
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Chem. , 04 May 2022
Sec. Inorganic Chemistry
Volume 10 - 2022 | https://doi.org/10.3389/fchem.2022.900660
This article is part of the Research Topic Inorganic Materials for Energy and Environmental Catalysis View all 18 articles
The reduction of fluoride concentrations in water is one of many concerns. Adsorption is the most widely used technology for fluoride removal and the center to development of adsorption technology is the improvement of adsorbents. This review classifies the typical fluoride removal adsorbents into four types: metal oxides/hydroxides, biopolymers, carbon-based, and other adsorbents. The exploitation of new materials and the synthesis of composite materials are two ways of developing new adsorbents. In comparison to the discovery of novel adsorbents for fluoride adsorption, research into the composite synthesis of different types of conventional adsorbents has proliferated in recent years. The traditional adsorbents used the earliest, metal oxides, can act as active centers in a wide range of applications for modifying and compounding with other types of adsorbents. This study emphasizes reviewing the research on fluoride removal by composite adsorbents synthesized from different types of metal-modified materials. Seven factors were compared in terms of material characterization, initial fluoride concentration, adsorbent dose, pH, temperature, reaction time, and maximum adsorption capacity. The modification of composite adsorbents is facile and the synergistic effect of the different types of adsorbents significantly improves fluoride adsorption capacity. Metal composite adsorbents are synthesized by facile coprecipitation, hydrothermal, or impregnation modification methods. The adsorption mechanisms involve electrostatic attraction, ion exchange, complexation, and hydrogen bonding. The fluoride adsorption capacity of composite adsorbents has generally improved, indicating that most modifications are successful and have application prospects. However, to achieve significant breakthroughs in practical applications, numerous issues such as cost, separation/regeneration performance, and safety still need to be considered.
Fluoride ions in water have a strong affinity with positively charged elements such as calcium (Bhatnagar et al., 2011), which is a major component of human bone and tooth structure (Rehman et al., 2015). Low concentrations of fluoride in drinking water (0.5–1.5 mg/L) (Chen et al., 2017) can strengthen bones and prevent dental caries, while excessive concentrations of fluoride (4–10 mg/L) can cause diseases such as fluorosis, osteoporosis, brittle bones, brain damage, and several thyroid disorders (Kumar et al., 2020). Excessive fluoride concentrations in water have become a public health concern in developing countries. High concentration (>10 mg/L) fluorinated wastewater is easier to treat and can be removed or reduced by coagulation, precipitation, electrochemistry, and other methods. The treatment of low concentration (2–10 mg/L) fluorinated wastewater is relatively difficult and is also a current research hotspot. The treatment methods include adsorption (Lin et al., 2016), membrane separation, ion exchange (Xu et al., 2017), nanofiltration (Wang A. et al., 2018), reverse osmosis (Ye et al., 2019), and electrodialysis (Fan et al., 2019). Among them, the adsorption method has the advantages of low cost, high flexibility, simple operation, and high efficiency (Sarkar et al., 2019). It is the most widely used and the treatment effect is more satisfactory.
The treatment effectiveness of the adsorption method is influenced by a number of factors, including adsorbent properties, fluoride ion selectivity, compatibility, solution pH, temperature, co-existing ions, and contact time (Pigatto et al., 2020). It mainly depends on the adsorbent properties such as particle size, pore size structure, zero charge point (pHPZC), and specific surface area (SBET) (Biswas et al., 2017). High specific surface area (developed pore structure) and ideal chemical surface (abundant functional groups) are two essentials for effective removal of fluoride by adsorbents. Although not systematically categorized, this review found that the main traditional sorbents frequently used for fluoride removal are metal oxides/hydroxides (Dhillon et al., 2017), low-cost carbon materials (Zhang X. et al., 2021), biomolecular materials (Jia et al., 2018), and others such as clay, hydroxyapatite, and graphite. This review classifies the more researched fluoride removal adsorbents into four categories: metal oxide/hydroxide adsorbents, biopolymer adsorbents, carbon-based adsorbents, and other adsorbents (industrial waste, minerals, etc.).
For the development of new adsorbents, the discovery of novel adsorbents that have never been used before and the composite material synthesis by combining traditional adsorbents are the two main approaches to improve adsorption capacity. In comparison to the discovery of novel adsorbents that have never been used before, research into the composite synthesis of different types of conventional adsorbents for fluoride adsorption has proliferated in recent years. The emphasis of this study is placed on the fluoride adsorption effect of this adsorbent compounded from different types of conventional adsorbents. The study of other types of metal-modified adsorbents accounts for a major part. A total of seven factors were compared in terms of material characterization, initial fluoride concentration, adsorbent dose, pH, temperature, reaction time, and maximum adsorption capacity.
Metal oxide/hydroxide nanoparticles were reported to show an affinity for fluoride and high performance in fluoride removal. The high reactivity (Lanas et al., 2016), specificity, specific surface area (Rathore and Mondal 2017), stability, and self-assembly potential have attracted attention in fluoride removal studies. Nanoscale dimensions with desirable physicochemical properties, such as high density of hydroxyl ions on the high specific surface area, will further enhance the fluoride adsorption capacity.
Aluminum oxide/hydroxide was the earliest studied and used adsorbents for fluoride removal (Chinnakoti et al., 2016a). Typically, aluminum hydroxide is first prepared by electrolysis or pyrolysis and then partially converted to aluminum oxide by calcination. One of the advantages of aluminum oxide/hydroxide adsorbents is the large specific surface area (Hafshejani et al., 2017), as shown in Table 1, and in general, SBET > 200 m2/g. Generally, high pHPZC allows its surface to appear positively charged in water (Dhawane et al., 2018). Several studies have reported that the mechanism of fluoride adsorption by alumina mainly consists of electrostatic attraction and ion exchange (Rathore and Mondal 2017), as shown in Figure 1A; the monodentate complex Al-F is the major formation after adsorption (Kang et al., 2018; Lin et al., 2020). Kang et al. (2018) synthesized an amorphous alumina microsphere using solvothermal reaction and calcination, with SBET = 400 m2/g and a maximum adsorption capacity of 129.4 mg/g; they proposed that the adsorption mechanism involves chemical reaction and pore filling in addition to ion exchange and electrostatic attraction. However, aluminum is easily leached out in aqueous solutions, especially under acidic conditions (Lin et al., 2020), leading to high concentration of aluminum residues in drinking water, which is also a major threat to human health.
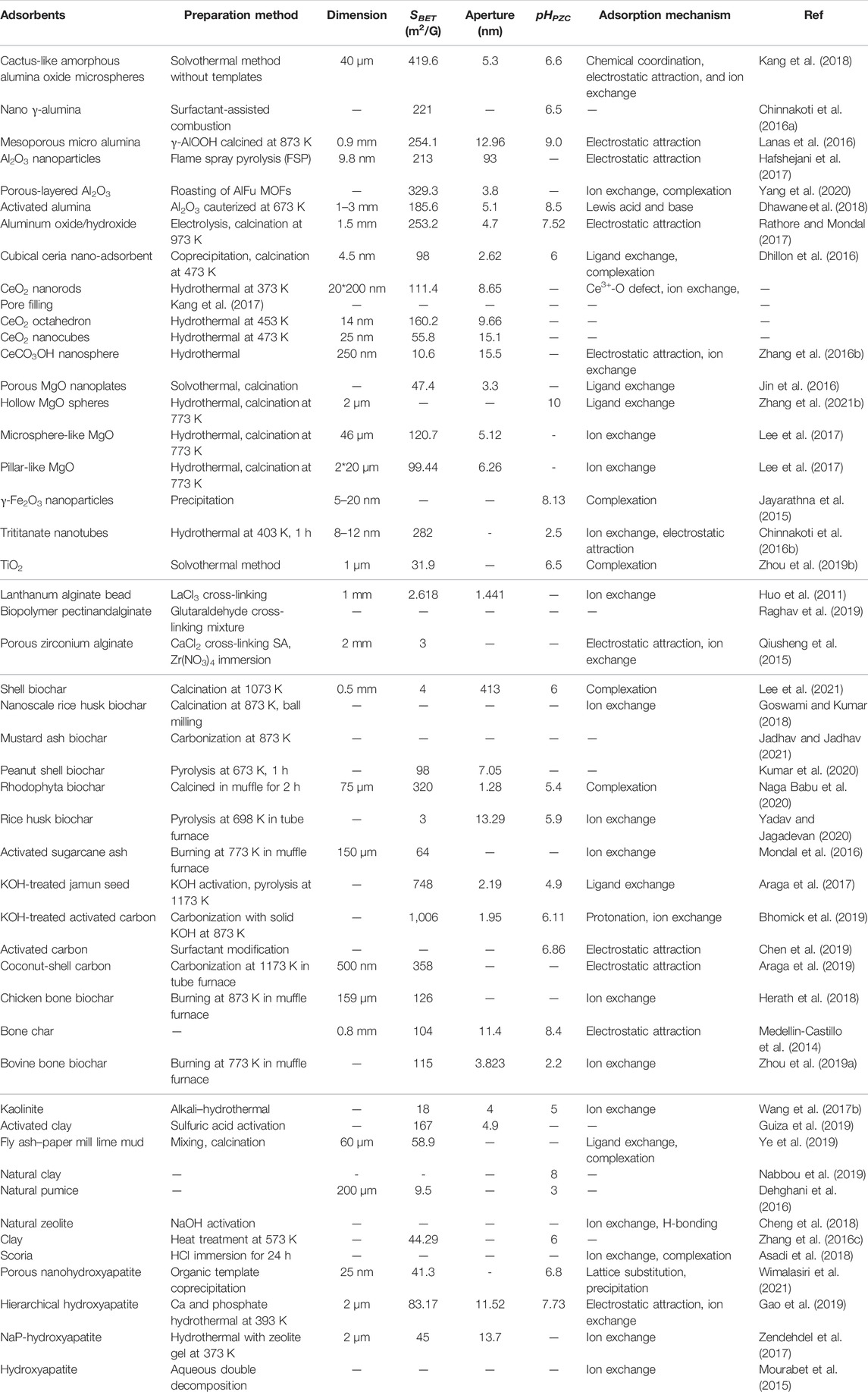
TABLE 1. Summary of the preparation methods, characteristics, and adsorption mechanisms of four traditional adsorbents.
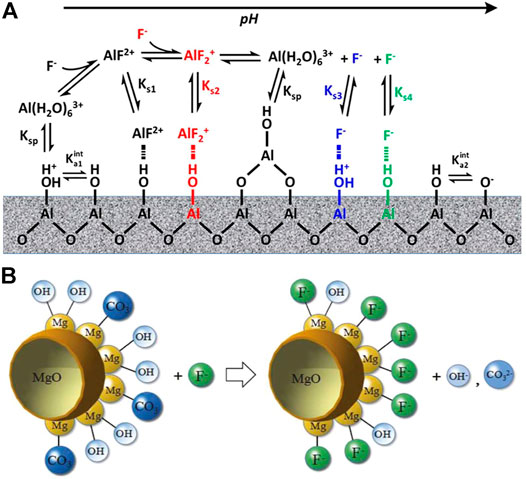
FIGURE 1. Mechanism of fluoride adsorption by activated alumina (A) (Lin et al., 2020) and MgO (B) (Zhang Y. et al., 2021).
Compared to aluminum, the rare earth metals (cerium, titanium, lanthanum, etc.) have further affinity for fluoride due to the ability to stabilize in the +3 or +4 valence state with a few numbers of outermost electrons; therefore, sufficient empty orbitals are available for fluoride ions (Zhang K. et al., 2016). The solubility of rare earth metals is relatively limited over a wide pH range (Dhillon et al., 2016), so rare earth oxides/hydroxides have been increasingly investigated as substitution for aluminum in recent years. Among these, CeO2 readily forms oxygen vacancies and, therefore, has particularly high oxygen storage/release capacity with high adsorption capacity (Kullgren et al., 2014; Wu and Gong 2016; Kang et al., 2017). Kang et al. (2017) compared the physicochemical characteristics and adsorption performance of different morphologies of CeO2 (nanorods, octahedrons, and nanocubes) prepared under different hydrothermal conditions. The different morphologies of CeO2 were found to expose distinct crystalline surfaces and proportions of oxygen defects, leading to significant differences in fluoride adsorption capacity, with CeO2 nanorods having the largest Qmax (71.5 mg/g). However, rare earth metal oxides are costly, prone to agglomeration, and high leaching concentrations can be toxic to water (Yu et al., 2015).
MgO is less dissolved, nontoxic, abundant in reserves compared to other metals, and has an affinity for fluoride (Jin et al., 2016), giving it an opportunity to be used. It has been reported that MgO has a high isoelectric point and relies on electrostatic attraction to adsorb fluoride (Suzuki et al., 2013). Y. Zhang et al. tested the zeta potential of hollow MgO spheres of pHPZC = 10, which is the highest value reported. In order to improve the morphology, Z. Jin et al. used a typical solvothermal method followed by calcination to form porous MgO nanoplates with an increased maximum adsorption capacity from 115.5 mg/g to 185.5 mg/g. They suggested that the mechanism of adsorption mainly consists of ligand exchange between fluoride and hydroxyl groups and carbonates on the surface of MgO (Figure 1B). Thus, the presence of carbonate in the solution can affect the fluoride adsorption capacity of MgO.
The most significant problem concerning metal oxide/hydroxide nanoparticles is low structural stability and the tendency to leach in water causing secondary contamination (Lin et al., 2020).
Biopolymers are the natural macromolecular materials derived from cellular or extracellular substances with properties such as biodegradability, nontoxicity, low waste generation, low leaching, biocompatibility, and hydrophilicity. The most researched fluoride removal biopolymer adsorbents in recent years include sodium alginate (SA), pectin, chitosan (CS), and carboxymethyl cellulose (CMC) (Araga and Sharma 2019). Hydrogels formed by chemical or physical cross-linking of biopolymers have hydrophobic, three-dimensional network structures, which are easier to separate compared to the powder state, making them an environment-friendly adsorbent.
Sodium alginate (SA) and pectin are both natural polysaccharides in colloidal form. Sodium alginate contains large numbers of -OH and -COOH groups on the main chain. The -COOH in the M unit is more bound by the surrounding electron cloud, while the -COOH in the G unit is arranged in the corner of the peak consisting of two adjacent carbon atoms; thus, G unit is more reactive (Wu T. et al., 2017). In the ionic cross-linking process (Figure 2A), when the dissolved colloidal sodium alginate is dropped into the solution of high-valent metal cations (Ca2+, Ce3+, Fe3+, Al3+, La3+, etc.), the high-valent cations in the solution will rapidly replace Na+ (Wu et al., 2016a). The embedded high-valent cations form ligand chelate crosslinks with the oxygen atoms in the carboxyl and hydroxyl groups of the G-units, which form irreversible hydrogel-like microbeads (Qiusheng et al., 2015). The thermal stability and acid resistance of sodium alginate are further improved after the formation of the gel, while some of the carboxyl functional groups are occupied by high-valent metal cations, so the active sites with an affinity for fluoride ions are increased. Huo et al. (2011) used ionic cross-linking to prepare lanthanum alginate with stable skeletal junctions. SEM showed cracks in the dense surface structure after adsorption, with Qmax = 197.2 mg/g.
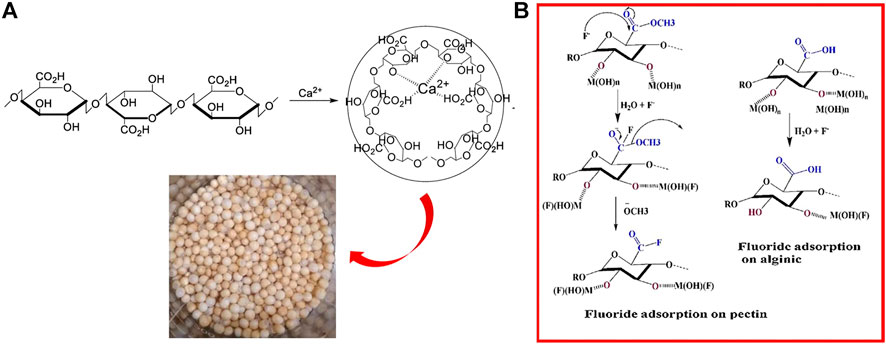
FIGURE 2. Ionic cross-linking procedure of sodium alginate (A) (Sharma et al., 2019) and fluoride adsorption mechanism of pectin (B) (Raghav and Kumar 2019).
Pectin is also rich in -COOH and -COOCH3 groups. The active sites of sodium alginate and pectin are essentially identical, the only difference being the presence of -COOCH3 in pectin (Sharma et al., 2019), whereas sodium alginate contains only -COOH. The ester group chelates better with metals through its lone pair of electron contribution. The carboxyl group is present in a dimeric form due to the conjugation effect, with the lone pair participating in the conjugation. Therefore, the ester group has a nucleophilic reaction to F− (Figure 2B), providing more active sites, and the pectin should have a higher fluoride removal capacity in comparison (Raghav and Kumar 2019). SA and pectin hydrogels accomplish adsorption by exchanging hydroxyl groups in the structure with fluoride.
Carbon-based adsorbents have developed pore structures, large specific surface areas, stable chemical properties, easily adjustable surface properties, good regenerability, and widely available and general waste, which is of low cost with promising applications.
Biochar (BC) is made from waste biomass from a wide range of sources such as reed (Singh and Majumder 2018), rice husks (Yadav and Jagadevan 2020), straw (Angelin et al., 2021), teak peel, and algae. BC is a carbon-rich, fine-grained, porous, and highly aromatized material and well suited as an adsorbent for the resource utilization of waste. BC contains lignocellulosic components capable of effectively adsorbing fluoride (Yadav and Jagadevan 2020). The pyrolysis temperature is a key factor in controlling the number of functional groups on the surface of BC (Wang et al., 2021). Generally, biochar prepared by hydrothermal pyrolysis below 573 K is rich in oxygen-containing functional groups (-COOH, -OH, etc.) and has stronger ion exchange capacity (Naga Babu et al., 2020). As the pyrolysis temperature increases, the abundance of hydroxyl, amino, and carboxyl groups decrease and the degree of carbonation increases (Kumar et al., 2020). Biochar prepared at 673–973 K has developed porosity (Figure 3A) and thermal stability (Goswami and Kumar 2018). Brunson and Sabatini (2015) recorded a significant increase in specific surface area (from 0.9 m2/g to 327 m2/g) and surface zero charge point (from pHPZC = 5.8 to pHPZC = 9.4) when the pyrolysis temperature of charcoal was increased from 573 to 873 K. BC also contains minerals such as potassium, calcium, magnesium, and phosphorus, which can be complex with fluoride ions or precipitate. Lee et al. (2021) found that the shell biochar could contain up to 56.9% CaCO3, and when the pyrolysis temperature was raised to 1073 K, CaCO3 was converted to Ca(OH)2; the structure was more conducive to the adsorption of fluoride. The adsorption mechanism was outer-sphere complexation between Ca and F. Although shell biochar has low carbon content and small specific surface area (SBET = 4.363 m2/g), the maximum fluoride adsorption capacity of their prepared shell biochar MCS-800 could reach 82.93 mg/g. The biochar obtained by pyrolysis alone has an average low adsorption effect but has the advantage of being easily modified (Wang et al., 2021).
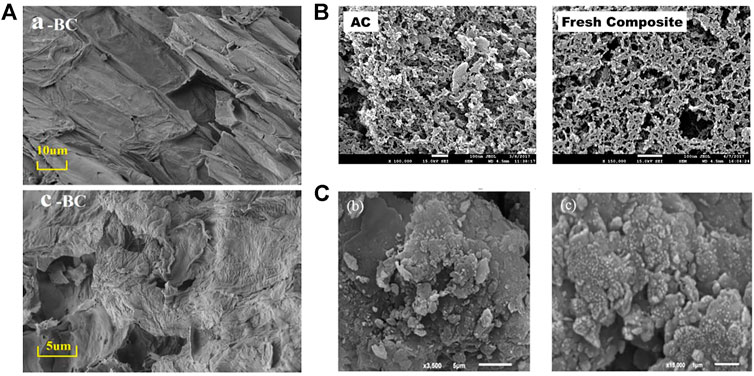
FIGURE 3. Rough surface of biochar (A) (Zhang X. et al., 2021), activated carbon (B) (Mullick and Neogi 2018), and clay (C) (Mobarak et al., 2018).
Activated carbon (AC) is usually made from coconut shells, hard cores, bamboo, coal, wood, and other raw materials (Collivignarelli et al., 2020), and the pyrolysis temperature is generally higher than 1173 K (Araga et al., 2019). After pyrolysis, further physical or chemical activation is required (Chen et al., 2019). Chemical activation has high activation yield but is highly corrosive to the equipment (Tomar et al., 2014). As shown in Figure 3B, AC has the advantage of high porosity and large specific surface area. Araga et al. (2017) and Bhomick et al. (2019) used KOH to activate mustard seed activated carbon and commercially available activated carbon, respectively, and the modified specific surface area reached 747 m2/g and 1,005 m2/g, respectively. The -OH group on the AC surface is protonated with fluoride at pH < pHPZC (acidic media). Numerous studies have demonstrated that fluoride adsorption on AC consists of the mechanism for the deprotonation of -OH functional groups on carbon surfaces (He et al., 2020).
Bone char is the charring product of animal bones and generally contains about 20% carbon and 80% hydroxyapatite (HAp) (Medellin-Castillo et al., 2014), with the content of each component varying slightly depending on the charring temperature. When the charring temperature is below 573 K, more organic matter remains in the bones, but the specific surface area and pore structure is not well developed (Zhou J. et al., 2019). The charring temperatures above 873 K may change the structure of the hydroxyapatite and also lead to reduction in fluoride adsorption capacity. The fluoride adsorption by bone char is reported to be mainly carried out by hydroxyapatite. Medellin-Castillo et al. (2014) found that fluoride in aqueous solutions was mainly adsorbed to HAp in bone char but not to other components. HAp in bone char contains most of the OH− that can be replaced by F−; the adsorption mechanism includes ion exchange and chemical precipitation. The detailed mechanism of fluoride adsorption by HAp is described in the following section.
Other materials such as natural mineral clays (clay (Zhang S. et al., 2016), bentonite (Mudzielwana et al., 2017), etc), industrial solid waste (zeolite (Ghosal and Gupta 2018), etc), and hydroxyapatite are also used for fluoride adsorption. Natural clay (Figure 3C) contains the main compounds SiO2 and Al2O3 and has the chemical potential to adsorb fluoride (Guiza et al., 2019). Zeolite is an aqueous skeletal structure composed of aluminosilicate minerals with the lattice of many pores and channels that have the structural potential to adsorb fluoride. However, these two types of materials usually show weak fluoride adsorption capacities (Table 2) and are generally modified by chemical activation or metal loading.
Hydroxyapatite [(Ca10(PO4)6(OH)2, HAp] is also a promising inorganic material for fluoride adsorption, with excellent biocompatibility, stability, and mechanical properties. Due to its unique crystal structure, HAp has a porous surface, large specific surface area, and high ion exchange capacity. The hydroxyl group in HAp is prone to rapid exchange with anion and has a strong binding capacity with fluoride (Raghav et al., 2018). F− replaces OH−, fills in the lattice of HAp forming insoluble fluorapatite (FAp), and OH− is released into solution. When high concentrations of fluoride ions are present in the solution, Ca2+ in HAp reacts with F− forming CaF2 precipitate, and phosphate is correspondingly released into the solution. Various forms of HAp have been reported for fluoride adsorption in water, such as nano-hydroxyapatite (Mourabet et al., 2015; Zendehdel et al., 2017), porous hydroxyapatite (Nijhawan et al., 2020), and layered hollow hydroxyapatite (Gao et al., 2019). The mechanism of fluoride adsorption by HAp mainly consists of the following: 1) electrostatic attraction by the surface of HAp to F−. 2) Anion exchange between OH− or PO42- and F−. 3) Complexation reaction of Ca2+ with F− ligates and forms surface precipitation. 4) F− can also form hydrogen bonds with OH− in the HAp lattice.
Over long periods of use and development, traditional adsorbents have gradually revealed the unique application value and drawbacks. Compared to the exploitation of novel adsorbents, research tends more to synthesize complexes of two or more adsorbents to produce synergistic fluoride adsorption. The synthesis of metal modifications to other types of adsorbents accounts for the majority.
Different metal oxide adsorbents have their individual strengths and weaknesses for fluoride removal, so recently there have been research studies using multi-metal oxide/hydroxide adsorbents (Chen et al., 2018). Compared to conventional metal oxides, various valence cations are often present in one multi-metal oxide, providing more chemisorption sites (Raghav and Kumar 2018). The tunability of the chemistry of each element ensures an abundance of active sites, and the components can be adjusted to each other, possessing different outstanding properties and therefore having unique quantum coupling effect and synergistic effects, resulting in more than doubling or tripling of the adsorption capacity. There are two common types of multi-metal oxide adsorbents. One is prepared by compounding each metal element in a certain ratio (Wu K. et al., 2017) such as layered double/triple hydroxides (Wu P. et al., 2017), and the other is to modify one metal oxide with others; the ones mostly reported are modified alumina (He et al., 2019) or magnetic iron oxides.
A series of layered double/triple hydroxides (LDHs) have a high affinity for anions with high ion exchange capacity and high adsorption volume, which are often used as anion exchangers and trapping agents. LDHs are two-dimensional layered materials whose structural formula can be expressed as
Al- and Fe-based oxides are mostly doped or load-modified by another metal. Metal-modified Al2O3 generally adsorbs fluoride by complexation and ion exchange (Figure 4A). Iron oxides (Fe3O4 or γ-Fe2O3), in addition to adsorption advantages, provide strong magnetic properties and large magnetic response with easy separability and controllability; thus, there have been more reported recently. Magnetic iron oxide nanoparticles can be used directly for fluoride adsorption or as a nucleus material for core-shell particles. The magnetic core particles are generally combined with metal oxide nano-shells by methods such as surface coating to ensure stronger magnetic response, more functional groups, and better properties (Zhang C. et al., 2016). Recent research has mostly used rare earth metals to modify magnetite. Rare earth ions are greater in radius than other elements in the iron oxides; doping with the appropriate amount of rare earth elements to replace some of the other elements in the iron oxides with a smaller ion radius distorts the lattice and can improve the physical activity. Most importantly, the hard Lewis acid nature of the rare earth metal ions (especially La) has a strong affinity for fluoride (Figure 4B) (Han et al., 2019). Ce can promote the dispersion of nanoparticles, giving the adsorbent a larger specific surface area, pore volume, and more active functional sites. Correspondingly, magnetic particles can attenuate the agglomeration effect of rare earth metal oxides, reducing the amount of precious metals used and improving the separation characteristics by magnetic assistance to reduce residues in water and avoid rare earth metal toxicity (Abo Markeb et al., 2017). Han et al. (2019) used VSM tests to show that Fe3O4 and Fe3O4@La-Ce were both superparamagnetic, and it was easy to separate the particles from the solution using the external magnetic field. The adsorption amount of Fe3O4@La-Ce was increased up to 20 times compared to Fe3O4.
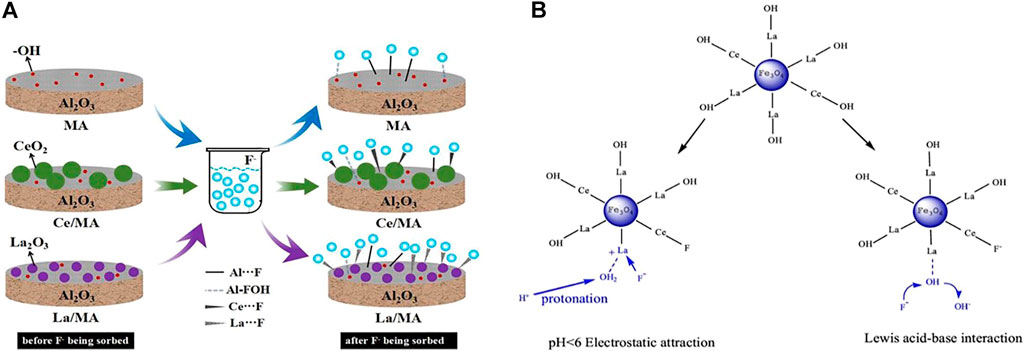
FIGURE 4. Schematic principle of modification on Al2O3 (A) (He et al., 2019) and Fe3O4 (B) (Han et al., 2019) by La, Ce, and fluoride adsorption mechanism.
Large-scale applications for fluoride removal in aqueous systems require the development of composite hydrogel materials with good mechanical properties and stability. For hydrogels with poor adsorption properties, the cross-linking of composites by cementing other high performance adsorbent materials onto biopolymers can effectively reduce the degradation of properties. The preparation of biopolymer-based composites is divided into three types: 1) doping of metals/metal oxides (Sapna et al., 2018), 2) blending with inorganic materials (Wang et al., 2020), and 3) mixing between polymeric organic substances (Preethi and Meenakshi 2018). This section summarizes the doping by metal oxide modification studies.
The biocompatibility and biodegradability of natural polymeric materials make sodium alginate and pectin effective substrates for the incorporation of multivalent metal ions. Studies have reported to dope SA with metals; co-mingling and cross-linking to form a stable gel structure can improve both the stability and mechanical properties of SA (Wu T. et al., 2017), while having an anchoring effect on metal oxides, reducing the agglomeration and leaching of metal oxides and maximizing the adsorption properties (He et al., 2020). Furthermore, the doping of metals can increase the metal active sites in the porous structure and combine the properties of organic and inorganic components to improve the adsorption capacity (Zhao et al., 2021). Mono and multi-metal doping options are available. Recent research has focused on the doping of sodium alginate and pectin with multi-metals. Compared to monometals, the multi-metals provide an enhanced abundance of active sites as mentioned earlier. In addition, the multi-metals used in the studies tend to be the composite of +2 valent and higher valent cations. The addition of +3 and +4 valent metal ions, especially rare earth metals, can improve the stability, recyclability, and adsorption capacity. Raghav and Kumar (2019) obtained a high adsorption capacity (Table 3) for all the composite hydrogels prepared by SA and pectin embedding Fe-Al-Ni (285 mg/g and 200 mg/g) and SA/pectin co-embedding Fe-Al-Ce (142.9 mg/g) (Raghav et al., 2019). Figure 5 shows the reaction process of multi-metal–modified SA and pectin and the exchange sites for fluoride adsorption.
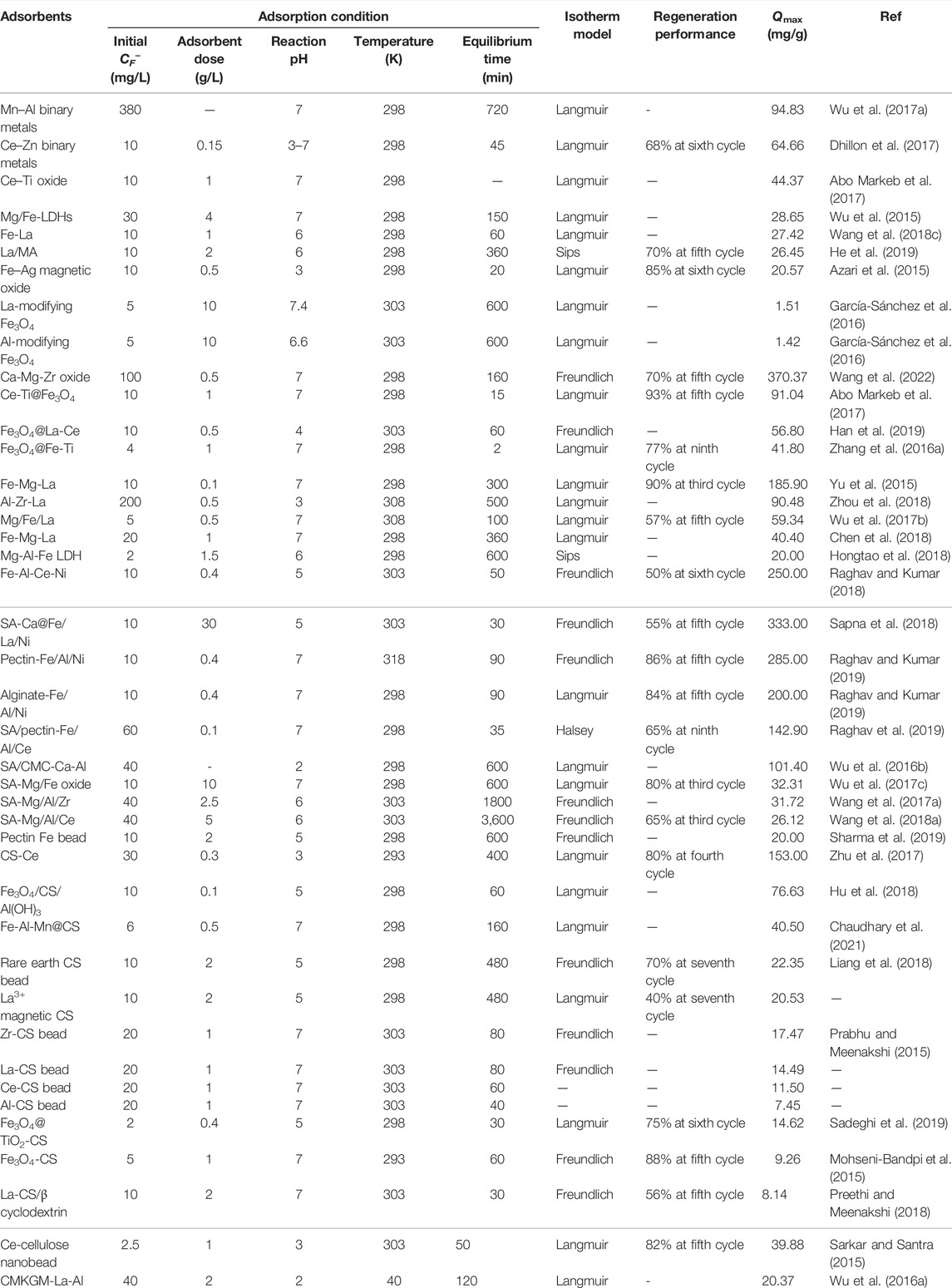
TABLE 3. Adsorption conditions and performance of fluoride by muti-metal and metal-biopolymer composite adsorbents.
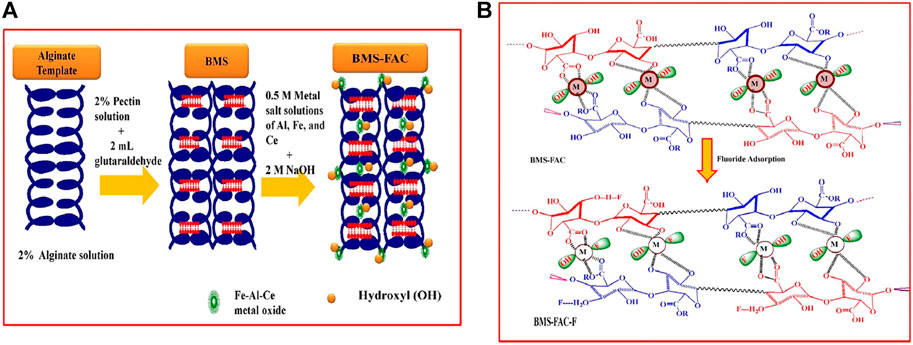
FIGURE 5. Schematic principle (A) and fluoride adsorption mechanism (B) of multi-metal–modified sodium alginate and pectin (Raghav et al., 2019).
Chitosan (CS) is an N-deacetylated derivative of the natural polysaccharide chitin and is rich in free amino acids. The -NH2 group in chitosan is more reactive (Zhu et al., 2017), easy to be chemically modified (Chaudhary et al., 2021), and exhibits high adsorption potential. Despite the numerous advantages such as biodegradability, biocompatibility, flexibility, hydrophilicity, and versatility, CS tends to be readily soluble in acidic solutions and has a weak chemical resistance (Dong and Wang 2016), especially in column continuous flow adsorption. Current research into the adsorption of fluoride ions by CS is also mostly metal-doped, but unlike sodium alginate and pectin, the modification of CS is more oriented toward monometallic impregnation followed by cross-linking using glutaraldehyde (Table 4). The size of the beads formed is much smaller, typically in the micron range (Prabhu and Meenakshi 2015). One of the top research hotspots is the magnetic modification of Fe3O4, mainly because the hydroxyl group on the surface of Fe3O4 can interact with the amino and hydroxyl groups of CS through hydrogen bonding (Sadeghi et al., 2019), enabling CS to remain stable under acidic conditions. The CS composite adsorbent is also endowed with magnetic ease of separation properties (Mohseni-Bandpi et al., 2015). Hu et al. (2018) obtained nano-microsphere Fe3O4/CS/Al(OH)3 beads by facile impregnation, which can rapidly accomplish high capacity adsorption of fluoride and rapid sedimentation under low magnetic fields.
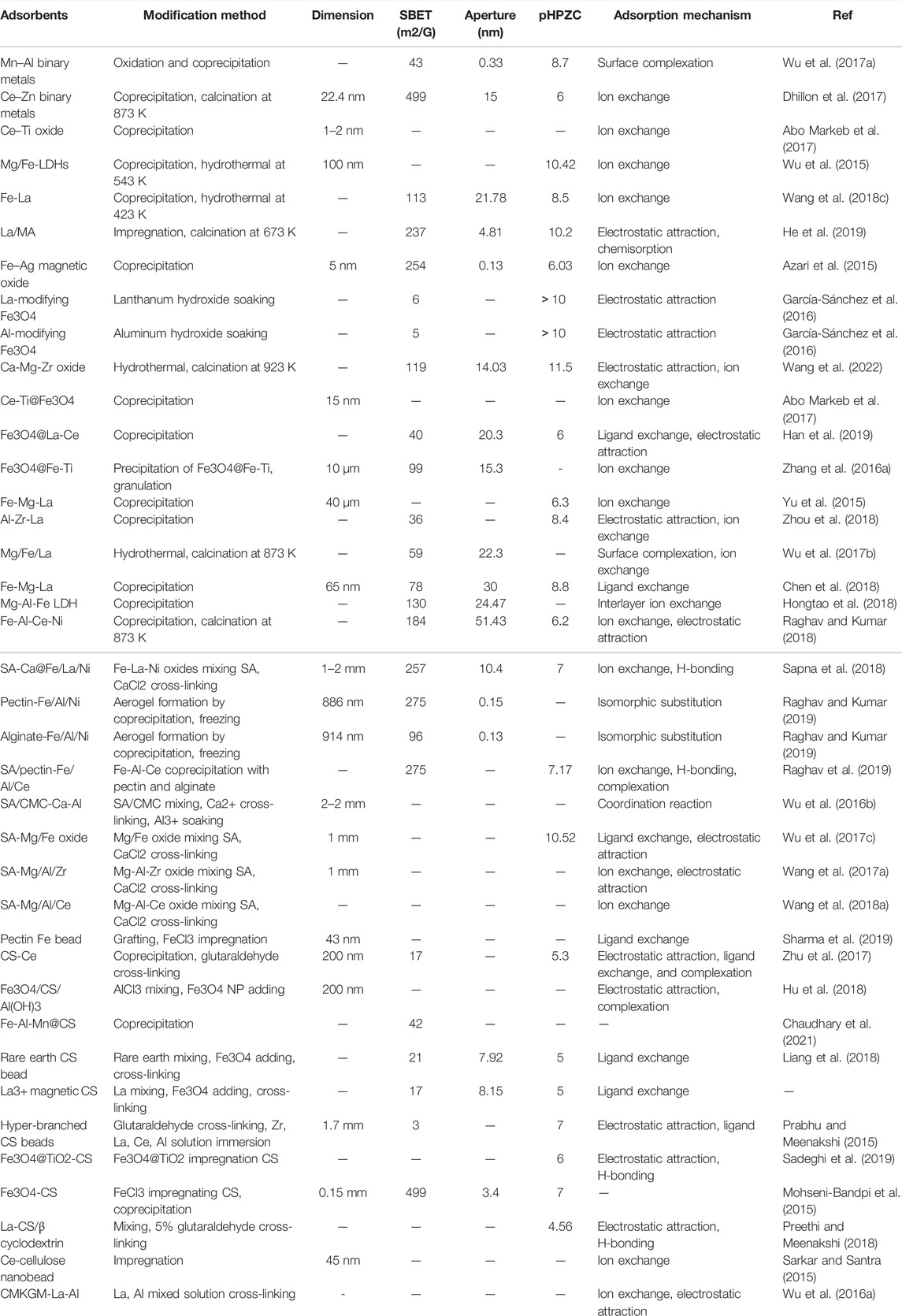
TABLE 4. Summary of modification methods, characteristics, and adsorption mechanisms of muti-metal and metal-biopolymer composite adsorbents.
Carbon-based adsorbents have the advantage of large specific surface area and rich pore structure (Shang et al., 2022), but they have low adsorption capacity for fluoride removal alone and require some modification. Research on carbon composites has focused on the doping or surface loading of carbon with nano-metal oxides/hydroxides (Dehghani et al., 2018). The affinity between fluoride ions and highly valent cations such as Al3+, Fe3+, Ca2+, and Mg2+ can improve the selectivity of carbon to fluoride. When the two are compounded, on the one hand, the metal nanoparticles provide a large number of active sites (Mohanta and Ahmaruzzaman 2018) to compensate for the absence of functional groups that can interact with fluoride ions after high temperature carbonization. On the other hand, the carbon-based adsorbent has large specific surface area and pores, which can act as carriers and dispersants to avoid agglomeration of the metal nanoparticles (Cai et al., 2022). More individual metal loadings are used, and multi-metal modifications are also available. More active sites enhance the adsorption performance, and the modified adsorbent surface is richer in specific types of adsorption sites, which may further increase the adsorption capacity.
The ability of biochar to remove pollutants is greatly influenced by the nature of the raw material, preparation technology, and pyrolysis conditions. Unsuitable pyrolysis conditions tend to under-carbonize or over-carbonize BC, so the adsorption performance of unmodified BC is limited. The raw biochar has a relatively poor adsorption effect on anions as the negative charge occupies the majority of the functional groups (Mei et al., 2020). Highly valent metal cations can provide sufficient positive charge to effectively alter surface physicochemical properties (Wang et al., 2019c). AlCl3 has been reported to generally increase the anion exchange capacity in all BC. Brunson and Sabatini (2015) studied the changes in charcoal surface area and surface chemistry following aluminum nitrate impregnation and found that the aluminum modification reduced the zero charge point of the charcoal in water (from pHPZC = 9.6 to pHPZC = 5.7) but significantly increased the adsorption capacity. Rare earth metal ions such as Ce3+, Zr4+, and La3+ are more alkaline, have a relatively low ionic potential, and show strong tendency to dissociate hydroxyl groups into ions. The possibility of ionic exchange with F− is higher and the affinity is stronger. Habibi et al. (2019) modified woody BC with LaCl3 and showed that the maximum adsorption capacity of 164.23 mg/g and adsorption equilibrium could be reached within 30 min. They concluded that H+ in functional groups such as carboxyl and sulfate groups on the surface of BC may exchange with La3+ ions. The presence of La3+ increased the adsorption mechanism with Lewis acid–base interaction and ion exchange. The F− adsorption rate of the adsorbent was still 80% at fifth recycling, indicating that the rare earth metals loaded on the BC are not easily leached. The modification of iron oxides can confer magnetic properties to BC, improving the separation and recovery performance. BC has good electrical conductivity, which is conducive to electron transfer and reduction of Fe3+. The stronger synergistic effect can further promote the fluoride adsorption performance (Wang et al., 2019c).
Activated carbon has large specific surface area and pores, which can act as carriers and dispersants to avoid agglomeration of the metal nanoparticles (Cai et al., 2022). More individual metal loadings are used, and multi-metal modifications are also available. More active sites enhance the adsorption performance, and the modified adsorbent surface is richer in specific types of adsorption sites, which may further increase the adsorption capacity. Li et al. (2018) precipitated Ti(OH)4 on the surface of AC, which further increased the specific surface area of Ti-AC to 1700 m2/g, providing more adsorption sites for fluoride ions. They confirmed that the adsorption capacity of Ti-AC was produced by Ti(OH)4 loaded on AC. The saturation adsorption capacity of Ti(OH)4 in Ti-AC was 62.1 mg/g, which was much higher than that of Ti(OH)4. It has also been reported that the loading of different metal oxides/hydroxides can form new functional groups on the AC surface with high affinity for fluoride adsorption, significantly improving the adsorption efficiency. A et al. used ultrasonically assisted polymetallic impregnation of AC (Mohanta and Ahmaruzzaman 2018; Mullick and Neogi 2018). The specific surface area decreased after modification, but the pHPZC increased to 11.9 and the adsorption capacity increased by four times compared to monometallic impregnation (Mullick and Neogi 2019).
Graphene oxide (GO) is a two-dimensional honeycomb carbon nanomaterial formed by the close packing of carbon atoms in a sp-hybridization pattern (Kanrar et al., 2016). GO carries various functional oxygen-containing groups (such as -OH, -COOH, C=O, and -CH(O)CH-) and provides active sites to connect to other substances (Jeyaseelan et al., 2021). GO generally adsorbs fluoride through electrostatic attraction, π-π stacking, and hydrogen bonding. It has been reported to have a huge theoretical specific surface area (up to 2,630 m2/g) (Mohan et al., 2017) and can be an excellent host for metal nanoparticles. In turn, nanometallic particles provide structural rigidity by inhibiting the restacking of different layers of GO and provide a higher surface area and many active centers (Mohan et al., 2017). Mohan et al. (2017) hydrothermally synthesized ZrO2/GO with SBET = 632 m2/g. The fixed-bed continuous flow experiments showed that the desorption elution efficiency of the adsorption column regenerated with 10% NaOH solution was greater than 95% for F− within three cycles, indicating the role of the ion exchange mechanism in the adsorption of F−. Nehra et al. (2019) hydrothermally synthesized TiO2/GO with a maximum fluoride adsorption capacity of 342 mg/g, which is the highest reported capacity available. Ti4+ forms strong bonds with the oxygen-containing functional groups of GO by electrostatic attraction and reacts with NaOH on the GO side to form basic titanium hydroxide on the GO layer. The adsorption mechanism for fluoride consists of a complexation reaction with Ti and F and an ion exchange between OH− and F−.
Studies have also reported on composite adsorbents synthesized from three or more types of materials, with combinations of metal-modified biopolymers and inorganic materials making up the bulk of the adsorbents (Table 5). The compound of metallic, inorganic, and biomaterials effectively combine the advantages of different types and can yield further synergistic effects. He et al. (2018) achieved a maximum adsorption capacity of 288.96 mg/g for yttrium-based GO/SA hydrogels prepared by sol–gel. Wei et al. (2022) mixed reed biomass powder with SA, cross-linked with CeCl3 solution, and then calcined to obtain cerium alginate biochar beads. The composite adsorbent RBM-Ce has greatly improved the maximum adsorption capacity, SBET, pHPZC, and stability compared to individual components.
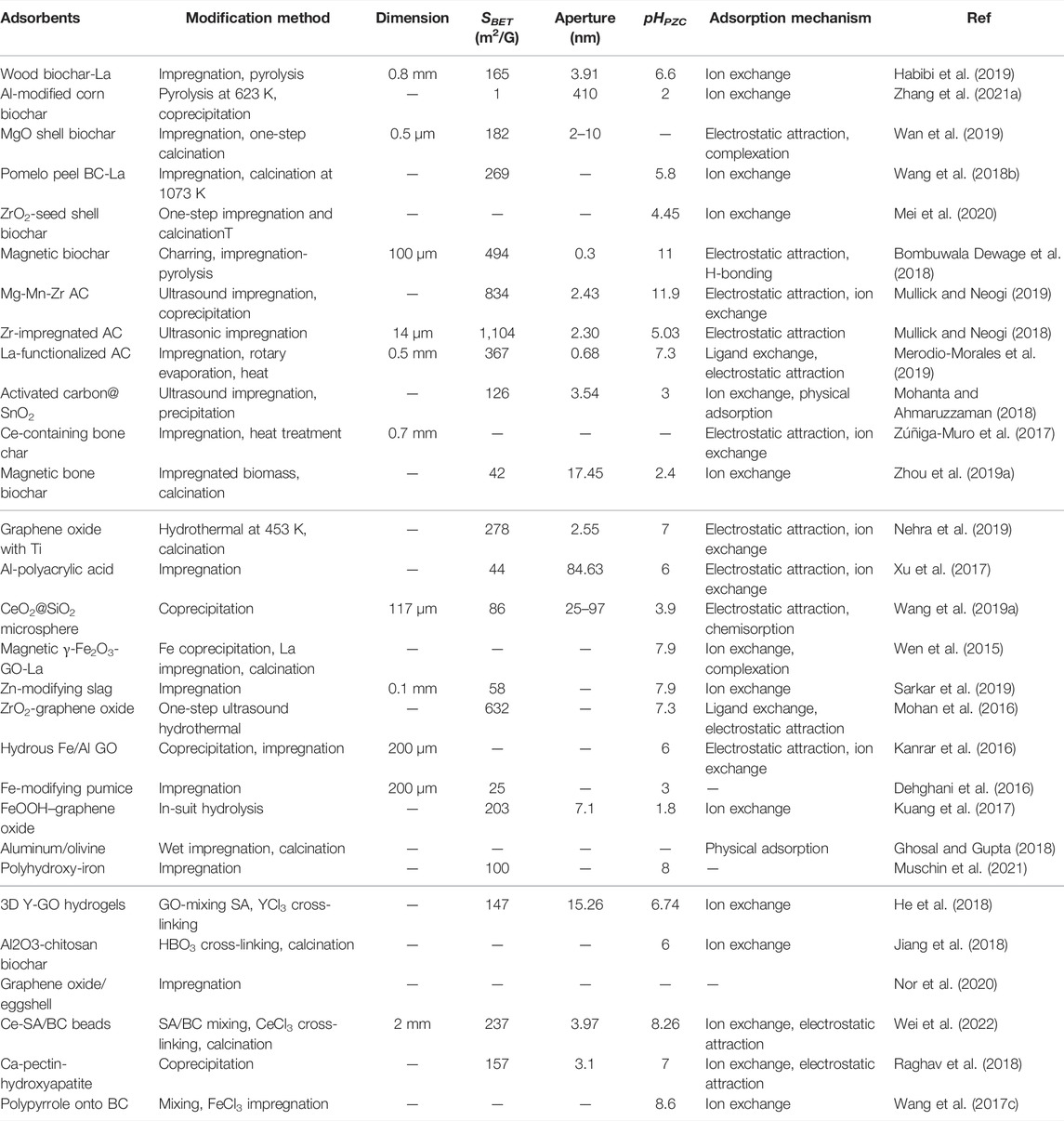
TABLE 5. Summary of modification methods, characteristics, and adsorption mechanisms of metal-modified carbon and other adsorbents.
In summary of the aforementioned composite adsorbent synthesis methods (Tables 4, 5), it can be seen that the general principle of metal-modifying adsorbents is to coat the opposing surface with metal salts. The main methods of modification are chemical coprecipitation, impregnation, and hydrothermal methods, which are described here.
Chemical coprecipitation is a common method for the preparation of multi-metals (Table 4) and metal-modified inorganic adsorbents (Table 5). In the preparation of multi-metal nanoparticles, the chemical coprecipitation method first mixes each metal salt solution in proportion to the atoms of the target product to be prepared to form an aqueous solution of the metal ions. The metal ions are then simultaneously precipitated out of the solution by the addition of appropriate precipitants to form hydroxide precipitate (Wu et al., 2013). The precipitates are separated out and then dried or calcined to obtain powdered multi-metal hydroxide/oxide nanoparticle adsorbents (Zhou et al., 2018). When modifying inorganic adsorbents with metals, the inorganic material is first put into a metal salt solution via methods such as adjusting the pH of the solution; the metal ions in the solution are induced to produce nano-metal particles that precipitate and load onto the surface and pore paths of the inorganic material, which are then dried or calcined to form composite adsorbents (Figure 6) (Wang et al., 2019b). The coprecipitation method is simple and not time-consuming. However, due to the different precipitation rates of different elements, there is sometimes a stratification of the precipitation, which makes the precipitate not uniformly dispersed and the composition of the product somewhat biased.
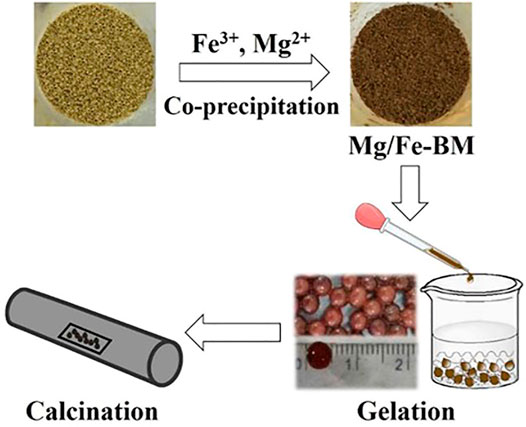
FIGURE 6. Coprecipitation method for preparation of MgFe2O4-doped biochar and ionic cross-linking process of composite sols (Wang et al., 2020).
The hydrothermal method is also commonly used for the preparation of multi-metals (Table 4), and some studies have also been used for the modification of other materials by metals. Similarly, the metal ions are first prepared in a mixed solution in a certain proportion and then placed in a hydrothermal reactor at 423–573 K for a specific time. The principle of the hydrothermal method is that in a closed reaction environment, the precursor undergoes high temperature and pressure to fully dissolve in the solvent (Nehra et al., 2019). Then hydrolysis and nucleation according to a certain crystallization mode to grow nano-microcrystalline particles, to obtain a uniform particle size and good dispersion of composite powder (Figure 7). The nanomaterials prepared by the hydrothermal method have homogeneous morphology and the products are well dispersed. However, high pressure and temperature-resistant instrumentation are required, with long reaction times and production cycles, which are not conducive to mass production. Thus, it is often used in the laboratory to prepare nanomaterials with special morphologies for research. By controlling the crystallization time, crystallization temperature, and other factors, nanopowders with different morphologies can be prepared.
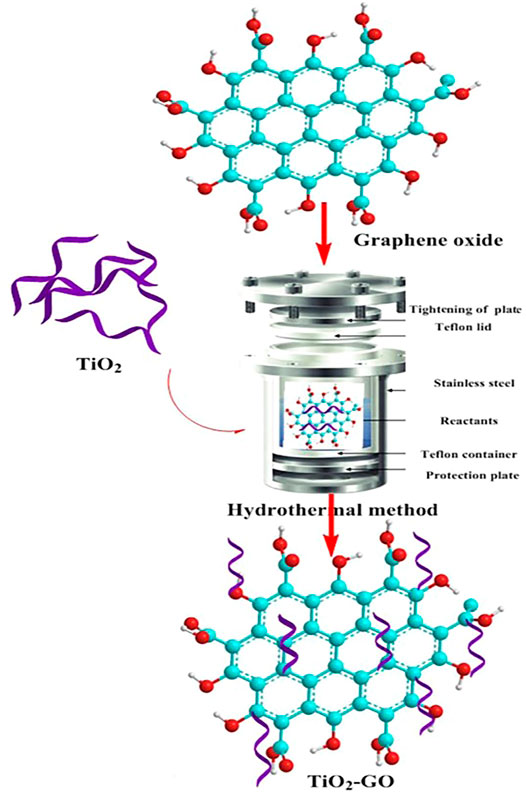
FIGURE 7. Hydrothermal for the preparation of TiO2-modified graphite (Nehra et al., 2019).
Impregnation is commonly used for metal-modified inorganic materials (carbon, clay, GO, etc. Table 5). The powdered inorganic material is first pre-treated by immersion in a metal salt solution (AlCl3, CaCl2, FeCl3, LaCl3, etc.). The metal ions in the solution can be loaded on an inorganic surface or internally via auxiliary heating and ultrasonic dispersion. The composite adsorbent is then dried or calcined (Figure 8). The impregnation preparation method is also facile but slightly more time-consuming, relying on the specific surface area of the inorganic material and bonding of the active sites on the surface to the metal.
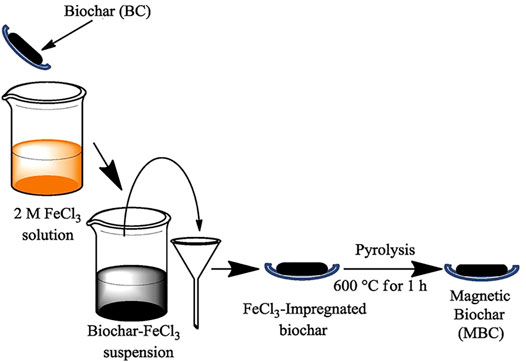
FIGURE 8. Preparation of magnetic biochar by impregnation and subsequent calcination (Bombuwala Dewage et al., 2018).
Some preparations are calcined after coprecipitation, impregnation, or hydrothermal treatment (Figures 6–8). For example, metal-modified biochar is calcined to form metal oxide nanoparticles on the surface of BC, which further enhances the adsorption capacity (Table 5). LDH is also sometimes calcined (Table 4). During heating, LDH can be transformed into mixed metal oxides as the interlayer anions are eliminated by thermal decomposition. After the adsorbent is put into a fluoride solution, it will undergo a rehydration process. During rehydration, these oxides are in turn rebuilt into original layered structures by adsorbing various anions from the aqueous solution, known as the “memory effectˮ (Wu P. et al., 2017). The specific surface area and anion exchange capacity of LDH increases further after calcination. After coprecipitation or impregnation, the modification of biopolymers is generally achieved by the sol–gel method for the preparation of hydrogels (Wang A. et al., 2017).
The adsorption mechanism can be divided into physical adsorption and chemisorption. Physical adsorption is generally considered to be caused by van der Waals forces, which are nonselective and reversible, and can be desorbed under certain conditions. Current research on the physical adsorption of fluoride ions is mainly based on electrostatic attraction and hydrogen bonding. Chemisorption is mainly the formation of chemical bonds between molecules and is described by the Langmuir model; the adsorption is selective and irreversible and desorption is more difficult. Physical adsorption depends mainly on the active pore volume and specific surface area (Wang H. et al., 2017), while chemisorption depends more on chemical or electro-affinity. The fluoride adsorption mechanism by various metal-modified adsorbents is summarized in Tables 4, 5. A total of four main adsorption mechanisms can be found: electrostatic attraction, ion exchange, hydrogen bonding, and complexation. The actual adsorption process is usually accompanied by several mechanisms (Figure 9).
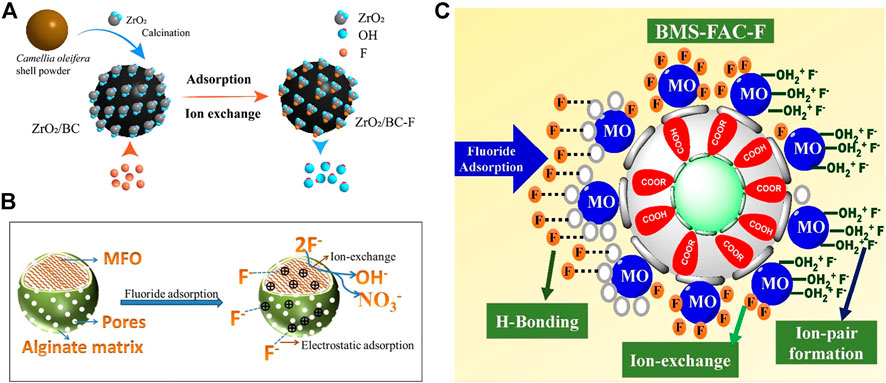
FIGURE 9. Respective adsorption mechanisms of composite adsorbents from different metal-modified materials. (A): ion exchange (Mei et al., 2020), (B): electrostatic attraction (Wu T. et al., 2017), (C): ion pair (Raghav et al., 2019).
When metal oxide enters the aqueous solution, the hydrogen ions are attracted to lone pair electrons of the oxygen element in metal oxide, forming a hydroxyl ligand (Zhang and Jia 2016). The fluoride removal by metal oxides and metal-modified composite adsorbents exploits the large number of hydroxyl groups on the surface (Figure 9A). When the solution pH is less than zero charge point (pHPZC) of the composite adsorbent, hydroxyl functional groups become protonated, forming OH2+ and are positively charged. The positive charge surface attracts negatively charged fluoride ions by electrostatic attraction (Figure 9B).
Fluoride is attracted to the surface of composite adsorbents for immobilization, but ion pairs (Figure 9C) are weakly interacting with each other and easily desorbed. Several studies have confirmed the involvement of hydroxyl groups in the adsorption reaction by FTIR and XPS characterization. F− has the same charge and similar radius composition as OH− and can replace OH− in the structure of composite adsorbents (Figure 9A). F− is bonded to a metal-occupying active site, OH− is released, and the solution pH rises after adsorption. Most studies have been based on the anion exchange mechanism. As pH rises above the pHPZC of the adsorbent, there is no significant decrease in adsorption. indicating that adsorption is mainly controlled by ion exchange. Generally, when the solution pH > 10, the large amount of free OH− in the solution competes with F−, resulting in a significant decrease in adsorption capacity. Complexation between metals and fluoride has also been suggested (Suzuki et al., 2013).
Metal-modified composite adsorbents often have polar functional groups containing hydrogen, such as -OH, -COOH, and -NH2 (Yang et al., 2017). The shared electron pairs of polar functional groups are strongly biased toward oxygen or nitrogen, leaving the hydrogen atom almost naked. The lone pair electrons of electronegative fluoride will interact with the hydrogen atom forming a hydrogen bond with a bond angle of 180° and immobilize (Figure 9C).
Comparison of Table 2 with Tables 3, 6 reveals an overall increase in fluoride adsorption capacity of metal-modified composites. It indicates that most of the modifications are successful with application prospects. However, there are still many issues that need to be considered to achieve a big breakthrough in practical applications. The multi-metals enrich active sites for fluoride, but agglomeration and easy leaching are still problems, and individual preparation still requires some cost. Metal-modified biopolymers improve the stability of hydrogels, and metals can also be dispersed and immobilized in the macromolecular structure. However, it is reported that the dense surface of hydrogel makes it difficult for fluoride ions to enter the internal pores of beads, and beads sink easily so they have a limited contact area with fluoride. Metal-modified carbon, mineral clay, and other inorganic materials can also improve the dispersion and immobilization of metals to some extent, but there are still problems of dissolution, and loaded metals are easily dislodged and poorly recycled. Low-cost inorganic materials balance the price of rare earth metals and reduce the amount of metals, but at the same time, present the safety risk of waste use. The metal and inorganic materials are both in powder form, and the issue of separation and recycling has not been addressed. Studies combining metals, inorganic materials, and biopolymers appear to address the agglomeration and immobilization of metals, expanding the pore space and fluoride contact area of hydrogel beads, while improving the separation and recovery properties of inorganic materials. However, more than 90% of studies mentioned in this review avoided exploring metal dissolution concentrations and less than 10% of adsorbents were able to achieve more than 80% fluoride adsorption at the fifth cycle. Future studies will need to pay attention to the simplicity, efficiency, and cost of preparation procedure. Overall, the search for future defluoridation adsorbents is not limited to the requirement for increasing adsorption capacity. More important is the attention to cost levels, regeneration performance, separation and recovery, and safety issues for practical applications.
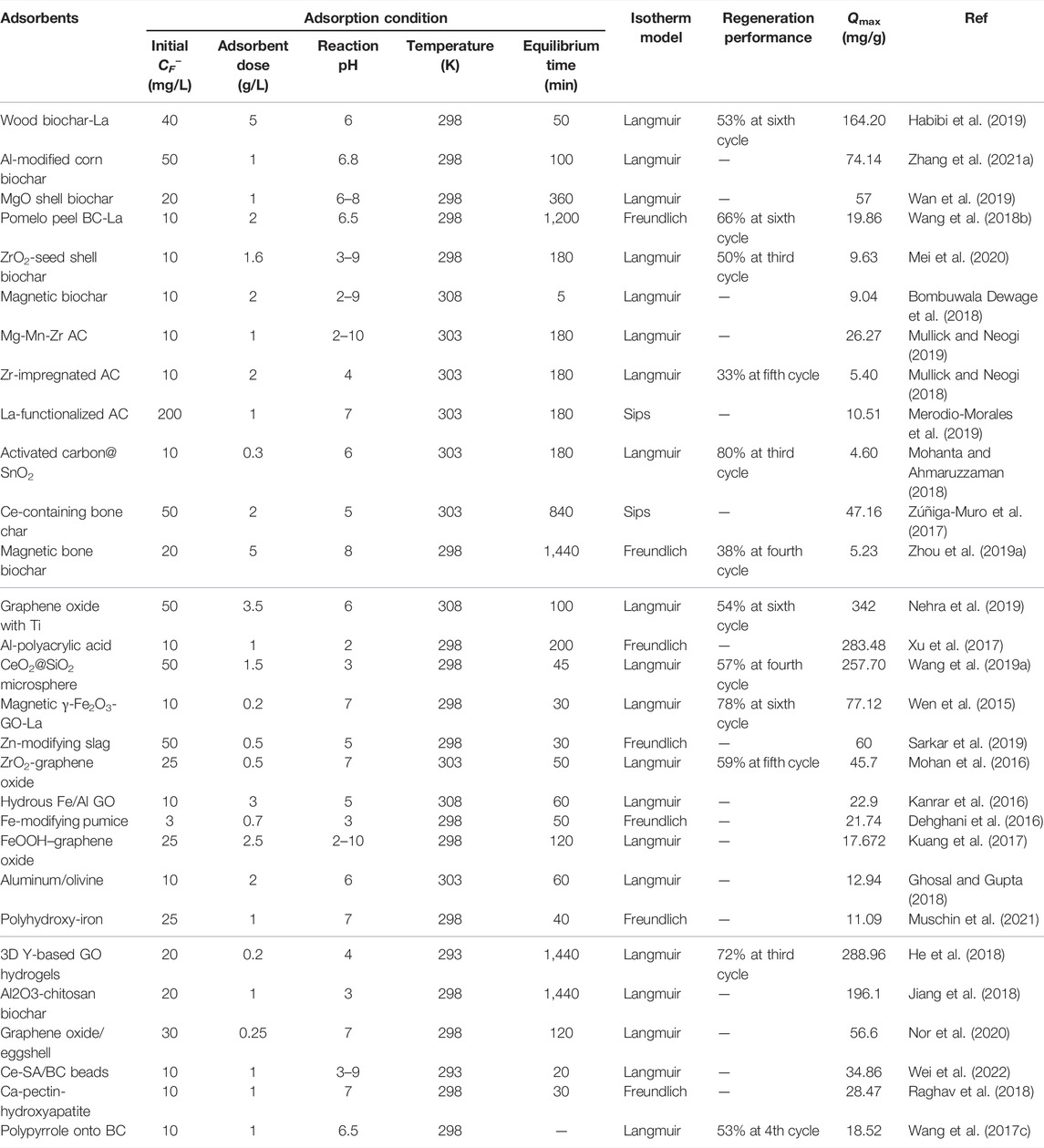
TABLE 6. Adsorption conditions and performance of fluoride by metal-modified carbon and other adsorbents.
YW summarized and wrote the article under the guidance of LW. All authors contributed to conceptualizing, editing, commenting on, and reviewing the manuscript.
This work is supported by the National Natural Science Foundation of China (51908457).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors thank Zijun Ren from Xi’an Jiaotong University for assistance with SEM analysis.
Abo Markeb, A., Alonso, A., Sánchez, A., and Font, X. (2017). Adsorption Process of Fluoride from Drinking Water with Magnetic Core-Shell Ce-Ti@Fe 3 O 4 and Ce-Ti Oxide Nanoparticles. Sci. Total Environ. 598, 949–958. doi:10.1016/j.scitotenv.2017.04.191
Angelin, A., Kalpana, M., Govindan, K., and Kavitha, S. (2021). Characterizations and Fluoride Adsorption Performance of Wattle Humus Biosorbent. Environ. Sci. Pollut. Res. Int. doi:10.1007/s11356-021-14864-9
Araga, R., Kali, S., and Sharma, C. S. (2019). Coconut‐Shell‐Derived Carbon/Carbon Nanotube Composite for Fluoride Adsorption from Aqueous Solution. CLEAN. – Soil Air Water 47 (5). doi:10.1002/clen.201800286
Araga, R., and Sharma, C. S. (2019). Amine Functionalized Electrospun Cellulose Nanofibers for Fluoride Adsorption from Drinking Water. J. Polym. Environ. 27 (4), 816–826. doi:10.1007/s10924-019-01394-2
Araga, R., Soni, S., and Sharma, C. S. (2017). Fluoride Adsorption from Aqueous Solution Using Activated Carbon Obtained from KOH-Treated Jamun (Syzygium Cumini) Seed. J. Environ. Chem. Eng. 5 (6), 5608–5616. doi:10.1016/j.jece.2017.10.023
Asadi, A., Sharafi, K., Mohammadi, H., and Pirsaheb, M. (2018). Fluoride and Nitrate Adsorption from Water by Fe(III)-doped Scoria: Optimizing Using Response Surface Modeling, Kinetic and Equilibrium Study. Water Supply 18 (3), 1117–1132.
Azari, A., Kalantary, R. R., Ghanizadeh, G., Kakavandi, B., Farzadkia, M., and Ahmadi, E. (2015). Iron-silver Oxide Nanoadsorbent Synthesized by Co-precipitation Process for Fluoride Removal from Aqueous Solution and its Adsorption Mechanism. RSC Adv. 5 (106), 87377–87391. doi:10.1039/c5ra17595j
Bhatnagar, A., Kumar, E., and Sillanpää, M. (2011). Fluoride Removal from Water by Adsorption-A Review. Chem. Eng. J. 171 (3), 811–840. doi:10.1016/j.cej.2011.05.028
Bhomick, P. C., Supong, A., Karmaker, R., Baruah, M., Pongener, C., and Sinha, D. (2019). Activated Carbon Synthesized from Biomass Material Using Single-step KOH Activation for Adsorption of Fluoride: Experimental and Theoretical Investigation. Korean J. Chem. Eng. 36 (4), 551–562. doi:10.1007/s11814-019-0234-x
Biswas, G., Kumari, M., Adhikari, K., and Dutta, S. (2017). A Critical Review on Occurrence of Fluoride and its Removal through Adsorption with an Emphasis on Natural Minerals. Curr. Pollut. Rep 3 (2), 104–119. doi:10.1007/s40726-017-0054-8
Bombuwala Dewage, N., Liyanage, A. S., Pittman, C. U., Mohan, D., and Mlsna, T. (2018). Fast Nitrate and Fluoride Adsorption and Magnetic Separation from Water on α-Fe2O3 and Fe3O4 Dispersed on Douglas Fir Biochar. Bioresour. Technology 263, 258–265. doi:10.1016/j.biortech.2018.05.001
Brunson, L. R., and Sabatini, D. A. (2015). Role of Surface Area and Surface Chemistry during an Investigation of Eucalyptus Wood Char for Fluoride Adsorption from Drinking Water. J. Environ. Eng. 141 (2). doi:10.1061/(asce)ee.1943-7870.0000891
Cai, P., Zhao, J., Zhang, X., Zhang, T., Yin, G., Chen, S., et al. (2022). Synergy between Cobalt and Nickel on NiCo2O4 Nanosheets Promotes Peroxymonosulfate Activation for Efficient Norfloxacin Degradation. Appl. Catal. B: Environ. 306. doi:10.1016/j.apcatb.2022.121091
Chaudhary, M., Jain, N., and Maiti, A. (2021). A Comparative Adsorption Kinetic Modeling of Fluoride Adsorption by Nanoparticles and its Polymeric Nanocomposite. J. Environ. Chem. Eng. 9 (5). doi:10.1016/j.jece.2021.105595
Chen, C.-L., Park, S.-W., Su, J. F., Yu, Y.-H., Heo, J.-e., Kim, K.-d., et al. (2019). The Adsorption Characteristics of Fluoride on Commercial Activated Carbon Treated with Quaternary Ammonium Salts (Quats). Sci. Total Environ. 693, 133605. doi:10.1016/j.scitotenv.2019.133605
Chen, J., Shu, C., Wang, N., Feng, J., Ma, H., and Yan, W. (2017). Adsorbent Synthesis of polypyrrole/TiO2 for Effective Fluoride Removal from Aqueous Solution for Drinking Water Purification: Adsorbent Characterization and Adsorption Mechanism. J. Colloid Interf. Sci. 495, 44–52. doi:10.1016/j.jcis.2017.01.084
Chen, P., Wang, T., Xiao, Y., Tian, E., Wang, W., Zhao, Y., et al. (2018). Efficient Fluoride Removal from Aqueous Solution by Synthetic Fe Mg La Tri-metal Nanocomposite and the Analysis of its Adsorption Mechanism. J. Alloys Compounds 738, 118–129. doi:10.1016/j.jallcom.2017.12.142
Cheng, L., Guan, Z., Si, L., Mang, C., Weng, X., Zhang, Q., et al. (2018). Fluoride Ion Adsorption from Wastewater Using Magnesium(II), Aluminum(III) and Titanium(IV) Modified Natural Zeolite: Kinetics, Thermodynamics, and Mechanistic Aspects of Adsorption. J. Water Reuse Desalination 8 (4), 479–489.
Chinnakoti, P., Chunduri, A. L. A., Vankayala, R. K., Patnaik, S., and Kamisetti, V. (2016a). Enhanced Fluoride Adsorption by Nano Crystalline γ-alumina: Adsorption Kinetics, Isotherm Modeling and Thermodynamic Studies. Appl. Water Sci. 7 (5), 2413–2423. doi:10.1007/s13201-016-0437-9
Chinnakoti, P., Vankayala, R. K., Chunduri, A. L. A., Nagappagari, L. R., Muthukonda, S. V., and Kamisetti, V. (2016b). Trititanate Nanotubes as Highly Efficient Adsorbent for Fluoride Removal from Water: Adsorption Performance and Uptake Mechanism. J. Environ. Chem. Eng. 4 (4), 4754–4768. doi:10.1016/j.jece.2016.11.007
Collivignarelli, M. C., Abbà, A., Carnevale Miino, M., Torretta, V., Rada, E. C., Caccamo, F. M., et al. (2020). Adsorption of Fluorides in Drinking Water by Palm Residues. Sustainability 12 (9). doi:10.3390/su12093786
Dehghani, M. H., Faraji, M., Mohammadi, A., and Kamani, H. (2016). Optimization of Fluoride Adsorption onto Natural and Modified Pumice Using Response Surface Methodology: Isotherm, Kinetic and Thermodynamic Studies. Korean J. Chem. Eng. 34 (2), 454–462. doi:10.1007/s11814-016-0274-4
Dehghani, M. H., Farhang, M., Alimohammadi, M., Afsharnia, M., and McKay, G. (2018). Adsorptive Removal of Fluoride from Water by Activated Carbon Derived from CaCl2-Modified Crocus Sativus Leaves: Equilibrium Adsorption Isotherms, Optimization, and Influence of Anions. Chem. Eng. Commun. 205 (7), 955–965. doi:10.1080/00986445.2018.1423969
Dhawane, S. H., Khan, A. A., Singh, K., Tripathi, A., Hasda, R., and Halder, G. (2018). Insight into Optimization, Isotherm, Kinetics, and Thermodynamics of Fluoride Adsorption onto Activated Alumina. Environ. Prog. Sustainable Energ. 37 (2), 766–776. doi:10.1002/ep.12814
Dhillon, A., Kumar Sharma, T., Soni, S. K., and Kumar, D. (2016). Fluoride Adsorption on a Cubical Ceria Nanoadsorbent: Function of Surface Properties. RSC Adv. 6 (92), 89198–89209. doi:10.1039/c6ra16962g
Dhillon, A., Soni, S. K., and Kumar, D. (2017). Enhanced Fluoride Removal Performance by Ce-Zn Binary Metal Oxide: Adsorption Characteristics and Mechanism. J. Fluorine Chem. 199, 67–76. doi:10.1016/j.jfluchem.2017.05.002
Dong, S., and Wang, Y. (2016). Characterization and Adsorption Properties of a Lanthanum-Loaded Magnetic Cationic Hydrogel Composite for Fluoride Removal. Water Res. 88, 852–860. doi:10.1016/j.watres.2015.11.013
Fan, Z., Gao, Y., Ning, X., Pan, F., and Ming, J. (2019). Adsorption of Fluoride Ions from Water by SF/PP Nonwoven Fabrics. Fibers Polym. 20 (4), 863–867. doi:10.1007/s12221-019-1085-0
Gao, M., Wang, W., Yang, H., and Ye, B.-C. (2019). Hydrothermal Synthesis of Hierarchical Hollow Hydroxyapatite Microspheres with Excellent Fluoride Adsorption Property. Microporous Mesoporous Mater. 289. doi:10.1016/j.micromeso.2019.109620
García-Sánchez, J. J., Solache-Ríos, M., Martínez-Gutiérrez, J. M., Arteaga-Larios, N. V., Ojeda-Escamilla, M. C., and Rodríguez-Torres, I. (2016). Modified Natural Magnetite with Al and La Ions for the Adsorption of Fluoride Ions from Aqueous Solutions. J. Fluorine Chem. 186, 115–124.
Ghosal, P. S., and Gupta, A. K. (2018). Thermodynamics of Fluoride Adsorption on Aluminum/Olivine Composite (AOC): Influence of Temperature on Isotherm, Kinetics, and Adsorption Mechanism. Water Air Soil Pollut. 229 (11). doi:10.1007/s11270-018-4003-y
Goswami, R., and Kumar, M. (2018). Removal of Fluoride from Aqueous Solution Using Nanoscale rice Husk Biochar. Groundwater Sustainable Development 7, 446–451. doi:10.1016/j.gsd.2017.12.010
Guiza, S., Hajji, H., and Bagane, M. (2019). External Mass Transport Process during the Adsorption of Fluoride from Aqueous Solution by Activated clay. Comptes Rendus Chim. 22 (2-3), 161–168. doi:10.1016/j.crci.2019.02.001
Habibi, N., Rouhi, P., and Ramavandi, B. (2019). Modification of Tamarix Hispida Biochar by Lanthanum Chloride for Enhanced Fluoride Adsorption from Synthetic and Real Wastewater. Environ. Prog. Sustainable Energ. 38 (s1), S298–S305.
Hafshejani, L. D., Tangsir, S., Daneshvar, E., Maljanen, M., Lähde, A., Jokiniemi, J., et al. (2017). Optimization of Fluoride Removal from Aqueous Solution by Al 2 O 3 Nanoparticles. J. Mol. Liquids 238, 254–262. doi:10.1016/j.molliq.2017.04.104
Han, M., Zhang, J., Hu, Y., and Han, R. (2019). Preparation of Novel Magnetic Microspheres with the La and Ce-Bimetal Oxide Shell for Excellent Adsorption of Fluoride and Phosphate from Solution. J. Chem. Eng. Data 64 (8), 3641–3651. doi:10.1021/acs.jced.9b00434
He, J., Cui, A., Ni, F., Deng, S., Shen, F., and Yang, G. (2018). A Novel 3D Yttrium Based-Graphene Oxide-Sodium Alginate Hydrogel for Remarkable Adsorption of Fluoride from Water. J. Colloid Interf. Sci. 531, 37–46. doi:10.1016/j.jcis.2018.07.017
He, J., Yang, Y., Wu, Z., Xie, C., Zhang, K., Kong, L., et al. (2020). Review of Fluoride Removal from Water Environment by Adsorption. J. Environ. Chem. Eng. 8 (6). doi:10.1016/j.jece.2020.104516
He, Y., Zhang, L., An, X., Wan, G., Zhu, W., and Luo, Y. (2019). Enhanced Fluoride Removal from Water by Rare Earth (La and Ce) Modified Alumina: Adsorption Isotherms, Kinetics, Thermodynamics and Mechanism. Sci. Total Environ. 688, 184–198. doi:10.1016/j.scitotenv.2019.06.175
Herath, H. M. A. S., Kawakami, T., and Tafu, M. (2018). The Extremely High Adsorption Capacity of Fluoride by Chicken Bone Char (CBC) in Defluoridation of Drinking Water in Relation to its Finer Particle Size for Better Human Health. Healthcare (Basel) 6 (4). doi:10.3390/healthcare6040123
Hongtao, L., Shuxia, L., Hua, Z., Yanling, Q., Daqiang, Y., Jianfu, Z., et al. (2018). Comparative Study on Synchronous Adsorption of Arsenate and Fluoride in Aqueous Solution onto MgAlFe-LDHs with Different Intercalating Anions. RSC Adv. 8 (58), 33301–33313. doi:10.1039/c8ra05968c
Hu, H., Yang, L., Lin, Z., Xiang, X., Jiang, X., and Hou, L. (2018). Preparation and Characterization of Novel Magnetic Fe3O4/chitosan/Al(OH)3 Beads and its Adsorption for Fluoride. Int. J. Biol. Macromolecules 114, 256–262. doi:10.1016/j.ijbiomac.2018.03.094
Huo, Y., Ding, W., Huang, X., Xu, J., and Zhao, M. (2011). Fluoride Removal by Lanthanum Alginate Bead: Adsorbent Characterization and Adsorption Mechanism. Chin. J. Chem. Eng. 19 (3), 365–370. doi:10.1016/s1004-9541(09)60222-6
Jadhav, A. S., and Jadhav, M. V. (2021). Utilization of Black Mustard Husk Ash for Adsorption of Fluoride from Water. Korean J. Chem. Eng. 38 (10), 2082–2090. doi:10.1007/s11814-021-0913-2
Jayarathna, L., Bandara, A., Ng, W. J., and Weerasooriya, R. (2015). Fluoride Adsorption on γ − Fe2O3 Nanoparticles. J. Environ. Health Sci. Engineer 13, 54. doi:10.1186/s40201-015-0210-2
Jeyaseelan, A., Katubi, K. M. M., Alsaiari, N. S., Naushad, M., and Viswanathan, N. (2021). Design and Fabrication of Sulfonic Acid Functionalized Graphene Oxide for Enriched Fluoride Adsorption. Diamond Relat. Mater. 117. doi:10.1016/j.diamond.2021.108446
Jia, Z., Hao, S., and Lu, X. (2018). Exfoliated Mg-Al-Fe Layered Double Hydroxides/polyether Sulfone Mixed Matrix Membranes for Adsorption of Phosphate and Fluoride from Aqueous Solutions. J. Environ. Sci. 70, 63–73. doi:10.1016/j.jes.2017.11.012
Jiang, X., Xiang, X., Hu, H., Meng, X., and Hou, L. (2018). Facile Fabrication of Biochar/Al2O3 Adsorbent and its Application for Fluoride Removal from Aqueous Solution. J. Chem. Eng. Data 64 (1), 83–89. doi:10.1021/acs.jced.8b00556
Jin, Z., Jia, Y., Zhang, K.-S., Kong, L.-T., Sun, B., Shen, W., et al. (2016). Effective Removal of Fluoride by Porous MgO Nanoplates and its Adsorption Mechanism. J. Alloys Compounds 675, 292–300. doi:10.1016/j.jallcom.2016.03.118
Kang, D., Yu, X., Ge, M., Lin, M., Yang, X., and Jing, Y. (2018). Insights into Adsorption Mechanism for Fluoride on Cactus-like Amorphous Alumina Oxide Microspheres. Chem. Eng. J. 345, 252–259. doi:10.1016/j.cej.2018.03.174
Kang, D., Yu, X., and Ge, M. (2017). Morphology-dependent Properties and Adsorption Performance of CeO2 for Fluoride Removal. Chem. Eng. J. 330, 36–43. doi:10.1016/j.cej.2017.07.140
Kanrar, S., Debnath, S., De, P., Parashar, K., Pillay, K., Sasikumar, P., et al. (2016). Preparation, Characterization and Evaluation of Fluoride Adsorption Efficiency from Water of Iron-Aluminium Oxide-Graphene Oxide Composite Material. Chem. Eng. J. 306, 269–279. doi:10.1016/j.cej.2016.07.037
Kuang, L., Liu, Y., Fu, D., and Zhao, Y. (2017). FeOOH-graphene Oxide Nanocomposites for Fluoride Removal from Water: Acetate Mediated Nano FeOOH Growth and Adsorption Mechanism. J. Colloid Interf. Sci. 490, 259–269. doi:10.1016/j.jcis.2016.11.071
Kullgren, J., Wolf, M. J., Castleton, C. W. M., Mitev, P., Briels, W. J., and Hermansson, K. (2014). Oxygen Vacancies versus Fluorine atCeO2(111): A Case of Mistaken Identity? Phys. Rev. Lett. 112 (15), 156102. doi:10.1103/physrevlett.112.156102
Kumar, P., Prajapati, A. K., Dixit, S., and Yadav, V. L. (2020). Adsorption of Fluoride from Aqueous Solution Using Biochar Prepared from Waste Peanut hull. Mater. Res. Express 6 (12). doi:10.1088/2053-1591/ab6ca0
Lanas, S. G., Valiente, M., Aneggi, E., Trovarelli, A., Tolazzi, M., and Melchior, A. (2016). Efficient Fluoride Adsorption by Mesoporous Hierarchical Alumina Microspheres. RSC Adv. 6 (48), 42288–42296. doi:10.1039/c5ra27371d
Lee, J.-I., Kang, J.-K., Hong, S.-H., Lee, C.-G., Jeong, S., and Park, S.-J. (2021). Thermally Treated Mytilus coruscus Shells for Fluoride Removal and Their Adsorption Mechanism. Chemosphere 263, 128328. doi:10.1016/j.chemosphere.2020.128328
Lee, S. G., Ha, J.-W., Sohn, E.-H., Park, I. J., and Lee, S.-B. (2017). Synthesis of Pillar and Microsphere-like Magnesium Oxide Particles and Their Fluoride Adsorption Performance in Aqueous Solutions. Korean J. Chem. Eng. 34 (10), 2738–2747. doi:10.1007/s11814-017-0160-8
Li, Y., Zhang, C., Jiang, Y., and Wang, T.-J. (2018). Electrically Enhanced Adsorption and green Regeneration for Fluoride Removal Using Ti(OH)4-loaded Activated Carbon Electrodes. Chemosphere 200, 554–560. doi:10.1016/j.chemosphere.2018.02.112
Liang, P., An, R., Li, R., and Wang, D. (2018). Comparison of La3+ and Mixed Rare Earths-Loaded Magnetic Chitosan Beads for Fluoride Adsorption. Int. J. Biol. Macromolecules 111, 255–263. doi:10.1016/j.ijbiomac.2017.12.151
Lin, J.-Y., Chen, Y.-L., Hong, X.-Y., Huang, C., and Huang, C. P. (2020). The Role of Fluoroaluminate Complexes on the Adsorption of Fluoride onto Hydrous Alumina in Aqueous Solutions. J. Colloid Interf. Sci. 561, 275–286. doi:10.1016/j.jcis.2019.10.085
Lin, K.-Y. A., Liu, Y.-T., and Chen, S.-Y. (2016). Adsorption of Fluoride to UiO-66-NH 2 in Water: Stability, Kinetic, Isotherm and Thermodynamic Studies. J. Colloid Interf. Sci. 461, 79–87. doi:10.1016/j.jcis.2015.08.061
Medellin-Castillo, N. A., Leyva-Ramos, R., Padilla-Ortega, E., Perez, R. O., Flores-Cano, J. V., and Berber-Mendoza, M. S. (2014). Adsorption Capacity of Bone Char for Removing Fluoride from Water Solution. Role of Hydroxyapatite Content, Adsorption Mechanism and Competing anionsRole of Hydroxyapatite Content, Adsorption Mechanism and Competing Anions. J. Ind. Eng. Chem. 20 (6), 4014–4021. doi:10.1016/j.jiec.2013.12.105
Mei, L., Qiao, H., Ke, F., Peng, C., Hou, R., Wan, X., et al. (2020). One-step Synthesis of Zirconium Dioxide-Biochar Derived from Camellia Oleifera Seed Shell with Enhanced Removal Capacity for Fluoride from Water. Appl. Surf. Sci. 509. doi:10.1016/j.apsusc.2019.144685
Merodio-Morales, E. E., Reynel-Ávila, H. E., Mendoza-Castillo, D. I., Duran-Valle, C. J., and Bonilla-Petriciolet, A. (2019). Lanthanum- and Cerium-Based Functionalization of Chars and Activated Carbons for the Adsorption of Fluoride and Arsenic Ions. Int. J. Environ. Sci. Technol. 17 (1), 115–128. doi:10.1007/s13762-019-02437-w
Mobarak, M., Selim, A. Q., Mohamed, E. A., and Seliem, M. K. (2018). Modification of Organic Matter-Rich clay by a Solution of Cationic surfactant/H2O2: A New Product for Fluoride Adsorption from Solutions. J. Clean. Prod. 192, 712–721. doi:10.1016/j.jclepro.2018.05.044
Mohan, S., Kumar, V., Singh, D. K., and Hasan, S. H. (2016). Synthesis and Characterization of rGO/ZrO2 Nanocomposite for Enhanced Removal of Fluoride from Water: Kinetics, Isotherm, and Thermodynamic Modeling and its Adsorption Mechanism. RSC Adv. 6 (90), 87523–87538. doi:10.1039/c6ra15460c
Mohan, S., Singh, D. K., Kumar, V., and Hasan, S. H. (2017). Effective Removal of Fluoride Ions by rGO/ZrO2 Nanocomposite from Aqueous Solution: Fixed Bed Column Adsorption Modelling and its Adsorption Mechanism. J. Fluorine Chem. 194, 40–50. doi:10.1016/j.jfluchem.2016.12.014
Mohanta, D., and Ahmaruzzaman, M. (2018). Bio-inspired Adsorption of Arsenite and Fluoride from Aqueous Solutions Using Activated carbon@SnO2 Nanocomposites: Isotherms, Kinetics, Thermodynamics, Cost Estimation and Regeneration Studies. J. Environ. Chem. Eng. 6 (1), 356–366. doi:10.1016/j.jece.2017.11.076
Mohseni-Bandpi, A., Kakavandi, B., Kalantary, R. R., Azari, A., and Keramati, A. (2015). Development of a Novel Magnetite-Chitosan Composite for the Removal of Fluoride from Drinking Water: Adsorption Modeling and Optimization. RSC Adv. 5 (89), 73279–73289. doi:10.1039/c5ra11294j
Mondal, N. K., Bhaumik, R., and Datta, J. K. (2016). Fluoride Adsorption by Calcium Carbonate, Activated Alumina and Activated Sugarcane Ash. Environ. Process. 3 (1), 195–216. doi:10.1007/s40710-016-0130-x
Mourabet, M., El Rhilassi, A., El Boujaady, H., Bennani-Ziatni, M., El Hamri, R., and Taitai, A. (2015). Removal of Fluoride from Aqueous Solution by Adsorption on Hydroxyapatite (HAp) Using Response Surface Methodology. J. Saudi Chem. Soc. 19 (6), 603–615. doi:10.1016/j.jscs.2012.03.003
Mudzielwana, R., Gitari, W. M., Akinyemi, S. A., and Msagati, T. A. M. (2017). Synthesis, Characterization, and Potential Application of Mn2+-Intercalated Bentonite in Fluoride Removal: Adsorption Modeling and Mechanism Evaluation. Appl. Water Sci. 7 (8), 4549–4561. doi:10.1007/s13201-017-0608-3
Mullick, A., and Neogi, S. (2018). Acoustic Cavitation Induced Synthesis of Zirconium Impregnated Activated Carbon for Effective Fluoride Scavenging from Water by Adsorption. Ultrason. Sonochem. 45, 65–77. doi:10.1016/j.ultsonch.2018.03.002
Mullick, A., and Neogi, S. (2019). Ultrasound Assisted Synthesis of Mg-Mn-Zr Impregnated Activated Carbon for Effective Fluoride Adsorption from Water. Ultrason. Sonochem. 50, 126–137. doi:10.1016/j.ultsonch.2018.09.010
Muschin, T., Zulchin, H., and Jia, M. (2021). Adsorption Behavior of Polyhydroxy‐Iron‐Modified Coal‐Bearing Kaolin for Fluoride Removal. ChemistrySelect 6 (13), 3075–3083. doi:10.1002/slct.202100226
Nabbou, N., Belhachemi, M., Boumelik, M., Merzougui, T., Lahcene, D., Harek, Y., et al. (2019). Removal of Fluoride from Groundwater Using Natural clay (Kaolinite): Optimization of Adsorption Conditions. Comptes Rendus Chim. 22 (2-3), 105–112. doi:10.1016/j.crci.2018.09.010
Naga Babu, A., Srinivasa Reddy, D., Suresh Kumar, G., Ravindhranath, K., and Krishna Mohan, G. V. (2020). Sequential Synergetic Sorption Analysis of Gracilaria Rhodophyta Biochar toward Aluminum and Fluoride: A Statistical Optimization Approach. Water Environ. Res. 92 (6), 880–898. doi:10.1002/wer.1283
Nehra, S., Nair, M., and Kumar, D. (2019). Hydrothermally Shape-Controlled Synthesis of TiO2/Graphene for Fluoride Adsorption Studies. J. Chem. Eng. Data 64 (12), 5373–5384. doi:10.1021/acs.jced.9b00591
Nijhawan, A., Butler, E. C., and Sabatini, D. A. (2020). Fluoride Adsorption on Porous Hydroxyapatite Ceramic Filters: A Study of Kinetics. Environ. Eng. Sci. 37 (6), 409–416. doi:10.1089/ees.2019.0392
Nor, N. M., Kamil, N. H. N., Mansor, A. I., and Maarof, H. I. (2020). Adsorption Analysis of Fluoride Removal Using Graphene Oxide/Eggshell Adsorbent. Indonesian J. Chem. 20 (3). doi:10.22146/ijc.43481
Pigatto, R. S., Franco, D. S. P., Netto, M. S., Carissimi, É., Oliveira, L. F. S., Jahn, S. L., et al. (2020). An Eco-Friendly and Low-Cost Strategy for Groundwater Defluorination: Adsorption of Fluoride onto Calcinated Sludge. J. Environ. Chem. Eng. 8 (6). doi:10.1016/j.jece.2020.104546
Prabhu, S. M., and Meenakshi, S. (2015). A Dendrimer-like Hyper Branched Chitosan Beads toward Fluoride Adsorption from Water. Int. J. Biol. Macromolecules 78, 280–286. doi:10.1016/j.ijbiomac.2015.04.002
Preethi, J., and Meenakshi, S. (2018). Fabrication of La3+ Impregnated Chitosan/β-Cyclodextrin Biopolymeric Materials for Effective Utilization of Chromate and Fluoride Adsorption in Single Systems. J. Chem. Eng. Data 63 (3), 723–731. doi:10.1021/acs.jced.7b00889
Qiusheng, Z., Xiaoyan, L., Jin, Q., Jing, W., and Xuegang, L. (2015). Porous Zirconium Alginate Beads Adsorbent for Fluoride Adsorption from Aqueous Solutions. RSC Adv. 5 (3), 2100–2112. doi:10.1039/c4ra12036a
Raghav, S., and Kumar, D. (2018). Adsorption Equilibrium, Kinetics, and Thermodynamic Studies of Fluoride Adsorbed by Tetrametallic Oxide Adsorbent. J. Chem. Eng. Data 63 (5), 1682–1697. doi:10.1021/acs.jced.8b00024
Raghav, S., and Kumar, D. (2019). Comparative Kinetics and Thermodynamic Studies of Fluoride Adsorption by Two Novel Synthesized Biopolymer Composites. Carbohydr. Polym. 203, 430–440. doi:10.1016/j.carbpol.2018.09.054
Raghav, S., Nehra, S., and Kumar, D. (2019). Biopolymer Scaffold of Pectin and Alginate for the Application of Health Hazardous Fluoride Removal Studies by Equilibrium Adsorption, Kinetics and Thermodynamics. J. Mol. Liquids 284, 203–214. doi:10.1016/j.molliq.2019.03.155
Raghav, S., Sapna, , and Kumar, D. (2018). Cubical-Shaped Rods of Pectin-Hydroxyapatite Composite for Adsorption Studies of Fluoride by Statistical Method and Adsorption Experiments. ACS Omega 3 (8), 9675–9688. doi:10.1021/acsomega.8b01330
Rathore, V. K., and Mondal, P. (2017). Competitive Adsorption of Arsenic and Fluoride onto Economically Prepared Aluminum Oxide/Hydroxide Nanoparticles: Multicomponent Isotherms and Spent Adsorbent Management. Ind. Eng. Chem. Res. 56 (28), 8081–8094. doi:10.1021/acs.iecr.7b01139
Rehman, M. A., Yusoff, I., and Alias, Y. (2015). Fluoride Adsorption by Doped and Un-doped Magnetic Ferrites CuCexFe2-xO4: Preparation, Characterization, Optimization and Modeling for Effectual Remediation Technologies. J. Hazard. Mater. 299, 316–324. doi:10.1016/j.jhazmat.2015.06.030
Sadeghi, A., Tajbakhsh, M., and Abri, A. (2019). Adsorption of Fluoride on a Chitosan-Based Magnetic Nanocomposite: Equilibrium and Kinetics Studies. Water Supply 19 (1), 40–51.
Sapna, S., Raghav, S., Nair, M., and Kumar, D. (2018). Trimetallic Oxide Entrapped in Alginate Polymeric Matrix Employed for Adsorption Studies of Fluoride. Surf. Inter. 13, 112–132. doi:10.1016/j.surfin.2018.08.005
Sarkar, C., Basu, J. K., and Samanta, A. N. (2019). Experimental and Kinetic Study of Fluoride Adsorption by Ni and Zn Modified LD Slag Based Geopolymer. Chem. Eng. Res. Des. 142, 165–175. doi:10.1016/j.cherd.2018.12.006
Sarkar, M., and Santra, D. (2015). Modeling Fluoride Adsorption on Cerium-Loaded Cellulose Bead—Response Surface Methodology, Equilibrium, and Kinetic Studies. Water Air Soil Pollut. 226 (3). doi:10.1007/s11270-015-2307-8
Shang, Y., Duan, X., Wang, S., Yue, Q., Gao, B., and Xu, X. (2022). Carbon-based Single Atom Catalyst: Synthesis, Characterization, DFT Calculations. Chin. Chem. Lett. 33 (2), 663–673. doi:10.1016/j.cclet.2021.07.050
Sharma, P., Sen, K., Thakur, P., Chauhan, M., and Chauhan, K. (2019). Spherically Shaped Pectin-G-Poly(amidoxime)-Fe Complex: A Promising Innovative Pathway to Tailor a New Material in High Amidoxime Functionalization for Fluoride Adsorption. Int. J. Biol. Macromolecules 140, 78–90. doi:10.1016/j.ijbiomac.2019.08.098
Singh, T. P., and Majumder, C. B. (2018). Batch and Column Performance of Fluoride Adsorption by Java Plum Seeds. J. Hazard. Tox. Radioactive Waste 22 (3). doi:10.1061/(asce)hz.2153-5515.0000394
Suzuki, T., Nakamura, A., Niinae, M., Nakata, H., Fujii, H., and Tasaka, Y. (2013). Immobilization of Fluoride in Artificially Contaminated Kaolinite by the Addition of Commercial-Grade Magnesium Oxide. Chem. Eng. J. 233, 176–184. doi:10.1016/j.cej.2013.08.042
Tomar, V., Prasad, S., and Kumar, D. (2014). Adsorptive Removal of Fluoride from Aqueous media Using Citrus Limonum (Lemon) Leaf. Microchemical J. 112, 97–103. doi:10.1016/j.microc.2013.09.010
Wan, S., Lin, J., Tao, W., Yang, Y., Li, Y., and He, F. (2019). Enhanced Fluoride Removal from Water by Nanoporous Biochar-Supported Magnesium Oxide. Ind. Eng. Chem. Res. 58 (23), 9988–9996. doi:10.1021/acs.iecr.9b01368
Wang, A., Zhou, K., Chen, W., Zhang, C., Liu, X., Chen, Q., et al. (2018a). Adsorption of Fluoride by the Calcium Alginate Embedded with Mg-Al-Ce Trimetal Oxides. Korean J. Chem. Eng. 35 (8), 1636–1641. doi:10.1007/s11814-018-0056-2
Wang, A., Zhou, K., Liu, X., Liu, F., Zhang, C., and Chen, Q. (2017a). Granular Tri-metal Oxide Adsorbent for Fluoride Uptake: Adsorption Kinetic and Equilibrium Studies. J. Colloid Interf. Sci. 505, 947–955. doi:10.1016/j.jcis.2017.06.074
Wang, F., Wang, K., Muhammad, Y., Wei, Y., Shao, L., and Wang, X. (2019a). Preparation of CeO2@SiO2 Microspheres by a Non-sintering Strategy for Highly Selective and Continuous Adsorption of Fluoride Ions from Wastewater. ACS Sustainable Chem. Eng. 7 (17), 14716–14726. doi:10.1021/acssuschemeng.9b02643
Wang, H., Feng, Q., Liu, K., Li, Z., Tang, X., and Li, G. (2017b). Highly Efficient Fluoride Adsorption from Aqueous Solution by Nepheline Prepared from Kaolinite through Alkali-Hydrothermal Process. J. Environ. Manage. 196, 72–79. doi:10.1016/j.jenvman.2017.03.015
Wang, J., Chen, N., Feng, C., and Li, M. (2018b). Performance and Mechanism of Fluoride Adsorption from Groundwater by Lanthanum-Modified Pomelo Peel Biochar. Environ. Sci. Pollut. Res. 25 (16), 15326–15335. doi:10.1007/s11356-018-1727-6
Wang, J., Chen, N., Li, M., and Feng, C. (2017c). Efficient Removal of Fluoride Using Polypyrrole-Modified Biochar Derived from Slow Pyrolysis of Pomelo Peel: Sorption Capacity and Mechanism. J. Polym. Environ. 26 (4), 1559–1572. doi:10.1007/s10924-017-1061-y
Wang, J., Wu, L., Li, J., Tang, D., and Zhang, G. (2018c). Simultaneous and Efficient Removal of Fluoride and Phosphate by Fe-La Composite: Adsorption Kinetics and Mechanism. J. Alloys Compounds 753, 422–432. doi:10.1016/j.jallcom.2018.04.177
Wang, L., Wang, J., He, C., Lyu, W., Zhang, W., Yan, W., et al. (2019b). Development of Rare Earth Element Doped Magnetic Biochars with Enhanced Phosphate Adsorption Performance. Colloids Surf. A: Physicochemical Eng. Aspects 561, 236–243. doi:10.1016/j.colsurfa.2018.10.082
Wang, L., Wang, J., Wang, Z., Feng, J., Li, S., and Yan, W. (2019c). Synthesis of Ce-Doped Magnetic Biochar for Effective Sb(V) Removal: Performance and Mechanism. Powder Technology 345, 501–508. doi:10.1016/j.powtec.2019.01.022
Wang, L., Wang, J., and Wei, Y. (2021). Facile Synthesis of Eggshell Biochar Beads for superior Aqueous Phosphate Adsorption with Potential Urine P-Recovery. Colloids Surf. A: Physicochemical Eng. Aspects 622. doi:10.1016/j.colsurfa.2021.126589
Wang, L., Wang, J., Yan, W., He, C., and Shi, Y. (2020). MgFe2O4-biochar Based Lanthanum Alginate Beads for Advanced Phosphate Removal. Chem. Eng. J. 387. doi:10.1016/j.cej.2019.123305
Wang, X., Pfeiffer, H., Wei, J., Dan, J., Wang, J., and Zhang, J. (2022). 3D Porous Ca-Modified Mg-Zr Mixed Metal Oxide for Fluoride Adsorption. Chem. Eng. J. 428. doi:10.1016/j.cej.2021.131371
Wei, Y., Wang, L., and Wang, J. (2022). Cerium Alginate Cross-Linking with Biochar Beads for Fast Fluoride Removal over a Wide pH Range. Colloids Surf. A: Physicochemical Eng. Aspects 636. doi:10.1016/j.colsurfa.2021.128161
Wen, S., Wang, Y., and Dong, S. (2015). Performance and Characteristics of Fluoride Adsorption Using Nanomagnetite Graphite-La Adsorbent. RSC Adv. 5 (109), 89594–89602. doi:10.1039/c5ra15215a
Wimalasiri, A. K. D. V. K., Fernando, M. S., Williams, G. R., Dissanayake, D. P., de Silva, K. M. N., and de Silva, R. M. (2021). Microwave Assisted Accelerated Fluoride Adsorption by Porous Nanohydroxyapatite. Mater. Chem. Phys. 257. doi:10.1016/j.matchemphys.2020.123712
Wu, K., Zhang, N., Liu, T., Ma, C., Jin, P., Zhang, F., et al. (2017a). Competitive Adsorption Behaviors of Arsenite and Fluoride onto Manganese-Aluminum Binary Adsorbents. Colloids Surf. A: Physicochemical Eng. Aspects 529, 185–194. doi:10.1016/j.colsurfa.2017.05.039
Wu, L., Lin, X., Wu, J., Zhou, X., and Luo, X. (2016a). Adsorption Behavior of Carboxymethyl Konjac Glucomannan Microspheres for Fluoride from Aqueous Solution. RSC Adv. 6 (92), 89417–89429. doi:10.1039/c6ra17183d
Wu, L., Lin, X., Zhou, X., and Luo, X. (2016b). Removal of Uranium and Fluorine from Wastewater by Double-Functional Microsphere Adsorbent of SA/CMC Loaded with Calcium and Aluminum. Appl. Surf. Sci. 384, 466–479. doi:10.1016/j.apsusc.2016.05.056
Wu, P., Wu, J., Xia, L., Liu, Y., Xu, L., and Song, S. (2017b). Adsorption of Fluoride at the Interface of Water with Calcined Magnesium-Ferri-Lanthanum Hydrotalcite-like Compound. RSC Adv. 7 (42), 26104–26112. doi:10.1039/c7ra04382a
Wu, T., Mao, L., and Wang, H. (2017c). Adsorption of Fluoride from Aqueous Solution by Using Hybrid Adsorbent Fabricated with Mg/Fe Composite Oxide and Alginate via a Facile Method. J. Fluorine Chem. 200, 8–17. doi:10.1016/j.jfluchem.2017.05.005
Wu, T., Mao, L., and Wang, H. (2015). Adsorption of Fluoride on Mg/Fe Layered Double Hydroxides Material Prepared via Hydrothermal Process. RSC Adv. 5 (30), 23246–23254. doi:10.1039/c4ra16839a
Wu, X. P., and Gong, X. Q. (2016). Clustering of Oxygen Vacancies at CeO2(111): Critical Role of Hydroxyls. Phys. Rev. Lett. 116 (8), 086102. doi:10.1103/PhysRevLett.116.086102
Wu, X., Zhang, Y., Dou, X., Zhao, B., and Yang, M. (2013). Fluoride Adsorption on an Fe-Al-Ce Trimetal Hydrous Oxide: Characterization of Adsorption Sites and Adsorbed Fluorine Complex Species. Chem. Eng. J. 223, 364–370. doi:10.1016/j.cej.2013.03.027
Xu, W., He, Q., Zhang, S., and Zhang, W. (2017). Adsorption of Fluoride from Aqueous Solutions by Polyacrylic Acid Modified with Aluminium. Polym. Bull. 75 (3), 1171–1184. doi:10.1007/s00289-017-2082-3
Yadav, K., and Jagadevan, S. (2020). Effect of Pyrolysis of Rice Husk–Derived Biochar on the Fuel Characteristics and Adsorption of Fluoride from Aqueous Solution. BioEnergy Res. 14, 964–977. doi:10.1007/s12155-020-10189-6
Yang, K., Li, Y., Zhao, Z., Tian, Z., and Lai, Y. (2020). Amorphous Porous Layered-Al2O3 Derived from AlFu MOFs as an Adsorbent for Removing Fluorine Ions in Industrial ZnSO4 Solution. Chem. Eng. Res. Des. 153, 562–571. doi:10.1016/j.cherd.2019.11.019
Yang, W., Tian, S., Tang, Q., Chai, L., and Wang, H. (2017). Fungus Hyphae-Supported Alumina: An Efficient and Reclaimable Adsorbent for Fluoride Removal from Water. J. Colloid Interf. Sci. 496, 496–504. doi:10.1016/j.jcis.2017.02.015
Ye, C., Yan, B., Ji, X., Liao, B., Gong, R., Pei, X., et al. (2019). Adsorption of Fluoride from Aqueous Solution by Fly Ash Cenospheres Modified with Paper Mill Lime Mud: Experimental and Modeling. Ecotoxicology Environ. Saf. 180, 366–373. doi:10.1016/j.ecoenv.2019.04.086
Yu, Y., Yu, L., and Paul Chen, J. (2015). Adsorption of Fluoride by Fe-Mg-La Triple-Metal Composite: Adsorbent Preparation, Illustration of Performance and Study of Mechanisms. Chem. Eng. J. 262, 839–846. doi:10.1016/j.cej.2014.09.006
Zendehdel, M., Shoshtari-Yeganeh, B., Khanmohamadi, H., and Cruciani, G. (2017). Removal of Fluoride from Aqueous Solution by Adsorption on NaP:HAp Nanocomposite Using Response Surface Methodology. Process Saf. Environ. Prot. 109, 172–191. doi:10.1016/j.psep.2017.03.028
Zhang, C., Li, Y., Wang, T.-J., Jiang, Y., and Wang, H. (2016a). Adsorption of Drinking Water Fluoride on a Micron-Sized Magnetic Fe3O4@Fe-Ti Composite Adsorbent. Appl. Surf. Sci. 363, 507–515. doi:10.1016/j.apsusc.2015.12.071
Zhang, K., Wu, S., He, J., Chen, L., Cai, X., Chen, K., et al. (2016b). Development of a Nanosphere Adsorbent for the Removal of Fluoride from Water. J. Colloid Interf. Sci. 475, 17–25. doi:10.1016/j.jcis.2016.04.037
Zhang, S., Lyu, Y., Su, X., Bian, Y., Yu, B., and Zhang, Y. (2016c). Removal of Fluoride Ion from Groundwater by Adsorption on Lanthanum and Aluminum Loaded clay Adsorbent. Environ. Earth Sci. 75 (5). doi:10.1007/s12665-015-5205-x
Zhang, X., Qi, Y., Chen, Z., Song, N., Li, X., Ren, D., et al. (2021a). Evaluation of Fluoride and Cadmium Adsorption Modification of Corn Stalk by Aluminum Trichloride. Appl. Surf. Sci. 543. doi:10.1016/j.apsusc.2020.148727
Zhang, Y.-X., and Jia, Y. (2016). Fluoride Adsorption onto Amorphous Aluminum Hydroxide: Roles of the Surface Acetate Anions. J. Colloid Interf. Sci. 483, 295–306. doi:10.1016/j.jcis.2016.08.054
Zhang, Y., Jin, Z., Min-He, L., Guang-Song, X., Min-Da, X., Cheng, H., et al. (2021b). Facile Synthesis of Hollow MgO Spheres and Their Fluoride Adsorption Properties. Adv. Condensed Matter Phys. 2021, 1–10. doi:10.1155/2021/6655593
Zhao, X., Yu, X., Wang, X., Lai, S., Sun, Y., and Yang, D. (2021). Recent Advances in Metal-Organic Frameworks for the Removal of Heavy Metal Oxoanions from Water. Chem. Eng. J. 407. doi:10.1016/j.cej.2020.127221
Zhou, J., Liu, Y., Han, Y., Jing, F., and Chen, J. (2019a). Bone‐derived Biochar and Magnetic Biochar for Effective Removal of Fluoride in Groundwater: Effects of Synthesis Method and Coexisting Chromium. Water Environ. Res. 91 (7), 588–597. doi:10.1002/wer.1068
Zhou, J., Zhu, W., Yu, J., Zhang, H., Zhang, Y., Lin, X., et al. (2018). Highly Selective and Efficient Removal of Fluoride from Ground Water by Layered Al-Zr-La Tri-metal Hydroxide. Appl. Surf. Sci. 435, 920–927. doi:10.1016/j.apsusc.2017.11.108
Zhou, Z., Yu, Y., Ding, Z., Zuo, M., and Jing, C. (2019b). Competitive Adsorption of Arsenic and Fluoride on {2 0 1} TiO2. Appl. Surf. Sci. 466, 425–432. doi:10.1016/j.apsusc.2018.10.052
Zhu, T., Zhu, T., Gao, J., Zhang, L., and Zhang, W. (2017). Enhanced Adsorption of Fluoride by Cerium Immobilized Cross-Linked Chitosan Composite. J. Fluorine Chem. 194, 80–88. doi:10.1016/j.jfluchem.2017.01.002
Keywords: fluoride adsorption, metal oxides/hydroxides, carbon-based adsorbents, biopolymer, modification
Citation: Wei Y, Wang L, Li H, Yan W and Feng J (2022) Synergistic Fluoride Adsorption by Composite Adsorbents Synthesized From Different Types of Materials—A Review. Front. Chem. 10:900660. doi: 10.3389/fchem.2022.900660
Received: 21 March 2022; Accepted: 07 April 2022;
Published: 04 May 2022.
Edited by:
Suqing Wu, Wenzhou University, ChinaCopyright © 2022 Wei, Wang, Li, Yan and Feng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li Wang, d2FuZ2xpLTIwMTVAeGp0dS5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.