
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Chem. , 09 May 2022
Sec. Analytical Chemistry
Volume 10 - 2022 | https://doi.org/10.3389/fchem.2022.904688
This article is part of the Research Topic Innovators in Analytical Chemistry View all 5 articles
Multimodal imaging is a powerful strategy for combining information from multiple images. It involves several fields in the acquisition, processing and interpretation of images. As multimodal imaging is a vast subject area with various combinations of imaging techniques, it has been extensively reviewed. Here we focus on Matrix-assisted Laser Desorption Ionization Mass Spectrometry Imaging (MALDI-MSI) coupling other imaging modalities in multimodal approaches. While MALDI-MS images convey a substantial amount of chemical information, they are not readily informative about the morphological nature of the tissue. By providing a supplementary modality, MALDI-MS images can be more informative and better reflect the nature of the tissue. In this mini review, we emphasize the analytical and computational strategies to address multimodal MALDI-MSI.
Matrix-assisted Laser Desorption Ionization Mass Spectrometry Imaging (MALDI-MSI) has established itself as one of the most attractive ex vivo techniques for the spatial characterization of molecules (Science, 2021). Marked by a steady stream of incremental improvements over the last 30 years, MALDI-MSI has become a premiere tool for biomedical researchers. It has been applied to numerous fields of study, from pharmacokinetics, to tumor detection and even sub-cellular metabolomics. MALDI-MSI excels in chemical specificity without prior molecular tagging, however it requires specific sample preparation and its spatial resolving capabilities relative to microscopy and even other MSI techniques are considered low. This unique set of compromises has pushed the field to combine MALDI-MSI with other imaging modalities (Masyuko et al., 2013; Matsumoto et al., 2021; Tuck et al., 2021; Lauwerends et al., 2022), whether they be other MSI techniques, other multidimensional modalities like vibrational spectroscopy, high spatial resolution imaging such as immunofluorescence microscopy (IF) or Imaging mass cytometry (IMC) (Hoch et al., 2022), and even in vivo techniques commonly seen in the clinic like magnetic resonance imaging (MRI). Any of these imaging techniques can supplement the information given by MALDI-MSI and alleviate its limitations. The goal is usually to perform the joint statistical analysis of the combined images, establishing spatial correlations between them. Complementary images convey different types of information, processing workflows are often suited to specific needs, with limited generalizability. The multimodal integration can be performed by various computational methods, and it is challenging to make an educated choice. Here, we attempt to review the advancements in MALDI-MSI and how they pertain to the nascent field of multimodal imaging. We classify computational methods so as to facilitate decision-making at the data processing stage.
MALDI-MSI has the ability to simultaneously detect thousands of ions in a single acquisition in situ, including proteins, peptides, glycans/polysaccharides, lipids, metabolites and pharmaceuticals (Buchberger et al., 2018). The multiplexed nature of the technique is afforded without tagging or prior knowledge of the tissue and with a mild sample preparation. Time-of-flight secondary ion MS (TOF-SIMS, Massonnet and Heeren (2019), laser ablation inductively-coupled plasma (LA-ICP, Perry et al. (2020b)), desorption electrospray ionization (DESI, Soudah et al. (2021) are other MS imaging techniques, however MALDI-MSI has emerged as the most popular as it strikes a favorable balance between sample preparation, chemical specificity/sensitivity and spatial resolution. Since its inception (Hillenkamp et al., 1975; Spengler and Hubert, 1994; Caprioli et al., 1997), MALDI-MSI has advanced incrementally in spatial resolution (Heiles et al., 2020), acquisition time (Prentice and Caprioli, 2016), and mass resolution (Spraggins et al., 2016). And yet, despite its continued amelioration, it remains shy of its potential as a molecular microscope, articulated in its earliest days (Caprioli, 2014).
MALDI-MSI involves a simple but carefully considered sample preparation, particularly in multimodal experiments. Tissue sections 10–20 µm thick are thaw-mounted to a suitable substrate, typically a glass/metal slide. The surface of the sample should be flat to ensure optimal laser focus, yet a height profile can be used to account for topographical changes. (Bartels et al., 2017). A chemical matrix is then applied. Matrix choice and application is always a compromise between preferred analyte classes, their extraction and potential delocalization. The matrix might affect the ionization mode, adducts seen, and size of detectable analytes (Yang et al., 2018). Matrix is commonly applied by spraying or sublimation, which might affect spatial resolution via crystal size, analyte extraction/delocalization and reproducibility. Matrices have been thoroughly interrogated (Perry et al., 2020a) and application methods reviewed (Calvano et al., 2018).
MALDI is widely viewed as qualitative. Indeed, the intensities of two MALDI-MS images cannot be directly compared because of the physics of ionization and the nature of complex tissue: the ionization efficiencies for different analyte classes differ and the signal can be suppressed due to different (biological) matrix effects (Unsihuay et al., 2021). Many challenges are not unique to MALDI-MSI; Kertesz and Cahill (2021) review quantitation of various MSI technologies.
Researchers have devised increasingly diligent approaches towards quantitative MSI (qMSI). This includes normalization/in-solution strategies like externally-sprayed standards as reference peaks or tissue extinction coefficient (TEC) (Hamm et al., 2012; Dilmetz et al., 2021), and computational strategies such as “virtual calibration” (Song et al., 2019). Also, in experimental approaches, calibration curves are employed to correlate signal intensity to known concentration of standards. Calibration curves in mimetic tissue, though more laborious, have seen good agreement with liquid chromatography-MS (Barry et al., 2019). The thoroughness of the mimetic model has made it attractive to many qMSI groups, particularly in the small molecule realm.
MALDI-MSI was conceived on TOF instrumentation but has since benefited from high mass resolving power provided by Fourier transform mass spectrometry (FTMS). Higher resolving power results in better analyte annotation, more peaks detected and less chemical matrix interference (Bowman et al., 2020). The addition of ion mobility has been a major advancement of MALDI-MSI as this enables the separation of isobaric species (Spraggins et al., 2019; Soltwisch et al., 2020).
Spatial resolution is another front for MALDI-MSI advancement. Alterations in laser geometry pushed the bounds of possibility, with commercial sources now capable of 10 µm spatial resolution that can often be tuned further to near one micron (Kompauer et al., 2016). Even subcellular resolutions have been achieved with transmission geometry (Zavalin et al., 2012). The spatial resolution of MALDI-MS images depends on the laser diameter and the stage step-size. It can be assessed precisely by determining the size and shape of the laser ablation marks on a grid with a resolution pattern (Fagerer et al., 2015).
Increased spatial resolution comes at the expense of sensitivity. MALDI-2 was developed to address such concerns (Soltwisch et al., 2015). Here, a secondary laser is directed at the MALDI plume and pulsed after an initial MALDI event. This ionizes neutrals and can increase sensitivity 100-fold for some molecular species (Barré et al., 2019; Soltwisch et al., 2020). This technology sparked renewed interests in transmission geometry, where researchers recently obtained ultra-high spatial resolutions, down to 600nm, with informative sensitivity by employing MALDI-2 (Niehaus et al., 2019; Spivey et al., 2019). MALDI-2 has also been combined with ion mobility (Soltwisch et al., 2020).
MALDI-MS images are large datasets and enclose complex data. Various approaches are designed to reduce their complexity. For instance, peak picking and alignment reduce the dataset size while preserving relevant information along the spectral dimension (Alexandrov, 2012). As for their visualization, there are multiple commercial and open-source software available (Weiskirchen et al., 2019), many of which handle the standardized imzML data format (Römpp et al., 2011; Schramm et al., 2012).
Spectral visualization strategies, such as Kendrick mass defect analysis, have also been used to deconvolute imaging analysis (Kune et al., 2019; Blanc et al., 2021). These efforts confront a major challenge in MALDI-MSI: a lack of chemical separation.
Data analysis involves methods which facilitate the interpretation and comparison of images. Method choice depends on the experimental design, the nature of the signal, and the scientific goal of the experiment.
Dimension reduction techniques, such as principal component analysis (PCA, Trindade et al. (2018a)), non-negative matrix factorization (NMF, Trindade et al. (2018b)) or t-distributed stochastic neighbor embedding (t-SNE, van der Maaten and Hinton (2008)) can further abate the data. PCA and NMF are linear matrix decomposition techniques, whereas t-SNE is a probabilistic, non-linear dimension reduction technique. PCA and NMF decompose the original, large, MALDI-MS image into two smaller matrices, whose product approximates the original image. Common global information shared across ion images are stored in so-called component images. Component images usually highlight important structures in the tissue. PCA loadings are weights that attribute more or less importance to ion images with each component image, enacting the coupling of spatial and spectral information. NMF differs from PCA in that it imposes a non-negativity constraint on the components, making them easier to interpret. By contrast to PCA and NMF, t-SNE retains local structures by preserving distances between points in a lower-dimensional embedding. This reduction makes for visualizations that are faithful to the original image data. However, the resulting mapping is different across multiple runs and requires larger computational resources than PCA. Abdelmoula et al. (2018) use a variant of t-SNE, called hierarchical SNE (Pezzotti et al., 2016), to visualize structures with different levels of details across ion images.
Clustering methods give simplified representations of the datasets, where similar spectra are grouped together. The output can be visualized as a 2D image where each pixel has an intensity equal to its cluster number. Similarities between spectra can be estimated by various metrics, such as the Euclidean distance, which captures similarities among raw intensities, or the cosine distance, which captures similar trends in the spectra. The k-means algorithm groups spectra according to a predefined number of clusters (Palmer et al., 2015), whereas hierarchical clustering yields a tree of subregions, arranged from broad structures to finer details (Urbini et al., 2017). Alexandrov and Kobarg (2011) proposed a clustering algorithm which limits the impact of noise in MALDI-MS images, where pixels are grouped by the similarities between their neighborhoods.
Supervised machine learning methods, i.e., methods that use annotated datasets, can recognize co-localized ions from manually annotated images. Ovchinnikova et al. (2020) found spatial correlations between ion images with a deep learning model using visual similarities.
Univariate analysis can be used to find differently-abundant ions across conditions, such as different regions in the images or different samples. T-test is used to highlight statistical differences for a specific m/z between two different samples (Yajima et al., 2018), or regions within a tissue section, whereas the analysis of variance (ANOVA) test can be used to compare three or more regions (Blanc et al., 2018). When the normality assumption is not verified, typical in MSI datasets, non-parametric tests, such as Mann-Whitney U-test or Wilcoxon signed-rank tests can be used (Guo et al., 2014). To determine if an ion is a biomarker for a condition, Receiver Operating Characteristic (ROC) curve analysis can be applied (Hoo et al., 2017).
Data analysis usually returns several m/z features of interest. Metabolite databases can be polled to infer their chemical structure. The choice of the database depends on the type of analyte (e.g., LipidMaps for lipids, UniProt for proteins, CSDB for glycans, etc.). A website dedicated to MSI called METASPACE2020 allows for the interrogation of different databases.
Balanced by optimism in MALDI-MSI and caution with its limitations, researchers have turned to other imaging modalities to compensate. In addition to previously mentioned limitations, MALDI-MSI ion images are not directly indicative of the histological setting, as immunohistochemistry (IHC) could be. Consequently, MALDI-MSI will tend to move toward multimodal imaging which can compensate for these limitations by combining chemical and histological information from various modalities, and which results in a more informative dataset.
Multimodal imaging can be used for guided acquisitions. For instance, the acquisition of MALDI-MS images can be guided with microscopy images, so as to restrict the imaged area and reduce the acquisition time and data size (Patterson et al., 2018a; Blanc et al., 2018). Rabe et al. (2018) guide the MALDI-MSI acquisition by performing a segmentation of brain tissue based on Fourier transform infrared spectroscopy (FT-IR), reducing time and data size by nearly 98%.
Multimodal imaging often combines several imaging modalities and yields integrated multimodal datasets, opening new possibilities for data mining. The methods used depend on the complementary modality associated with MALDI-MSI, the processing strategies and scientific goal (Table 1). Integrated multimodal datasets are typically obtained by a three-step-workflow: multimodal acquisition, registration, or the spatial alignment of imaging datasets, and data analysis to highlight correlations between imaging datasets (Figure 1). The objective is to use information from both modalities to evidence complementarities and correlations with different data processing strategies, as described by Bemis et al. (2019): (1) class discovery to find commonalities (spectral or spatial) without a priori information, (2) class prediction, using existing information to infer spatial or biological knowledge in a new dataset and (3) class comparison, which compares intensity averages in regions of interest (ROIs) to find differentially-abundant ions.
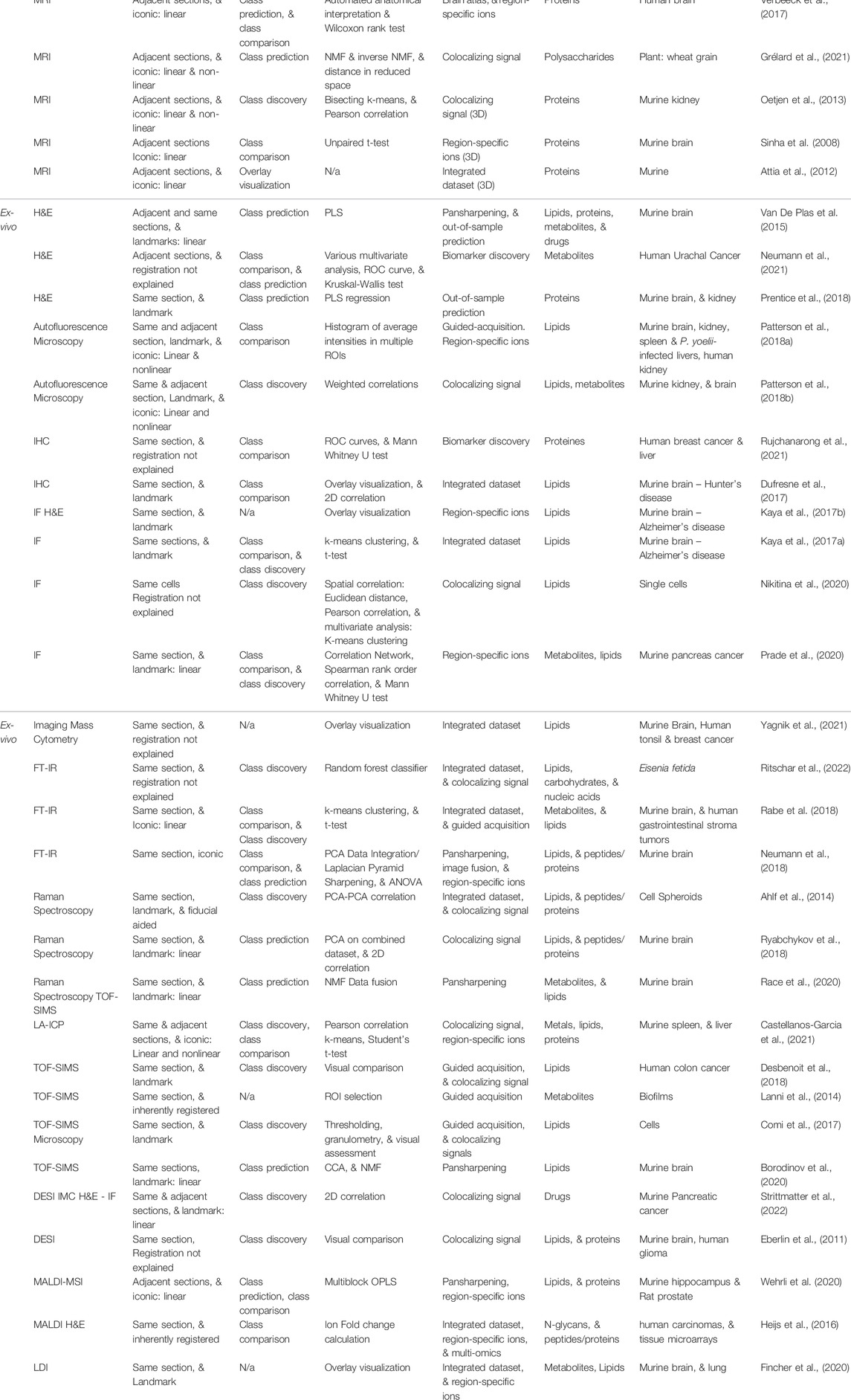
TABLE 1. Collection of publications that combine MALDI-MSI with another modality. It attempts to classify each paper by its multimodal strategy and scientific goals. The list is separated in vivo/ex vivo and grouped by the spectral nature of its complementary modality, i.e., whether it is mono-, multi-channel, i.e., spectra with discrete bands, or hyperspectral, i.e., spectra with continuous bands.
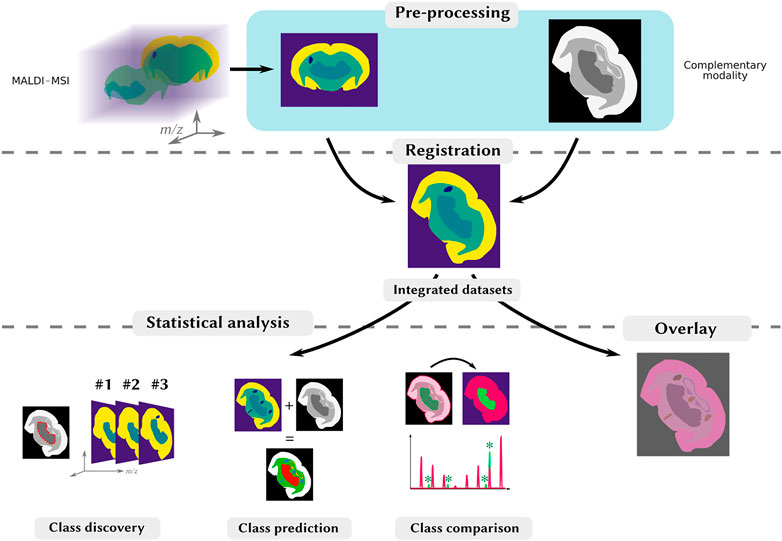
FIGURE 1. Typical processing and analysis workflow for multimodal MALDI-MSI. Top-row: the MALDI image (on the left), and the complementary images (on the right) are processed so as to have comparable shapes, through various specific processing steps, including segmentation. Middle-row: the images are spatially aligned through registration. Bottom-row: once the images are registered, they can be visualized on-top of each other to reveal similar spatial distributions (on the right). Patterns can be objectified by a joint statistical analysis to, e.g., find spatial correlations, by investigating spatial clusters (class discovery), produce an enriched dataset from both modalities (class prediction) or find region-specific ions in two different ROIs (class comparison).
Biological questions drive the choice of modalities and their implementation. Multimodal acquisitions involve some critical parameters to be considered in terms of analytical strategy: the use of a same sample section or adjacent section, the level of destructiveness of complementary modalities and the order of operations in which the acquisitions occur. Use of same section is advised to establish cellular or subcellular correlations. Adjacent sections are advised when all modalities are destructive, or when it is desirable to keep the sample preparation processes separate. However, the resulting integrated dataset is not reliably interpretable.
The significance of MALDI-MSI sample preparation in multimodal experiments cannot be overstated as there will be consequences for downstream analysis. Due to sample preparation, MALDI-MSI is often conducted after a less destructive imaging modality. In some cases, complementary images can be acquired after MALDI-MSI to facilitate the registration process. Neumann et al. (2018), Patterson et al. (2018b), and Jones et al. (2020) demonstrated multimodal imaging of MALDI-MSI followed by another technique, which is unintuitive in most instances.
Registration is usually the first step of multimodal computational workflow. It consists in aligning two or more images, such that the enclosed objects are superimposed.
Before registration, images should be comparable and have the same number of spatial dimensions. Standardization ensures that the images are in the same intensity range. Images involved in the registration process may come from unprocessed images (e.g., ion image in MALDI) or from segmentation methods, which extract objects of interest in images. For instance, segmentation maps from dimension reduction techniques (e.g., component image), or from clustering, can be used. Segmentation methods are frequently based on pixel intensities or use the geometrical properties of the objects. In a multimodal context, MALDI-MSI segmentation can involve simple thresholding (Anyz et al., 2017), region growing (Grélard et al., 2021), methods assisting in selecting spatially coherent ion images (Alexandrov and Bartels, 2013), or deep learning (Abdelmoula et al., 2022).
Registration methods aim at estimating the transformation which maps a moving, or deformable image onto a fixed, or reference image. If possible, the moving image is chosen as the one with the lowest resolution, so as to preserve the data from the fixed image with higher resolution. MALDI-MSI datasets are large and it is desirable not to interpolate new signal intensities. Thus, MALDI-MSI are generally chosen as the fixed image. The transformation can be rigid (translation and rotation), affine (rigid with scaling, and shearing), projective (affine with perspective) or non-linear. Non-linear transformations account for local deformations in the image. They are advised for adjacent sections and to accurately model the damages during sample preparation.
Registration methods can be classified in two categories, depending on the information they use: landmarks, or pixel intensities (iconic methods).
Landmarks are features in the image that are salient and easily recognizable. Landmarks can be extraneous marks added during sample preparation or during acquisition, or unique features visible in both images. They can be selected automatically or manually. The minimum number of landmarks depends on the transformation model and the dimensionality: for the 2D case, a minimum of three points is necessary for an affine transformation, and up to four for a projective transformation. Manual methods require user intervention in selecting landmarks. They are useful for images with different spatial resolutions, or when it is difficult to find intensity similarities. It is also the preferred method for partial registration, as the registration of an image subset onto an image depicting a larger part of the tissue. Borodinov et al. (2020) registered MALDI images onto a TOF-SIMS image by manually selecting matching pairs of fiducial markers etched by an ion beam. Ryabchykov et al. (2018) registered Raman images onto larger MALDI-MS images by manually selecting common features from PCA component images.
Iconic methods are based on similarities in pixel intensities. Transformation parameters are modified iteratively until a local optimum of a function, called similarity metric, is reached. The similarity metric measures how well two images match after applying a deformation. Typical similarity metrics are the sum of squared intensity differences (SSD), normalized cross-correlation, and mutual information, which estimates the statistical independence of the intensity distributions of the two images. SSD can be applied when both images have the same intensity range and similar contrast. Normalized cross-correlation or mutual information are employed when images have different contrast or dynamic range. Transformation parameters are updated by an optimization algorithm involving the similarity metric. For instance, linear gradient descent updates transformation parameters based on the slope of the metric. The resulting transformation strongly depends on the initialization parameters.
Iconic methods are used in numerous MALDI-MSI registration tasks. Anyz et al. (2017) aligned histological staining (Hematoxylin Eosin, H&E) and MALDI-MS images. Both images are different in terms of pixel intensities, so the authors extract binary masks of the sample. These masks are registered by an affine transformation, with an iterative gradient descent optimization of the SSD metric. Castellanos-Garcia et al. (2021) combined MALDI-MS with LA-ICP-MS images, so as to merge the extensive molecular information with supplemental metals analysis. They select matching ion images manually in order to estimate both linear, and non-linear transformations. Abdelmoula et al. (2019) registered several 2D MALDI slices with a 3D MRI image in order to obtain a volumetric representation of the MALDI dataset. The MALDI image is reduced by the t-SNE algorithm. The corresponding 2D MR image is selected from the 3D volume manually. Then, both linear and non-linear registration methods are applied, using mutual information as a similarity metric.
Hybrid approaches use both iconic and landmark-based registration methods. Patterson et al. (2018a) registered microscopy and MALDI-MS images, as follows: two microscopy images, before and after MALDI-MSI, were acquired. As they are the same modality, the authors use iconic approaches to register them. The microscopy images are then registered onto an upsampled version of the MALDI-MS image using laser ablation marks as landmarks. This workflow avoids introducing deformation artifacts in the MSI datasets, and preserves the resolution of microscopy images.
The quality of the registration determines the reliability of the results of the subsequent data analysis step. Registration evaluation can be achieved through various metrics, such as the Dice coefficient or the F-measure, which both estimate the proportion of overlapping pixels between the deformed and fixed images. When using non-linear transformations, it is important to select parameters such as to obtain a compromise between shape-matching and intensity fidelity. For instance, Grélard et al. (2021) estimated the shape matching by F-measure, and the intensity fidelity by computing the mutual information between the images before and after applying non-linear registration.
The last step of a multimodal workflow is usually the joint analysis of the images. This involves using information from both modalities to evidence complementarities and correlations. The algorithms depend on the scientific goal and can be classified in three classes, as described previously: (1) class discovery, (2) class prediction, and (3) class comparison.
Class discovery finds spatial or spectral similarities in an image, and does not require supplementary information from a complementary modality. It can be achieved by data dimension reduction methods or clustering mentioned previously. Abdelmoula et al. (2019) found segmentation maps of registered MALDI-MS images by a hierarchical variant of the t-SNE algorithm. Then, spatial correlations are established by Pearson correlation coefficient between the segmentation map and the ion images. Ryabchykov et al. (2018) found spatial correlations between Raman and MALDI-MS images by analyzing PCA component images and loadings. This analysis highlights changes in lipid distributions.
Class prediction consists in inferring spatial or spectral information, by using extraneous information which usually come from expert annotations or from regions found in the complementary modality. Borodinov et al. (2020) sharpened MALDI-MS images, i.e., they enhance their spatial resolution, by combining them with TOF-SIMS images. They used Canonical Correlation Analysis (CCA) on NMF component images to find similarities. This decomposition and transformation enact the reconstruction of MALDI ion images from the high-resolution TOF-SIMS images. van de Plas et al. (2015) sharpened MALDI-MS images by microscopy images using Partial Least Squares (PLS) regression. They used these images to find anatomical distributions of ions in the sample, as well as predict distributions out of the sample. Prentice et al. (2018) used out-of-sample prediction to correlate microscopy to MALDI-MSI when the acquisition is not practically obtainable. Wehrli et al. (2020) combined Raman and MALDI-MS images and extract a high-resolution MALDI-MS image. Since both images are hyperspectral, they use a multiblock method derived from Orthogonal PLS.
Class comparison consists in comparing two or more ROIs in an image to highlight patterns that have different intensity distributions across the ROIs. This can typically be achieved by univariate analysis. Verbeeck et al. (2017) automatically identified the spatial distributions observed in registered ion images as a combination of the anatomical structures described in the atlas built from MR images. They then used Wilcoxon rank test to find differently expressed ions between healthy and diseased brain hemispheres. Patterson et al. (2018a) found spatial correlations between two MS datasets by weighing abundance correlations by their degree of pixel overlap. Bemis et al. (2019) proposed several models based on hierarchical Bayesian spatial models to find differently abundant ions. They modeled the fact that neighboring pixels have similar spectra, which retrieves differently abundant ions that are missed by other models.
Class discovery, prediction and comparison can be integrated in the same workflow. Jones et al. (2019) used various methods encompassing all these analyses. They combined MALDI-MS images with various microscopy images (fluorescence and H&E). First, they manually selected ions that visibly colocalize with ROIs in the tissue. This approach was insufficient as it led to subjective results, and was not tractable on large datasets. They performed class discovery by the spatially shrunken centroid clustering algorithm and analyzed the clustering results further by looking up ions that were representative of a cluster of interest. Class prediction achieved by PLS regression, which allows to approximate MALDI ion images from a linear combination of fluorescence microscopy channels. The resulting regression made it easy to find biomarkers that were specifically distributed in a specific fluorescence channel. Finally, they performed class comparison on weighted averages in ROIs by Student t-test.
Multimodal imaging strategies involving MALDI-MSI alleviate technological issues related to scientific questions, leading to a better understanding of the living. It offers new opportunities in biology by allowing direct correlation between cellular and molecular information, but requires diligent approaches for acquisition, registration and computational data analysis to combine and get the best of the different modalities. Once achieved, these combinations raise new questions and initiate new studies, which would not have been possible with a single modality as shown in Table 1.
After highlighting relevant methodological developments, multimodal imaging better contributes or provides evidence towards the biological understanding. Grélard et al. (2021) correlated water distribution given by MR images with the distribution of specific polysaccharides in MS images. This study hints at the role of these molecules in the cell-wall porosity on tissue, and corroborates previous observations made in vitro. Strittmatter et al. (2022) combined MALDI and DESI to study the distribution of a drug and its different metabolites, which cannot be established by a single modality. Then, they combined MSI and IMC to show that drug metabolites co-localize with immunohistochemistry markers for DNA damage. Prade et al. (2020) correlated multiplexed fluorescent immunohistochemical staining with MS images to identify ions involved in metabolic networks. They showed discrepancies in metabolic distributions according to different cell populations.
The capabilities achieved by multimodal imaging workflows are numerous, and many are yet to be fully exploited. The technical progress enacting higher spatial resolution for MALDI-MS images is met with larger dataset sizes. These datasets do not necessarily fit in memory, thus memory requirements become a bottleneck to several research groups. Bemis and Vitek (2017) developed an R package which loads larger-than-memory datasets, using on-disk data structures. This effort should be furthered in other programming languages as well. This involves adapting the existing computational methods such that it complies with these data structures, which would require significant work and collaboration from the community.
The variety of processing algorithms makes it difficult to pick suitable methods. Deep learning could facilitate the processing and joint analysis of MS datasets. Abdelmoula et al. (2022) used a deep learning architecture to classify tumor and healthy pixels in MS datasets. One of the benefits of this approach is that it does not rely on spectral and signal processing. More generally, it could facilitate the interpretation of MSI datasets and their integration with other modalities.
What is clear, however, is that we are at the beginning of an exciting new era of analytical chemistry and biomedical research. Groups have laid the groundwork for what we hope will lead to new understandings, new discoveries and new kinds of questions in medicine and biochemistry. And, in turn, these new questions will be met with the emergence of new technologies.
All authors listed have made a direct, intellectual and equal contribution to the work and approved it for publication.
This work was financially supported by the Agence National de la Recherche (France, Grants ANR-19-CE29-0010601 “MultiRaMaS”), the CNRS (International Emerging Actions (IEA) “MultIRaMaS”, and Groupement de Recherche “GdR-MSI”, GDR2125). Landry BLANC is supported by a grant from Gates Foundation (INV-004704).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abdelmoula, W. M., Pezzotti, N., Hölt, T., Dijkstra, J., Vilanova, A., McDonnell, L. A., et al. (2018). Interactive Visual Exploration of 3D Mass Spectrometry Imaging Data Using Hierarchical Stochastic Neighbor Embedding Reveals Spatiomolecular Structures at Full Data Resolution. J. Proteome Res. 17, 1054–1064. doi:10.1021/acs.jproteome.7b00725
Abdelmoula, W. M., Regan, M. S., Lopez, B. G. C., Randall, E. C., Lawler, S., Mladek, A. C., et al. (2019). Automatic 3D Nonlinear Registration of Mass Spectrometry Imaging and Magnetic Resonance Imaging Data. Anal. Chem. 91, 6206–6216. doi:10.1021/acs.analchem.9b00854
Abdelmoula, W. M., Stopka, S. A., Randall, E. C., Regan, M., Agar, J. N., Sarkaria, J. N., et al. (2022). massNet: Integrated Processing and Classification of Spatially Resolved Mass Spectrometry Data Using Deep Learning for Rapid Tumor Delineation. Bioinformatics 38, 2015–2021. doi:10.1093/bioinformatics/btac032
Ahlf, D. R., Masyuko, R. N., Hummon, A. B., and Bohn, P. W. (2014). Correlated Mass Spectrometry Imaging and Confocal Raman Microscopy for Studies of Three-Dimensional Cell Culture Sections. Analyst 139, 4578–4585. doi:10.1039/c4an00826j
Alexandrov, T., and Bartels, A. (2013). Testing for Presence of Known and Unknown Molecules in Imaging Mass Spectrometry. Bioinformatics 29, 2335–2342. doi:10.1093/bioinformatics/btt388
Alexandrov, T., and Kobarg, J. H. (2011). Efficient Spatial Segmentation of Large Imaging Mass Spectrometry Datasets with Spatially Aware Clustering. Bioinformatics 27, i230–i238. doi:10.1093/bioinformatics/btr246
Alexandrov, T. (2012). MALDI Imaging Mass Spectrometry: Statistical Data Analysis and Current Computational Challenges. BMC Bioinforma. 13 (Suppl. 1), S11. doi:10.1186/1471-2105-13-s16-s11
Anyz, J., Vyslouzilova, L., Vaculovic, T., Tvrdonova, M., Kanicky, V., Haase, H., et al. (2017). Spatial Mapping of Metals in Tissue-Sections Using Combination of Mass-Spectrometry and Histology through Image Registration. Sci. Rep. 7. doi:10.1038/srep40169
Attia, A. S., Schroeder, K. A., Seeley, E. H., Wilson, K. J., Hammer, N. D., Colvin, D. C., et al. (2012). Monitoring the Inflammatory Response to Infection through the Integration of MALDI IMS and MRI. Cell Host Microbe 11, 664–673. doi:10.1016/j.chom.2012.04.018
Barré, F. P. Y., Paine, M. R. L., Flinders, B., Trevitt, A. J., Kelly, P. D., Ait-Belkacem, R., et al. (2019). Enhanced Sensitivity Using Maldi Imaging Coupled with Laser Postionization (Maldi-2) for Pharmaceutical Research. Anal. Chem. 91, 10840–10848. doi:10.1021/acs.analchem.9b02495
Barry, J. A., Groseclose, M. R., and Castellino, S. (2019). Quantification and Assessment of Detection Capability in Imaging Mass Spectrometry Using a Revised Mimetic Tissue Model. Bioanalysis 11, 1099–1116. doi:10.4155/bio-2019-0035
Bartels, B., Kulkarni, P., Danz, N., Böcker, S., Saluz, H. P., and Svatoš, A. (2017). Mapping Metabolites from Rough Terrain: Laser Ablation Electrospray Ionization on Non-flat Samples. RSC Adv. 7, 9045–9050. doi:10.1039/c6ra26854d
Bemis, K. A., Guo, D., Harry, A. J., Thomas, M., Lanekoff, I., Stenzel-Poore, M. P., et al. (2019). Statistical Detection of Differentially Abundant Ions in Mass Spectrometry-Based Imaging Experiments with Complex Designs. Int. J. Mass Spectrom. 437, 49–57. doi:10.1016/j.ijms.2018.07.006
Bemis, K. A., and Vitek, O. (2017). Matter: An R Package for Rapid Prototyping with larger-Than-memory Datasets on Disk. Bioinformatics 33, 3142–3144. doi:10.1093/bioinformatics/btx392
Blanc, L., Daudelin, I. B., Podell, B. K., Chen, P.-Y., Zimmerman, M., Martinot, A. J., et al. (2018). High-resolution Mapping of Fluoroquinolones in TB Rabbit Lesions Reveals Specific Distribution in Immune Cell Types. Elife 7, 41115. doi:10.7554/eLife.41115
Blanc, L., Ferraro, G. B., Tuck, M., Prideaux, B., Dartois, V., Jain, R. K., et al. (2021). Kendrick Mass Defect Variation to Decipher Isotopic Labeling in Brain Metastases Studied by Mass Spectrometry Imaging. Anal. Chem. 93, 16314–16319. doi:10.1021/acs.analchem.1c03916
Borodinov, N., Lorenz, M., King, S. T., Ievlev, A. V., and Ovchinnikova, O. S. (2020). Toward Nanoscale Molecular Mass Spectrometry Imaging via Physically Constrained Machine Learning on Co-registered Multimodal Data. npj Comput. Mat. 6. doi:10.1038/s41524-020-00357-9
Bowman, A. P., Blakney, G. T., Hendrickson, C. L., Ellis, S. R., Heeren, R. M. A., and Smith, D. F. (2020). Ultra-High Mass Resolving Power, Mass Accuracy, and Dynamic Range MALDI Mass Spectrometry Imaging by 21-T FT-ICR MS. Anal. Chem. 92, 3133–3142. doi:10.1021/acs.analchem.9b04768
Buchberger, A. R., DeLaney, K., Johnson, J., and Li, L. (2018). Mass Spectrometry Imaging: A Review of Emerging Advancements and Future Insights. Anal. Chem. 90, 240–265. doi:10.1021/acs.analchem.7b04733
Calvano, C. D., Monopoli, A., Cataldi, T. R. I., and Palmisano, F. (2018). MALDI Matrices for Low Molecular Weight Compounds: an Endless Story? Anal. Bioanal. Chem. 410, 4015–4038. doi:10.1007/s00216-018-1014-x
Caprioli, R. M., Farmer, T. B., and Gile, J. (1997). Molecular Imaging of Biological Samples: Localization of Peptides and Proteins Using MALDI-TOF MS. Anal. Chem. 69, 4751–4760. doi:10.1021/ac970888i
Caprioli, R. M. (2014). Imaging Mass Spectrometry: Molecular Microscopy for Enabling a New Age of Discovery. Proteomics 14, 807–809. doi:10.1002/pmic.201300571
Castellanos-Garcia, L. J., Sikora, K. N., Doungchawee, J., and Vachet, R. W. (2021). LA-ICP-MS and MALDI-MS Image Registration for Correlating Nanomaterial Biodistributions and Their Biochemical Effects. Analyst 146, 7720–7729. doi:10.1039/d1an01783g
Comi, T. J., Neumann, E. K., Do, T. D., and Sweedler, J. V. (2017). microMS: A Python Platform for Image-Guided Mass Spectrometry Profiling. J. Am. Soc. Mass Spectrom. 28, 1919–1928. doi:10.1007/s13361-017-1704-1
Desbenoit, N., Walch, A., Spengler, B., Brunelle, A., and Römpp, A. (2018). Correlative Mass Spectrometry Imaging, Applying Time-Of-Flight Secondary Ion Mass Spectrometry and Atmospheric Pressure Matrix-Assisted Laser Desorption/ionization to a Single Tissue Section. Rapid Commun. Mass Spectrom. 32, 159–166. doi:10.1002/rcm.8022
Dilmetz, B. A., Lee, Y. R., Condina, M. R., Briggs, M., Young, C., Desire, C. T., et al. (2021). Novel Technical Developments in Mass Spectrometry Imaging in 2020: A Mini Review. Anal. Sci. Adv. 2, 225–237. doi:10.1002/ansa.202000176
Dufresne, M., Guneysu, D., Patterson, N. H., Marcinkiewicz, M. M., Regina, A., Demeule, M., et al. (2017). Multimodal Detection of GM2 and GM3 Lipid Species in the Brain of Mucopolysaccharidosis Type II Mouse by Serial Imaging Mass Spectrometry and Immunohistochemistry. Anal. Bioanal. Chem. 409, 1425–1433. doi:10.1007/s00216-016-0076-x
Eberlin, L. S., Liu, X., Ferreira, C. R., Santagata, S., Agar, N. Y. R., and Cooks, R. G. (2011). Desorption Electrospray Ionization Then MALDI Mass Spectrometry Imaging of Lipid and Protein Distributions in Single Tissue Sections. Anal. Chem. 83, 8366–8371. doi:10.1021/ac202016x
Fagerer, S. R., Römpp, A., Jefimovs, K., Brönnimann, R., Hayenga, G., Steinhoff, R. F., et al. (2015). Resolution Pattern for Mass Spectrometry Imaging. Rapid Commun. Mass Spectrom. 29, 1019–1024. doi:10.1002/rcm.7191
Fincher, J. A., Korte, A. R., Yadavilli, S., Morris, N. J., and Vertes, A. (2020). Multimodal Imaging of Biological Tissues Using Combined MALDI and NAPA-LDI Mass Spectrometry for Enhanced Molecular Coverage. Analyst 145, 6910–6918. doi:10.1039/d0an00836b
Grélard, F., Legland, D., Fanuel, M., Arnaud, B., Foucat, L., and Rogniaux, H. (2021). Esmraldi: Efficient Methods for the Fusion of Mass Spectrometry and Magnetic Resonance Images. BMC Bioinforma. 22. doi:10.1186/s12859-020-03954-z
Guo, S., Qiu, L., Wang, Y., Qin, X., Liu, H., He, M., et al. (2014). Tissue Imaging and Serum Lipidomic Profiling for Screening Potential Biomarkers of Thyroid Tumors by Matrix-Assisted Laser Desorption/ionization-Fourier Transform Ion Cyclotron Resonance Mass Spectrometry. Anal. Bioanal. Chem. 406, 4357–4370. doi:10.1007/s00216-014-7846-0
Hamm, G., Bonnel, D., Legouffe, R., Pamelard, F., Delbos, J.-M., Bouzom, F., et al. (2012). Quantitative Mass Spectrometry Imaging of Propranolol and Olanzapine Using Tissue Extinction Calculation as Normalization Factor. J. Proteomics 75, 4952–4961. doi:10.1016/j.jprot.2012.07.035
Heijs, B., Holst, S., Briaire-De Bruijn, I. H., Van Pelt, G. W., De Ru, A. H., Van Veelen, P. A., et al. (2016). Multimodal Mass Spectrometry Imaging of N-Glycans and Proteins from the Same Tissue Section. Anal. Chem. 88, 7745–7753. doi:10.1021/acs.analchem.6b01739
Heiles, S., Kompauer, M., Müller, M. A., and Spengler, B. (2020). Atmospheric-Pressure MALDI Mass Spectrometry Imaging at 213 Nm Laser Wavelength. J. Am. Soc. Mass Spectrom. 31, 326–335. doi:10.1021/jasms.9b00052
Hillenkamp, F., UNSöLD, E., Kaufmann, R., and Nitsche, R. (1975). Laser Microprobe Mass Analysis of Organic Materials. Nature 256, 119–120. doi:10.1038/256119a0
Hoch, T., Schulz, D., Eling, N., Gómez, J. M., Levesque, M. P., and Bodenmiller, B. (2022). Multiplexed Imaging Mass Cytometry of the Chemokine Milieus in Melanoma Characterizes Features of the Response to Immunotherapy. Sci. Immunol. 7. doi:10.1126/sciimmunol.abk1692
Hoo, Z. H., Candlish, J., and Teare, D. (2017). What Is an ROC Curve? Emerg. Med. J. 34, 357–359. doi:10.1136/emermed-2017-206735
Jones, M. A., Cho, S. H., Patterson, N. H., Van de Plas, R., Spraggins, J., Boothby, M. R., et al. (2019, Multimodal Fusion of Lipid MALDI IMS with a Transgenic Fluorophore). doi:10.26434/chemrxiv.9853856.v1
Jones, M. A., Cho, S. H., Patterson, N. H., Van De Plas, R., Spraggins, J. M., Boothby, M. R., et al. (2020). Discovering New Lipidomic Features Using Cell Type Specific Fluorophore Expression to Provide Spatial and Biological Specificity in a Multimodal Workflow with MALDI Imaging Mass Spectrometry. Anal. Chem. 92, 7079–7086. doi:10.1021/acs.analchem.0c00446
Kaya, I., Brinet, D., Michno, W., Syvänen, S., Sehlin, D., Zetterberg, H., et al. (2017a). Delineating Amyloid Plaque Associated Neuronal Sphingolipids in Transgenic Alzheimer's Disease Mice (tgArcSwe) Using MALDI Imaging Mass Spectrometry. ACS Chem. Neurosci. 8, 347–355. doi:10.1021/acschemneuro.6b00391
Kaya, I., Michno, W., Brinet, D., Iacone, Y., Zanni, G., Blennow, K., et al. (2017b). Histology-Compatible MALDI Mass Spectrometry Based Imaging of Neuronal Lipids for Subsequent Immunofluorescent Staining. Anal. Chem. 89, 4685–4694. doi:10.1021/acs.analchem.7b00313
Kertesz, V., and Cahill, J. F. (2021). Spatially Resolved Absolute Quantitation in Thin Tissue by Mass Spectrometry. Anal. Bioanal. Chem. 413, 2619–2636. doi:10.1007/s00216-020-02964-3
Kompauer, M., Heiles, S., and Spengler, B. (2016). Atmospheric Pressure MALDI Mass Spectrometry Imaging of Tissues and Cells at 1.4-μm Lateral Resolution. Nat. Methods 14, 90–96. doi:10.1038/nmeth.4071
Kune, C., McCann, A., Raphaël, L. R., Arias, A. A., Tiquet, M., Van Kruining, D., et al. (2019). Rapid Visualization of Chemically Related Compounds Using Kendrick Mass Defect as a Filter in Mass Spectrometry Imaging. Anal. Chem. 91, 13112–13118. doi:10.1021/acs.analchem.9b03333
Lanni, E. J., Masyuko, R. N., Driscoll, C. M., Aerts, J. T., Shrout, J. D., Bohn, P. W., et al. (2014). MALDI-guided SIMS: Multiscale Imaging of Metabolites in Bacterial Biofilms. Anal. Chem. 86, 9139–9145. doi:10.1021/ac5020222
Lauwerends, L. J., Abbasi, H., Bakker Schut, T. C., Van Driel, P. B. A. A., Hardillo, J. A. U., Santos, I. P., et al. (2022). The Complementary Value of Intraoperative Fluorescence Imaging and Raman Spectroscopy for Cancer Surgery: Combining the Incompatibles. Eur. J. Nucl. Med. Mol. Imaging. doi:10.1007/s00259-022-05705-z
Massonnet, P., and Heeren, R. M. A. (2019). A Concise Tutorial Review of TOF-SIMS Based Molecular and Cellular Imaging. J. Anal. At. Spectrom. 34, 2217–2228. doi:10.1039/c9ja00164f
Masyuko, R., Lanni, E. J., Sweedler, J. V., and Bohn, P. W. (2013). Correlated Imaging - a Grand Challenge in Chemical Analysis. Analyst 138, 1924–1939. doi:10.1039/c3an36416j
Matsumoto, K.-i., Mitchell, J. B., and Krishna, M. C. (2021). Multimodal Functional Imaging for Cancer/tumor Microenvironments Based on MRI, EPRI, and PET. Molecules 26, 1614. doi:10.3390/molecules26061614
Neumann, E. K., Comi, T. J., Spegazzini, N., Mitchell, J. W., Rubakhin, S. S., Gillette, M. U., et al. (2018). Multimodal Chemical Analysis of the Brain by High Mass Resolution Mass Spectrometry and Infrared Spectroscopic Imaging. Anal. Chem. 90, 11572–11580. doi:10.1021/acs.analchem.8b02913
Neumann, J. M., Niehaus, K., Neumann, N., Knobloch, H. C., Bremmer, F., Krafft, U., et al. (2021). A New Technological Approach in Diagnostic Pathology: Mass Spectrometry Imaging-Based Metabolomics for Biomarker Detection in Urachal Cancer. Lab. Invest. 101, 1281–1288. doi:10.1038/s41374-021-00612-7
Niehaus, M., Soltwisch, J., Belov, M. E., and Dreisewerd, K. (2019). Transmission-mode MALDI-2 Mass Spectrometry Imaging of Cells and Tissues at Subcellular Resolution. Nat. Methods 16, 925–931. doi:10.1038/s41592-019-0536-2
Nikitina, A., Huang, D., Li, L., Peterman, N., Cleavenger, S. E., Fernández, F. M., et al. (2020). A Co-registration Pipeline for Multimodal MALDI and Confocal Imaging Analysis of Stem Cell Colonies. J. Am. Soc. Mass Spectrom. 31, 986–989. doi:10.1021/jasms.9b00094
Oetjen, J., Aichler, M., Trede, D., Strehlow, J., Berger, J., Heldmann, S., et al. (2013). MRI-compatible Pipeline for Three-Dimensional MALDI Imaging Mass Spectrometry Using PAXgene Fixation. J. Proteomics 90, 52–60. doi:10.1016/j.jprot.2013.03.013
Ovchinnikova, K., Stuart, L., Rakhlin, A., Nikolenko, S., and Alexandrov, T. (2020). ColocML: Machine Learning Quantifies Co-localization between Mass Spectrometry Images. Bioinformatics 36, 3215–3224. doi:10.1093/bioinformatics/btaa085
Palmer, A. D., Bunch, J., and Styles, I. B. (2015). The Use of Random Projections for the Analysis of Mass Spectrometry Imaging Data. J. Am. Soc. Mass Spectrom. 26, 315–322. doi:10.1007/s13361-014-1024-7
Patterson, N. H., Tuck, M., Lewis, A., Kaushansky, A., Norris, J. L., Van De Plas, R., et al. (2018a). Next Generation Histology-Directed Imaging Mass Spectrometry Driven by Autofluorescence Microscopy. Anal. Chem. 90, 12404–12413. doi:10.1021/acs.analchem.8b02885
Patterson, N. H., Tuck, M., Van De Plas, R., and Caprioli, R. M. (2018b). Advanced Registration and Analysis of MALDI Imaging Mass Spectrometry Measurements through Autofluorescence Microscopy. Anal. Chem. 90, 12395–12403. doi:10.1021/acs.analchem.8b02884
Perry, W. J., Patterson, N. H., Prentice, B. M., Neumann, E. K., Caprioli, R. M., and Spraggins, J. M. (2020a). Uncovering Matrix Effects on Lipid Analyses in MALDI Imaging Mass Spectrometry Experiments. J. Mass Spectrom. 55, e4491–2. doi:10.1002/jms.4491
Perry, W. J., Weiss, A., Van de Plas, R., Spraggins, J. M., Caprioli, R. M., and Skaar, E. P. (2020b). Integrated Molecular Imaging Technologies for Investigation of Metals in Biological Systems: A Brief Review. Curr. Opin. Chem. Biol. 55, 127–135. doi:10.1016/j.cbpa.2020.01.008
Pezzotti, N., Höllt, T., Lelieveldt, B., Eisemann, E., and Vilanova, A. (2016). Hierarchical Stochastic Neighbor Embedding. Comput. Graph. Forum 35, 21–30. doi:10.1111/cgf.12878
Prade, V. M., Kunzke, T., Feuchtinger, A., Rohm, M., Luber, B., Lordick, F., et al. (2020). De Novo discovery of Metabolic Heterogeneity with Immunophenotype-Guided Imaging Mass Spectrometry. Mol. Metab. 36, 100953. doi:10.1016/j.molmet.2020.01.017
Prentice, B. M., and Caprioli, R. M. (2016). The Need for Speed in Matrix-Assisted Laser Desorption/Ionization Imaging Mass Spectrometry. Jpr 4, 3–13. doi:10.14304/surya.jpr.v4n3.1
Prentice, B. M., Ryan, D. J., Van De Plas, R., Caprioli, R. M., and Spraggins, J. M. (2018). Enhanced Ion Transmission Efficiency up to M/z 24 000 for MALDI Protein Imaging Mass Spectrometry. Anal. Chem. 90, 5090–5099. doi:10.1021/acs.analchem.7b05105
Rabe, J.-H., A. Sammour, D., Schulz, S., Munteanu, B., Ott, M., Ochs, K., et al. (2018). Fourier Transform Infrared Microscopy Enables Guidance of Automated Mass Spectrometry Imaging to Predefined Tissue Morphologies. Sci. Rep. 8, 1–11. doi:10.1038/s41598-017-18477-6
Race, A. M., Rae, A., Vorng, J.-L., Havelund, R., Dexter, A., Kumar, N., et al. (2020). Correlative Hyperspectral Imaging Using a Dimensionality-Reduction-Based Image Fusion Method. Anal. Chem. 92, 10979–10988. doi:10.1021/acs.analchem.9b05055
Ritschar, S., Schirmer, E., Hufnagl, B., Löder, M. G. J., Römpp, A., and Laforsch, C. (2022). Classification of Target Tissues of Eisenia fetida Using Sequential Multimodal Chemical Analysis and Machine Learning. Histochem. Cell Biol. 157, 127–137. doi:10.1007/s00418-021-02037-1
Römpp, A., Schramm, T., Hester, A., Klinkert, I., Both, J.-P., Heeren, R. M. A., et al. (2011). “imzML: Imaging Mass Spectrometry Markup Language: A Common Data Format for Mass Spectrometry Imaging,” in Data Mining in Proteomics. Methods in Molecular Biology (Methods and Protocols). Hamacher M., Eisenacher M., Stephan C. (eds.) (Clifton, N.J.: Humana Press. Vol. 696), 205–224. doi:10.1007/978-1-60761-987-1_12
Rujchanarong, D., Lefler, J., Saunders, J. E., Pippin, S., Spruill, L., Bethard, J. R., et al. (2021). Defining the Tumor Microenvironment by Integration of Immunohistochemistry and Extracellular Matrix Targeted Imaging Mass Spectrometry. Cancers 13, 4419. doi:10.3390/cancers13174419
Ryabchykov, O., Popp, J., and Bocklitz, T. (2018). Fusion of MALDI Spectrometric Imaging and Raman Spectroscopic Data for the Analysis of Biological Samples. Front. Chem. 6. doi:10.3389/fchem.2018.00257
Schramm, T., Hester, Z., Klinkert, I., Both, J.-P., Heeren, R. M. A., Brunelle, A., et al. (2012). ImzML - A Common Data Format for the Flexible Exchange and Processing of Mass Spectrometry Imaging Data. J. Proteomics 75, 5106–5110. doi:10.1016/j.jprot.2012.07.026
Science, C. P. (2021). “MALDI Mass Spectrometry Imaging,” in MALDI Mass Spectrometry Imaging and Orbitrap. Editor T. Porta Siegel (Cambridge: Royal Society of Chemistry). doi:10.1039/9781839165191
Sinha, T. K., Khatib-Shahidi, S., Yankeelov, T. E., Mapara, K., Ehtesham, M., Cornett, D. S., et al. (2008). Integrating Spatially Resolved Three-Dimensional MALDI IMS with In Vivo Magnetic Resonance Imaging. Nat. Methods 5, 57–59. doi:10.1038/nmeth1147
Soltwisch, J., Heijs, B., Koch, A., Vens-Cappell, S., Höhndorf, J., and Dreisewerd, K. (2020). MALDI-2 on a Trapped Ion Mobility Quadrupole Time-Of-Flight Instrument for Rapid Mass Spectrometry Imaging and Ion Mobility Separation of Complex Lipid Profiles. Anal. Chem. 92, 8697–8703. doi:10.1021/acs.analchem.0c01747
Soltwisch, J., Kettling, H., Vens-Cappell, S., Wiegelmann, M., Müthing, J., and Dreisewerd, K. (2015). Mass Spectrometry Imaging with Laser-Induced Postionization. Science 348, 211–215. doi:10.1126/science.aaa1051
Song, X., He, J., Pang, X., Zhang, J., Sun, C., Huang, L., et al. (2019). Virtual Calibration Quantitative Mass Spectrometry Imaging for Accurately Mapping Analytes across Heterogenous Biotissue. Anal. Chem. 91, 2838–2846. doi:10.1021/acs.analchem.8b04762
Soudah, T., Zoabi, A., and Margulis, K. (2021). Desorption Electrospray Ionization Mass Spectrometry Imaging in Discovery and Development of Novel Therapies. Mass Spec. Rev., 21736. doi:10.1002/mas.21736
Spengler, B., and Hubert, M., K. R. (1994). “MALDI Ion Imaging and Biological Ion Imaging with a New Scanning UV-Laser Microprobe,” in Proc. 42nd Annu. Conf. Mass Spectrom. Allied Top., 1041.
Spivey, E. C., McMillen, J. C., Ryan, D. J., Spraggins, J. M., and Caprioli, R. M. (2019). Combining MALDI-2 and Transmission Geometry Laser Optics to Achieve High Sensitivity for Ultra-high Spatial Resolution Surface Analysis. J. Mass Spectrom. 54, 366–370. doi:10.1002/jms.4335
Spraggins, J. M., Djambazova, K. V., Rivera, E. S., Migas, L. G., Neumann, E. K., Fuetterer, A., et al. (2019). High-Performance Molecular Imaging with MALDI Trapped Ion-Mobility Time-Of-Flight (timsTOF) Mass Spectrometry. Anal. Chem. 91, 14552–14560. doi:10.1021/acs.analchem.9b03612
Spraggins, J. M., Rizzo, D. G., Moore, J. L., Noto, M. J., Skaar, E. P., and Caprioli, R. M. (2016). Next-generation Technologies for Spatial Proteomics: Integrating Ultra-high Speed MALDI-TOF and High Mass Resolution MALDI FTICR Imaging Mass Spectrometry for Protein Analysis. Proteomics 16, 1678–1689. doi:10.1002/pmic.201600003
Strittmatter, N., Richards, F. M., Race, A. M., Ling, S., Sutton, D., Nilsson, A., et al. (2022). Method to Visualize the Intratumor Distribution and Impact of Gemcitabine in Pancreatic Ductal Adenocarcinoma by Multimodal Imaging. Anal. Chem. 94, 1795–1803. doi:10.1021/acs.analchem.1c04579
Trindade, G. F., Abel, M.-L., Lowe, C., Tshulu, R., and Watts, J. F. (2018a). A Time-Of-Flight Secondary Ion Mass Spectrometry/Multivariate Analysis (ToF-SIMS/MVA) Approach to Identify Phase Segregation in Blends of Incompatible but Extremely Similar Resins. Anal. Chem. 90, 3936–3941. doi:10.1021/acs.analchem.7b04877
Trindade, G. F., Abel, M.-L., and Watts, J. F. (2018b). simsMVA: A Tool for Multivariate Analysis of ToF-SIMS Datasets. Chemom. Intelligent Laboratory Syst. 182, 180–187. doi:10.1016/j.chemolab.2018.10.001
Tuck, M., Blanc, L., Touti, R., Patterson, N. H., Van Nuffel, S., Villette, S., et al. (2021). Multimodal Imaging Based on Vibrational Spectroscopies and Mass Spectrometry Imaging Applied to Biological Tissue: A Multiscale and Multiomics Review. Anal. Chem. 93, 445–477. doi:10.1021/acs.analchem.0c04595
Unsihuay, D., Mesa Sanchez, D., and Laskin, J. (2021). Quantitative Mass Spectrometry Imaging of Biological Systems. Annu. Rev. Phys. Chem. 72, 307–329. doi:10.1146/annurev-physchem-061020-053416
Urbini, M., Petito, V., de Notaristefani, F., Scaldaferri, F., Gasbarrini, A., and Tortora, L. (2017). ToF-SIMS and Principal Component Analysis of Lipids and Amino Acids from Inflamed and Dysplastic Human Colonic Mucosa. Anal. Bioanal. Chem. 409, 6097–6111. doi:10.1007/s00216-017-0546-9
Van De Plas, R., Yang, J., Spraggins, J., and Caprioli, R. M. (2015). Image Fusion of Mass Spectrometry and Microscopy: A Multimodality Paradigm for Molecular Tissue Mapping. Nat. Methods 12, 366–372. doi:10.1038/nmeth.3296
Van Der Maaten, L., and Hinton, G. (2008). Visualizing Data Using T-SNE. J. Mach. Learn. Res. 9, 2579–2625. Available at: http://jmlr.org/papers/v9/vandermaaten08a.html.
Verbeeck, N., Spraggins, J. M., Murphy, M. J. M., Wang, H.-d., Deutch, A. Y., Caprioli, R. M., et al. (2017). Connecting Imaging Mass Spectrometry and Magnetic Resonance Imaging-Based Anatomical Atlases for Automated Anatomical Interpretation and Differential Analysis. Biochimica Biophysica Acta (BBA) - Proteins Proteomics 1865, 967–977. doi:10.1016/j.bbapap.2017.02.016
Wehrli, P., Michno, W., Guerard, L., Fernandez-Rodriguez, J., Bergh, A., Blennow, K., et al. (2020). Spatial Chemometrics and Comprehensive Chemical Imaging Based Molecular Histopathology Delineates Anatomical Heterogeneity at Single Pixel Resolution. ChemRxiv, 1–32. doi:10.26434/chemrxiv.11800209.v1
Weiskirchen, R., Weiskirchen, S., Kim, P., and Winkler, R. (2019). Software Solutions for Evaluation and Visualization of Laser Ablation Inductively Coupled Plasma Mass Spectrometry Imaging (LA-ICP-MSI) Data: A Short Overview. J. Cheminform. 11, 16. doi:10.1186/s13321-019-0338-7
Yagnik, G., Liu, Z., Rothschild, K. J., and Lim, M. J. (2021). Highly Multiplexed Immunohistochemical MALDI-MS Imaging of Biomarkers in Tissues. J. Am. Soc. Mass Spectrom. 32, 977–988. doi:10.1021/jasms.0c00473
Yajima, Y., Hiratsuka, T., Kakimoto, Y., Ogawa, S., Shima, K., Yamazaki, Y., et al. (2018). Region of Interest Analysis Using Mass Spectrometry Imaging of Mitochondrial and Sarcomeric Proteins in Acute Cardiac Infarction Tissue. Sci. Rep. 8, 7493. doi:10.1038/s41598-018-25817-7
Yang, J., Norris, J. L., and Caprioli, R. (2018). Novel Vacuum Stable Ketone-Based Matrices for High Spatial Resolution MALDI Imaging Mass Spectrometry. J. Mass Spectrom. 53, 1005–1012. doi:10.1002/jms.4277
Keywords: MALDI mass spectrometry imaging, multimodal imaging, analytical strategy, computational strategy, biological applications
Citation: Tuck M, Grélard F, Blanc L and Desbenoit N (2022) MALDI-MSI Towards Multimodal Imaging: Challenges and Perspectives. Front. Chem. 10:904688. doi: 10.3389/fchem.2022.904688
Received: 25 March 2022; Accepted: 14 April 2022;
Published: 09 May 2022.
Edited by:
Nicole J. Jaffrezic-Renault, Université Claude Bernard Lyon 1, FranceReviewed by:
Alfredo J. Ibáñez, Pontificia Universidad Católica del Perú, PeruCopyright © 2022 Tuck, Grélard, Blanc and Desbenoit. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nicolas Desbenoit, bi5kZXNiZW5vaXRAY2Jtbi51LWJvcmRlYXV4LmZy
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.