- 1State Key Laboratory Breeding Base of Green Pesticide and Agricultural Bioengineering, Ministry of Education, Guizhou University, Huaxi District, Guiyang, China
- 2Key Laboratory of Green Pesticide and Agricultural Bioengineering, Ministry of Education, Guizhou University, Huaxi District, Guiyang, China
- 3School of Biological Sciences, Guizhou Education University, Wudang District, Guiyang, China
- 4School of Biologi and Engineering, Guizhou Medical University, Huaxi District, Guiyang, China
The crop loss caused by bacteria has increased year by year due to the lack of effective control agents. In order to develop efficient, broad-spectrum, and structurally simple agricultural bactericide, the structure of piperonylic acid was modified and a series of novel piperonylic acid derivatives containing a sulfonic acid ester moiety was synthesized. Bioassay results indicated the compounds exhibited significantly antibacterial activities. Among them, compound 41 exhibited excellent antibacterial activities against Pseudomonas syringae pv. Actinidiae (Psa), with inhibitory value 99 and 85% at 100 μg/ml and 50 μg/ml, respectively, which was higher than that of thiodiazole-copper (84 and 77%) and bismerthiazol (96 and 78%). In addition, some compounds also showed moderate insecticidal activity against Spodoptera frugiperda. The abovementioned results confirm the broadening of the application of piperonylic acid, with reliable support for the development of novel agrochemical bactericide.
1 Introduction
Crop diseases caused by bacteria are considered as the second largest disease in agriculture, second only to fungal diseases, and cause major agricultural losses every year (Abdullahiab et al., 2020; Wang et al., 2021). Although there are some agents widely used to control bacterial diseases, such as bismerthiazol, streptomycin, and copper compounds (Chen M. H. et al., 2021), due to long-term and large-scale use for many years, it not only caused resistance in bacteria but also caused serious environmental problems. Pests were also an important culprit in reducing crop yields. In addition to fed directly on crops, pests also transmitted many viruses and bacteria during migration and feeding. Therefore, it was very necessary to develop an efficient and broad-spectrum agricultural bactericide (Wang et al., 2022).
Due to its characteristics of unique mechanism of action, novel scaffolds, and easy derivation, the natural products have always been a valuable source for lead compounds discovery in agricultural chemistry. Piperonylic acid is an aromatic acid mainly found in black pepper (Moreira et al., 2021). Lots of research results revealed members of the piperonylic acid family had a range of biological activities and were further developed into a commercial drug and widely used in the field of medicine, such as oxolinic acid (Yamazawa et al., 2021; Boycov et al., 2022; Quan et al., 2022), kakuol (Jang et al., 2020; Matsumoto et al., 2020; Sui et al., 2020), and miloxacin (Horie and Nakazawa, 1992; Ueno and Aoki, 1996; Ueno et al., 2001). In addition, piperonylic acid derivatives also showed good activity against bacteria (Umadevi et al., 2013). Sulfonic acid groups are widely used in the field of medicine mainly in the form of sulfonate derivatives. Such as apatinib mesylate (Guo Q. et al., 2020; Chen M. et al., 2021; Kou et al., 2021; Zheng et al., 2021), donafenib tosylate (Wang et al., 2017), and dabrafenib mesylate (Carlos et al., 2015; Liu et al., 2019; Rai et al., 2020) that have been widely used to treat cancer, gemifloxacin mesylate for antibacterial (Chai et al., 2019), and pradefovir mesylate for antiviral (Tuerkova and Zdrazil, 2020). However, many research results revealed that sulfonic acid ester derivatives also had very extensive and excellent biological activities, especially the antibacterial activity was impressive. Guo et al. (2019) and Guo T. et al. (2020) had reported that by splicing a sulfonic acid ester moiety into the backbone of 1,4-pentadien-3-one and chalcone, respectively, the two series of derivatives obtained showed excellent inhibitory activities against bacteria such as Xanthomonas axonopodis pv. citri (Xac), Ralstonia solanacearum (Rs), and Xanthomonas oryzae pv. oryzae (Xoo). Inspired by the results of these studies, the present work aims to incorporate a sulfonic acid ester moiety into the piperonylic acid backbone to synthesize a series of novel derivatives, and further evaluate their antibacterial and insecticidal activity, and hope to obtain piperonylic acid derivatives with good antibacterial activities.
2 Experimental
2.1 Chemistry
All starting materials and reagents were commercially available and used without further purification, except as indicated. The 1H NMR and 13C NMR spectra were recorded on a Bruker DPX 400 MHz (Bruker BioSpin GmbH, Rheinstetten, Germany) NMR spectrometer with CDCl3 as the solvent. The following abbreviations were used to explain the multiplicities: s, singlet; d, doublet; t, triplet; m, multiplet; and br, broadened. The melting points were determined on a WRX-4 microscope melting point apparatus (YiCe Apparatus & Equipment co., LTD, Shanghai, China). High-resolution mass spectrometry (HRMS) was conducted using a Thermo Scientific Q Exactive (Thermo Fisher Scientific, Massachusetts, America). The X-ray crystallographic data were determined on a D8 Quest X-ray diffractometer (Bruker BioSpin GmbH, Rheinstetten, German).
2.1.1 General Procedures for Preparing Compounds
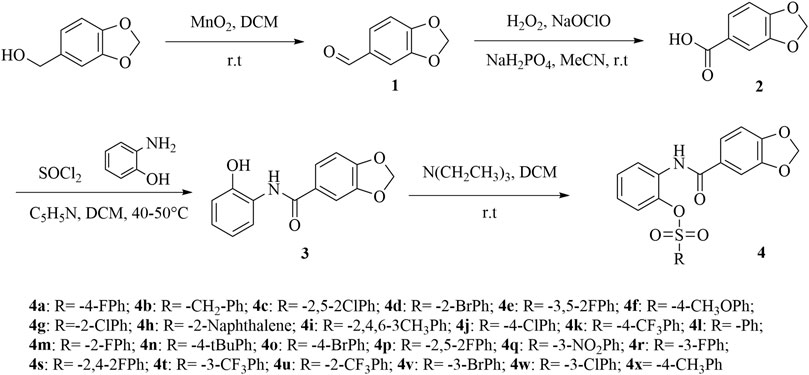
The synthetic route for the final compounds 4a–4x were depicted in Scheme 1. Intermediates 1–2 were synthesized according to a previously reported method (Dam and Madsen, 2009; Zazeri et al., 2020). Intermediate 3 was prepared according to literature method (Joseph et al., 2019). Target compounds 4a–4x were synthesized by condensation of different sulfonyl chloride which contained different substituent group and intermediate 3 at room temperature condition. Intermediate 2 equivalent of triethylamine was added to the system as a catalyst to neutralize the HCl generated by the reaction so that the reaction can proceed smoothly. After approximately about 4 h, the solvent was removed, and the residue was purified by flash chromatography on silica gel with petroleum n-hexane/ethyl acetate (volume ratio 5:1) to obtain the pure product.
2.1.1.1 N-(2-((4-fluorophenyl)sulfonyl)phenyl)benzo[d][1,3]dioxole-5-carboxamide (4a)
Light yellow powder, yield 82%. m.p 133.4–134.7°C. 1H NMR (400 MHz, CDCl3) δ 8.32 (dd, J = 8.3, 1.6 Hz, 1H, Ph-H), 8.26 (s, 1H, -NH-), 7.87 (dd, J = 9.0, 4.9 Hz, 2H, Ph-H), 7.40 (dd, J = 8.1, 1.9 Hz, 1H, Ph-H), 7.36–7.29 (m, 2H, Ph-H), 7.21–7.10 (m, 2H, Ph-H), 7.06–7.02 (m, 1.6 Hz, 1H, Ph-H), 6.94–6.87 (m, 2H, Ph-H), 6.09 (s, 2H, -OCH2O-). 13C NMR (100 MHz, CDCl3) δ 164.4, 151.0, 148.3, 139.2, 131.5, 131.4, 128.2, 124.5, 123.3, 122.9, 122.0, 117.1, 116.8, 108.3, 107.7, 102.0. HRMS (ESI): calculated for C20H14FNO6S [M + Na]+: 438.0526, found: 438.0418.
2.1.1.2 2-(Benzo[d][1,3]dioxole-5-carboxamido)phenyl Phenyl Methanesulfonate (4b)
Light yellow powder, yield 80%. m.p 127.5–128.5°C. 1H NMR (400 MHz, CDCl3) δ 7.48 (dd, J = 7.8, 1.8 Hz, 1H, -NH-), 7.29 (s, 2H, Ph-H), 7.13–7.01 (m, 6H, Ph-H), 6.95 (dd, J = 8.2, 1.5 Hz, 1H, Ph-H), 6.83 (d, J = 8.7 Hz, 1H, Ph-H), 6.07 (s, 2H, -OCH2O-), 4.65 (s, 2H, -CH2-PH). 13C NMR (100 MHz, CDCl3) δ 164.6, 150.8, 148.1, 138.0, 131.8, 130.9, 129.6, 129.2, 128.4, 128.2, 126.8, 124.6, 123.3, 123.0, 122.1, 108.2, 107.8, 101.8, 57.8. HRMS (ESI): calculated for C21H17NO6S [M + Na]+: 434.0777, found: 434.0667.
2.1.1.3 2-(Benzo[d][1,3]dioxole-5-carboxamido)phenyl 2,5-Dichlorobenzenesulfonate (4c)
Light yellow powder, yield 83%. m.p 149.7–152.7°C. 1H NMR (400 MHz, CDCl3) δ 8.67 (s, 1H, -NH-), 8.30–7.76 (m, 2H, Ph-H), 7.69–7.33 (m, 2H, Ph-H, Ph-H), 7.21–6.74 (m, 6H, Ph-H), 6.08 (s, 2H, -OCH2O-). 13C NMR (100 MHz, CDCl3) δ 166.4 151.3, 148.8, 127.2, 125.7, 122.4, 122.3, 120.6, 119.8, 108.3, 107.9, 102.1. HRMS (ESI): calculated for C20H13Cl2NO6S [M + Na]+: 487.9732, found: 487.9705.
2.1.1.4 2-(Benzo[d][1,3]dioxole-5-carboxamido)phenyl 2-Bromobenzenesulfonate (4d)
Light yellow powder, yield 85%. m.p 130.5–132.5°C. 1H NMR (400 MHz, CDCl3) δ 8.58 (s, 1H, -NH-), 8.37 (dd, J = 8.3, 1.6 Hz, 1H, Ph-H), 8.11–8.04 (m, 1H, Ph-H), 7.78 (d, J = 7.5 Hz, 1H, Ph-H), 7.56–7.46 (m, 3H, Ph-H), 7.41 (d, J = 1.8 Hz, 1H, Ph-H), 7.31–7.29 (m, 1H, Ph-H), 7.16–6.98 (m, 2H, Ph-H), 6.90 (d, J = 8.1 Hz, 1H, Ph-H), 6.08 (s, 2H, -OCH2O-). 13C NMR (100 MHz, CDCl3) δ 164.7, 151.0, 148.2, 138.8, 135.9, 135.6, 132.6, 128.2, 128.0, 124.5, 123.3, 122.8, 122.3, 121.3, 108.2, 108.0, 101.9. HRMS (ESI): calculated for C20H14BrNO6S [M + Na]+: 497.9617, found: 497.9614.
2.1.1.5 2-(Benzo[d][1,3]dioxole-5-carboxamido)phenyl 3,5-Difluorobenzenesulfonate (4e)
Light yellow powder, yield 81.5%. m.p 152.7–153.8°C. 1H NMR (400 MHz, CDCl3) δ 8.32 (dd, J = 8.3, 1.6 Hz, 1H, Ph-H), 8.17 (s, 1H, -NH-), 7.46–7.31 (m, 5H, Ph-H), 7.17–6.82 (m, 4H, Ph-H), 6.09 (s, 2H, -OCH2O-). 13C NMR (100 MHz, CDCl3) δ 164.4, 161.6, 151.1, 148.4, 139.1, 131.2, 128.5, 128.1, 124.8, 123.6, 122.6, 121.9, 112.3, 112.0, 110.6, 108.3, 107.7, 102.0. HRMS (ESI): calculated for C20H13F2NO6S [M + Na]+: 456.0324, found: 456.0319.
2.1.1.6 2-(Benzo[d][1,3]dioxole-5-carboxamido)phenyl 4-Methoxybenzenesulfonate (4f)
Light yellow powder, yield 89%. m.p 94.3–95.2°C. 1H NMR (400 MHz, CDCl3) δ 8.34 (s, 1H, -NH-), 8.32 (d, J = 2.0 Hz, 1H, Ph-H), 7.75 (d, J = 9.0 Hz, 2H, Ph-H), 7.39 (dd, J = 8.1, 1.9 Hz, 1H, Ph-H), 7.34–7.28 (m, 2H, Ph-H), 7.01 (dd, J = 7.5, 1.6 Hz, 1H, Ph-H), 6.95–6.87 (m, 4H, Ph-H), 6.08 (s, 2H, -OCH2O-), 3.86 (s, 3H, -OCH3). 13C NMR (100 MHz, CDCl3) δ 164.6, 164.4, 150.9, 139.3, 130.8, 127.9, 125.7, 124.4, 123.1, 122.9, 122.0, 114.7, 108.2, 107.8, 101.9, 55.8. HRMS (ESI): calculated for C21H17NO7S [M + Na]+: 450.0618, found: 450.0619.
2.1.1.7 2-(Benzo[d][1,3]dioxole-5-carboxamido)phenyl 2-Chlorobenzenesulfonate (4g)
Light yellow powder, yield 88%. m.p 101.8–103.6°C. 1H NMR (400 MHz, CDCl3) δ 8.53 (s, 1H, -NH-), 8.40 (dd, J = 8.3, 1.6 Hz, 1H, Ph-H), 7.93–7.90 (m, 1H, Ph-H), 7.73–7.67 (m, 1H, Ph-H), 7.49 (dd, J = 8.1, 1.9 Hz, 1H, Ph-H), 7.42 (d, J = 1.8 Hz, 1H, Ph-H), 7.36–7.29 (m, 2H, Ph-H), 7.24–7.16 (m, 1H, Ph-H), 7.09–7.05 (m, 1H, Ph-H), 6.91 (d, J = 8.2 Hz, 1H, Ph-H), 6.08 (s, 2H, -OCH2O-). 13C NMR (100 MHz, CDCl3) δ 164.6, 160.8, 158.2, 151.0, 148.2, 138.4, 137.5, 137.4, 131.5, 131.4, 128.4, 128.2, 124.8, 124.5, 123.0, 122.8, 122.2, 117.7, 117.5, 108.2, 107.9, 101.9. HRMS (ESI): calculated for C20H14ClNO6S [M + Na]+: 454.0210, found: 424.0146.
2.1.1.8 2-(Benzo[d][1,3]dioxole-5-carboxamido)phenyl Naphthalene-2-sulfonate (4h)
Light yellow powder, yield 83%. m.p 145.6–147.1°C. 1H NMR (400 MHz, CDCl3) δ 8.44 (s, 1H, Ph-H), 8.32 (dd, J = 8.3, 1.4 Hz, 1H, Ph-H), 8.22 (s, 1H, -NH-), 7.90 (q, J = 8.4 Hz, 3H, Ph-H), 7.75–7.58 (m, 3H, Ph-H), 7.32–7.27 (m, 1H, Ph-H), 7.24–7.16 (m, 2H, Ph-H), 6.77 (d, J = 8.1 Hz, 1H, -OCH2O-). 13C NMR (100 MHz, CDCl3) δ 164.3, 150.8, 148.1, 139.3, 135.6, 131.8, 131.6, 131.4, 130.5, 129.9, 129.5, 128.1, 128.0, 124.4, 123.1, 123.0, 122.4, 121.7, 108.1, 107.7, 101.8. HRMS (ESI): calculated for C24H17Cl2NO6S [M + Na]+: 470.0777, found: 470.6777.
2.1.1.9 2-(Benzo[d][1,3]dioxole-5-carboxamido)phenyl 2,4,6-Trimethylbenzenesulfonate (4i)
Light yellow powder, yield 84%. m.p 130.5–131.6°C. 1H NMR (400 MHz, CDCl3) δ 8.60 (s, 1H, -NH-), 8.38 (dd, J = 8.3, 1.6 Hz, 1H, Ph-H), 7.47 (dd, J = 8.1, 1.8 Hz, 1H, Ph-H), 7.41 (d, J = 1.9 Hz, 1H, Ph-H), 7.29 (d, J = 1.4 Hz, 1H, Ph-H), 7.02–6.84 (m, 4H, Ph-H), 6.67 (dd, J = 8.2, 1.5 Hz, 1H, Ph-H), 6.07 (s, 2H, -OCH2O-), 2.56 (s, 6H, -2CH3), 2.34 (s, 3H, -CH3). 13C NMR (100 MHz, CDCl3) δ 164.6, 150.9, 148.2, 144.7, 140.7, 139.1, 132.1, 132.0, 129.8, 128.7, 127.8, 124.3, 123.2, 122.5, 122.0, 108.2, 107.9, 101.8, 22.9, 21.2. HRMS (ESI): calculated for C23H21NO6S [M + Na]+: 462.0982, found: 462.0977.
2.1.1.10 2-(Benzo[d][1,3]dioxole-5-carboxamido)phenyl 4-Chlorobenzenesulfonate (4j)
Light yellow powder, yield 85%. m.p 134.6–137.0°C. 1H NMR (400 MHz, CDCl3) δ 8.31 (dd, J = 8.3, 1.6 Hz, 1H, Ph-H), 8.19 (s, 1H, -NH-), 7.77 (d, J = 8.6 Hz, 2H, Ph-H), 7.44 (d, J = 8.6 Hz, 2H, Ph-H), 7.37 (dd, J = 8.1, 1.9 Hz, 1H, Ph-H), 7.33–7.29 (m, 2H, Ph-H), 7.13–7.02 (m, 1H, Ph-H), 6.95 (dd, J = 8.2, 1.5 Hz, 1H, Ph-H), 6.90 (d, J = 8.1 Hz, 1H, Ph-H), 6.09 (s, 2H, -2CH3). 13C NMR (100 MHz, CDCl3) δ 164.3, 151.1, 148.3, 141.8, 139.2, 133.1, 131.3, 129.9, 129.8, 128.2, 124.6, 123.3, 122.9, 121.9, 108.3, 107.7, 102.0. HRMS (ESI): calculated for C20H14ClNO6S [M + Na]+: 454.0122, found: 454.0119.
2.1.1.11 2-(Benzo[d][1,3]dioxole-5-carboxamido)phenyl 4-(Trifluoromethyl)benzenesulfonate (4k)
Light yellow powder, yield 82.3%. m.p 120.8–122.5°C. 1H NMR (400 MHz, CDCl3) δ 8.31 (dd, J = 8.3, 1.6 Hz, 1H, Ph-H), 8.17 (s, 1H, -NH-), 8.05–7.66 (m, 4H, Ph-H), 7.46–7.29 (m, 3H, Ph-H), 7.10–6.86 (m, 3H, Ph-H), 6.09 (s, 2H, -OCH2O-). 13C NMR (100 MHz, CDCl3) δ 164.2, 151.1, 148.3, 139.2, 138.3, 131.2, 129.0, 128.4, 128.1, 126.6, 126.7, 124.7, 123.6, 122.8, 121.9, 108.2, 107.7, 102.0. HRMS (ESI): calculated for C21H14F3NO6S [M + Na]+: 488.0386, found: 488.0386.
2.1.1.12 2-(Benzo[d][1,3]dioxole-5-carboxamido)phenyl Benzenesulfonate (4l)
Light yellow powder, yield 86.2%. m.p 140.0–140.9°C. 1H NMR (400 MHz, CDCl3) δ 8.34 (dd, J = 8.3, 1.6 Hz, 1H, Ph-H), 8.31 (s, 1H, -NH-), 7.86 (dd, J = 8.5, 1.3 Hz, 2H, Ph-H), 7.69–7.65 (m, 1H, Ph-H), 7.52–7.48 (m, 2H, Ph-H), 7.40–7.37 (m, 1H, Ph-H), 7.35–7.28 (m, 2H, Ph-H), 7.04–7.02 (m, 1H, Ph-H), 6.95–6.86 (m, 2H, Ph-H), 6.08 (s, 2H, -OCH2O-). 13C NMR (100 MHz, CDCl3) δ 164.4, 151.0, 148.2, 139.2, 134.9, 131.4, 129.5, 128.4, 128.0, 124.4, 123.0, 122.9, 122.0, 108.2, 107.8, 101.9. HRMS (ESI): calculated for C20H15NO6S [M + Na]+: 420.0512, found: 420.0511.
2.1.1.13 2-(Benzo[d][1,3]dioxole-5-carboxamido)phenyl 2-Fluorobenzenesulfonate (4m)
Light yellow powder, yield 80%. m.p 93.7–94.7°C. 1H NMR (400 MHz, CDCl3) δ 8.58 (s, 1H, -NH-), 8.37 (dd, J = 8.3, 1.6 Hz, 1H, Ph-H), 8.05 (dd, J = 8.0, 1.6 Hz, 1H, Ph-H), 7.62–7.54 (m, 2H, Ph-H), 7.49 (dd, J = 8.1, 1.8 Hz, 1H, Ph-H), 7.41 (d, J = 1.8 Hz, 1H, Ph-H), 7.36–7.27 (m, 2H, Ph-H), 7.14–7.02 (m, 2H, Ph-H), 6.91 (d, J = 8.1 Hz, 1H, Ph-H), 6.08 (s, 2H, Ph-H). 13C NMR (100 MHz, CDCl3) δ 164.7, 151.0, 138.7, 135.7, 132.4, 131.6, 128.2, 127.4, 124.5, 123.2, 122.8, 122.2, 108.2, 108.0, 101.9. HRMS (ESI): Calculated for C20H14FNO6S [M + K]+: 454.0163, found: 454.0120.
2.1.1.13 2-(Benzo[d][1,3]dioxole-5-carboxamido)phenyl 4-(tert-butyl) Benzenesulfonate (4n)
Light yellow powder, yield 82%. m.p 107.7–109.3°C. 1H NMR (400 MHz, CDCl3) δ 8.36 (dd, J = 8.3, 1.6 Hz, 1H, Ph-H), 8.33 (s, 1H, -NH-), 7.64 (dd, J = 111.7, 8.7 Hz, 4H, Ph-H), 7.39 (dd, J = 8.1, 1.9 Hz, 1H, Ph-H), 7.33–7.27 (m, 2H, Ph-H), 7.06–6.96 (m, 2H, Ph-H), 6.90 (d, J = 8.1 Hz, 1H, Ph-H), 6.08 (s, 2H, -OCH2O-), 1.31 (s, 9H, -CH3 × 3). 13C NMR (100 MHz, CDCl3) δ 164.2, 159.2, 150.9, 148.2, 139.2, 131.5, 128.4, 128.3, 128.0, 126.5, 124.3, 123.0, 122.8, 122.0, 108.2, 107.8, 101.9, 35.4, 30.9. HRMS (ESI): calculated for C24H23Cl2NO6S [M + Na]+: 476.1144, found: 476.1138.
2.1.1.14 2-(Benzo[d][1,3]dioxole-5-carboxamido)phenyl 4-Bromobenzenesulfonate (4o)
Light yellow powder, yield 83%. m.p 122.2–125.1°C. 1H NMR (400 MHz, CDCl3) δ 8.31 (dd, J = 8.3, 1.6 Hz, 1H, Ph-H), 8.17 (s, 1H, -NH-), 7.73–7.58 (m, 4H, Ph-H), 7.43–7.30 (m, 3H, Ph-H), 7.06 (td, J = 7.9, 1.6 Hz, 1H, Ph-H), 6.96 (dd, J = 8.2, 1.5 Hz, 1H, Ph-H), 6.90 (d, J = 8.1 Hz, 1H, Ph-H), 6.09 (s, 2H, -OCH2O-). 13C NMR (100 MHz, 7.30 (m, 3H) δ 164.3, 151.1, 148.3, 139.2, 133.6, 132.9, 131.2, 130.5, 129.8, 128.2, 124.6, 123.3, 122.9, 121.9, 108.3, 107.7, 102.0. HRMS (ESI): calculated for C20H14BrNO6S [M + Na]+: 497.9617, found: 497.9612.
2.1.1.15 2-(Benzo[d][1,3]dioxole-5-carboxamido)phenyl 2,5-Difluorobenzenesulfonate (4p)
Light yellow powder, yield 88%. m.p 109.9–121.6°C. 1H NMR (400 MHz, CDCl3) δ 8.45 (s, 1H, -NH-), 8.39 (dd, J = 8.3, 1.6 Hz, 1H, Ph-H), 7.66–7.57 (m, 1H, Ph-H), 7.48 (dd, J = 8.1, 1.9 Hz, 1H, Ph-H), 7.44–7.30 (m, 3H, Ph-H), 7.24–7.17 (m, 2H, Ph-H), 7.10 (ddd, J = 8.2, 7.4, 1.6 Hz, 1H, Ph-H), 6.92 (d, J = 8.1 Hz, 1H, Ph-H), 6.08 (s, 2H, -OCH2O-). 13C NMR (100 MHz, CDCl3) δ 164.6, 151.1, 148.3, 138.4, 131.4, 128.5, 124.6, 123.2, 122.7, 122.1, 118.2, 108.3, 107.8, 101.9. HRMS (ESI): calculated for C20H13F2NO6S [M + Na]+: 456.0323, found: 456.0322.
2.1.1.16 2-(Benzo[d][1,3]dioxole-5-carboxamido)phenyl 3-Nitrobenzenesulfonate (4q)
Light yellow powder, yield 81%. m.p 116.2–127.1°C. 1H NMR (400 MHz, CDCl3) δ 8.44 (s, 1H, -NH-), 8.24 (dd, J = 8.3, 1.6 Hz, 1H, Ph-H), 7.86 (dd, J = 7.8, 1.5 Hz, 1H, Ph-H), 7.74 (td, J = 7.7, 1.5 Hz, 1H, Ph-H), 7.66 (td, J = 7.7, 1.4 Hz, 1H, Ph-H), 7.53 (dd, J = 7.9, 1.4 Hz, 1H, Ph-H), 7.43–7.29 (m, 3H, Ph-H), 7.21 (d, J = 1.9 Hz, 1H, Ph-H), 7.15 (ddd, J = 8.3, 7.5, 1.6 Hz, 1H, Ph-H), 6.86 (d, J = 8.1 Hz, 1H, Ph-H), 6.07 (s, 2H, -OCH2O-). 13C NMR (100 MHz, CDCl3) δ 164.6, 151.0, 148.0, 138.9, 135.7, 132.5, 132.1, 131.0, 128.4, 128.2, 127.8, 124.9, 124.7, 123.6, 123.2, 122.4, 108.1, 108.0, 101.9. HRMS (ESI): calculated for C20H14Cl2N2O8S [M + Na]+: 465.0363, found: 465.0362.
2.1.1.17 2-(Benzo[d][1,3]dioxole-5-carboxamido)phenyl 3-Fluorobenzenesulfonate (4r)
Light yellow powder, yield 86.6%. m.p 160.0–162.1°C. 1H NMR (400 MHz, CDCl3) δ 8.33 (dd, J = 8.3, 1.6 Hz, 1H, Ph-H), 8.23 (s, 1H, -NH-), 7.67–7.62 (m, 1H, Ph-H), 7.59 (ddd, J = 7.7, 2.6, 1.7 Hz, 1H, Ph-H), 7.50 (td, J = 8.1, 5.1 Hz, 1H, Ph-H), 7.41–7.31 (m, 4H, Ph-H), 7.05 (ddd, J = 8.9, 7.4, 1.6 Hz, 1H, Ph-H), 6.96 (dd, J = 8.2, 1.5 Hz, 1H, Ph-H), 6.91 (d, J = 8.1 Hz, 1H, Ph-H), 6.09 (s, 2H, -OCH2O-). 13C NMR (100 MHz, CDCl3) δ 164.4, 151.0, 148.3, 139.1, 131.4, 131.3, 128.3, 128.2, 124.6, 124.3, 123.3, 122.8, 122.4, 122.2, 121.9, 116.0, 115.7, 108.3, 107.7, 101.9. HRMS (ESI): calculated for C20H14FNO6S [M + Na]+: 438.0418, found: 438.0419.
2.1.1.18 2-(Benzo[d][1,3]dioxole-5-carboxamido)phenyl 2,4-Difluorobenzenesulfonate (4s)
Light yellow powder, yield 83.2%. m.p 95.6–98.4°C. 1H NMR (400 MHz, CDCl3) δ 8.46 (s, 1H, -NH-), 8.38 (dd, J = 8.3, 1.6 Hz, 1H, Ph-H), 7.97–7.87 (m, 1H, Ph-H), 7.49 (dd, J = 8.1, 1.9 Hz, 1H, Ph-H), 7.41 (d, J = 1.9 Hz, 1H, Ph-H), 7.35–7.29 (m, 1H, Ph-H), 7.21–7.14 (m, 1H, Ph-H), 7.12–6.87 (m, 4H, Ph-H), 6.09 (s, 2H, -OCH2O-). 13C NMR (100 MHz, CDCl3) δ 164.6, 151.0, 148.3, 138.5, 133.4, 133.3, 131.4, 128.4, 124.6, 123.3, 122.8, 122.1, 112.7, 108.3, 107.8, 106.3, 101.9. HRMS (ESI): calculated for C20H13F2NO6S [M + Na]+: 456.0324, found: 456.0326.
2.1.1.19 2-(Benzo[d][1,3]dioxole-5-carboxamido)phenyl 3-(Trifluoromethyl)benzenesulfonate (4t)
Light yellow powder, yield 84%. m.p 120.9–123.6°C. 1H NMR (400 MHz, CDCl3) δ 8.33 (dd, J = 8.3, 1.6 Hz, 1H, Ph-H), 8.23 (s, 1H, -NH-), 8.14 (s, 1H, Ph-H), 8.06–7.90 (m, 2H, Ph-H), 7.67 (t, J = 7.9 Hz, 1H, Ph-H), 7.43–7.31 (m, 3H, Ph-H), 7.06 (ddd, J = 9.1, 7.4, 1.6 Hz, 1H, Ph-H), 6.94 (dd, J = 8.3, 1.5 Hz, 1H, Ph-H), 6.90 (d, J = 8.1 Hz, 1H, Ph-H), 6.08 (s, 2H, -OCH2O-). 13C NMR (100 MHz, CDCl3) δ 164.3, 151.1, 148.3, 139.0, 135.9, 131.6, 131.2, 130.4, 128.4, 128.1, 125.5, 124.6, 123.5, 122.7, 121.9, 108.3, 107.7, 102.0. HRMS (ESI): calculated for C21H14F3NO6S [M + Na]+: 488.0386, found: 488.0396.
2.1.1.20 2-(Benzo[d][1,3]dioxole-5-carboxamido)phenyl 2-(Trifluoromethyl)benzenesulfonate (4u)
Light yellow powder, yield 87%. m.p 101.3–105.4°C. 1H NMR (400 MHz, CDCl3) δ 8.36 (s, 1H, -NH-), 8.34 (d, J = 4.1 Hz, 1H, Ph-H), 8.13 (d, J = 7.9 Hz, 1H, Ph-H), 7.90 (d, J = 8.3 Hz, 1H, Ph-H), 7.85–7.69 (m, 2H, Ph-H), 7.42 (dd, J = 8.1, 1.8 Hz, 1H, Ph-H), 7.35 (d, J = 1.9 Hz, 1H, Ph-H), 7.33–7.28 (m, 1H, Ph-H), 7.08–7.04 (m, 2H, Ph-H), 6.90 (d, J = 8.1 Hz, 1H, Ph-H), 6.08 (s, 2H, -OCH2O-). 13C NMR (100 MHz, CDCl3) δ 148.2, 138.8, 134.8, 132.9, 132.6, 131.4, 128.3, 124.5, 123.2, 123.0, 122.1, 108.2, 107.8, 101.9. HRMS (ESI): calculated for C21H14F3NO6S [M + Na]+: 488.0386, found: 488.0388.
2.1.1.21 2-(Benzo[d][1,3]dioxole-5-carboxamido)phenyl 3-Bromobenzenesulfonate (4v)
Light yellow powder, yield 84%. m.p 158.0–158.5°C. 1H NMR (400 MHz, CDCl3) δ 8.34 (dd, J = 8.3, 1.6 Hz, 1H, Ph-H), 8.20 (s, 1H, -NH-), 8.04 (t, J = 1.9 Hz, 1H, Ph-H), 7.80–7.71 (m, 2H, Ph-H), 7.44–7.30 (m, 4H, Ph-H), 7.09–7.05 (m, 1H, Ph-H), 6.98 (dd, J = 8.2, 1.6 Hz, 1H, Ph-H), 6.91 (d, J = 8.1 Hz, 1H, Ph-H), 6.09 (s, 2H, -OCH2O-). 13C NMR (100 MHz, CDCl3) δ 151.1, 148.4, 139.1, 137.9, 136.4, 131.3, 131.1, 130.9, 128.3, 128.2, 127.0, 124.6, 123.5, 123.3, 122.8, 121.9, 108.3, 107.8, 101.9. HRMS (ESI): calculated for C20H14BrNO6S [M + Na]+: 497.9617, found: 497.9619.
2.1.1.22 2-(Benzo[d][1,3]dioxole-5-carboxamido)phenyl 3-Chlorobenzenesulfonate (4w)
Light yellow powder, yield 86%. m.p 148.4–150.1°C. 1H NMR (400 MHz, CDCl3) δ 8.33 (dd, J = 8.2, 1.6 Hz, 1H, Ph-H), 8.20 (s, 1H, -NH-), 7.88 (t, J = 1.9 Hz, 1H, Ph-H), 7.74–7.67 (m, 1H, Ph-H), 7.61 (dd, J = 2.1, 1.0 Hz, 1H, Ph-H), 7.44 (t, J = 8.0 Hz, 1H, Ph-H), 7.39–7.30 (m, 3H, Ph-H), 7.07 (ddd, J = 9.0, 7.4, 1.6 Hz, 1H, Ph-H), 6.98 (dd, J = 8.2, 1.5 Hz, 1H, Ph-H), 6.91 (d, J = 8.1 Hz, 1H, Ph-H), 6.08 (s, 2H, -OCH2O-). 13C NMR (100 MHz, CDCl3) δ 164.3, 151.1, 148.3, 139.1, 136.3, 135.9, 135.0, 131.3, 130.7, 128.3, 126.5, 124.6, 123.3, 122.8, 121.9, 108.3, 107.8, 101.9. HRMS (ESI): calculated for C20H14ClNO6S [M + Na]+: 454.0168, found: 454.0121.
2.1.1.23 2-(Benzo[d][1,3]dioxole-5-carboxamido)phenyl 4-Methylbenzenesulfonate (4x)
Light yellow powder, yield 85%. m.p 130.0–131.1°C. 1H NMR (400 MHz, CDCl3) δ 8.33 (dd, J = 8.3, 1.6 Hz, 1H, Ph-H), 8.28 (s, 1H, -NH-), 7.72 (d, J = 8.4 Hz, 2H, Ph-H), 7.38 (dd, J = 8.1, 1.8 Hz, 1H, Ph-H), 7.32–7.27 (m, 3H, Ph-H), 7.26 (s, 1H, Ph-H), 7.02 (td, J = 7.8, 7.4, 1.6 Hz, 1H, Ph-H), 6.94 (dd, J = 8.2, 1.5 Hz, 1H, Ph-H), 6.90 (d, J = 8.1 Hz, 1H, Ph-H), 6.08 (s, 2H, -OCH2O-), 2.42 (s, 3H, -CH3). 13C NMR (100 MHz, CDCl3) δ 164.3, 150.9, 148.2, 146.3, 139.2, 131.6, 131.4, 130.1, 128.4, 128.0, 124.4, 123.0, 122.9, 122.0, 108.2, 107.8, 101.9, 21.8. HRMS (ESI): calculated for C21H17NO6S [M + Na]+: 434.0669, found: 434.0673.
2.2 Antimicrobial Assay
The antimicrobial activity of the derivatives (4a–4x) was tested using the turbidimeter test, the commercial agricultural bactericide bismerthiazol, thiodiazole-copper and lead compound piperonylic acid used as control. The test compounds were dissolved in 150 μL of dimethylformamide (DMF) and diluted with 0.1% (v/v) Tween-20 to prepare two concentrations of 100 and 50 μg/ml. One milliliter of the liquid sample was added to the 40 ml non-toxic nutrient broth medium (NB: 1.5 g of beef extract, 2.5 g of peptone, 0.5 g of yeast powder, 5.0 g of glucose, and 500 ml of distilled water, pH 7.0–7.2). Then, 40 μL of NB medium containing bacteria was added to 5 ml of solvent NB containing the test compounds or thiodiazole–copper. The inoculated test tubes were incubated at 30 ± 1°C with continuous shaking at 180 rpm for 48 h. The culture growth was monitored spectrophotometrically by measuring the optical density at 600 nm (OD600) and expressed as corrected turbidity. The relative inhibition rates Inhibition (%) were calculated as the following equation, where Ctur was the corrected turbidity value of bacterial growth on untreated NB and Ttur was the corrected turbidity value of bacterial growth on treated NB.
2.3 Insecticidal Activity Assay
Divide 20 second-instar larvae of Spodoptera frugiperda into 20 small cups and starve for 3–4 h. Cut the fresh corn leaves into small leaf discs of 1 cm × 1 cm with scissors, and then soak them in each test solution for 5 s, and then air dry them naturally. Then put them in a cup with Spodoptera frugiperda and keep it under the conditions of temperature of 25 ± 1°C, relative humidity of 60∼70%, and a light-dark cycle of L: D = 14 h: 10 h. Feed normal fresh corn leaf discs after 12 h and record the number of dead insects at 12, 24, and 36 h.
3 Results and Discussion
3.1 Chemistry
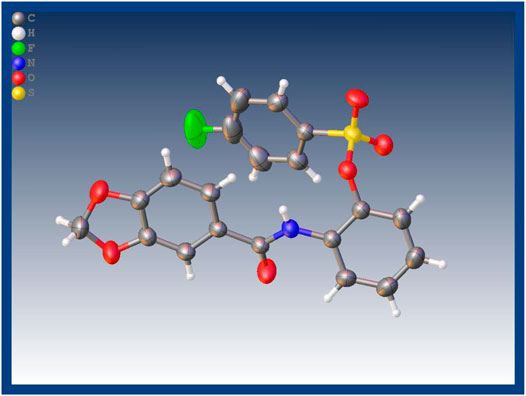
The synthetic route for the target compounds 4a–4x was shown in Scheme 1. Intermediates 2–3 were prepared according to previously reported procedures, and the yield of all compounds was satisfactory, usually higher than 80%. In the syntheses target compounds of 4a–4x, the yield when using inorganic base, such as K2CO3 or KHCO3 as catalyst was usually only approximately 30%. When inorganic base was replaced with organic base triethylamine as the catalyst, the yield was considerably greater usually more than 80%. It was worth noting that when the intermediates 3 were synthesized using acid and 2-amino phenol the carboxyl group might have reacted with hydroxyl group to form an ester, or it may have reacted with the amino group to form an amide. These two structures of isomers were difficult to confirm through HRMS or NMR. In order to get the exact structure of the target compound, we used X-ray to confirm the structure of compound 4a, and the results are show in Figure 1 (CCDC 2131244). Crystal data of 4a indicated that target compounds were in the form of carbonamide instead of carbonate.
3.2 In Vitro Antibacterial Activity
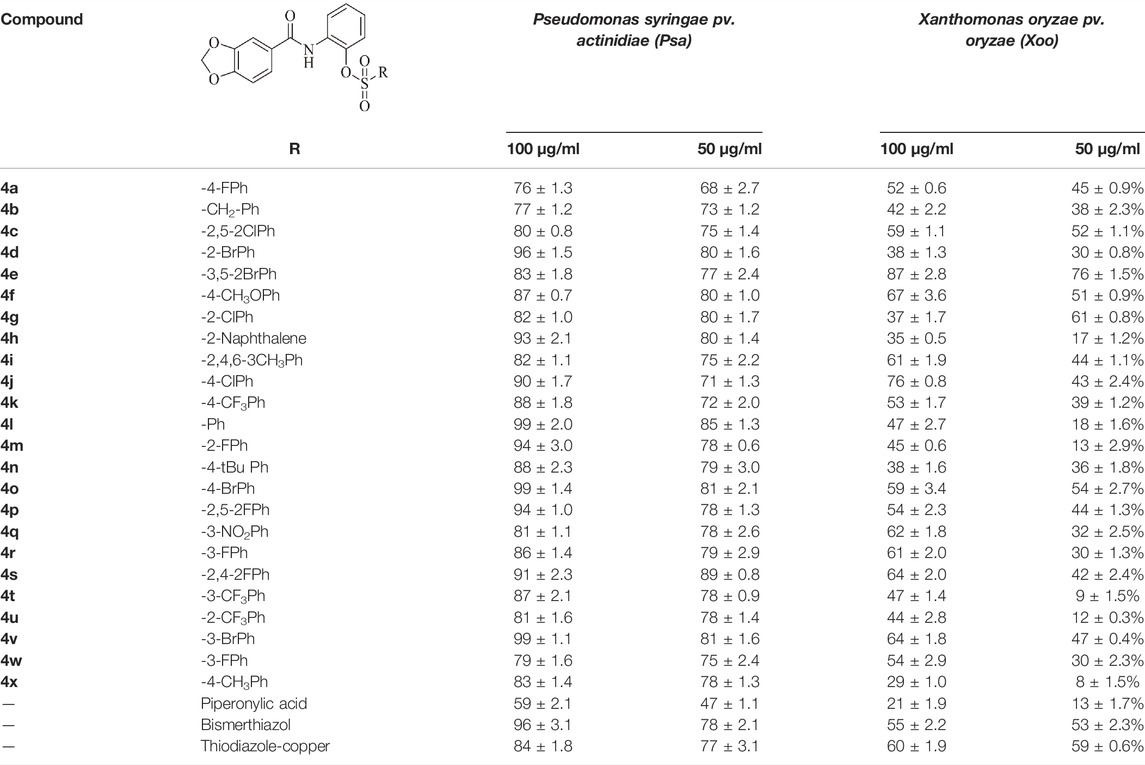
Antibacterial activities of target compounds 4a–4x against agriculturally important pathogenic bacteria Psa and Xoo were determined in vitro via the turbidimetric method, using the commercialized bismerthiazol, thiodiazole-copper, and piperonylic acid as a control agent. The bactericide which was used to make the bioassay was provided by Guizhou Tea Institute, and the results of the bioassay against Psa and Xoo are shown in Table 1 and indicated that most of the title compounds exhibited good to excellent activities in vitro. Compounds 4l, 4o, and 4v showed excellent activities against Psa at 100 μg/ml with inhibition rates of 99%, which were higher than those of thiodiazole-copper (84%), bismerthiazol (96%), and lead compound piperonylic acid (59%), respectively. In particular, even at a concentration as low as 50 μg/ml, compound 4l was found to still possess a pronounced anti-Pas efficacy of 85%. Moreover, compounds 4e, 4f, and 4j exhibited higher activities (i.e., 87, 67 and 76%, respectively) against Xoo than that of thiodiazole-copper (60%), bismerthiazol (55%), and piperonylic acid (21%) at 100 μg/ml even at a concentration as low as 50 μg/ml, compound 4e was found to still possess a pronounced anti-Xoo effifcacy of 76%, which was significantly higher than that of control agent. It is worth mentioning that whether for Psa or Xoo, the activities of almost all target compounds were significantly higher than that of the lead compound piperonylic acid. This indicated that incorporation of a sulfonic acid ester moiety into the piperonylic acid backbone could significantly improve its antibacterial activity.
3.3 Insecticidal Activity Assay Against Spodoptera frugiperda
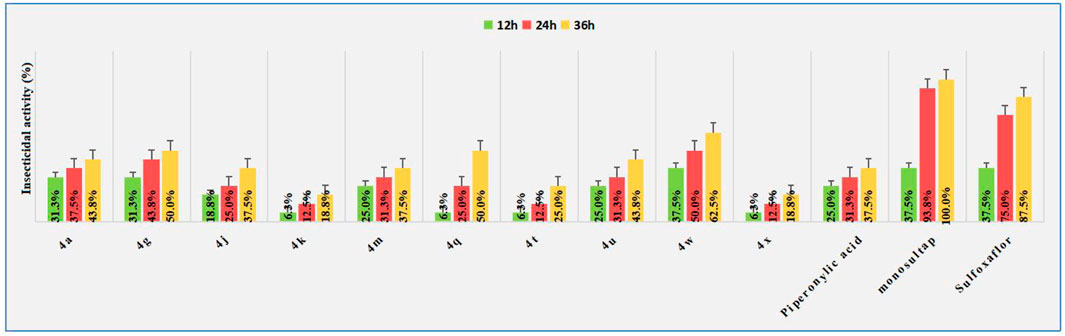
In view of the literature which reported that piperonylic acid has certain insecticidal activities, we also evaluated the activity of some title compounds against Spodoptera frugiperda at 50 μg/ml, The Spodoptera frugiperda used in the biological tests were collected from fields in Luodian County, Guizhou Province, China, and bred in a greenhouse. The pesticidal results are shown in Figure 2. Although most compounds exhibited certain insecticidal effect on Spodoptera frugiperda, such as the lethal rate of compound 4g, 4q, and 4w on to the second instar larvae of the insect reached 50.0, 50.0 and 62.5% at the 36 h, respectively, which was significantly higher than the lead structure piperonylic acid (37.5%), but still lower than the commercial insecticide monosultap (100%) and sulfoxaflor (87.5%).
4 Conclusion
In summary, in order to seek new efficiency, broad-spectrum, and structure simple agricultural bactericide, a series of novel piperonylic acid derivatives containing a sulfonic acid ester moiety was synthesized. The structures of the title compounds were verified by 1H NMR, 13C NMR, and HRMS. The bioassay results revealed that these compounds showed good inhibition activity against Xoo and Psa, and some compounds even exhibited higher antibacterial activity than those of commercial bactericide which are widely used. The title compounds showed weaker activity against |Spodoptera frugiperda compared with commercial pesticides. Thus, we recommend these newly designed and synthesized scaffolds should be used as a bactericide lead compound rather than an insecticide lead compound for further optimization and research.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
Author Contributions
DX and XH contributed to the synthesis, purification, and characterization of all compounds and prepared the original manuscript. XH and ZY performed the biological activity research. DX and XR analyzed the experimental results and drafted the first and second version of the manuscript. All authors discussed, edited, and approved the final version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors gratefully acknowledge the National Key R&D Program of China (2019YFD0300103) and Science Foundation of Guizhou Province [ZK (2021) 143]. The authors are deeply grateful to Professor Lei of Guizhou Tea Research Institute for his support in this study.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fchem.2022.913003/full#supplementary-material
References
Abdullahiab, A., Khairulmazmibce, A., Yasmeen, S., Ismail, I. S., Norhayu, A., Sulaiman, M. R., et al. (2020). Phytochemical Profiling and Antimicrobial Activity of Ginger (Zingiber Officinale) Essential Oils against Important Phytopathogens. Arab. J. Chem. 13, 8012–8025. doi:10.1016/j.arabjc.2020.09.031
Boycov, D. E., Manin, A. N., Drozd, K. V., Churakov, A. V., and Perlovich, G. L. (2022). Thermal Method Usage Features for Multicomponent Crystal Screening. CrystEngComm 24, 2280–2290. doi:10.1039/d1ce01717a
Carlos, G., Anforth, R., Clements, A., Menzies, A. M., Carlino, M. S., Chou, S., et al. (2015). Cutaneous Toxic Effects of bRAF Inhibitors Alone and in Combination with MEK Inhibitors for Metastatic Melanoma. JAMA Dermatol 151, 1103–1109. doi:10.1001/jamadermatol.2015.1745
Chai, F., Zhao, X., Gao, H., Zhao, Y., Huang, H., and Gao, Z. (2019). Effective Removal of Antibacterial Drugs from Aqueous Solutions Using Porous Metal-Organic Frameworks. J. Inorg. Organomet. Polym. 29, 1305–1313. doi:10.1007/s10904-019-01094-3
Chen, M. H., Zhang, X., Lu, D. W., Luo, H. R., Zhou, Z. Y., Qi, X. F., et al. (2021). Synthesis and Bioactivities of Novel 1,3,4-thiadiazole Derivatives of Glucosides. Front. Chem. 9, 1–8. doi:10.3389/fchem.2021.645876
Chen, M., Wu, S., He, Z., Cheng, Z., Duan, S., Jiang, H., et al. (2021). Apatinib Combined with Concurrent Chemoradiotherapy in Patients with Subglottic Small Cell Carcinoma: a Case Report. J. Int. Med. Res. 49, 030006052110161. doi:10.1177/03000605211016146
Dam, J. H., and Madsen, R. (2009). Convergent Synthesis of Pancratistatin from Piperonal and Xylose. Eur. J. Org. Chem. 2009, 4666–4673. doi:10.1002/ejoc.200900719
Guo, Q., Sun, Y., Kong, E., Rao, L., Chen, J., Wu, Q., et al. (2020). Apatinib Combined with Chemotherapy or Concurrent Chemo-Brachytherapy in Patients with Recurrent or Advanced Cervical Cancer. Medicine 99, e19372. doi:10.1097/md.0000000000019372
Guo, T., Xia, R., Chen, M., He, J., Su, S., Liu, L., et al. (2019). Biological Activity Evaluation and Action Mechanism of Chalcone Derivatives Containing Thiophene Sulfonate. RSC Adv. 9, 24942–24950. doi:10.1039/c9ra05349b
Guo, T., Xia, R., Chen, M., Su, S., He, J., He, M., et al. (2020). Biological Activity Evaluation and Action Mechanism of 1,4-Pentadien-3-One Derivatives Containing Thiophene Sulfonate. Phosphorus, Sulfur, Silicon Relat. Elem. 195, 123–130. doi:10.1080/10426507.2019.1655418
Horie, M., and Nakazawa, H. (1992). Determination of Miloxacin and its Metabolite in Fish by High Performance Liquid Chromatography with Fluorescence and UV Detection. J. Liq. Chromatogr. 15, 2057–2070. doi:10.1080/10826079208016325
Jang, S., Park, S. H., and Kim, H. K. (2020). Simultaneous Determination of 6 Antiallergic Components in Asarum Sieboldii Using High-Performance Liquid Chromatography. Nat. Prod. Commun. 15, 1–10. doi:10.1177/1934578x20966191
Joseph, J., Dixit, S. R., and Pujar, G. V. (2019). Design, Synthesis and In-Vitro Evaluation of Aryl Amides as Potent Inhibitors against Mycobacterium tuberculosis. J. Pharm. Sci. Res. 11, 3166–3173. http://www.jpsr.pharmainfo.in/Documents/Volumes/vol11issue09/jpsr11091913.pdf.
Kou, S.-B., Lin, Z.-Y., Wang, B.-L., Shi, J.-H., and Liu, Y.-X. (2021). Evaluation of the Interaction of Novel Tyrosine Kinase Inhibitor Apatinib Mesylate with Bovine Serum Albumin Using Spectroscopies and Theoretical Calculation Approaches. J. Biomol. Struct. Dyn. 39, 4795–4806. doi:10.1080/07391102.2020.1782767
Liu, C., Zhang, J., and You, G. (2019). Interaction of Anticancer Drugs with Human Organic Anion Transporter hOAT4. J. Oncol. 2019, 1951786. doi:10.1155/2019/1951786
Matsumoto, T., Takiyama, M., Sanechika, S., Nakayama, A., Aoki, K., Ohbuchi, K., et al. (2020). In Vivo pharmacokinetic Analysis Utilizing Non-targeted and Targeted Mass Spectrometry and In Vitro Assay against Transient Receptor Potential Channels of Maobushisaishinto and its Constituent Asiasari Radix. Molecules 25, 4283. doi:10.3390/molecules25184283
Moreira, K. G., do Prado, T. P., Mendes, N. F., de Medeiros Bezerra, R., Jara, C. P., Melo Lima, M. H., et al. (2021). Accelerative Action of Topical Piperonylic Acid on Mice Full Thickness Wound by Modulating Inflammation and Collagen Deposition. PLoS One 16, e0259134. doi:10.1371/journal.pone.0259134
Quan, X., Xu, X., and Yan, B. (2022). Facile Fabrication of Tb3+-Functionalized COF Mixed-Matrix Membrane as a Highly Sensitive Platform for the Sequential Detection of Oxolinic Acid and Nitrobenzene. J. Hazard. Mater. 427, 127869. doi:10.1016/j.jhazmat.2021.127869
Rai, S. K., Gunnam, A., Mannava, M. K. C., and Nangia, A. K. (2020). Improving the Dissolution Rate of the Anticancer Drug Dabrafenib. Cryst. Growth & Des. 20, 1035–1046. doi:10.1021/acs.cgd.9b01365
Sui, G., Xu, D., Luo, T., Guo, H., Sheng, G., Yin, D., et al. (2020). Design, Synthesis and Antifungal Activity of Amide and Imine Derivatives Containing a Kakuol Moiety. Bioorg. Med. Chem. Lett. 30, 126774. doi:10.1016/j.bmcl.2019.126774
Tuerkova, A., and Zdrazil, B. (2020). An Integrative Drug Repurposing Pipeline Using KNIME and Programmatic Data Access: a Case Study on COVID-19 Data. ChemRxiv 6, 1–32. doi:10.26434/chemrxiv.12678488
Ueno, R., and Aoki, T. (1996). High-performance Liquid Chromatographic Method for the Rapid and Simultaneous Determination of Sulfamonomethoxine, Miloxacin and Oxolinic Acid in Serum and Muscle of Cultured Fish. J. Chromatogr. B Biomed. Sci. Appl. 682, 179–181. doi:10.1016/0378-4347(96)00078-3
Ueno, R., Okada, Y., and Tatsuno, T. (2001). Pharmacokinetics and Metabolism of Miloxacin in Cultured Eel. Aquaculture 193, 11–24. doi:10.1016/s0044-8486(00)00475-0
Umadevi, P., Deepti, K., and Venugopal, D. V. R. (2013). Synthesis, Anticancer and Antibacterial Activities of Piperine Analogs. Med. Chem. Res. 22, 5466–5471. doi:10.1007/s00044-013-0541-4
Wang, J., Lü, B. H., Dai, X. J., Zhang, Y. F., Chen, X. Y., and Zhong, D. F. (2017). Simultaneous Determination of Donafenib and its N-Oxide Metabolite in Human Plasma by Liquid Chromatography-Tandem Mass Spectrometry. Yao Xue Xue Bao 52, 443–448. (In Chinese). doi:10.16438/j.0513-4870.2016-0954
Wang, X., Wang, X., Zhou, B., Long, J., and Li, P. (2021). Design, Synthesis, and Evaluation of New 4( 3 H )‐quinazolinone Derivatives Containing a Pyrazole Carboxamide Moiety. J. Heterocycl. Chem. 58, 2109–2116. doi:10.1002/jhet.4334
Wang, Y., Zhou, R., Sun, N., He, M., Wu, Y., and Xue, W. (2022). Synthesis and Antibacterial Activity of Novel 1,4‐pentadien‐3‐one Derivatives Bearing a Benzothiazole Moiety. J. Heterocycl. Chem 59, 533–542. doi:10.1002/jhet.4399
Yamazawa, T., Kobayashi, T., Kurebayashi, N., Konishi, M., Noguchi, S., Inoue, T., et al. (2021). A Novel RyR1-Selective Inhibitor Prevents and Rescues Sudden Death in Mouse Models of Malignant Hyperthermia and Heat Stroke. Nat. Commun. 12, 4293. doi:10.1038/s41467-021-24644-1
Zazeri, G., Povinelli, A. P. R., Le Duff, C. S., Tang, B., Cornelio, M. L., and Jones, A. M. (2020). Synthesis and Spectroscopic Analysis of Piperine- and Piperlongumine-Inspired Natural Product Scaffolds and Their Molecular Docking with IL-1β and NF-Κb Proteins. Molecules 25, 2841–2848. doi:10.3390/molecules25122841
Keywords: piperonylic acid, sulfonic acid esters, synthesis, antibacterial activities, insecticidal activity
Citation: Xie D, Hu X, Ren X and Yang Z (2022) Synthesis and Bioactivities of Novel Piperonylic Acid Derivatives Containing a Sulfonic Acid Ester Moiety. Front. Chem. 10:913003. doi: 10.3389/fchem.2022.913003
Received: 09 April 2022; Accepted: 19 April 2022;
Published: 31 May 2022.
Edited by:
Pei Li, Kaili University, ChinaReviewed by:
Xiaobin Shi, Hunan Academy of Agricultural Sciences, ChinaMeihang Chen, Tongren University, China
Hongbo Li, Guizhou Academy of Agricultural Sciences, China
Copyright © 2022 Xie, Hu, Ren and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dandan Xie, eGRkeGVkQDE2My5jb20=
 Dandan Xie
Dandan Xie Xin Hu3
Xin Hu3