- Colleges of Material and Chemistry Engineering, Tongren University, Tongren, China
A series of novel galactoside derivatives containing 1,3,4-thiadiazole moiety were synthesized, and the structure of them was verified by spectroscopy of NMR and HRMS, and antifungal and antibacterial activities of them were screened. The results showed that the newly synthesized compounds had good antifungal activities. Among them, Ⅲ16, Ⅲ17, and Ⅲ19 exhibited satisfactory activities against Phytophthora infestans (P. infestans), with EC50 values of 5.87, 4.98, and 6.17 μg/ml, respectively, which were similar to those of dimethomorph (5.52 μg/ml). Meanwhile, the title compounds also possessed certain antibacterial activities.
Introduction
Galactoside and its derivatives are widely found in litchi, laver, seaweed, and snails (Choucry et al., 2021; Kumar, 2020) and had anticancer (Tacke et al., 2017; Oueslati et al., 2020), antiviral (Cai et al., 2006; Abu-Zaied et al., 2021), and antibacterial (Upadhyay et al., 2010) activities. In addition, it was found that novel galactoside derivatives containing a pyrimidine moiety possessed good antifungal activities against Gibberella zeae (G. zeae), Botryosphaeria dothidea (B. dothidea), Phytophthora infestans (P. infestans), Thanatephorus cucumeris (T. cucumeris), and Phompsis sp in the preliminary working of our group (Chen M. H. et al., 2021; Chen et al., 2022). Moreover, it has been reported that glycosylation can improve the properties of active lead compounds, such as solubility, stability, and bioactivity (Wu et al., 2014; Gurung et al., 2017).
It is known that nitrogen-containing heterocyclic compounds have not only a broad spectrum of biological activity and diversity of structure changes but also low toxicity to most warm-blooded animals, birds, fish, and bees (Mermer et al., 2021). 1,3,4-Thiadiazole derivatives, important nitrogen-containing heterocyclic compounds, showed a wide range of bioactivities, such as antifungal (Bhinge et al., 2015; Chudzik et al., 2019), antibacterial (Wu et al., 2021), anticancer (Abas et al., 2021; Avvaru et al., 2021), and antiviral (Yu et al., 2017) activities. In our previous working, 1,3,4-thiadiazole derivatives of glucosides showed good antibacterial and antifungal activities (Chen M. et al., 2021).
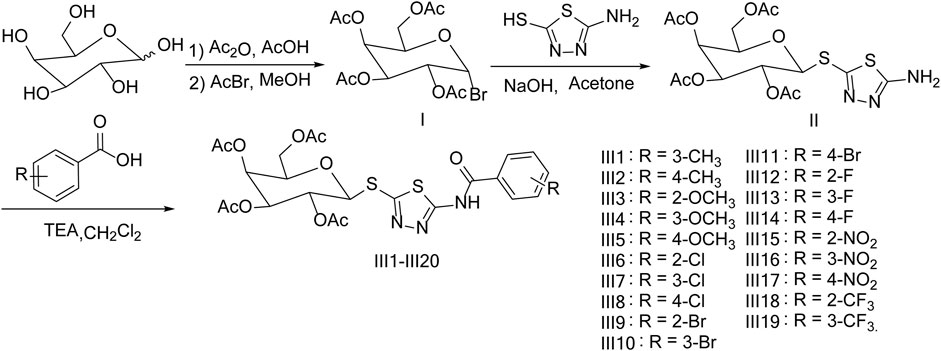
In order to find novel structure and effective biological activity of galactoside derivatives, 19 novel galactoside derivatives containing 1,3,4-thiadiazole moiety were synthesized by five reactions and were designed under the guidance of the active substructure splicing method by retaining a part of 1,3,4-thiadiazole and replacing the original glucoside with galactoside on the basis of our previous working (Figure 1). Then, the newly synthesized title compounds are tested for antibacterial and antifungal activities.
Experimental
Materials and Instruments
All solvents and reagents were purchased from commercial suppliers and met the standards. 1H NMR and 13C NMR spectra were obtained using Bruker DPX 400 MHz and Bruker DPX 600 MHz spectrometers (Bruker, Germany) in DMSO-d6 or CDCl3 solution. High-resolution mass spectrometry (HRMS) of the title compounds was performed using an Agilent Technologies mass spectrometer (Agilent Technologies, United States).
Chemistry
General Synthesis Procedures for Intermediate Ⅱ
Intermediate Ⅰ was synthesized by referring to the method of the literature (Kamat et al., 2007; Chen M. et al., 2021). The crude product of intermediate Ⅰ is used directly for the next step of the reaction. To 2-amino-5-mercapto-1,3,4-thiadiazole (1.33 g, 10.0 mmol), 50 ml acetone and 40% sodium hydroxide solution (10 ml) were added successively into a 100-ml two-necked bottle. Then, a solution of intermediate Ⅰ (4.11 g, 10.0 mmol) in acetone (5 ml) was added and maintained under stirring for about 30 min (Scattolin et al., 2020; Chen M. et al., 2021). After the reaction was completed, the mixture was concentrated, and 30 ml of water was added and extracted with dichloromethane (3 × 20 ml), and the organic layer was concentrated and recrystallized with ethyl acetate to afford the intermediate Ⅱ (3.9 g, yield: 84%) as a white solid. 1H NMR (600 MHz, DMSO-d6) δ 7.48 (s, 2H, NH2), 5.33 (s, 1H, H-1´), 5.30–5.21 (m, 2H, H-2´, H-3´), 5.05 (t, J = 9.9 Hz, 1H, H-4´), 4.33 (t, J = 6.2 Hz, 1H, H-5´), 4.13–4.00 (m, 2H, H-6´, H-6´´), 2.14 (s, 3H, CH3), 2.07 (s, 3H, CH3), 2.02 (s, 3H, CH3), and 1.93 (s, 3H, CH3).
General Synthesis Procedures for Title Compounds Ⅲ1-Ⅲ19
Substituted benzoic acid (2.4 mmol) was added to 4 ml thionyl chloride in batches with magnetic stirring and refluxed for 2.0 h (monitored by TLC). The solvent was removed under negative pressure; dichloromethane (2 ml) was added into the residue to give a light yellow solution, which was added dropwise into a mixture of the intermediate Ⅱ (0.93 g, 2.0 mmol), 15 ml dichloromethane, and triethylamine (0.24 g, 2.4 mmol) (Chen M. et al., 2021). After the reaction was completed, 10 ml water was added into the mixture and divided, and the organic layer was concentrated to the crude product. The crude product was recrystallized with isopropanol to afford the title compounds Ⅲ1–Ⅲ19. The characterization details of the title compounds Ⅲ2–Ⅲ19 are presented in the Supplemental Material.
(2R,3S,4S,5R,6S)-2-(acetoxymethyl)-6-((5-(3-methylbenzamido)-1,3,4-thiadiazol-2-l)thio)tetrahydro-2H-pyran-3,4,5-triyltriacetate (Ⅲ1): white solid, yield 75.0%, m.p. 159–161°C; 1H NMR (400 MHz, CDCl3) δ 12.33 (s, 1H, NH), 8.01 (s, 1H, Ar-H), 7.95 (d, J = 7.3 Hz, 1H, Ar-H), 7.55–7.37 (m, 2H, Ar-H), 5.48 (d, J = 2.9 Hz, 1H, H-3´), 5.38 (t, J = 10.0 Hz, 1H, H-1´), 5.11 (dd, J = 10.0, 3.3 Hz, 1H, H-2´), 5.03 (d, J = 10.1 Hz, 1H, H-4´), 4.20 (d, J = 7.6 Hz, 2H, H-5´, H-6´), 3.99 (t, J = 6.4 Hz, 1H, H-6´´), 2.48 (s, 3H, CH3), 2.20 (s, 3H, CH3), 2.10 (s, 6H, 2×CH3), and 2.00 (s, 3H, CH3). 13C NMR (150 MHz, CDCl3) δ 172.48, 170.58, 170.45, 169.89, 169.80, 154.72, 138.62, 134.24, 131.59, 129.35, 129.11, 126.02, 83.10, 74.70, 71.16, 68.06, 67.32, 62.39, 40.41, 40.27, 40.13, 39.99, 39.85, 39.71, 39.57, 21.35, 20.90, and 20.78; HRMS [M+H]+ calculated for C24H27N3O10S2: m/z 582.1216, found 582.1210.
Antifungal Activity In Vitro
The antifungal activity of the title compounds Ⅲ1–Ⅲ19 against G. zeae, B. dothidea, Phompsis sp., P. infestans, and T. cucumeris in vitro were tested by a mycelia growth method at 50 μg/ml (Maddila et al., 2016; Chen M. H. et al., 2021; Chen M. et al., 2021; Chen et al., 2022). Dimethomorph was used as a positive control, and DMSO was used as a negative control, and each treatment was operated in three replicates. Subsequently, the title compounds Ⅲ16, Ⅲ17, and Ⅲ19 were further evaluated for their corresponding antifungal EC50 values with three replicates and used dimethomorph as the positive controls.
Antibacterial Activity In Vitro
The antibacterial activity of the title compounds Ⅲ1–Ⅲ19 against Xcc and Xoo in vitro was tested using the turbidimeter test at 200 and 100 μg/ml (Dalgaard et al., 1994; Yu et al., 2017; Chen M. H. et al., 2021; Chen et al., 2022). Thiodiazole-copper was used as a positive control, and DMF was used as a negative control, and each treatment was operated in three replicates.
Result and Discussion
Synthesis
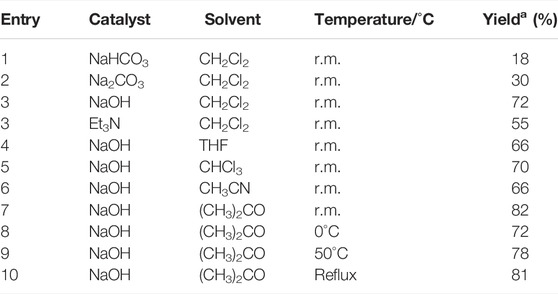
The method of the synthesis for title compounds was listed as follows: Intermediate Ⅰ was synthesized by galactose acetylation and bromination, and then intermediate Ⅰ reacted with 2-amino-5-mercapto-1,3,4-thiadiazole to give intermediate Ⅱ; substituted benzoic acids were chlorinated by thionyl chloride and reacted with intermediate Ⅱ to produce the title compounds Ⅲ1–Ⅲ19. Moreover, to optimize the reaction conditions of the key intermediate Ⅱ, the influence of catalyst, temperature, and solvent were tested and are listed in Table 1. The results indicated that the catalyst, solvent, and temperature had a pronounced effect on the yield, and a maximum yield of 82% was achieved when sodium hydroxide was used as a catalyst and acetone as a solvent for 0.5 h at room temperature.
Antifungal Activity In Vitro
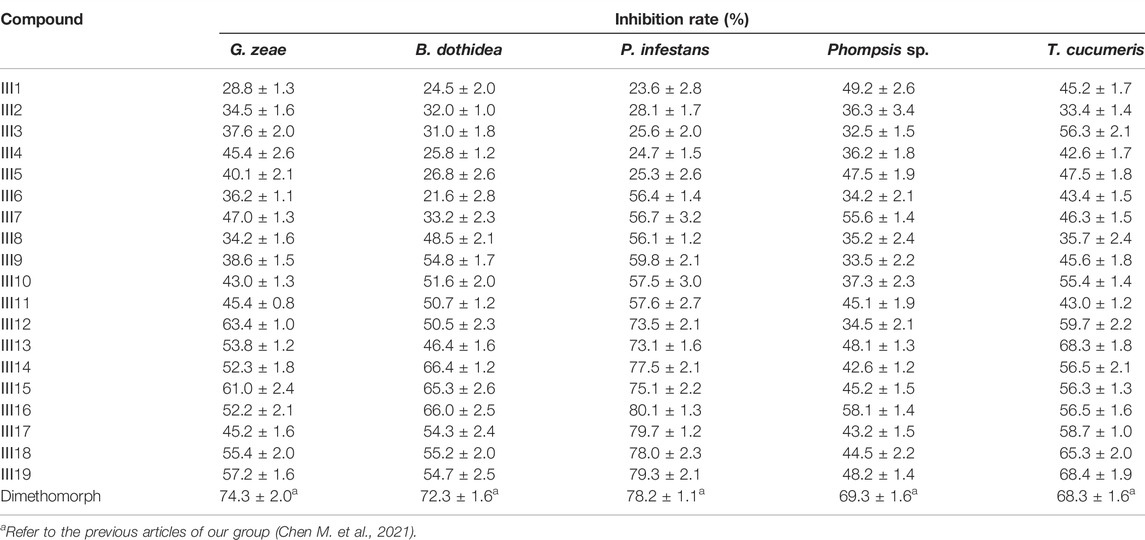
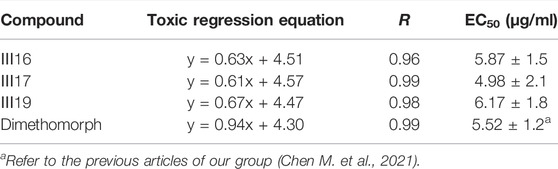
The antifungal activity of the title compounds Ⅲ1–Ⅲ19 against G. zeae, B. dothidea, P. infestans, Phompsis sp., and T. cucumeris are listed in Table 2. Table 2 indicated that Ⅲ1–Ⅲ19 showed good antifungal activities, with the inhibition rates of 21.5%–63.4%, 21.6%–66.0%, 23.6%–80.1%, 32.5%–58.1%, and 33.4–68.4% at 50 μg/ml, respectively. Among them, Ⅲ16, Ⅲ17, and Ⅲ19 exhibited satisfactory in vitro antifungal activities against P. infestans, with the inhibition rates of 80.1, 79.7, and 79.3%, respectively, which were equal to those of dimethomorph (78.2%). Based on the aforementioned results, the EC50 values of Ⅲ16, Ⅲ17, and Ⅲ19 were tested and are shown in Table 3. Table 3 indicated that Ⅲ16, Ⅲ17, and Ⅲ19 showed good antifungal activities against P. infestans, with EC50 values of 5.87, 4.98, and 6.17 μg/ml, respectively, which were similar to those of dimethomorph (5.52 μg/ml) (Chen et al., 2022), and which were comparable to those of the previously found inhibitory activity of glucosides derivatives containing 4-fluorobenzamido-1,3,4-thiadiazole against P. infestans (3.43 μg/ml) (Chen M. et al., 2021).
Antibacterial Activity In Vivo
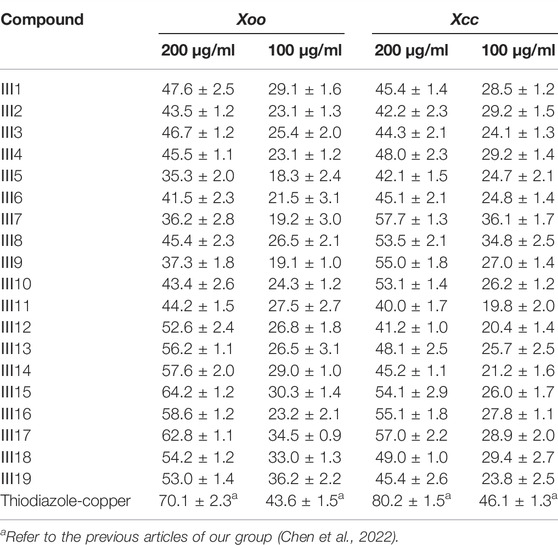
Moreover, the antibacterial activities of the title compounds against Xcc and Xoo were tested at 200 and 100 μg/ml and are listed in Table 4. Table 4 indicated that the title compounds Ⅲ1–Ⅲ19 exhibited certain antibacterial activities against Xoo and Xcc at 200 and 100 μg/ml, with the inhibition rates of 31.5%–64.2% and 40.8%–57.7% and 18.3%–36.2% and 19.8%–36.1%, respectively, which were lower than those of thiodiazole-copper (70.1, 43.6, and 46.1%), and which were comparable to that of the previously found novel glucoside derivatives containing 1,3,4-thiadiazole moiety with antibacterial activity (Chen M. et al., 2021). Based on the aforementioned results, it was demonstrated that the antifungal and antibacterial activities of compounds replacing the original glucoside with galactoside did not show any improvement, that is, the configuration of the third on the six-member sugar ring has little influence on the antifungal and antibacterial activities.
Conclusion
A total of 19 novel galactoside derivatives containing 1,3,4-thiadiazole moiety were designed under the guidance of the active substructure splicing method and synthesized by five reactions. The bioactivity results indicated that the title compounds exhibited good antibacterial and antifungal activities, while some of them showed excellent antifungal activities. Therefore, it was demonstrated that the galactoside derivatives containing 1,3,4-thiadiazole moiety can be used to develop potential agrochemicals in the future.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
YS and MC performed the synthesis and bioactivity of all compounds. DL and HL contributed to the original manuscript. ZZ, AL, and JL analyzed the results. JHY, XH, and JQY drafted the version of the manuscript.
Funding
The research was supported by the National Natural Science Foundation of China (No. 21762037), the Guizhou Science and Technology Planning Project (No. (2019)1454 and (2020)4Y097), the Key Laboratory Project of Guizhou Province ((2020)2003), and the Major Research Project for Innovative Group of Education Department of Guizhou Province (No. QJHKYZ(2020)029).
Conflict of Interest
The handling editor PL declared a past co-authorship with the author MC.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fchem.2022.910710/full#supplementary-material
References
Abas, M., Bahadur, A., Ashraf, Z., Iqbal, S., Rajoka, M. S. R., Rashid, S. G., et al. (2021). Designing Novel Anticancer Sulfonamide Based 2,5-Disubstituted-1,3,4-Thiadiazole Derivatives as Potential Carbonic Anhydrase Inhibitor. J. Mol. Struct. 1246, 131145. doi:10.1016/j.molstruc.2021.131145
Abu-Zaied, M. A., Elgemeie, G. H., and Mahmoud, N. M. (2021). Anti-covid-19 Drug Analogues: Synthesis of Novel Pyrimidine Thioglycosides as Antiviral Agents against SARS-COV-2 and Avian Influenza H5N1 Viruses. ACS Omega 6 (26), 16890–16904. doi:10.1021/acsomega.1c01501
Avvaru, S. P., Noolvi, M. N., More, U. A., Chakraborty, S., Dash, A., Aminabhavi, T. M., et al. (2021). Synthesis and Anticancer Activity of Thiadiazole Containing Thiourea, Benzothiazole and Imidazo[2,1-B][1,3,4]thiadiazole Scaffolds. Mc 17, 750–765. doi:10.2174/1573406416666200519085626
Bhinge, S. D., Chature, V., and Sonawane, L. V. (2015). Synthesis of Some Novel 1,3,4-thiadiazole Derivatives and Biological Screening for Anti-microbial, Antifungal and Anthelmintic Activity. Pharm. Chem. J. 49, 367–372. doi:10.1007/s11094-015-1287-8
Cai, S.-Q., Wang, R., Yang, X., Shang, M., Ma, C., and Shoyama, Y. (2006). Antiviral Flavonoid-TypeC-Glycosides from the Flowers of Trollius Chinensis. Chem. Biodivers. 3, 343–348. doi:10.1002/cbdv.200690037
Chen, M. H., Lu, D. W., Zhang, X., Chen, M. Y., and Luo, H. R. (2021). Synthesis and Biological Activities of Novel S-β-D-Glucopyranoside Derivatives of 1,2,4-triazole. Phosphorus Sulfur, 1–6. doi:10.1080/10426507.2021.1901704
Chen M., M., Zhang, X., Lu, D., Luo, H., Zhou, Z., Qin, X., et al. (2021). Synthesis and Bioactivities of Novel 1,3,4-thiadiazole Derivatives of Glucosides. Front. Chem. 9, 645876. doi:10.3389/fchem.2021.645876
Chen, M., Zhang, X., Luo, H., Zhou, Z., Chen, M., Wu, W., et al. (2022). Synthesis and Antifungal and Antibacterial Evaluation of Novel Pyrimidine Derivatives with Glycoside Scaffolds. Chem. Pap. 76, 1321–1328. doi:10.1007/s11696-021-01907-1
Chudzik, B., Bonio, K., Dabrowski, W., Pietrzak, D., Niewiadomy, A., Olender, A., et al. (2019). Synergistic Antifungal Interactions of Amphotericin B with 4-(5-Methyl-1,3,4-Thiadiazole-2-Yl) Benzene-1,3-Diol. Sci. Rep. 9, 12945–12959. doi:10.1038/s41598-019-49425-1
Dalgaard, P., Ross, T., Kamperman, L., Neumeyer, K., and Mcmeekin, T. A. (1994). Estimation of Bacterial Growth Rates from Turbidimetric and Viable Count Data. Int. J. Food Microbiol. 23, 391–404. doi:10.1016/0168-1605(94)90165-1
Gurung, R. B., Gong, S. Y., Dhakal, D., Le, T. T., Jung, N. R., Jung, H. J., et al. (2017). Synthesis of Curcumin Glycosides with Enhanced Anticancer Properties Using One-Pot Multienzyme Glycosylation Technique. J. Microbiol. Biotechnol. 27, 1639–1648. doi:10.4014/jmb.1701.01054
Kamat, M. N., Rath, N. P., and Demchenko, A. V. (2007). Versatile Synthesis and Mechanism of Activation of S-Benzoxazolyl Glycosides. J. Org. Chem. 72 (18), 6938–6946. doi:10.1021/jo0711844
Maddila, S., Gorle, S., Sampath, C., and Lavanya, P. (2016). Synthesis and Anti-inflammatory Activity of Some New 1,3,4-thiadiazoles Containing Pyrazole and Pyrrole Nucleus. J. Saudi Chem. Soc. 20, S306–S312. doi:10.1016/j.jscs.2012.11.007
Mermer, A., Keles, T., and Sirin, Y. (2021). Recent Studies of Nitrogen Containing Heterocyclic Compounds as Novel Antiviral Agents: a Review. Bioorg. Chem. doi:10.1016/j.bioorg.2021.105076
Oueslati, M. H., Tahar, L. B., and Harrath, A. H. (2020). Catalytic, Antioxidant and Anticancer Activities of Gold Nanoparticles Synthesized by Kaempferol Glucoside from lotus Leguminosae. Arabian J. Chem. 13, 3112–3122. (in press). doi:10.1016/j.arabjc.2018.09.003
Scattolin, T., Bortolamiol, E., Rizzolio, F., Demitri, N., and Visentin, F. (2020). Allyl Palladium Complexes Bearing Carbohydrate‐based N ‐heterocyclic Carbenes: Anticancer Agents for Selective and Potent In Vitro Cytotoxicity. Appl. Organomet. Chem. 34, 5876. doi:10.1002/aoc.5876
Tacke, M., Zhu, X., Werner, C., Schmidt, C., Sanchez-Sanz, G., Ott, I., et al. (2017). In Vitro and In Vivo Investigations into the Carbene Gold Chloride and Thioglucoside Anticancer Drug Candidates NHC-AuCl and NHC-AuSR. Lett. Drug Des. Discov. 14 (2), 125–134. doi:10.2174/1570180813666160826100158
Upadhyay, N. K., Yogendra Kumar, M. S., and Gupta, A. (2010). Antioxidant, Cytoprotective and Antibacterial Effects of Sea Buckthorn (Hippophae Rhamnoides l.) Leaves. Food Chem. Toxicol. 48, 3443–3448. doi:10.1016/j.fct.2010.09.019
Wu, M., Han, G., Meng, C., Wang, Z., Liu, Y., and Wang, Q. (2014). Design, Synthesis, and Anti-tobacco Mosaic Virus (TMV) Activity of Glycoconjugates of Phenanthroindolizidines Alkaloids. Mol. Divers. 18, 25–37. doi:10.1007/s11030-013-9484-4
Wu, S., Shi, J., Chen, J., Hu, D., Zang, L., and Song, B. (2021). Synthesis, Antibacterial Activity, and Mechanisms of Novel 6-Sulfonyl-1,2,4-Triazolo[3,4-B][1,3,4]thiadiazole Derivatives. J. Agric. Food Chem. 69 (16), 4645–4654. doi:10.1021/acs.jafc.1c01204
Keywords: galactoside, thiadiazole, aromatic amide, synthesis, bioactivity
Citation: Shu Y, Chen M, Lu D, Zhou Z, Yu J, Hu X, Yang J, Li A, Liu J and Luo H (2022) Synthesis and Bioactivities of Novel Galactoside Derivatives Containing 1,3,4-Thiadiazole Moiety. Front. Chem. 10:910710. doi: 10.3389/fchem.2022.910710
Received: 01 April 2022; Accepted: 19 April 2022;
Published: 19 May 2022.
Edited by:
Pei Li, Kaili University, ChinaCopyright © 2022 Shu, Chen, Lu, Zhou, Yu, Hu, Yang, Li, Liu and Luo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Meihang Chen, Y2hlbm1laWhhbmcwMTIzQDEyNi5jb20=
 Yafei Shu
Yafei Shu Meihang Chen
Meihang Chen