
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
PERSPECTIVE article
Front. Chem. , 20 May 2022
Sec. Supramolecular Chemistry
Volume 10 - 2022 | https://doi.org/10.3389/fchem.2022.870769
 Bing Chen1,2†
Bing Chen1,2† Chengchao Chu2†
Chengchao Chu2† En Ren2
En Ren2 Huirong Lin2
Huirong Lin2 Yang Zhang2
Yang Zhang2 Peiyu Wang2
Peiyu Wang2 Hong Yao1
Hong Yao1 Ailin Liu1
Ailin Liu1 Gang Liu2*
Gang Liu2* Xinhua Lin1*
Xinhua Lin1*Metal-ion-based self-assembly supramolecular theranostics exhibit excellent performance in biomedical applications owing to their potential superiorities for simultaneous precise diagnosis, targeted drug delivery, and monitoring the response to therapy in real-time. Specially, the rational designed systems could achieve specific in vivo self-assembly through complexation or ionic interaction to improve tissue-specific accumulation, penetration, and cell internalization, thereby reducing toxicities of drugs in diagnostics and therapy. Furthermore, such imaging traceable nanosystems could provide real-timely information of drug accumulation and therapeutic effects in a non-invasive and safe manner. Herein, the article highlights the recent prominent applications based on the metal ions self-assembly in cancer treatment. This strategy may open up new research directions to develop novel drug delivery systems for cancer theranostics.
The usefulness of small-molecule therapeutic dyes has been widely studied in various therapeutic paradigms (e.g., phototherapy, sonodynamic therapy, and chemodynamic therapy); however, their efficacy is limited by instability, rapid clearance, and low tissue selectivity (Li et al., 2021). Supramolecular self-assembly which incorporate the functional moieties into supramolecular systems can improve their own performance such as prolonged half-life, enhanced stability and tissue-specific accumulation, is a promising strategy for a personalized therapeutic regimen (Wang et al., 2020; Chen Y. X et al., 2021). In addition, such supramolecular nanostructures can be used for multiple applications simultaneously, including diagnosis, drug delivery, and monitoring the spontaneous response to therapy. (Ren et al., 2017; Chen S et al., 2021). Based on these multiple advantages, an ever-expanding set of supramolecular architectures have been extensively explored, including block copolymers, metal-organic complexes (MOCs), and rationally designed peptides and so on (Dong et al., 2021). However, most of previously reported molecular self-assembly drug delivery systems have little availability in clinical practice, primarily due to the hurdles that can be associated with the biotoxic materials and biological side-effects. Supramolecular self-assembly based on therapeutic agents that are safe and close to clinical translation, which can avoid the potential safety issues and have significant potential for disease theranostics. Indeed, these theranostic systems have garnered considerable attention in multidisciplinary fields, and further progress toward their clinical application is expected in the near future. (Moore and Jokerst, 2019; Gao J et al., 2020).
Metal ions (e.g., iron, zinc, and calcium ions) play important roles in numerous physiological functions such as cellular homeostasis and enzymatic activities, and can also coordinate with functional molecules to spontaneously form an organized self-assembled supramolecular nanostructure with excellent biostability, biocompatibility, and safety (Qian and Xu, 2015). Iron, an essential element, is required for most living systems because it is the key constituent of fundamental cellular and organismal processes. Either too much or too little iron ions will cause serious health problems. For example, excess free iron accumulates in the liver when hepatic diseases (e.g., chronic hepatitis, hepatic fibrosis, and hepatocellular carcinoma) and hemochromatosis occur, which can cause oxidative damage to lipids, proteins, and DNA (Asare et al., 2006; Brissot et al., 2018; Wang and Babitt, 2019). Magnetic resonance imaging (MRI), a non-invasive imaging modality, has been widely employed for the clinical detection of the liver structure and functioning. However, the low specificity, iron quantification sensitivity, and the different iron forms (e.g., ferritin, hemosiderin, and labile iron) distinction inability limit the application of MRI in precision liver iron concentration detection (Wáng, 2021). Metal coordination-driven self-assembling photosensitizers in supramolecular systems can improve phototherapy efficacy and the safety of photosensitizers. The tumor environment-triggered coassembly strategy can overcome the critical issues of precise delivery and address the requirements to monitor therapeutic effects with non-invasive quantitative diagnosis (Chu et al., 2017). Ferric (Fe3+), zinc (Zn2+), and manganese (Mn2+) ions have been confirmed to interact with sulfonic acid and Lewis base groups at the terminal of Indocyanine green (ICG), a US FDA-approved theranostic dye for clinical use, to self-assemble into multi-level supramolecular systems (Shang et al., 2017; Chu et al., 2019; Zhang et al., 2021). Such metal-assisted multi-level self-assembly strategies offer significant advantages with respect to fabrication of hybrid imaging techniques that combine therapy for multimodal imaging-guided theranostics (Zhang et al., 2020; Du et al., 2021). For example, ICG interacting with an RGD peptide-modified ZnII-dipicolylamine-Arg-Gly-Asp (Bis(DPA-Zn)-RGD) complex showed more efficient ability to carry surviving small interfering RNA and to target pathological corneal tissues. Moreover, this ICG-linked complex, can self-assemble into a metal-organic nanostructures (MONs) to realize multimodal imaging-guided phototherapy and gene therapy, thus improving the corneal neovascularization synergistic therapy effect (Chu et al., 2020). Furthermore, in situ self-assembly of supramolecular systems can improve tissue-specific accumulation, multimodal imaging signals, and therapy efficacy, which possess good biocompatibility and stability. Additionally, the study of metal-ion-guided in vivo self-assembly is also helpful to understand the assembly characteristic of disease-related metal ions and the process of natural self-assembled supramolecular nanostructures in living systems (Guo et al., 2021).
Lin et al. developed an in situ self-assembly ICG-based supramolecular system for simultaneous detection and treatment of hepatocellular carcinoma or other iron-overload disorders. Fe3+ was confirmed to coordinate with the sulfonic acid from ICG and form supramolecular nanoassemblies, which significantly decreased the single-MRI intensity and enabled rapid clearance from the body (approximately 50-fold lower than that of free Fe3+), thereby, addressing the clinical requirement for high-performance iron quantification while facilitating excess iron drainage (Figure 1A). The ICG-Lecithin (ICG/Leci) chelates with endogenous Fe3+ ions, which could more significantly reduce serum ferritin levels and increase iron excretion than free ICG and deferoxamine (DFO, a clinically prescribed iron depletion drug) (Lin et al., 2019). Meanwhile, ICG and ICG/Leci could significantly decrease the T1 signal intensity ratio (T1SIR) of Fe3+, producing a marked correlation between the T1SIR and the inner Fe3+ concentration, which could be repurposed as an MRI contrast and quantitative liver iron concentration measure (Lin et al., 2021). The metal-coordination-assisted in situ self-assembly supramolecular systems with the theranostic dye can promote iron ion-induced aggregation and further trigger unique optical properties (Ren et al., 2019). After incubation with Fe3+, the fluorescence intensity of ICG/Leci was reduced due to its coordination with Fe3+. As shown in Figures 1B–D, compared with ICG and DFO, ICG/leci showed that the optical absorption band appeared on a unique spectroscopic peak at 890 nm and obviously decreased from 550 to 880 nm, which resulted from the surface modification of ICG/Leci by free Fe3+. By taking advantage of photoacoustic imaging (PAI) of ICG/Leci-assembly of Fe3+ and MRI contrast changes (Figures 1E,F), which could provide valuable anatomical and functional information in a non-invasive manner, to observe iron depletion and quantitation, and comprehensively understand the therapeutic effects of in vivo self-assembly supramolecular on iron-overload disorders (Gao H et al., 2020). Furthermore, the ability of DFO, ICG, and ICG/Leci to promote iron excretion and elimination was evaluated in Hfe−/− mice, a model of hereditary hemochromatosis. The ICG/Leci supramolecular system exhibited significantly greater removal of excess iron in vivo without inducing renal injury. Based on the advantages of deep tissue diagnosis and real-time monitoring of therapeutic effects in vivo, the precise localization and multimodal imaging-guided therapy efficacy, the ICG/Leci supramolecular system is a promising theranostic agent for future research and clinical translation (Chen et al., 2020). Collectively, a supramolecular system was introduced to encapsulate Fe3+/ICG/leci through in situ self-assembly, which is a highly adaptable platform that integrates multimodal probes for fluorescence imaging, MRI, PAI, and synergetic therapy.
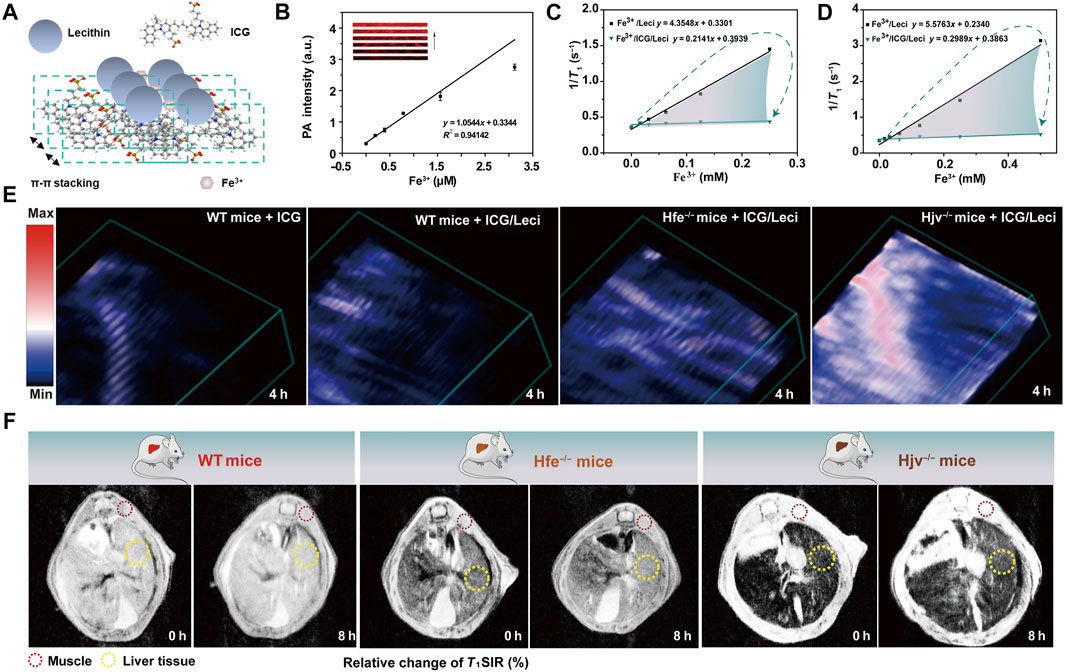
FIGURE 1. (A) Schematic illustration of Fe3+/ICG/Leci formation; (B) photoacoustic response of the probes at 890 nm to Fe3+ (0–3.12 μM) at pH 4.5; (C) r1 of Fe3+/Leci and Fe3+/ICG/Leci was measured in 1.5-T and (D) 9.4-T magnetic fields at pH 4.5; (E) representative photoacoustic images and (F) MRI images of mice models before and after intravenous injection of free ICG and ICG/Leci (ICG dose, 2.5 mg/kg) (Lin et al., 2021). Science Advances.
In addition to endogenous metal irons, exogenous essential mineral ions have also been explored for the design of a tissue environment-triggered supramolecular system with in vivo self-assembly. Chu et al. proposed a tumor stimuli-responsive co-assembly strategy to improve cancer therapy by in situ oxygen and generation of the novel sinoporphyrin sodium (DVDMS) nanotheranostic photosensitizer (nanoDVD). DVDMS is widely applied in phototherapy due to its ability to transform near-infrared (NIR) laser into heat and generate copious amounts of reactive oxygen species (ROS) (Ai et al., 2016). However, a few deficiencies still limit its application, such as non-selectively and low tumor penetrability. The MnO2 nanosheet has excellent efficiency for DVDMS loading, which can release out Mn2+, O2 and DVDMS under GSH and H2O2/H+ reduction in the tumor environment, to produce copious singlet state O2 (1O2) production, thus improving the phototherapy effect. Forthermore, as shown in Supplementary Figure S1 MnO2/DVDMS can self-assemble into nanoDVD in vivo, which can be monitored by activated photoacoustic/fluorescence/magnetic resonance imaging (Xuan et al., 2020; Yu et al., 2021). The tumor microenvironment-triggered supramolecular system has been demonstrated overall improved cancer therapy effect through the consumption of GSH, the production of O2 and ROS.
Furthermore, metal ion-guided supramolecular self-assembly nanoplatforms have multiple desirable functions, which can overcome the two major obstacles of drug delivery: the blood-brain barrier (BBB) and the blood–brain tumor barrier (Shi et al., 2017). Gao et al. developed an in situ assembled nanoplatform to achieve precise orthotopic multimodal imaging and imaging-guided thermal ablation through coordination-driven self-assembly supramolecular systems (Supplementary Figure S2A). The authors demonstrated that intelligent self-assemblies by upconversion nanocrystals could improve tumor-specific accumulation/retention for the phototherapy effect without damaging the skull and scalp in rodent models of orthotopic glioma (Supplementary Figures S2B,C). Specifically, Bis(DPA-Zn)-RGD nano-components exhibited good biocompatibility, improved neovascular-targeting, and excellent photothermal properties (Supplementary Figures S2B,C). Simultaneously, the tumor site of the photoacoustic signal increase in the glioma region of the mice was consistent with the determined using MRI (Supplementary Figures S2D,E). Moreover, nanoscale ICG-based nanoscale gold particles have been extensively applied in drug delivery and molecular imaging because of their tunable size, outstanding optical properties, and nontoxicity. Considering the ultra-small size ( ∼ 7 nm) of gold nanoparticles and the positive charge of Bis(DPA-Zn)-RGD with angiogenesis targeting, these materials can successfully cross the BBB to reach the tumor site, resulting in an unexpected therapeutic effect for orthotopic glioma.
In summary, rationally designed nanotheranostics that harness the metal-coordination-assisted molecular assembly of therapeutic agents can simultaneously diagnose, deliver drugs, and monitor the response to therapy in real-time. The high specificity and sensitivity of theranostic dye-bearing supramolecular nanoplatforms integrate multimodal imaging functions as well as synergetic therapy, which demonstrates significant potential for clinical early-stage diagnosis and effective treatment (Figure 2). Compared with traditional methods for preparing an ICG-based supramolecular therapeutic, metal ion-guided self-assembly supramolecular systems have advantages such as improved drug loading and tissue-specific targeting capacity, resulting in precise orthotopic multimodal imaging-guided drug delivery. It is believed that this carrier-free delivery system, which exhibits improved biocompatibility and outstanding performance in terms of biostability and non-invasive molecular imaging, can be applied from beach to bedsides. However, the comprehensive pharmacokinetic behavior and safety evaluation of metal-organic nanoparticles are needed before the clinical application.
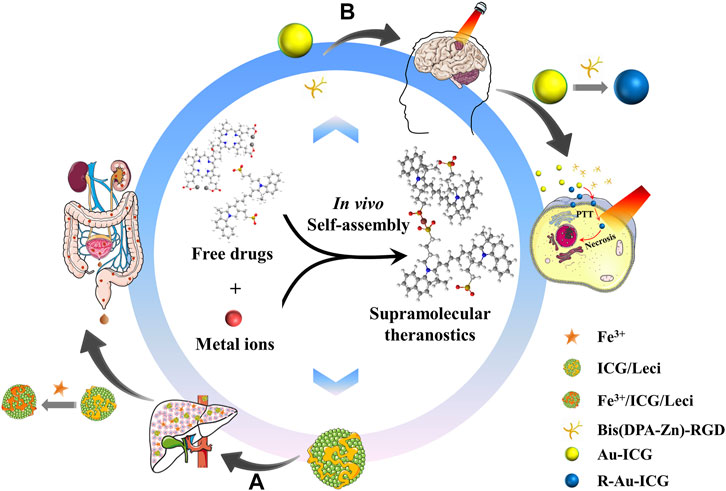
FIGURE 2. Schematic graph of metal-ion-guided in vivo self-assembly supramolecular for theranostic application. (A) Iron chelators of ICG/Leci will capture endogenous hepatic iron and self-assembled in situ, which can efficiently removing excess iron; (B) Positive charges and neovascular targeting properties of Bis(DPA-Ze)-RGD and ultrasmall particle size of Au-ICG could successfully cross the BBB and BBTB, and in situ assembled for imaging and therapy of orthotopic glioma.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Conceptualization, BC, CC, and ER; Original draft preparation, BC, CC, ER, HL, and YZ; Review and editing, BC, PW, HY, and AL; Supervision, GL and XL; Project administration, BC, GL, and XL; Funding acqusition, GL and XL. All authors have read and agreed to the published version of the manuscript.
This work was supported by the Major State Basic Research Development Program of China (2017YFA0205201), the National Natural Science Foundation of China (81925019, 81901878, 22074017 and U1705281), Natural Science Foundation of Fujian province (2020J01631 and 2021J02034) and Joint Funds for the innovation of science and Technology of Fujian province (2018Y9076), and Open Research Fund of Key Laboratory of Nanomedical Technology (Education Department of Fujian Province) (2022KLNT203).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fchem.2022.870769/full#supplementary-material.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
BBB, Blood-brain barrier; BBTB, Blood-brain tumor barrier; DVDMS, Sinoporphyrin sodium; ICG, Indocyanine green; MOCs, Metal-organic complexes; PAI, Photoacoustic imaging; ROS, Reactive oxygen species; T1SIR, T1 signal intensity ratio.
Ai, X., Ho, C. J. H., Aw, J., Attia, A. B. E., Mu, J., Wang, Y., et al. (2016). In Vivo covalent Cross-Linking of Photon-Converted Rare-Earth Nanostructures for Tumour Localization and Theranostics. Nat. Commun. 7, 10432. doi:10.1038/ncomms10432
Asare, G. A., Mossanda, K. S., Kew, M. C., Paterson, A. C., Kahler-Venter, C. P., and Siziba, K. (2006). Hepatocellular Carcinoma Caused by Iron Overload: a Possible Mechanism of Direct Hepatocarcinogenicity. Toxicology 219, 41–52. doi:10.1016/j.tox.2005.11.006
Brissot, P., Pietrangelo, A., Adams, P. C., De Graaff, B., Mclaren, C. E., and Loréal, O. (2018). Haemochromatosis. Nat. Rev. Dis. Prim. 4, 18016. doi:10.1038/nrdp.2018.16
Chen, H., Cheng, H., Dai, Q., Cheng, Y., Zhang, Y., Li, D., et al. (2020). A Superstable Homogeneous Lipiodol-ICG Formulation for Locoregional Hepatocellular Carcinoma Treatment. J. Control. Release 323, 635–643. doi:10.1016/j.jconrel.2020.04.021
Chen, S., Costil, R., Leung, F. K. C., and Feringa, B. L. (2021). Self‐Assembly of Photoresponsive Molecular Amphiphiles in Aqueous Media. Angew. Chem. Int. Ed. 60, 11604–11627. doi:10.1002/anie.202007693
Chen, Y. X., Zhang, W. W., Liang, C. H., Zheng, D. B., Wang, Y. H., Li, X. Y., et al. (2021). Supramolecular Nanofibers with Superior Anti-angiogenesis and Antitumor Properties by Enzyme-Instructed Self-Assembly (EISA). Chem. Eng. J. 425, 7. doi:10.1016/j.cej.2021.130531
Chu, C., Lin, H., Liu, H., Wang, X., Wang, J., Zhang, P., et al. (2017). Tumor Microenvironment-Triggered Supramolecular System as an In Situ Nanotheranostic Generator for Cancer Phototherapy. Adv. Mater 29, 7. doi:10.1002/adma.201605928
Chu, C., Yu, J., Ren, E., Ou, S., Zhang, Y., Wu, Y., et al. (2020). Multimodal Photoacoustic Imaging-Guided Regression of Corneal Neovascularization: A Non-invasive and Safe Strategy. Adv. Sci. (Weinh) 7, 2000346. doi:10.1002/advs.202000346
Chu, C., Ren, E., Zhang, Y., Yu, J., Lin, H., Pang, X., et al. (2019). Zinc(II)-Dipicolylamine Coordination Nanotheranostics: Toward Synergistic Nanomedicine by Combined Photo/Gene Therapy. Angew. Chem. Int. Ed. 58, 269–272. doi:10.1002/anie.201812482
Dong, J., Liu, Y., and Cui, Y. (2021). Supramolecular Chirality in Metal-Organic Complexes. Acc. Chem. Res. 54, 194–206. doi:10.1021/acs.accounts.0c00604
Du, Y., Liu, D., Sun, M., Shu, G., Qi, J., You, Y., et al. (2021). Multifunctional Gd-CuS Loaded UCST Polymeric Micelles for MR/PA Imaging-Guided Chemo-Photothermal Tumor Treatment. Nano Res. 15 (3), 2288–2299. doi:10.1007/s12274-021-3812-2
Gao, H., Chu, C., Cheng, Y., Zhang, Y., Pang, X., Li, D., et al. (2020). In Situ Formation of Nanotheranostics to Overcome the Blood-Brain Barrier and Enhance Treatment of Orthotopic Glioma. ACS Appl. Mat. Interfaces 12, 26880–26892. doi:10.1021/acsami.0c03873
Gao, J., Zhan, J., and Yang, Z. (2020). Enzyme-Instructed Self-Assembly (EISA) and Hydrogelation of Peptides. Adv. Mater 32, e1805798. doi:10.1002/adma.201805798
Guo, R. C., Zhang, X. H., Fan, P. S., Song, B. L., Li, Z. X., Duan, Z. Y., et al. (2021). In Vivo Self‐Assembly Induced Cell Membrane Phase Separation for Improved Peptide Drug Internalization. Angew. Chem. Int. Ed. 60, 25128–25134. doi:10.1002/anie.202111839
Li, D., Yang, Y., Li, D. F., Pan, J., Chu, C. C., and Liu, G. (2021). Organic Sonosensitizers for Sonodynamic Therapy: From Small Molecules and Nanoparticles toward Clinical Development. Small 17, 20. doi:10.1002/smll.202101976
Lin, H., Zhou, Y., Wang, J., Wang, H., Yao, T., Chen, H., et al. (2021). Repurposing ICG Enables MR/PA Imaging Signal Amplification and Iron Depletion for Iron-Overload Disorders. Sci. Adv. 7, eabl5862. doi:10.1126/sciadv.abl5862
Lin, H., Li, S., Wang, J., Chu, C., Zhang, Y., Pang, X., et al. (2019). A Single-step Multi-Level Supramolecular System for Cancer Sonotheranostics. Nanoscale Horiz. 4, 190–195. doi:10.1039/c8nh00276b
Moore, C., and Jokerst, J. V. (2019). Strategies for Image-Guided Therapy, Surgery, and Drug Delivery Using Photoacoustic Imaging. Theranostics 9, 1550–1571. doi:10.7150/thno.32362
Qian, X., and Xu, Z. (2015). Fluorescence Imaging of Metal Ions Implicated in Diseases. Chem. Soc. Rev. 44, 4487–4493. doi:10.1039/c4cs00292j
Ren, E., Chu, C., Pang, X., Lv, P., Lei, Z., and Liu, G. (2019). Mineral Iron Based Self-Assembling: Bridging the Small Molecular Drugs and Transformative Application. Sci. Bull. 64, 216–218. doi:10.1016/j.scib.2019.01.011
Ren, E., Wang, J., and Liu, G. (2017). Cell-surface Cascaded Landing Location for Nanotheranostics. Chin. Chem. Lett. 28, 1799–1800. doi:10.1016/j.cclet.2017.07.015
Shang, W., Zeng, C., Du, Y., Hui, H., Liang, X., Chi, C., et al. (2017). Core-Shell Gold Nanorod@Metal-Organic Framework Nanoprobes for Multimodality Diagnosis of Glioma. Adv. Mater 29, 8. doi:10.1002/adma.201604381
Shi, J., Kantoff, P. W., Wooster, R., and Farokhzad, O. C. (2017). Cancer Nanomedicine: Progress, Challenges and Opportunities. Nat. Rev. Cancer 17, 20–37. doi:10.1038/nrc.2016.108
Wang, C.-Y., and Babitt, J. L. (2019). Liver Iron Sensing and Body Iron Homeostasis. Blood 133, 18–29. doi:10.1182/blood-2018-06-815894
Wang, D., Wu, H., Phua, S. Z. F., Yang, G., Qi Lim, W., Gu, L., et al. (2020). Self-assembled Single-Atom Nanozyme for Enhanced Photodynamic Therapy Treatment of Tumor. Nat. Commun. 11, 357. doi:10.1038/s41467-019-14199-7
Wáng, Y. X. J. (2021). Physiological Variation of Liver Iron Concentration May Not Be Dominantly Responsible for the Liver T1rho Variations Associated with Age and Gender. Quant. Imaging Med. Surg. 11, 1668–1673. doi:10.21037/qims-20-1250
Xuan, W., Xia, Y., Li, T., Wang, L., Liu, Y., and Tan, W. (2020). Molecular Self-Assembly of Bioorthogonal Aptamer-Prodrug Conjugate Micelles for Hydrogen Peroxide and pH-independent Cancer Chemodynamic Therapy. J. Am. Chem. Soc. 142, 937–944. doi:10.1021/jacs.9b10755
Yu, J., Chu, C., Wu, Y., Liu, G., and Li, W. (2021). The Phototherapy toward Corneal Neovascularization Elimination: An Efficient, Selective and Safe Strategy. Chin. Chem. Lett. 32, 99–101. doi:10.1016/j.cclet.2020.11.025
Zhang, P., Wang, J., Chen, H., Zhao, L., Chen, B., Chu, C., et al. (2018). Tumor Microenvironment-Responsive Ultrasmall Nanodrug Generators with Enhanced Tumor Delivery and Penetration. J. Am. Chem. Soc. 140, 14980–14989. doi:10.1021/jacs.8b09396
Zhang, Y., Cheng, H., Chen, H., Xu, P., Ren, E., Jiang, Y., et al. (2021). A Pure nanoICG-Based Homogeneous Lipiodol Formulation: toward Precise Surgical Navigation of Primary Liver Cancer after Long-Term Transcatheter Arterial Embolization. Eur. J. Nucl. Med. Mol. Imaging 49, 1–13. doi:10.1007/s00259-021-05654-z
Keywords: supramolecular therapeutic systems, metal-coordination-assisted, in vivo self-assembly, tumor microenvironment, personalized medicine
Citation: Chen B, Chu C, Ren E, Lin H, Zhang Y, Wang P, Yao H, Liu A, Liu G and Lin X (2022) Metal Ion-Based Supramolecular Self-Assembly for Cancer Theranostics. Front. Chem. 10:870769. doi: 10.3389/fchem.2022.870769
Received: 08 February 2022; Accepted: 22 April 2022;
Published: 20 May 2022.
Edited by:
Jyotirmayee Mohanty, Bhabha Atomic Research Centre, IndiaReviewed by:
Harekrushna Sahoo, National Institute of Technology Rourkela, IndiaCopyright © 2022 Chen, Chu, Ren, Lin, Zhang, Wang, Yao, Liu, Liu and Lin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xinhua Lin, MTM5MDY5MDk2MzhAMTYzLmNvbQ==; Gang Liu, R2FuZ2xpdS5jbWl0bUB4bXUuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.