulfur vacancies at the proximal sites as evidenced by TPR results. More importantly, the DFT results showed that the adsorption energy of 4-methylphenol and the reaction energy barrier were significantly decreased on Co NPs-MoS2, which endowed the catalyst exhibit the lowest reaction temperature (120°C) up to now.
Ni is also a commonly used promoter to improve the catalytic performance of MoS2. However, Ni-promoted MoS2 catalysts usually have high hydrogenation activity because Ni is more capable of adsorbing and activating H2. Ni-Mo-S and NiS2//MoS2 catalysts with the same Ni/Mo molar ratio by one-step hydrothermal method and two-step hydrothermal method were prepared, respectively (Wang et al., 2015a). It was found that NiS2//MoS2 showed a much higher p-cresol conversion than Ni-Mo-S, which indicated that the synergistic effect between Ni promoters and MoS2 came from the spillover hydrogen rather than the Ni-Mo-S phase (Wang et al., 2016a). The optimized NiS2//MoS2 showed a p-cresol conversion of 95.8% and a methylcyclohexane selectivity of 55.6% at 275°C for 4 h. Although Fe is much cheaper than Co and Ni, Fe has attracted little research interest because of its poor performance in promoting MoS2. Recently, Fe-promoted MoS2 catalysts by one-step hydrothermal method, which exhibited separated FeS2 and MoS2 phases were prepared (Guo et al., 2019). However, the FeS2 was transformed into FeS after a pretreatment in H2 and the HDO activity was significantly enhanced. The authors concluded that FeS was a better promoter than FeS2 for MoS2, and acts as a donor of activated hydrogen in the HDO reaction.
According to a recent report, precious metals such as Pt can also play a synergistic role in promoting the HDO activity of unsupported MoS2 catalysts. A metal insertion-deinsertion strategy was adopted to prepare Pt-MoS2-x catalysts in which Pt4+ initially substituted the Mo4+ on the basal planes and then Pt species was deinserted to be anchored on the adjacent sites (Wu et al., 2022). As a result, a large number of edge sites were created at the proximal sites of the Pt-edge interface within the basal planes which are originally inert. Thus, the optimized Pt-MoS2-x catalyst displayed extraordinary catalytic activity in the HDO of p-cresol, which achieved a conversion of 100% and a methylcyclohexane selectivity of 96.3% at 120°C. The DFT calculation results showed that the metal-edge interface was beneficial for lowering both the adsorption energy of reactants and the energy barrier of the HDO reaction. We have summarized the HDO performance of some unsupported MoS2-based catalysts in the past decade utilizing p-cresol as a probe molecule (Table 1).
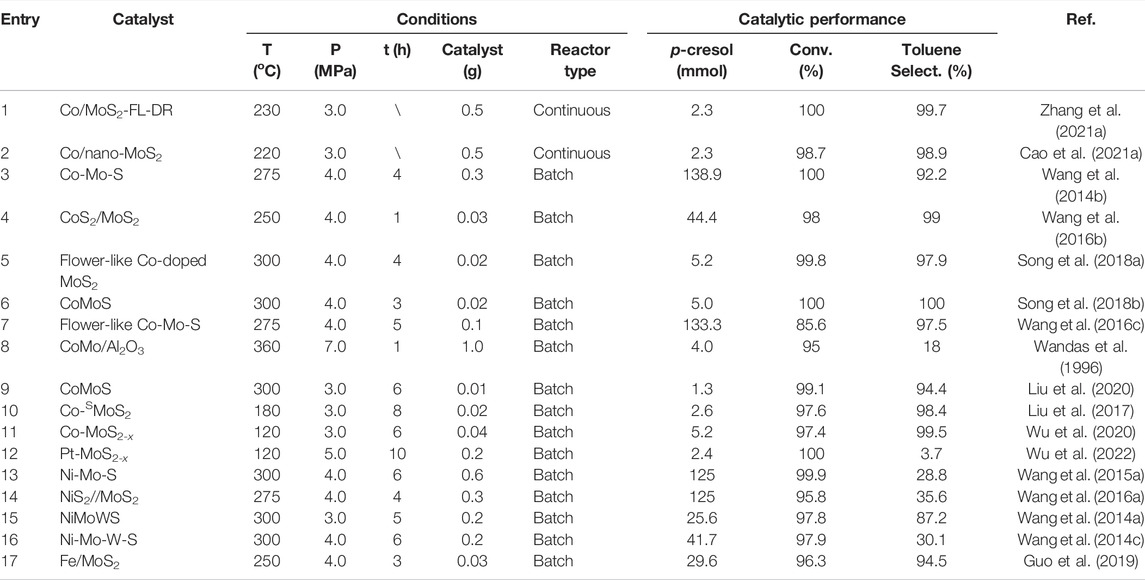
TABLE 1. Comparison of the catalytic performance in the HDO of p-cresol for MoS2-based catalysts from the literatures.
Deactivation Mechanism and Measures to Inhibit Deactivation
Deactivation is a serious problem when MoS2-based catalysts are applied in the HDO reactions, which include two main causes such as sulfur loss and coking (Figure 1D). As the bio-oil does not contain sulfur, sulfur will be gradually lost due to S-O exchange, and the presence of H2O product will aggravate this process. Therefore, the following strategies have been carried out in order to inhibit catalyst deactivation due to sulfur loss. The first type of method is the co-feeding of sulfur-containing compounds such as H2S or benzothiophene. Adding an appropriate amount of H2S to the reaction system could well maintain the activity and stability of NiMo and CoMo catalysts due to the increased concentration of -SH groups (Dabros et al., 2018). A small amount of benzothiophene in the feed is helpful to inhibit catalyst deactivation because the H2S molecules produced by HDS of benzothiophene will adsorb on the active sites and weaken the negative effect of water (Wang et al., 2018). However, excessive benzothiophene introduction resulted in a decrease in HDO activity due to competitive adsorption between H2S and p-cresol. More importantly, it should be noted that contamination of bio-oil products is inevitable by adding sulfiding agents. To overcome this shortcoming, a surface hydrophobic treatment strategy was proposed in which the generated water is removed from the surface immediately, thus improving the cyclic stability of MoS2-based HDO catalysts. For example, the surface hydrophobicity of MoS2 was enhanced significantly by silicomolybdic acid modification as revealed by the contact angle results (Wu et al., 2019). After recycling for three runs, the p-cresol conversion of the modified MoS2 catalyst only showed a slight loss of 0.4%, which is much lower than that of the original MoS2 (9.2%). Carbon coating is another surface hydrophobic modification method to prevent sulfur loss in the HDO process. Amorphous carbon-coated CoS2-MoS2 catalysts were prepared by adding polyvinylpyrrolidone (PVP) to the hydrothermal system (Wang et al., 2017). The hydrophobic carbon layer covered on the surface effectively prevented the oxidation caused by water, and the HDO activity of CoS2-MoS2 decreased by only 2.6% after recycling three times. Lowering the reaction temperature is also an effective way to inhibit sulfur loss. The Co-SMoS2 maintained excellent HDO activity and selectivity without sulfur loss after using seven cycles, which is attributed to the greatly decreased reaction temperature from 300°C of traditional CoMo/Al2O3 catalysts to 180°C (Liu et al., 2017). The Co NPs-MoS2 and Pt-MoS2-x catalysts reported (Wu et al., 2020; Wu et al., 2022) also showed superior stability in the HDO of p-cresol due to the further lowered operating temperature of 120°C.
Coking is a general problem for catalyst deactivation by decreasing the surface area and blocking the active sites. The total pore volume of the Co-MoS2/Al2O3 catalyst was reduced by 1/3 due to carbon deposition in the initial stage (Fonseca et al., 1996). In our previous works, the Raman characterization of spent Co/MoS2 catalysts after running for 72 h at 220°C showed typical peaks at 1,350 and 1,586 cm−1 attributed to graphitic carbon species. In addition, the coke content was 4.4 wt.% determined by CHNS analysis, and the combination of carbon deposition and sulfur loss jointly resulted in the decrease of HDO conversion by 2.8% (Zhang C. et al., 2021). In order to reduce the formation of coking, some measures could be adopted such as reducing the acidity of catalysts or increasing the hydrogen pressure.
Conclusion and Future Perspectives
In this mini-review, we summarized the recent progress of unsupported MoS2-based catalysts in the HDO of bio-oil. Reducing the layer number of MoS2, especially to a single-layer could significantly improve the HDO activity. Creating defects within the basal planes is also an effective way to enhance the HDO activity, especially for metal-promoted MoS2 catalysts because it provides additional anchoring sites. The regulation of HDO selectivity is easily realized by just adjusting the layer number of MoS2. Co and Pt doping can both significantly improve the HDO activity and stability of MoS2 catalysts, while Co-promoted catalysts are more potential due to the minimized H2 consumption. Four different active phases including Co-Mo-S, CoS2-MoS2, Co-S-Mo, and Co NPs-MoS2 have been proposed to construct highly efficient CoMo bimetallic catalysts. Sulfur loss and coking are the main factors to cause deactivation, which could be alleviated by co-feeding of sulfiding agents, surface hydrophobic treatment, and lowering the reaction temperature.
Although tremendous progress has been achieved in the construction of efficient MoS2-based HDO catalysts, there are still some challenges to be solved in future research. 1) Simple synthesis of monolayer MoS2 nanosheets with rich defects which are ideal supports to prepare CoMo bimetallic catalysts. 2) Experimental and theoretical comparison of the four different active phases of Co-promoted catalysts to figure out which model is best for designing highly efficient CoMo bimetallic catalysts. 3) Deactivation study by using real bio-oil as feedstock and determination of the sulfur loss and coke content.
Author Contributions
JC and YZ drafted the manuscript. LW and CoZ participated in the manuscript revision. CeZ revised the manuscript and provided the funding support.
Funding
This work was financially supported by the National Natural Science Foundation of China (Grant No. 22102055), the Natural Science Foundation of Hunan Province (Grant No. 2021JJ40222), and the Scientific Research Fund of Hunan Provincial Education Department (Grant No. 20B264).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Cao, J., Li, A., Zhang, Y., Mu, L., Huang, X., Li, Y., et al. (2021a). Highly Efficient Unsupported Co-doped Nano-MoS2 Catalysts for P-Cresol Hydrodeoxygenation. Mol. Catal. 505, 111507. doi:10.1016/j.mcat.2021.111507
Cao, J., Xia, J., Zhang, Y., Liu, X., Bai, L., Xu, J., et al. (2021b). Influence of the Alumina Crystal Phase on the Performance of CoMo/Al2O3 Catalysts for the Selective Hydrodesulfurization of Fluid Catalytic Cracking Naphtha. Fuel 289, 119843. doi:10.1016/j.fuel.2020.119843
Cao, J., Zhang, Y., Zhang, C., Cai, L., Li, Z., and Zhou, C. (2021c). Construction of Defect-Rich 1T-MoS2 towards Efficient Electrocatalytic Hydrogen Evolution: Recent Advances and Future Perspectives. Surfaces Interfaces 25, 101305. doi:10.1016/j.surfin.2021.101305
Daage, M., and Chianelli, R. R. (1994). Structure-function Relations in Molybdenum Sulfide Catalysts: The "Rim-Edge" Model. J. Catal. 149, 414–427. doi:10.1006/jcat.1994.1308
Dabros, T. M. H., Gaur, A., Pintos, D. G., Sprenger, P., Høj, M., Hansen, T. W., et al. (2018). Influence of H2O and H2S on the Composition, Activity, and Stability of Sulfided Mo, CoMo, and NiMo Supported on MgAl2O4 for Hydrodeoxygenation of Ethylene Glycol. Appl. Catal. A General 551, 106–121. doi:10.1016/j.apcata.2017.12.008
Fonseca, A., Zeuthen, P., and Nagy, J. B. (1996). 13C n.m.R. Quantitative Analysis of Catalyst Carbon Deposits. Fuel 75, 1363–1376. doi:10.1016/0016-2361(96)00106-8
Genuit, D., Afanasiev, P., and Vrinat, M. (2005). Solution Syntheses of Unsupported Co(Ni)-Mo-S Hydrotreating Catalysts. J. Catal. 235, 302–317. doi:10.1016/j.jcat.2005.08.016
Guo, X., Wang, W., Wu, K., Huang, Y., Shi, Q., and Yang, Y. (2019). Preparation of Fe Promoted MoS2 Catalysts for the Hydrodeoxygenation of P-Cresol as a Model Compound of Lignin-Derived Bio-Oil. Biomass Bioenergy 125, 34–40. doi:10.1016/j.biombioe.2019.04.014
Jiang, S., Ji, N., Diao, X., Li, H., Rong, Y., Lei, Y., et al. (2021). Vacancy Engineering in Transition Metal Sulfide and Oxide Catalysts for Hydrodeoxygenation of Lignin‐Derived Oxygenates. ChemSusChem 14, 4377–4396. doi:10.1002/cssc.202101362
Lai, W., Chen, Z., Zhu, J., Yang, L., Zheng, J., Yi, X., et al. (2016). A NiMoS Flower-like Structure with Self-Assembled Nanosheets as High-Performance Hydrodesulfurization Catalysts. Nanoscale 8, 3823–3833. doi:10.1039/c5nr08841k
Lauritsen, J. V., Nyberg, M., Nørskov, J. K., Clausen, B. S., Topsøe, H., Lægsgaard, E., et al. (2004). Hydrodesulfurization Reaction Pathways on MoS2 Nanoclusters Revealed by Scanning Tunneling Microscopy. J. Catal. 224, 94–106. doi:10.1016/j.jcat.2004.02.009
Li, L., Qin, Z., Ries, L., Hong, S., Michel, T., Yang, J., et al. (2019). Role of Sulfur Vacancies and Undercoordinated Mo Regions in MoS2 Nanosheets toward the Evolution of Hydrogen. ACS Nano 13, 6824–6834. doi:10.1021/acsnano.9b01583
Liu, G., Ma, H., Teixeira, I., Sun, Z., Xia, Q., Hong, X., et al. (2016). Hydrazine-Assisted Liquid Exfoliation of MoS2 for Catalytic Hydrodeoxygenation of 4-Methylphenol. Chem. Eur. J. 22, 2910–2914. doi:10.1002/chem.201504009
Liu, G., Robertson, A. W., Li, M. M.-J., Kuo, W. C. H., Darby, M. T., Muhieddine, M. H., et al. (2017). MoS2 Monolayer Catalyst Doped with Isolated Co Atoms for the Hydrodeoxygenation Reaction. Nat. Chem. 9, 810–816. doi:10.1038/nchem.2740
Liu, X., Hou, X., Zhang, Y., Yuan, H., Hong, X., and Liu, G. (2020). In Situ formation of CoMoS Interfaces for Selective Hydrodeoxygenation of P-Cresol to Toluene. Ind. Eng. Chem. Res. 59, 15921–15928. doi:10.1021/acs.iecr.0c03589
Mortensen, P. M., Grunwaldt, J.-D., Jensen, P. A., Knudsen, K. G., and Jensen, A. D. (2011). A Review of Catalytic Upgrading of Bio-Oil to Engine Fuels. Appl. Catal. A General 407, 1–19. doi:10.1016/j.apcata.2011.08.046
Qu, L., Jiang, X., Zhang, Z., Zhang, X.-g., Song, G.-y., Wang, H.-l., et al. (2021). A Review of Hydrodeoxygenation of Bio-Oil: Model Compounds, Catalysts, and Equipment. Green Chem. 23, 9348–9376. doi:10.1039/d1gc03183j
Song, W., Nie, T., Lai, W., Yang, W., and Jiang, X. (2018a). Tailoring the Morphology of Co-doped MoS2 for Enhanced Hydrodeoxygenation Performance of P-Cresol. CrystEngComm 20, 4069–4074. doi:10.1039/c8ce00510a
Song, W., Zhou, S., Hu, S., Lai, W., Lian, Y., Wang, J., et al. (2018b). Surface Engineering of CoMoS Nanosulfide for Hydrodeoxygenation of Lignin-Derived Phenols to Arenes. ACS Catal. 9, 259–268. doi:10.1021/acscatal.8b03402
Tao, L., Duan, X., Wang, C., Duan, X., and Wang, S. (2015). Plasma-engineered MoS2 Thin-Film as an Efficient Electrocatalyst for Hydrogen Evolution Reaction. Chem. Commun. 51, 7470–7473. doi:10.1039/C5CC01981H
Wandas, R., Surygala, J., and Śliwka, E. (1996). Conversion of Cresols and Naphthalene in the Hydroprocessing of Three-Component Model Mixtures Simulating Fast Pyrolysis Tars. Fuel 75, 687–694. doi:10.1016/0016-2361(96)00011-7
Wang, C., Wang, D., Wu, Z., Wang, Z., Tang, C., and Zhou, P. (2014a). Effect of W Addition on the Hydrodeoxygenation of 4-methylphenol over Unsupported NiMo Sulfide Catalysts. Appl. Catal. A General 476, 61–67. doi:10.1016/j.apcata.2014.02.010
Wang, L., Zhang, Y., Zhang, Y., Jiang, Z., and Li, C. (2009). Ultra-deep Hydrodesulfurization of Diesel Fuels on Trimetallic NiMoW Sulfide Catalysts. Chem. Eur. J. 15, 12571–12575. doi:10.1002/chem.200901997
Wang, W., Li, L., Tan, S., Wu, K., Zhu, G., Liu, Y., et al. (2016a). Preparation of NiS2//MoS2 Catalysts by Two-step Hydrothermal Method and Their Enhanced Activity for Hydrodeoxygenation of P-Cresol. Fuel 179, 1–9. doi:10.1016/j.fuel.2016.03.068
Wang, W., Li, L., Wu, K., Zhang, K., Jie, J., and Yang, Y. (2015a). Preparation of Ni-Mo-S Catalysts by Hydrothermal Method and Their Hydrodeoxygenation Properties. Appl. Catal. A General 495, 8–16. doi:10.1016/j.apcata.2015.01.041
Wang, W., Li, L., Wu, K., Zhu, G., Tan, S., Li, W., et al. (2015b). Hydrothermal Synthesis of Bimodal Mesoporous MoS2 Nanosheets and Their Hydrodeoxygenation Properties. RSC Adv. 5, 61799–61807. doi:10.1039/c5ra09690a
Wang, W., Li, L., Wu, K., Zhu, G., Tan, S., Liu, Y., et al. (2016b). Highly Selective Catalytic Conversion of Phenols to Aromatic Hydrocarbons on CoS2/MoS2 Synthesized Using a Two Step Hydrothermal Method. RSC Adv. 6, 31265–31271. doi:10.1039/c5ra27066a
Wang, W., Tan, S., Wu, K., Zhu, G., Liu, Y., Tan, L., et al. (2018). Hydrodeoxygenation of P-Cresol as a Model Compound for Bio-Oil on MoS2: Effects of Water and Benzothiophene on the Activity and Structure of Catalyst. Fuel 214, 480–488. doi:10.1016/j.fuel.2017.11.067
Wang, W., Wu, K., Tan, S., and Yang, Y. (2017). Hydrothermal Synthesis of Carbon-Coated CoS2-MoS2 Catalysts with Enhanced Hydrophobicity and Hydrodeoxygenation Activity. ACS Sustain. Chem. Eng. 5, 8602–8609. doi:10.1021/acssuschemeng.7b01087
Wang, W., Zhang, K., Li, L., Wu, K., Liu, P., and Yang, Y. (2014b). Synthesis of Highly Active Co-mo-S Unsupported Catalysts by a One-step Hydrothermal Method for P-Cresol Hydrodeoxygenation. Ind. Eng. Chem. Res. 53, 19001–19009. doi:10.1021/ie5032698
Wang, W., Zhang, K., Qiao, Z., Li, L., Liu, P., and Yang, Y. (2014c). Hydrodeoxygenation of P-Cresol on Unsupported Ni-W-Mo-S Catalysts Prepared by One Step Hydrothermal Method. Catal. Commun. 56, 17–22. doi:10.1016/j.catcom.2014.06.024
Wang, W., Zhang, K., Qiao, Z., Li, L., Liu, P., and Yang, Y. (2014d). Influence of Surfactants on the Synthesis of MoS2 Catalysts and Their Activities in the Hydrodeoxygenation of 4-Methylphenol. Ind. Eng. Chem. Res. 53, 10301–10309. doi:10.1021/ie500830f
Wang, W., Zhu, G., Li, L., Tan, S., Wu, K., Zhang, X., et al. (2016c). Facile Hydrothermal Synthesis of Flower-like Co-mo-S Catalysts and Their High Activities in the Hydrodeoxygenation of P-Cresol and Hydrodesulfurization of Benzothiophene. Fuel 174, 1–8. doi:10.1016/j.fuel.2016.01.074
Wang, X., Zhang, Y., Si, H., Zhang, Q., Wu, J., Gao, L., et al. (2020). Single-Atom Vacancy Defect to Trigger High-Efficiency Hydrogen Evolution of MoS2. J. Am. Chem. Soc. 142, 4298–4308. doi:10.1021/jacs.9b12113
Wu, K., Li, X., Wang, W., Huang, Y., Jiang, Q., Li, W., et al. (2022). Creating Edge Sites within the Basal Plane of a MoS2 Catalyst for Substantially Enhanced Hydrodeoxygenation Activity. ACS Catal. 12, 8–17. doi:10.1021/acscatal.1c03669
Wu, K., Wang, C., Chen, X., Wang, W., and Yang, Y. (2019). Facile Synthesis of Hydrophobic MoS2 and its Activity and Stability in the Hydrodeoxygenation Reaction. New J. Chem. 43, 2734–2739. doi:10.1039/C8NJ05980B
Wu, K., Wang, W., Guo, H., Yang, Y., Huang, Y., Li, W., et al. (2020). Engineering Co Nanoparticles Supported on Defect MoS2-X for Mild Deoxygenation of Lignin-Derived Phenols to Arenes. ACS Energy Lett. 5, 1330–1336. doi:10.1021/acsenergylett.0c00411
Yi, Y., Jin, X., Wang, L., Zhang, Q., Xiong, G., and Liang, C. (2011). Preparation of Unsupported Ni-Mo-S Catalysts for Hydrodesulfurization of Dibenzothiophene by Thermal Decomposition of Tetramethylammonium Thiomolybdates. Catal. Today 175, 460–466. doi:10.1016/j.cattod.2011.04.039
Yoosuk, B., Tumnantong, D., and Prasassarakich, P. (2012). Unsupported MoS2 and CoMoS2 Catalysts for Hydrodeoxygenation of Phenol. Chem. Eng. Sci. 79, 1–7. doi:10.1016/j.ces.2012.05.020
Zhang, C., Li, P., Liu, X., Liu, T., Jiang, Z., and Li, C. (2018a). Morphology-performance Relation of (Co)MoS2 Catalysts in the Hydrodesulfurization of FCC Gasoline. Appl. Catal. A General 556, 20–28. doi:10.1016/j.apcata.2018.02.026
Zhang, C., Liu, K., Zhang, Y., Mu, L., Zhang, Z., Huang, J., et al. (2021a). Co-promoted Few-Layer and Defect-Rich MoS2 for Enhanced Hydrodeoxygenation of P-Cresol. Appl. Catal. A General 621, 118175. doi:10.1016/j.apcata.2021.118175
Zhang, H., Lin, H., Zheng, Y., Hu, Y., and MacLennan, A. (2015). Understanding of the Effect of Synthesis Temperature on the Crystallization and Activity of Nano-MoS2 Catalyst. Appl. Catal. B Environ. 165, 537–546. doi:10.1016/j.apcatb.2014.10.046
Zhang, W., Xie, Z., Wu, X., Sun, M., Deng, X., Liu, C., et al. (2018b). Acid-engineered Defective MoS2 as an Efficient Electrocatalyst for Hydrogen Evolution Reaction. Mater. Lett. 230, 232–235. doi:10.1016/j.matlet.2018.07.108
Zhang, Y., Liu, T., Xia, Q., Jia, H., Hong, X., and Liu, G. (2021b). Tailoring of Surface Acidic Sites in Co-MoS2 Catalysts for Hydrodeoxygenation Reaction. J. Phys. Chem. Lett. 12, 5668–5674. doi:10.1021/acs.jpclett.1c01201
Keywords: bio-oil, hydrodeoxygenation, unsupported MoS2, morphology, defect, metal doping, deactivation
Citation: Cao J, Zhang Y, Wang L, Zhang C and Zhou C (2022) Unsupported MoS2-Based Catalysts for Bio-Oil Hydrodeoxygenation: Recent Advances and Future Perspectives. Front. Chem. 10:928806. doi: 10.3389/fchem.2022.928806
Received: 26 April 2022; Accepted: 11 May 2022;
Published: 17 June 2022.
Edited by:
Xianxiang Liu, Hunan Normal University, ChinaCopyright © 2022 Cao, Zhang, Wang, Zhang and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cen Zhang, Y2VuemhhbmdAaG5pc3QuZWR1LmNu
 Jing Cao
Jing Cao Cen Zhang
Cen Zhang