
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Chem. , 13 April 2022
Sec. Nanoscience
Volume 10 - 2022 | https://doi.org/10.3389/fchem.2022.850757
This article is part of the Research Topic Nanotechnology for Natural Products View all 7 articles
Ocular disorders comprising various diseases of the anterior and posterior segments are considered as the main reasons for blindness. Natural products have been identified as potential treatments for ocular diseases due to their anti-oxidative, antiangiogenic, and anti-inflammatory effects. Unfortunately, most of these beneficial compounds are characterised by low solubility which results in low bioavailability and rapid systemic clearance thus requiring frequent administration or requiring high doses, which hinders their therapeutic applications. Additionally, the therapeutic efficiency of ocular drug delivery as a popular route of drug administration for the treatment of ocular diseases is restricted by various anatomical and physiological barriers. Recently, nanotechnology-based strategies including polymeric nanoparticles, micelles, nanofibers, dendrimers, lipid nanoparticles, liposomes, and niosomes have emerged as promising approaches to overcome limitations and enhance ocular drug bioavailability by effective delivery to the target sites. This review provides an overview of nano-drug delivery systems of natural compounds such as thymoquinone, catechin, epigallocatechin gallate, curcumin, berberine, pilocarpine, genistein, resveratrol, quercetin, naringenin, lutein, kaempferol, baicalin, and tetrandrine for ocular applications. This approach involves increasing drug concentration in the carriers to enhance drug movement into and through the ocular barriers.

GRAPHICAL ABSTRACT. Application of nanostructures in ocular delivery of natural products.
The anatomy and physiological characteristics of the eye makes it a unique sensory organ. The eye can commonly be categorized into two main compartments: the front one-third of the eye between the cornea and the lens which is called the anterior segment and the back two-thirds of the eye from the lens to the optic nerve, including the vitreous humor which is called the posterior segment (Figure 1). There are various chronic and acute diseases that can affect the anterior and posterior segments of the eye. The most common chronic posterior segment diseases such as age-related macular degeneration (AMD), diabetic macular edema (DME), and diabetic retinopathy (DR) and some of the chronic anterior segment diseases such as glaucoma, uveitis, cataract, dry eye syndrome (DES) are the leading causes of vision loss (Joseph and Venkatraman, 2017). Most eye diseases are associated with aging, oxidative stress mechanism, and inflammatory responses. While natural products with the ability to scavenger reactive oxygen species (ROS) and suppress inflammatory mediators can be considered as a promising remedy for the prevention and treatment of ocular diseases.
Since early times, natural products containing active pharmacological ingredients with various molecular structures have been used for the treatment of numerous diseases and disorders. Many of these compounds possess strong anti-oxidative, anti-inflammatory, and anti-apoptotic effects. Natural products have a particular chemical and structural diversity (Figure 2), with less toxicity and currently, many of the modern drugs in use have their origin in natural compounds and their derivatives (Mathur and Hoskins, 2017). The alkaloids, flavonoids, and phenolic compounds are among the bioactive ingredients that exist in natural products. The chemical structures of natural compounds play a crucial role in their therapeutic effects and their biological properties. For example, lutein with two hydroxyl groups can effectively scavenge the free radicals and prevent the oxidation process, so it can be considered as a potential drug for the prevention and treatment of a posterior segment of the eye such as AMD (Koushan et al., 2013) or polyphenolic structure of curcumin makes it a promising candidate for the treatment of bacterial infection and inflammation (Gupta et al., 2012). Other studies also revealed that the replacement of the methoxy groups of curcumin with other groups change its anti-inflammatory effect and reduce this effect. These findings demonstrated the role of the aryl group of curcumin in its anti-inflammatory effect (Noureddin et al., 2019). Several studies have been performed to find herbal active ingredients such as curcumin, catechin, lutein, ginseng, resveratrol, quercetin, and many more to prevent or ameliorate sight-threatening eye diseases (Fathi et al., 2017; Kim et al., 2020). However, these compounds show low absorption ability, bioavailability, and efficiency due to their high molecular weight to pass through lipid membranes. However, the mechanisms of action of these ingredients are not fully investigated and few literature studies exist on the efficiency of natural compounds on human eye diseases, but there are many reasons to consider natural products that can work synergistically to enhance the activity of other drugs (Sulaiman et al., 2014).
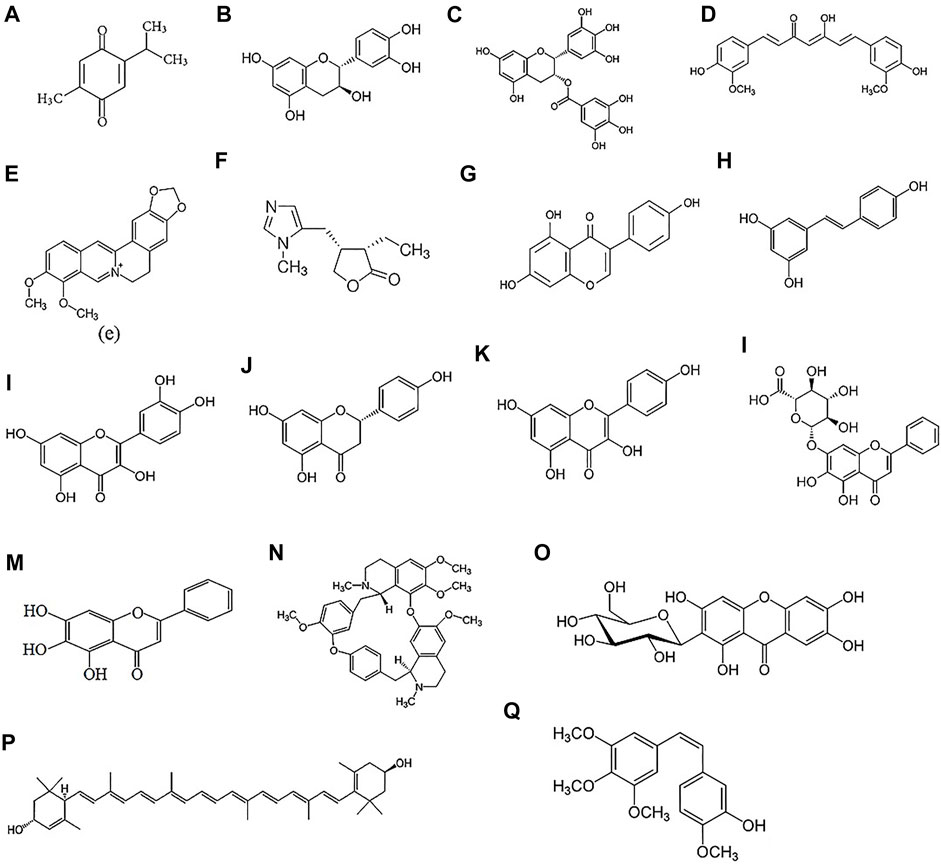
FIGURE 2. Chemical structure of natural products: Thymoquinone (A), Catechin (B), Epigalloctatin gallate (C), Curcumin (D), Berberine (E), Pilocarpine (F), Genistein (G), Resveratrol (H), Quercetin (I), Naringenin (J), Kaempferol (K), Baicalin (L), Baicalein (M), Tetrandrine (N), Mangiferin (O), Lutein (P), Combretastatin A4 (Q).
The eye is a well-protected organ in the body and has several protective barrier layers and complex structures with various defense mechanisms that defend it against harmful substances, microorganisms, and toxins. These barriers that are essential in protecting and preserving vision also restrain the entry and penetration of drug molecules to the inner ocular tissues (Joseph and Venkatraman, 2017). Thus ocular drug delivery remains a great challenge to researchers and ophthalmologists due to the presence of these complex barriers. There are various pathways for drug delivery to the anterior and posterior segments of the eye including topical, periocular, systemic, and intravitreal routes (Figure 1) (Varela-Fernández et al., 2020). The advantages and disadvantages of these routes are summarized in Table 1. Conventional delivery systems such as eye drops, injections, and implants have been the most extensively utilized, but they have some disadvantages including challenges to traverse the physiological barriers, enzymatic drug degradation, protein binding, poor targeting efficiency, low penetration and retention time, side effects and low bioavailability (Ingle et al., 2017; Sánchez-López et al., 2017). Numerous researchers have attempted to develop non-invasive, cost-effective, sustained release approaches with enhanced therapeutic efficacy over conventional systems. Due to the complex structure and ocular barriers, there is a need for the rational design of drug delivery carriers to provide effective treatment. Nanocarrier-based drug delivery systems are designed to deliver the drug to the target site by delivering small molecules either by improving their permeation or by extending residence time, prolonging the drug release profile, and reducing the injection frequency (Shen et al., 2015). Several nanomaterials have been explored to overcome ocular barriers and control the release of drugs (Ingle et al., 2017; Lakhani et al., 2018a). Even with the few available reports about the delivery of natural products, nanoparticles as promising carriers could entrap these natural products to make a safe and more promising alternative for the remedy of ocular diseases through the ocular routes. This review highlights the various challenges associated with drug delivery to the anterior and posterior segments of the eye, and provide an overview of novel nanomaterials with the potential for ocular delivery of natural products, and treatment of ocular diseases.
The anatomy and structure of eye are complex with two principle pathways for drugs to pass into ocular tissues and reach their target, namely the corneal and the conjunctiva-scleral (non-corneal) pathways. Drugs traversing each pathway encounter barriers such as pre-corneal (tear film), the cornea, the blood aqueous barrier (BAB), and the blood retinal barrier (BRB) (Figure 3) (Loftsson and Stefánsson, 2017). For easy reading abbreviations are listed in Table 2.
The main component of the pre-corneal barrier is through tear drainage (Ghate and Edelhauser, 2006; Gaudana et al., 2010). The conjunctival cul-de-sac can accommodate the low amount of topically administered eye drops (Lakhani et al., 2018a). Pre-corneal drainage causes the removal of the applied formulation and decreases the corneal residence time of the formulation (Nejima et al., 2005). In addition, tear fluid proteins can bind to the drug and lead to a reduced concentration of free drug in the tear fluid (Svitova and Lin, 2010).
The cornea is a transparent multilayered barrier limiting drug penetration into the aqueous humor through the corneal pathway. It is comprised of five-layers with alternating lipophilic and hydrophilic characteristics. The lipophilic epithelium permits the diffusion of particles with dimensions up to approximately 20 nm (Yellepeddi and Palakurthi, 2016; Bisht et al., 2018). Stroma is hydrophilic in nature and only hydrophilic molecules up to 500 kDa of size are amenable to diffusion, while the entry of most hydrophobic drugs is restricted (Yellepeddi and Palakurthi, 2016). Thus, for efficient permeation across the cornea both molecular weight and logP should be optimized. The leaky corneal endothelium provides minimal resistance to the movement of macromolecules between the stroma and aqueous humor (Cheruvu et al., 2008; Yellepeddi and Palakurthi, 2016). The two principal routes for drugs to cross the epithelium are transcellular and paracellular pathways. Generally, lipophilic drug molecules permeate via the transcellular route whereas hydrophilic molecules and small ions pass through the paracellular route (Raghava et al., 2004).
Topically administrated drugs may also pass through the conjunctiva and the sclera water channels/pores (ranging between 30 and 300 nm in size) facilitated by passive diffusion to get into the vitreous humor (Kompella et al., 2010). The conjunctiva is moist and highly vascularised epithelial tissue, so a significant amount of the drug molecules permeating through the conjunctival epithelium is eliminated via systemic absorption resulting in low drug bioavailability (Prausnitz and Noonan, 1998; Pearce et al., 2015; Battaglia et al., 2016).
The choroid and especially Bruch’s membrane are considered as important barriers for the penetration of drugs. The blood retinal barrier (BRB) is a significant barrier composed of tight junctions between the retinal endothelial blood vessel and retinal pigment epithelium (RPE) cells (Jo et al., 2019). The RPE acts as a rate-limiting permeation barrier particularly to hydrophilic molecules where the time of permeation increases with increasing the molecular weight (Mains and Wilson, 2013).
The beneficial therapeutic effects of natural products has attracted a significant attention in the treatment and prevention of diseases. They are considered effective agents for the treatment and prevention of various ocular disorders such as glaucoma, cataract, corneal and choroidal neovascularization, AMD, DR, DES (Radomska-Leśniewska et al., 2019). Besides their beneficial effects, most of these natural products suffer from low water solubility and low bioavailability due to rapid enzymatic degradation that hinder their medical applications (Chebil et al., 2007; Khan et al., 2015; Shim et al., 2019). Different techniques have been applied to overcome these limitations such as encapsulating flavonoids into polymeric carriers, covalent conjugation of flavonoids to hydrophilic polymers, such as dextran (Yee et al., 2017), poly (ethylene glycol) (PEG) (Liang et al., 2018), poly (allylamine), and gelatin (Spizzirri et al., 2009). The focus in this section will be on natural products that have been delivered using nanotechnology systems in the area of ophthalmology. The chemical structures of these compounds is depicted in Figure 2. The characteristics, properties and applications of various natural products used in ocular drug delivery are summarised in Table 3.
Thymoquinone (2-isopropyl-5-methyl-1,4-benzoquinone) is the major biologically active ingredient of the volatile oil that is isolated from the medicinal plant Nigella sativa (Darakhshan et al., 2015). It has been demonstrated that this herbal ingredient shows neuroprotective, anti-inflammatory, and antioxidant effects (Alhebshi et al., 2013) and is effective in the treatment of glaucoma (Fahmy et al., 2018). Fahmy et al. (2018) reported various liposomal formulations of thymoquinone (TQ) and latanoprost (LAT) in reducing Intraocular pressure (IOP). Addition, this study indicated the promising role of TQ in the amelioration of retinal damage and the inflammatory responses in glaucomatous rabbits (Fahmy et al., 2018).
Catechin (flavan-3-ol) is a member of the flavonoids, a class of natural polyphenols considered an antioxidant ingredient and found in various fruits, beverages, and tea. It has some biological advantages (Shimamura et al., 2007; Singh et al., 2011; Li et al., 2019). The therapeutic applications of catechin for ocular disease has been reported in various studies, including for dry eye, glaucoma, and various retinal disorders due to anti-inflammatory and anti-oxidative properties (Lee et al., 2017a). Lee et al. (2017a) investigated the nano-complex of PEG and catechin for enhancing the bioavailability and the therapeutical effect of catechin in the treatment of dry eye disease (DED). In another study Li and his coworkers employed a simple self-polymerization and self-assembly reaction to formulate a core-shell structure of polycatechin and gold nanoparticles (Au@Poly-CH NPs) as an eye drop to synergistically eliminate DED due to its antioxidant and anti-inflammatory effects (Li et al., 2019).
Epigallocatechin gallate (EGCG) as a major ingredient of green tea, exhibits anti-inflammatory effects and is extensively used in the treatment of various inflammatory diseases and for the treatment of ocular disorders, such as AMD, DR, and DES (Fangueiro et al., 2016). However, the corneal epithelium is an effective barrier for hydrophilic EGCG. Luo and Lai (2017) designed new biodegradable gelatin-g poly (N-isopropyl acrylamide) (GN) nanocarriers for the topical administration of EGCG on rabbit eyes in the treatment of DED with anti-oxidant activity and sustained release profile (Luo and Lai, 2017).
Curcumin [1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione] is the yellow-colored bioactive component of turmeric powder, extracted from the rhizome of the plant Curcuma longa (Aggarwal et al., 2003) with a wide verity of physiological and pharmacological characteristics (Sharma et al., 2005; Shishodia et al., 2005; Sadeghi Ghadi and Ebrahimnejad, 2019; Sadeghi-Ghadi et al., 2020). Curcumin (CUR) is also considered as an effective ingredient for the treatment and prevention of various ocular disorders such as glaucoma, cataract, corneal and choroidal neovascularization, AMD, DR, DES (Radomska-Leśniewska et al., 2019). It can be beneficial in the treatment of proliferative epithelial disorders, the proliferation of human lens epithelial cells, and protects retinal cells, retinal ganglion cells, and corneal epithelial cells (Beevers and Huang, 2011). One of the major challenges with curcumin is low stability and storage difficulties. In order to overcome these problems, Maharjan et al. (2019) investigated different approaches to formulating curcumin or tetrahydrocurcumin (THC)-loaded in various derivatives of hydroxypropyl (HP)-cyclodextrins (CD) by applying the spray drying technique. It was reported that the stability, bioavailability and corneal and retinal epithelial permeability of curcumin (or THC) was significantly enhanced by encapsulating into the HP-CDs (Maharjan et al., 2019).
Berberine (BBR), a type of isoquinoline alkaloid, is an active ingredient of Rhizome Coptidis and Cortex Phellodendri and extensively used in China for treating a variety of disorders (Lin et al., 2015; Choi, 2016; Wen et al., 2016). In order to improve the therapeutic effects, thermal stability of berberine and prevent it from oxidation, Lai and his coworkers investigated new liposomal formulations coated with G3 polyamidoamine dendrimer (PAMAM G3.0) for the treatment of AMD disease. They used berberine hydrochloride (BBH) and chrysophanol (CHR) in their formulations due to their anti-inflammatory and anti-angiogenesis effects for ocular drug delivery applications, respectively (Lai et al., 2019). According to previous studies, CHR can be beneficial in the treatment of retinal disorders due to its ability to suppress NF-κB/caspase-1 activation that leads to reduced inflammatory responses (Kim et al., 2010).
Pilocarpine, an alkaloid with an imidazole ring, is extracted from the leaves of the Jaborandi plant. It can be applied as a miotic agent for topical administration in glaucoma treatment. However, the corneal permeation of pilocarpine is restricted due to its high hydrophilicity that results in low ocular bioavailability. Nair et al. (2012) encapsulated pilocarpine in Poly (lactic-co-glycolic acid) (PLGA) nanoparticles to improve miotic effect and enhance the bioavailability and ocular retention time of pilocarpine.
Genistein (4,5,7-trihydroxyisoflavone) is a flavonoid that’s abundant in soy products and has numerous pharmacological properties such as anti-oxidative, anti-inflammatory, and anti- angiogenesic (Aldina et al., 2019). It also considered a beneficial factor in the treatment and prevention of eye diseases, including DED, DR, AMD, cataract formation, and glaucoma (Lin et al., 2016). Genistein (GEN) can protect the cornea through its anti-inflammatory effect by suppression of oxidative stress (Xiao et al., 2018). It can also suppress IL-1β in the dry-eye model rat. Genistein can be used to prevent posterior capsular opacification (PCO) that’s the most common complication that occurs after cataract surgery due to the remained epithelial cells in the capsular bag that’s proliferated or migrated after cataract surgery which causes blurred vision (Spalton, 1999). So, genistein as an inhibitor of the growth of epithelial cells, can effectively reduce the frequency of PCO and enhance patient satisfaction after cataract surgery (Zhang et al., 2013).
Resveratrol (trans-3,5,4′-trihydroxystilbene) is a non-flavonoid polyphenol compound that widely exists in dietary sources including grapes and peanuts. It has various therapeutic effects (Lee et al., 2011; Abengózar-Vela et al., 2019) and it also considered as a potential ingredient to prevent ocular disorders, including glaucoma, AMD, cataract, and DR (Bola et al., 2014) due to its free radical scavenging properties (Anisimova et al., 2011; Natesan et al., 2017). The small size and hydrophilic characteristics of resveratrol (RES) enables it to pass through the cornea and enter into the retina, however, the low bioavailability of RES restricts its applications. Several novel carriers have been explored for RES such as liposomes (Cote et al., 2015), β-cyclodextrin nanosponges (Abdallah et al., 2015), chitosan nanoparticles (Chuah et al., 2013), solid lipid nanoparticles (SLN) (Hippalgaonkar et al., 2013), protein complexes (Sahoo et al., 2011), poly-ε-caprolactone (PCL) (Wu et al., 2005), to overcome the low solubility, bioavailability, and stability issues of RES. Dong et al. (2019) synthesized gold nanoparticles (AuNPs) without utilising any harmful reductants. Resveratrol is used as a stabilizer and reducing agent in the fabrication of AuNPs. This formulation can reduce the permeability of the blood-retinal barrier in streptozotocin (STZ)-induced diabetic rats. According to the study’s findings, the number of retinal vessels and the expression of the vascular endothelial growth factor (VEGF-1) was decreased whilst the expression of renal pigment epithelium-derived factor (PEDF) increased in the retina of diabetic rats after administration of AuNPs (Dong et al., 2019).
Quercetin is the major prevalent flavonoid that is extensively found in various sources such as apples, tea, onions, nuts, berries, cauliflower, cabbage, and many seeds. Quercetin has many beneficial effects in the treatment and prevention of various diseases (Abengózar-Vela et al., 2019). It is beneficial in ocular disorders such as cataract, choroidal neovascularization (CNV), and AMD (Zhuang et al., 2011). It has antiangiogenic activity and a protective effect on human retinal pigment epithelium cells (Adelli et al., 2013). Subramanian et al. loaded RES and quercetin (QUR) in chitosan nanoparticles to reduce IOP in the glaucoma treatment (Natesan et al., 2017).
Naringenin [2,3-dihydro-5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one] is a flavonoid that belongs to the flavanones subgroup, is extensively found in several citrus fruits, figs, bergamot, and tomatoes. It has been shown to be useful in certain disorders (Salehi et al., 2019) including a beneficial effect on ocular disorders such as retinal pigment epithelium degeneration (RPE), choroidal neovascularization (CNV), and AMD and has attracted attention in recent years. Zhang et al. (2016) encapsulated Naringenin (NG) into sulfobutylether- β-cyclodextrin/chitosan nanoparticles (NG-CD/CS-NPs) for topical administration of the drug to treat AMD disorder.
Lutein is a hydrophobic carotenoid with anti-oxidative and anti-inflammatory properties (Chang et al., 2018) and is found in green leafy vegetables, yellow fruits, petals of the marigold flower, orange, broccoli, spinach, kale, cilantro, corn, and egg yolk (Wallace et al., 2015). Lutein is found at high concentrations in macular pigment in the retina and functions as a light filter to protect the macula from UV-light and anti-oxidative damage. It has a protective effects in the treatment and prevention of ocular diseases (especially posterior eye diseases) such as DR, macular degeneration (MD), neuronal injury, AMD, uveitis, choroidal neovascularization, retinal ischemia, retinitis, and cataract (Koushan et al., 2013; Buscemi et al., 2018). Despite all these beneficial effects, the low stability, bioavailability, and solubility of lutein hinders its medicinal applications (Toragall et al., 2020). Liu et al. (2014) developed lipid nanoparticles and cyclodextrin for topical administration of lutein. This formulation was more successful in accumulating and partitioning lutein in the cornea, enhanced drug loading efficiency, stability, and decreased cytotoxicity than nanoparticles without lutein (Liu et al., 2014).
Kaempferol [3,5,7- trihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one] is a natural flavonoid that is found extensively in edible plants and fruits with high anti-oxidative, anti-inflammatory, anticancer, anti-angiogenesis, and antimicrobial activities (Hung et al., 2017; Du et al., 2018). In ocular disorders, it has attracted interest for the topical treatment of corneal neovascularization and its protective effect on RPE cells from reactive oxygen. Chuang et al. (2019) applied Kaempferol (KA) to hinder vessel formation and treat corneal neovascularization. To increase the bioavailability and sustained release of KA, it was loaded into gelatin nanoparticles (GNP) for administration as eye drops for ocular drug delivery (Chuang et al., 2019).
Baicalin (5,6-dihydroxy7-O-glucuronide) is a flavonoid with low water solubility and stability and is extracted from the Scutellaria baicalensis Georgi plant (Ashraf et al., 2018). Baicalein, baicalin, and wogonin are among the major bioflavonoids extracted from it. They can be used for the treatment of various diseases (Natesan et al., 2017; Sowndhararajan et al., 2018). They exhibit a broad spectrum of biological activities in the eyes, such as anti-inflammatory, antibacterial, anti-cataract, antioxidant, and anti-angiogenesis effects and can be effective in the treatment of AMD, DR, and uveitis (Natesan et al., 2017).
Ashraf et al. (2018) formulated three different nanostructural systems to enhance baicalin (BN) pharmacological and physiological properties (Liu et al., 2009). In other studies, the efficiency of SLN and NLC for the delivery of baicalin for the treatment of cataractic rats was investigated (Liu et al., 2011).
Tetrandrine (6,6′,7,12-tetra methoxy-2,2′-dimethyl-1 beta-berbamane) is an alkaloid extracted from the Chinese medicinal herb Radix Stephania tetrandrae S with anti-inflammatory, immunologic and antiallergenic effects. It has beneficial effects on ocular disorders and can be used in the treatment of opacification of the posterior lens capsule, cataracts, glaucoma, chronic keratitis, retinopathy, and ocular inflammations (Huang et al., 2011). Li et al. loaded tetrandrine in cationic solid lipid nanoparticles (TET-CNP) and anionic solid lipid nanoparticles (TET-NP) to enhance the bioavailability of TET (Liu et al., 2016a). They demonstrated that negatively charged NPs are more efficiently uptaken into the cellular human lens compared to the cationic TET-CNP, thus the formulation could be effective in PCO treatment (Li et al., 2014).
Nanotechnology has been extensively explored in the medical field in recent years, in both the diagnosis and treatment of diseases (Mir and Ebrahimnejad, 2014; Jafari et al., 2016). The advent of nanotechnology promises to accelerate improvements in ophthalmologic drug delivery systems (Kamaleddin, 2017). These novel drug delivery systems aim to facilitate the efficient permeation of drugs through complex ocular barriers (Liu et al., 2016b; Cheng et al., 2019), thus enhancing the therapeutic effect (Lee et al., 2017a; Lee et al., 2017b) and bioavailability (Lee et al., 2017a; Lee et al., 2017b) compared to conventional drug delivery systems. Nanoparticles (NPs) can be designed to prevent drugs from degradation (Beloqui et al., 2016; Lee et al., 2017b), improve penetration through ocular barriers (Priwitaningrum et al., 2016), drug targeting (Hornung et al., 2015), and sustain drug release (Hornung et al., 2015; Yang et al., 2016) and thus enhance efficacy.
Nanotechnology introduces many novel nanocarriers for the treatment of ophthalmic disorders by modification and formulation of existing drugs that lead to an increase in the number of commercial nano-based drugs, in the ocular drug delivery area. Despite much progress in this field, there are few FDA-approved nanomedicine drugs in the market (Table 4), and many of them are in their early stage of clinical development (Reimondez-Troitiño et al., 2015).
In ocular drug delivery, the ability of NPs to adhere to an ocular tissue, mucosa, and epithelium is a major benefit and prevents the formulations from being washed away immediately by ocular defense mechanisms (Yu et al., 2020; Sai et al., 2020). Various types of nanotechnology have been investigated to improve the ocular drug delivery (Omerović and Vranić, 2019). Nanostructured carriers have emerged as minimally invasive drug delivery systems (Zhang et al., 2016; Wu et al., 2011), which can preserve therapeutic drug concentrations in the eye for extended times (Shen et al., 2015; Zhang et al., 2016), reducing the need for frequent administration (Battaglia et al., 2016), and reducing the side effects (Lai et al., 2019; Sultana et al., 2011). To date, various nanocarriers such as polymeric NPs, lipid NPs, liposomes, niosomes, micelles, dendrimers, and nanofibers (Figure 4) have emerged as novel technologies to overcome ocular barriers and improve drug delivery of therapeutic drugs to target sites with enhanced ocular bioavailability (Hironaka et al., 2009; Madni et al., 2017). The small size and adjustable physicochemical and functional properties provide advantages for delivering drugs to target sites (Patra et al., 2018). In this review we provide an overview of nanoparticles and nanofibers that have been explored for the ocular drug delivery of natural products. The various nanocarriers used in ocular drug delivery applications in the treatment of glaucoma, corneal diseases, corneal neovascularization, choroidal neovascularization, age-related macular degeneration, will be considered.
Polymeric nanoparticles (PNPs) are carriers composed of biodegradable and biocompatible natural or synthetic polymers, with or without mucoadhesive properties. Both synthetic polymers such as polyacrylamide, polyacrylate, PCL, PEG, and PLGA (Ebrahimnejad et al., 2009; Wilczewska et al., 2012) and natural polymers such as gelatin, albumin, DNA, sodium alginate, carboxymethylcellulose sodium (CMC), and chitosan can be used to produce PNPs (Varshochian et al., 2015; Madni et al., 2017). They can deliver drugs from either active ingredients adsorbed on the surface or by having it encapsulated into the particle itself. Nanoparticles can be classified as nanospheres (NSs) and nanocapsules (NCs). NSs represent a matrix delivery system where a drug is adsorbed on the surface of the matrix or dispersed within it (Khalili and Ebrahimnezhad, 2017; Omerović and Vranić, 2019). NCs are vesicular systems where the inner core has different properties to the outer polymeric layer and they consist of film polymeric cover wrapping around an oil-filled chamber with a size distribution typically in the range from 10 to 1,000 nm (Paolicelli et al., 2010). In these systems, a drug is commonly dispersed in the core of the particle, but it may also be adsorbed on the surface. The drug loading efficiency is dependent on the affinity between drug and polymers, and the number of functional groups in the polymers for interaction with drugs. In one study, Lee and his coworkers synthesized two types of nanoparticles for long-term and prolonged release of pilocarpine in glaucoma therapy. They used poly (ε-caprolactone) to prepare nanocapsules and nanospheres harboring or encapsulating drugs for ocular drug delivery. It was demonstrated that the loading efficiency of pilocarpine in the PCL NCs was significantly higher than that the PCL NSs and drug release followed a sustained release pattern. The bioavailability, degradation rate, and in vivo experiments on rabbit eyes indicated that PCL NCs are a promising carrier for the treatment of glaucoma, and most effectively reduced the intraocular pressure of rabbit eyes (Lee et al., 2017b). Ruginǎ et al. (2019) loaded RES as an anti-VEGF agent in micro/nanocapsules [composed of polyelectrolytes coated with rhodamine 6G (Rh6G)] to deliver RES into retina pigmented epithelial D407 cells to treat diabetic retinopathy. In another study, Kim et al. (2019) formulated nanospheres with bovine serum albumin and evaluated the potency of antioxidant protection of rosmarinic, ursolic acid, and curcumin in the retina epithelial cells. It was demonstrated that these formulations increased drug solubility and bioavailability and decreased the production of ROS in retina tissues, thus albumin nanospheres could be a promising carrier to deliver anti-oxidative drugs to the anterior and posterior chamber of the eye. There are a number of approaches that can be considered to enhance the absorption of nanoparticles by increasing the retention time such as the use of mucoadhesive polymers, and optimizing nanoparticle size (Ebrahimnejad,, 2009; Ebrahimnejad et al., 2011; Tahara et al., 2017; Sharifi et al., 2019). Bodoki et al. (2019) developed novel nano-formulation by using zein and PLGA nanoparticles to form nano-gels with mucoadhesive and thermosensitive properties for topical administration to enhance the bioavailability, stability, and retention time of lutein for ocular drug delivery. The efficiency of these formulations was evaluated on the selenite-induced rat model of cataracts. The obtained results demonstrated that topically applied lutein-NPs significantly reduced the cataract intensity in comparison to free ocular lutein and oral delivery (Bodoki et al., 2019). Recently, polymeric nanoparticles in the size range from 10–200 nm have gained considerable attention as carriers for ocular drug delivery (Sabzevari et al., 2013; Badiee et al., 2018), due to their ability to enhance bioavailability (Ogunjimi et al., 2017). This indicates that increasing the size of functionalized nanoparticles decreases bioavailability. Therefore, to target the posterior segment of the eye the functionalized nanoparticles size should be kept around 200 nm. The physicochemical properties of nanoparticles enhanced absorption and penetration to the retinal glial cells. Nanoparticle charge is an important parameter, for example changing the negative charge particle to become cationic resulted in NPs penetrating deeper into ocular tissues (Madni et al., 2017; Bisht et al., 2018).
PEGylation is one of the most frequently used approaches to modify the surface of carriers in order to influence the permeability, retention time, and absorption of drugs. Pandian et al. (2016) applied PEG to surface modify chitosan nanoparticles. Resveratrol (RES) was used as a drug model for glaucoma treatment and loaded into nanoparticles. The results indicated an excellent correlation between an increase in PEG concentration and the size and polydispersity index of formulated nanoparticles. The release profile of RES indicated an initial burst release that was followed by a sustained release profile. The irritation test of formulations was evaluated by Hen’s Egg test and results demonstrated the safety of these formulations for ocular drug delivery. These surface modified formulations significantly reduced the IOP within rabbit eyes and enhanced drug permeation through the cornea (Pandian et al., 2016). Chang et al. (2017) developed gelatin/epigalloccatechin-3-gallate nanoparticles coated with conjugated complex comprised of an arginine–glycine–aspartic acid (RGD) peptide grafted to hyaluronic acid (HA) to target αvβ3 integrin on human umbilical vein endothelial cells (HUVECs) for treatment of corneal neovascularization of mouse eye. Surface plasmon resonance was used to confirm to the binding of NPs to the integrin αvβ3. The drug release demonstrated a sustained release profile. These nanoparticles are considered a promising carrier to inhibit the vascular endothelial cells and target the specific site of action (Chang et al., 2017).
Micelles are composed of monolayers of amphiphilic agents (e.g., lipids, polymers) that can self-assemble in aqueous media. The particle size of micelles range between 10 and 100 nm. Micelles show more ordered structures than liposomes but exhibit various structures that depend on the hydrophobic and hydrophilic properties of molecules and solvents. The concentration of polymers in solution is a determining factor in the formation of micelles thus the critical micelles concentration should be attained in order to obtain core-shell nanocarriers with a hydrophobic core and a hydrophilic shell (Cagel et al., 2017). Hydrophobic drugs and active ingredients can be encapsulated and protected in the hydrophobic core of micelles in order to deliver them to the target site and enhance permeation of drugs through the epithelial layers which leads to reduced side effects and increased bioavailability. The hydrophobic shell can be utilized to control release and also specific targeting by immobilizing targeting moieties on the surface of micelle. Li et al. (2017) formulated curcumin nanomicelles as a topical ophthalmic formulation decorated with polyvinyl caprolactam-polyvinyl acetate-polyethylene glycol (PVCL-PVA-PEG) as a graft copolymer. This functionalized nanomicelles improved the solubility, stability, encapsulation efficiency, antioxidant properties of curcumin, and was well tolerated in rabbit eyes. Moreover, it enhanced the corneal permeation and anti-inflammatory properties of curcumin, indicating it as a promising carrier in ophthalmology (Li et al., 2017). Additionally, nanomicelles demonstrate a low critical micelles concentration, stability in solution, a high solubilization capacity, and low cytotoxicity (Mandal et al., 2017). The mucoadhesive nature and small size of polymeric micelles were evaluated and the results from both in vitro and in vivo animal studies indicate that polymeric micelles can enhance contact time with the ocular surface and improve drug transport through intraocular tissues via the paracellular route (Suri et al., 2020). Moreover, the hydrophilic nature of polymeric micelles produces clear solutions that can be used in the form of eye drops without any visual disturbance. Polymeric micelles can therefore be considered as one of the most promising techniques in ocular drug delivery for the treatment of both anterior and posterior segment of eye diseases such as DES, AMD, DR, glaucoma (Alviset et al., 2022), endophthalmitis, retinitis and corneal or conjunctival squamous cell carcinoma (Madni et al., 2017). Li et al. (2020a) evaluated a Soluplus micelle of resveratrol (SOL-RES) for corneal wound healing. In a separate study of cellular uptake and corneal permeation, coumarin-6 loaded within nanomicelles. The irritation test and histopathological observation of rabbit corneas were evaluated 24 h after eye drops instillation and results indicated good ocular tolerance and no eye irritation (Li et al., 2020a).
Nanofibers are fibers with diameters in the range of 1–100 nm. They provide a large surface area up to 1,000 m2 per Gram that can enhance drug loading capacity (Deepak et al., 2018). Various natural (e.g., chitosan, fibronectin, gelatin, collagen, silk, and ethylcellulose) or synthetic polymers (e.g., PLA, PGA, PLGA, PEO, PCL, and PVA), or combinations can be used to produce nanofibers through the electrospinning process. Nanofibers can be modified by varying parameters such as the concentration of polymer solution and drug, adjusting porosity (Goyal et al., 2016), morphology and the diameter of fibers. Moreover, they can be functionalized to modulate the drug release (Zong et al., 2022). They can provide sustained-release profile that results in a reduction in the frequency of administration and thus enhance patient compliance (Gelb et al., 2022). Attractive physical properties of nanofibers such as the high surface-area-to-volume ratio, high porosity, flexibility, high drug-loading capacity, biocompatibility, biodegradability and increasing the contact time of drug with target tissues make them a unique candidate for drug delivery applications, diagnosis and treatment of different diseases, particularly for chronic ocular diseases that require frequent drug administration. Moreover, they can provide a surface for growth, attachment, differentiation, and proliferation of cells (Goyal et al., 2016). Forouzideh et al. (2020) investigated the beneficial anti-angiogenesis effect of silk fibroin nanofibers (SFNF) loaded EGCG on corneal tissue. The nanofibers prepared by electrospinning technique were characterized, the drug release studies of nanofibers showed a controlled release pattern over 144 h, and drug loading of EGCG into the silk fibroin nanofiber reported at approximately 8.0%. MTT assay and human umbilical vein endothelial cells (HUVEC) were used to determine the toxicity and appropriate dose of the drug. Results demonstrated EGCG in nanofiber lead to inhibition of HUVEC and provide an appropriate environment for hosting and proliferation of limbal cells. Moreover, SFNFs with a rough surface provide good conditions for attachment and adhesion of cells on the surface of nanofiber that makes it a promising scaffold for corneal tissue engineering (Forouzideh et al., 2020).
Dendrimers are nano-sized, three-dimensional, hyperbranched, and typically star-shaped structures with many arms emerged symmetrically from a central core (Patri et al., 2002). The size of these structures is related to the various generations (G0, G1, and G2, etc.). Dendrimer nanoparticles can be produced by fast reduction and nucleation reactions (Crooks et al., 2001). The size of dendrimers is usually smaller than 100 nm. The synthetic dendrimers most commonly used in nanomedicine include polyamidoamines (PAMAM), poly (l-lysine) (PLL), polyesters (PGLSA-OH), polypropylimines (PPI), poly (2,2-bis (hydroxymethyl)propionic acid), and aminobis (methylenephosphonic acid) (Mignani et al., 2013). Hydrophobic drugs can be encapsulated in the core or entrapped among the branches of dendrimers based on the properties of polymers used in their construction. The surface of dendrimers can be modified by attaching molecules that may result in increasing the interaction of the dendrimer with biological membranes and high drug payloads. The small size, multi-functional properties, high drug loading ability, water-solubility, targeting ability by surface modification, bioavailability, and biocompatibility make dendrimers a promising candidate for drug delivery (Yavuz et al., 2015; Rodríguez Villanueva et al., 2016). Moreover, their low polydispersity index prevents them from uptake by the reticuloendothelial systems, thus enhancing drug permeation. PAMAM dendrimer have been the main family of dendrimers investigated for drug delivery (Chaplot and Rupenthal, 2014). Yang et al. (2012) indicated that the bioavailability of anti-glaucoma drugs in the cornea of rabbits was enhanced and intraocular pressure decreased by using a hybrid of PAMAM dendrimer hydrogel/PLGA formulation (Yang et al., 2012). PAMAM dendrimer could be used to reduce the frequency of topical ocular administration. The influence of size, molecular weight and various type of surface groups in poly PAMAM dendrimers was investigated in a controlled ocular drug delivery by Vandamme et al. Pilocarpine and tropicamide were loaded in different dendrimer formulations to evaluate the miotic and mydriatic activities, the tolerability, and residence time of dendrimer solutions on the ocular surface of rabbits. The obtained results indicated that the retention time of dendrimers with carboxylic and hydroxyl surface groups was longer than the other formulations. However, altering dendrimer concentration had no significant effect. Moreover, this study demonstrated the influence of size, molecular weight, charge, and geometry of dendrimers on ocular residence time (Vandamme and Brobeck, 2005).
Lipid nanoparticles (LNPs) can be considered as oil/water (o/w) emulsions where liquid lipids are replaced with solid lipids at room temperature. LNPs can provide prolonged drug release with negligible toxicity, so they may be explored as promising carriers for ocular therapeutics. LNPs are classified into two groups: solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (NLCs) (Omerović and Vranić, 2019). SLNs are colloidal lipid-based systems with an average diameter from 50 to 1,000 nm (Müller et al., 2000) and composed of high melting point lipids, water, surfactants, and cosurfactants that stabilize the liquid dispersion (Lingayat et al., 2017). A broad spectrum of lipids can be used to produce SLN include triglycerides, partial glycerides, fatty acids, steroids and waxes (Mehnert and Mäder, 2012; Dudhipala, 2019). Different methods can be applied to produce SLNs such as hot homogenization methods, micro-emulsion method, coacervation method, solvent evaporation, and solvent diffusion from emulsions, solvent injection method, ultrasonication, supercritical fluid extraction of emulsions, and precipitation method (Mukherjee et al., 2009; Silva et al., 2011; Battaglia et al., 2014; Naseri et al., 2015; Rajpoot, 2019). SLNs can be used for different routes of drug administration such as oral, rectal, topical, ophthalmic, parenteral, and other routes (Azhar Shekoufeh Bahari and Hamishehkar, 2016; Beloqui et al., 2016; Bhagurkar et al., 2017). SLNs have the ability to entrap hydrophilic and hydrophobic drugs, are physically stable, prevent the degradation of encapsulated drug, enhance drug bioavailability and biocompatibility (based on the kind of lipids used), and a production process that is simple and cost-effective (and without requiring organic solvents), and the ability to be sterilized and produced at an industrial scale (Beloqui et al., 2016). The biocompatibility and mucoadhesive properties of SLNs, cause to enhance their interaction with the eye mucosa and drug retention time on the eye surface and let it pass the corneal barrier. The negatively charged epithelium provides an opportunity for cationic SLN particles to enhance the drug retention time on the eye and increase its absorption (Bonilla et al., 2022). Despite the numerous advantages, SLNs suffer from numerous disadvantages such as limited drug loading due to the solid crystalline state of the nanoparticles and burst release of both hydrophilic and hydrophobic drugs to the solubility of the drug in the lipid melt (especially remarkable in hydrophilic drugs via adsorption to the surface of SLNs and in polar drugs via existence in outer surfactant layer). Thus, the second generation of lipid nanoparticles introduced to eliminate these drawbacks were NLCs that composed of a mixture of solid and liquid lipids. Different kinds of NLCs can be prepared by applying various concentrations of liquid lipids and different methods of production. Utilizing the liquid lipid in the NLCs leads to enhanced drug loading, increased drug solubility, and reduces the crystallization of solid lipid that minimises the burst release of drug (Tian et al., 2012). NLCs have been extensively applied for anterior and posterior segment ocular drug delivery via corneal and non-corneal pathways (Tian et al., 2013; Zahir‐Jouzdani et al., 2019). The size and surface charge of lipid nanoparticles have an important role in the potential targeting and the extent of drug permeation, in this approach reducing the size of LNPs increases trans-corneal absorption (Kalam et al., 2010) and a positive charge results in higher permeation than a neutral or negative charge and enhances the retention time of nanoparticles on the surface of the cornea (Tamilvanan and Kumar, 2011), however, cationic particles may cause irritation and have toxic effects on ocular tissue due to a greater electrostatic interaction with the anionic layer of ocular tissue thus non-ionic surfactants and lipids preferred (Naseri et al., 2015; Üstündağ Okur et al., 2015). Fangueiro et al. (2016) evaluated in vivo, ex vivo, and in vitro studies on EGCG loaded cationic lipid nanoparticles (LNPs) produced by the double-emulsion technique. The pharmacokinetic profile of the corneal permeation of EGCG loaded into two different formulations of LNPs were evaluated and obtained results of EGCG cetyltrimethylammonium bromide (CTAB) LNs and EGCG-dimethyl dioctadecyl ammonium bromide (DDAB) showed a Boltzmann sigmoidal profile and first-order kinetics respectively. They utilised natural lipid in the formulations that are considered safe, biocompatible, and biodegradable. These cationic lipids indicated high stability without making no irritation or any toxic effects. The positive charge of these LNPs can interact with negative charge of mucosa on the surface of the eye which leads to higher retention time and enhanced permeation through trans-scleral and trans-corneal pathways (Fangueiro et al., 2016; Bodoki et al., 2019). Yu et al. formulated a hybrid pH and thermo-sensitive hydrogel of NLCs for ocular delivery of quercetin. Carboxymethyl chitosan (CMC), and poloxamer 407 were used in hydrogel construction and genipin (GN) used as a crosslinker. Fluorescence imaging, confocal laser scanning microscopy (CLSM), and ex-vivo transcorneal experiments demonstrated that NLCs enhanced corneal permeability and retention time. To evaluate the cellular uptake an ex-vivo transcorneal study was undertaken. Coumarin 6 was used as a hydrophobic fluorescence marker that was administrated into rabbit eyes. Intraocular permeation and distribution of Coumarin 6 were imaged by CLSM after 30 and 120 min of drug instillation. According to the findings, the corneal retention time followed an order of: NLC-Gel > Gel > NLC > eye drops. Cytotoxicity tests and histological examination demonstrated the safety and cytocompatibility of the NLC-Gel formulation (Yu et al., 2018; Yu et al., 2019; Yu et al., 2020).
Liposomes are spherical vesicles with phospholipid bilayers surrounding an aqueous core. The encapsulated drug in these systems can be protected by the lipid bilayer that leads to controlled drug release (Fakhravar et al., 2016). Phosphatidylcholine, cholesterol, and lipid-conjugated hydrophilic polymers are among the common components found in their structures. The size of liposomes ranges from 25–2,500 nm (Akbarzadeh et al., 2013). Liposomes are biodegradable, biocompatible, and nontoxic carriers that can encapsulate both hydrophilic and hydrophobic drug molecules. Despite these superior properties, liposomes suffer from instability due to the presence of unsaturated lipids in their structures that may be hydrolyzed or oxidized and causes the leakage of encapsulated drug. Moreover, aggregation and fusion of liposomes prevent them from ocular tissue absorption. To overcome this limitation, positively charged liposomes were introduced to increase corneal absorption and resistance time. There are numerous methods for producing liposomes which include: thin-film hydration (Zhang, 2017; Zhao et al., 2017), size reduction sonication, reverse-phase evaporation (Shi and Qi, 2018), solvent injection (Sharma et al., 2018), detergent depletion (Salimi, 2018), supercritical fluid process (William et al., 2020), high-pressure homogenization (Ibišević et al., 2019), and low-pressure extrusion (Rameez et al., 2010). Liposomes can be considered as a good carrier for sustained and triggered drug release (Oude Blenke et al., 2013) and have the potential to use for ocular drug delivery (Vafaei et al., 2015; Goyal et al., 2016). They are able to increase the contact time within ocular tissues, thus improve drug absorption and enhance ocular bioavailability and also patient satisfaction due to reducing the dosing frequency. In liposomal systems, drugs can be protected against enzymatic degradation in tear film or/and corneal epithelium which results in a reduction in the clearance rate of the formulations (López-Cano et al., 2021). Mucoadhesive and permeation properties of liposomes can be enhanced by surface modification. Surface charge and size of liposomes have a great effect on ocular drug delivery and the degree of drug permeation into ocular tissues. The critical role of size and charge of liposome on corneal permeation was considered by Schaeffer and Krohn where they applied the formulations in rabbit models. They demonstrated that the permeation of topically administrated formulation through the cornea of rabbits increased in the order small cationic unilamellar vesicles (SUV+) > multilamellar anionic vesicles (MLV-) > small anionic unilamellar vesicles (SUV-) > SUV > MLV free drug (Lakhani et al., 2018a; Venkatraman et al., 2018). In another study, Hironaka et al. (2009) indicated that liposomes smaller than 200 nm show better absorption into retinal tissue whilst particles larger than 600 nm exhibit minimal absorption. According to the obtained results, liposomes can enhance the pharmacokinetic profile of drugs so they can be applied for the treatment of the anterior and posterior segment of the ocular diseases such as glaucoma, DME, ARD, DR, endophthalmitis, retinitis, and corneal or conjunctival squamous cell carcinoma (Kompella et al., 2013). However, the applicability of liposomes has been hindered by issues such as low stability and poor reproducibility, low encapsulation efficiency, uptake by the reticuloendothelial system during phagocytosis, and cause visual cloudiness when intravitreal injected. ER et al. (2021) loaded curcumin and rhodamine B (RhB) dye into multilamellar liposome (MLV) by the thin-film hydration method. They used sodium alginate (SA) and acrylic acid (AA) grafted to each other through a radical polymerization method. Since riboflavin (RB) works as a transporter through the blood retina barrier (BBB), it was conjugated to the produced SA-g-AA to facilitate efficient delivery into the retina region. The resultant product (SA-g-AA-RB) was coated on the surface of the produced MLV using the o/w emulsion method that followed by ionotropic gelation to construct MLV-SA-g-AA-RB carriers that are able to target the retinal region. This formulation produced a prolonged-release profile, good membrane permeability, cellular absorption, and good bioavailability. The size of MLV-SA-g-AA-RB was reported at 730.5 nm while the size of MLV-SA-g-AA with CUR and RhB was 981.7 nm. The results demonstrated that the small size of liposomes produce higher uptake by cells. The encapsulation efficiency (EE) of CUR and RhB was 61 and 66%, respectively, and both showed a controlled manner of drug release. These formulations can be considered as an appropriate carrier to target and deliver drugs to the retina tissue (ER et al., 2021). In another study, Ibrahim et al. (2019) evaluated the protective and anti-oxidative effects of liposomal forms of lutein in cisplatin-induced retinal injury in rabbit eyes. Liposome prepared by the thin-film hydration technique were injected into the peritoneal cavity. Intraperitoneal injections were repeated twice per week for 2 weeks. The rabbit retina was analyzed by the Comet assay, electroretinogram (ERG), and histopathological examination. The result demonstrated that liposomal lutein formulation could be beneficial to avoid the deleterious effects of cisplatin on the rabbits’ retina and prevent DNA and histopathological damage (Ibrahim et al., 2019).
Niosomes are formed by the self-assembly of non-ionic surfactants that form closed bilayer vesicles in aqueous media. They are biocompatible and biodegradable in nature. They are able to entrap both hydrophilic and hydrophobic drugs (Sadeghi Ghadi et al., 2019). They have enhanced chemical stability, mechanical rigidity, safety, bioavailability, and entrapment efficiency compared to liposomes (Gan et al., 2013). However, hydrolyzation and leakage of the drug are the major disadvantages of niosmes. To improve the stability of niosomes against enzymatic degradation, cholesterol can be used in their formulations. Solulan, chitosan, carbopol, and dicetylphosphate are among the non-ionic surfactants that are utilized for ocular formulations (Wadhwa et al., 2009). Niosomes are considered a promising system for topical drug delivery to the eye for the treatment of ocular disorders due to controlled drug release, ability to deliver drug to the target site with no ocular irritation or side effects, and enhanced bioavailability (Sultana et al., 2011). Jain et al. (2020) developed a niosomal gel formulation of pilocarpine instilled into the lower conjunctival sac of rabbit eyes for glaucoma treatment. Pilocarpine niosomes prepared by the ether injection technique, a nonionic surfactant such as Span 20, 60, 80, and various molar ratios of cholesterol evaluated to optimize the niosomal formulation. The optimized formulation integrated into Carbopol 934 and locust bean gum-based gels. Pilocarpine niosomal gel formulation enhances the bioavailability, stability, and precorneal retention time of niosomes. This study showed that this formulation effectively reduced the IOP of glaucomatous rabbits and prolonged the release profile of pilocarpine. Draize’s test demonstrated that these formulations were safe for ocular tissues, with no signs of irritation observed during the study period (Jain et al., 2020).
Aboali et al. (2020) investigated the anti-inflammatory effects of curcumin loaded in niosomal gel on rabbit eyes and found that the formulation reduced inflammation by the same amount produced by marketed corticosteroids (40%) with minimal side effects. Cremophore RH, lecithin, and cholesterol were used as nonionic surfactants in the preparation of pronisomes. These spherical and uniform proniosomes increased the corneal permeation and resistance time. The corneal permeation of proniosomal gel formulation was 3.22-fold higher than curcumin dispersion. The formulation efficiency in lowering ocular pressure and anti-inflammatory effects was evaluated by eye drop instillation every 4 h for 6 days. The measurement of IOP before and after 8 h of instillation indicated that marketed corticosteroids increased the IOP (1.5-fold) more than the curcumin pronisomal gel and curcumin suspension (Aboali et al., 2020).
Despite many developments of nanotechnology in ophthalmic drug delivery, the fate, toxicity, aggregation, long-term effect, and clearance of nanocarriers are in discussion and a few numbers of these drugs are in the market due to many limitations in their production from in vitro testing, in vivo animal studies to human studies (due to the difference between corneal mucoadhesion, mucus and tear productions 4 of rabbit eyes and human eyes). There are many factors that influence the toxicity of nanocarriers such as dose of administration, shape, size, surface charge, and functional groups of nanocarriers. So further investigations are needed to ensure the advantages and efficiency of nanocarriers in humans (Maulvi et al., 2021). On the other hand, natural products with numerous benefits such as safety, efficiency, and promising therapeutic effects suffer from low bioavailability, stability, degradation, and elimination. Moreover, most of their superior therapeutic effects in animal models are not the same as the human model in clinical trials.
For determining the safety and toxicity of ophthalmic nanoformulation, the Draize test on rabbit eyes and in-vivo test on corneal epithelium cells of the human eye have been performed. For instance, Zhang et al. (2014a) used NLC for ocular delivery of GEN and the Draize test results indicated no toxicity or eye irritation. On the other hand Prow et al. demonstrated the intravitreal injection of chitosan nanoparticles causes irritation of the eye (Prow, 2010). According to these data and other findings, we can summarize that LNPs and liposomes are more suitable, safe, and biocompatible carriers to interact with the biological membrane, use in ocular drug delivery and introduce into the market (Gorantla et al., 2020).
According to mentioned information, the structure, size, composition, and surface properties of nanocarriers have critical effects on corneal permeation and retention of nanoformulations. Particles under 10 µm in size can be better tolerated by the human eye. However, the most suitable size for ocular administration is between 50 and 400 nm provides more effective mucoadhesion and passes through ocular barriers to target the specific site, and causes less ocular irritation (Silva et al., 2021). Furthermore, the surface properties of nanocarriers are another determining factor in ocular drug delivery. Surfactants are commonly used in the production of most nanoparticles to enhance the dissolution and permeation of drugs across cellular membranes. Nonionic surfactants are mostly used in ophthalmic formulations to enhance stability, permeability, solubility, biocompatibility, and decrease the toxicity of nanoformulations. Some of the surfactants are more toxic than others and irritate the eye and should be eliminated once the nanocarriers are formulated. According to Leonardi et al. (2014) findings, some surfactants caused no irritation to the eye such as Kolliphor® P188, and some made irritation in a high concentration such as Tween® 80, and some of them like sodium dodecyl sulfate made severe irritation (Üner et al., 2021). Cationic nanocarriers in comparison to neutral or anionic carriers can bind more effectively to the negatively charged mucin (in corneal and conjunctival epithelium), enhance the retention time, and the therapeutic effect of the entrapped drugs. But the toxicity of cationic LNPs related to surfactants remains a concern. Silva et al. (2019) demonstrated that CTAB in comparison to DDAB is more toxic at the same concentration. Moreover, the structure of polymers or other compounds used in the nanocarriers preparation has a great effect on encapsulation efficiency, drug loading, drug release, and their stability against degrading enzymes, oxidative agents, hydrolysis, or light. Natural compounds can incorporate into the nanocarriers via the hydrophobic and electrostatic interactions or hydrogen bonding between natural compounds and polymers which is another determining factor in drug release and storage stability. The phenolic hydroxyl group that exists in flavonoids such as ECGC and catechin is used for fabricating many nanoparticles to enhance stability, biocompatibility, biodegradability, and safety. Polyphenols show a great tendency to polymers via noncovalent interactions (Guo et al., 2021). The presence of carbonyl and phenolic groups in the structure of natural compounds can induce noncovalent interactions with other compounds such as polymers. For instance, β-Cyclodextrin with a cone-shaped structure is hydrophilic at the outer surface due to the presence of many hydroxyl groups and hydrophobic at its cavity that can encapsulate hydrophobic drugs with suitable size such as curcumin (with phenolic and carbonyl groups) via hydrophobic interactions. Yallapu et al. (2010) demonstrated that β-cyclodextrin has higher entrapment efficiency in encapsulating curcumin in comparison to PLGA NPs. The hydrophilicity and hydrophobicity of drugs can affect the selection of nanocarriers. For example, in dual drug delivery of BBR (hydrophilic drug) and CHR (hydrophobic drug), the best vehicle to carry these drugs to the target site is liposomes to accommodate CHR in the hydrophilic core and BBR at its hydrophobic shell to control the release of drugs (Lai et al., 2019). The selection of appropriate nanocarrier in ocular drug delivery is directly related to the aim of the study such as the amount of permeation, nature of drugs, kind of ocular disease, and targeted tissues. Overly, among different nanocarriers used in ocular drug delivery, LNPs are delivery systems with both advantages of PNPs and liposomes due to their preparation methods without using toxic organic solvents. Moreover, the production process of liposomes is more expensive and complicated than LNPs production. The small size, stability, possibility to scale up, easy production, the ability of sterilization, increase corneal absorption with enhanced bioavailability and corneal retention time, positive charge of cationic LNPs (Wang et al., 2021), ability to penetrate different parts of ocular tissues especially posterior segment, and lipophilic nature of natural products (such as phenolic compounds), make LNPs as a promising carrier for ocular drug delivery of natural compounds (Faridi Esfanjani et al., 2018).
Ocular diseases as a vision-threatening disorder attracted the attention of scientists due to many challenges in conventional therapies. On the other hand, Natural compounds with many beneficial effects on the treatment and prevention of eye disorders encounter many limitations in their solubility, absorption, and bioavailability. In this review, we discussed the potency of nanotechnology to resolve these limitations and transfer therapeutic natural products to the target site. Nanotechnology merges with pharmaceutics to introduce novel compounds to solve the problems of conventional ocular drug delivery and treatment of ocular disorders. Despite a few documents on the delivery of natural products, the encapsulation of them in nanocarriers enhances their therapeutic effects, bioavailability, stability, efficiency, ocular tolerability, and reduce toxicity. However, the toxicity and scale-up production of these carriers in the industry remain a big challenge so more research on this topic needs to be undertaken for applying these nanoparticles in clinics. The aim of future studies is to enhance the therapeutic effect of natural compounds, drug targeting, bioavailability, safety, and reduce the frequency of administration by reducing the drug’s side effects and enhancing patient compliance. Moreover, drug delivery to the posterior segment of the eye is also challenging, and the most common method for drug delivery to the posterior segment is through intravitreal injection. Researchers aim to develop nanocarriers to overcome the ocular barriers and be helpful in the treatment of ocular diseases, especially those related to the posterior segment with more efficiency, safety, and reduced frequency of administrations.
A comprehensive search was carried out through the PubMed database on the articles using the combination of different terms in three various fields such as (ophthalmology, eye, ocular, cornea, retina, glaucoma, cataract, chemical burn, corneal injuries, and cornea chemical burns) AND (nano, liposomes, nanofibers, nanoparticles, nanospheres, nanocapsules, hydrogel, chitosan nanoparticles, polymeric nanoparticles, and ocular drug delivery), AND (herbs, natural products, curcumin, quercetin, and the other natural products in a row). For example combination of three terms such as eye AND nano AND curcumin, and the combination of two terms such as curcumin AND ocular drug delivery, etc., more than it, the other electronic databases used and the related articles extruded by applying the aforementioned terms noted in the references list. Only English articles published after 1990 were used, however for historical purposes a limited number of articles were included before this time.
MR: collecting data, writing, drawing pictures and editing the manuscript. PE: writing and editing the mauscript. YF: improve the picture of manuscript in final version (revised). AD’E: editing the manuscript. RD: supervisor, editing the manuscript.
The present study is part of a research project with grant number 8135, supported by Mazandaran University of Medical Sciences, Sari, Iran.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abd El-Rehim, H. A., Swilem, A. E., Klingner, A., Hegazy, E.-S. A., and Hamed, A. A. (2013). Developing the Potential Ophthalmic Applications of Pilocarpine Entrapped into Polyvinylpyrrolidone-Poly(acrylic Acid) Nanogel Dispersions Prepared by γ Radiation. Biomacromolecules 14 (3), 688–698. doi:10.1021/bm301742m
Abdallah, H. M., Al-Abd, A. M., El-Dine, R. S., and El-Halawany, A. M. (2015). P-glycoprotein Inhibitors of Natural Origin as Potential Tumor Chemo-Sensitizers: A Review. J. Adv. Res. 6 (1), 45–62. doi:10.1016/j.jare.2014.11.008
Abengózar-Vela, A., Schaumburg, C. S., Stern, M. E., and Calonge, M. (2019). Topical Quercetin and Resveratrol Protect the Ocular Surface in Experimental Dry Eye Disease. Ocul. Immunol. Inflamm. 27 (6), 1023–1032. doi:10.1080/09273948.2018.1497664
Aboali, F. A., Habib, D. A., Elbedaiwy, H. M., and Farid, R. M. (2020). Curcumin-loaded Proniosomal Gel as a Biofreindly Alternative for Treatment of Ocular Inflammation: In-Vitro and In-Vivo Assessment. Int. J. Pharmaceutics 589, 119835. doi:10.1016/j.ijpharm.2020.119835
Adelli, G. R., Srirangam, R., and Majumdar, S. (2013). Phytochemicals in Ocular Health: Therapeutic Potential and Delivery Challenges. Wjp 2 (1), 18–34. doi:10.5497/wjp.v2.i1.18
Aggarwal, B. B., Kumar, A., and Bharti, A. C. (2003). Anticancer Potential of Curcumin: Preclinical and Clinical Studies. Anticancer Res. 23 (1/A), 363–398.
Akbarzadeh, A., Rezaei-Sadabady, R., Davaran, S., Joo, S. W., Zarghami, N., Hanifehpour, Y., et al. (2013). Liposome: Classification, Preparation, and Applications. Nanoscale Res. Lett. 8 (1), 102. doi:10.1186/1556-276x-8-102
Aldina, R., Sujuti, H., Permatasari, N., and Widodo, M. A. (2019). The Effects of Genistein on Estrogen Receptor-β, IL-1β Levels, and MUC5AC Expression in Ovariectomized Rats with Dry Eye. Clin. Nutr. Exp. 27, 21–28. doi:10.1016/j.yclnex.2017.12.003
Alhebshi, A. H., Gotoh, M., and Suzuki, I. (2013). Thymoquinone Protects Cultured Rat Primary Neurons against Amyloid β-induced Neurotoxicity. Biochem. biophysical Res. Commun. 433 (4), 362–367. doi:10.1016/j.bbrc.2012.11.139
Alshamrani, M., Sikder, S., Coulibaly, F., Mandal, A., Pal, D., and Mitra, A. K. (2019). Self-Assembling Topical Nanomicellar Formulation to Improve Curcumin Absorption across Ocular Tissues. AAPS PharmSciTech 20 (7), 254. doi:10.1208/s12249-019-1404-1
Alviset, G., Corvis, Y., Hammad, K., Lemut, J., Maury, M., and Mignet, N. (2022). New Preservative-free Formulation for the Enhanced Ocular Bioavailability of Prostaglandin Analogues in Glaucoma. pharmaceutics 14 (2), 453. doi:10.3390/pharmaceutics14020453
Anisimova, NY, Kiselevsky, MV, Sosnov, AV, Sadovnikov, SV, Stankov, IN, and Gakh, AA (2011). Trans-, Cis-, and Dihydro-Resveratrol: A Comparative Study. Chem. Cent. J. 5 (1), 1–6.
Ashraf, O., Nasr, M., Nebsen, M., Said, A. M. A., and Sammour, O. (2018). In Vitro stabilization and In Vivo Improvement of Ocular Pharmacokinetics of the Multi-Therapeutic Agent Baicalin: Delineating the Most Suitable Vesicular Systems. Int. J. Pharm. 539 (1-2), 83–94. doi:10.1016/j.ijpharm.2018.01.041
Azhar Shekoufeh Bahari, L., and Hamishehkar, H. (2016). The Impact of Variables on Particle Size of Solid Lipid Nanoparticles and Nanostructured Lipid Carriers; a Comparative Literature Review. Adv. Pharm. Bull. 6 (2), 143–151. doi:10.15171/apb.2016.021
Badiee, P., Varshochian, R., Rafiee-Tehrani, M., Abedin Dorkoosh, F., Khoshayand, M. R., and Dinarvand, R. (2018). Ocular Implant Containing Bevacizumab-Loaded Chitosan Nanoparticles Intended for Choroidal Neovascularization Treatment. J. Biomed. Mater. Res. 106 (8), 2261–2271. doi:10.1002/jbm.a.36424
Battaglia, L., Gallarate, M., and Ugazio, E. (2014). Techniques for the Preparation of Solid Lipid Nano and Microparticles. Appl. nanotechnology Drug Deliv. 1, 51–75. doi:10.5772/58405
Battaglia, L., Serpe, L., Foglietta, F., Muntoni, E., Gallarate, M., Del Pozo Rodriguez, A., et al. (2016). Application of Lipid Nanoparticles to Ocular Drug Delivery. Expert Opin. Drug Deliv. 13 (12), 1743–1757. doi:10.1080/17425247.2016.1201059
Beevers, C. S., and Huang, S. (2011). Pharmacological and Clinical Properties of Curcumin. Botanics: Targets Ther. 1, 5–18.
Beloqui, A., Solinís, M. Á., Rodríguez-Gascón, A., Almeida, A. J., and Préat, V. (2016). Nanostructured Lipid Carriers: Promising Drug Delivery Systems for Future Clinics. Nanomedicine: Nanotechnology, Biol. Med. 12 (1), 143–161. doi:10.1016/j.nano.2015.09.004
Bhagurkar, A. M., Repka, M. A., and Murthy, S. N. (2017). A Novel Approach for the Development of a Nanostructured Lipid Carrier Formulation by Hot-Melt Extrusion Technology. J. Pharm. Sci. 106 (4), 1085–1091. doi:10.1016/j.xphs.2016.12.015
Bisht, R., Mandal, A., Jaiswal, J. K., and Rupenthal, I. D. (2018). Nanocarrier Mediated Retinal Drug Delivery: Overcoming Ocular Barriers to Treat Posterior Eye Diseases. Wiley Interdiscip. Rev. Nanomed Nanobiotechnol 10 (2), e1473. doi:10.1002/wnan.1473
Bodoki, E., Vostinaru, O., Samoila, O., Dinte, E., Bodoki, A. E., Swetledge, S., et al. (2019). Topical Nanodelivery System of Lutein for the Prevention of Selenite-Induced Cataract. Nanomedicine: Nanotechnology, Biol. Med. 15 (1), 188–197. doi:10.1016/j.nano.2018.09.016
Bola, C., Bartlett, H., and Eperjesi, F. (2014). Resveratrol and the Eye: Activity and Molecular Mechanisms. Graefes Arch. Clin. Exp. Ophthalmol. 252 (5), 699–713. doi:10.1007/s00417-014-2604-8
Bonilla, L., Espina, M., Severino, P., Cano, A., Ettcheto, M., Camins, A., et al. (2022). Lipid Nanoparticles for the Posterior Eye Segment. Pharmaceutics 14 (1), 90. doi:10.3390/pharmaceutics14010090
Buosi, F. S., Alaimo, A., Di Santo, M. C., Elías, F., García Liñares, G., Acebedo, S. L., et al. (2020). Resveratrol Encapsulation in High Molecular Weight Chitosan-Based Nanogels for Applications in Ocular Treatments: Impact on Human ARPE-19 Culture Cells. Int. J. Biol. Macromolecules 165, 804–821. doi:10.1016/j.ijbiomac.2020.09.234
Buscemi, S., Corleo, D., Di Pace, F., Petroni, M., Satriano, A., and Marchesini, G. (2018). The Effect of Lutein on Eye and Extra-eye Health. Nutrients 10 (9), 1321. doi:10.3390/nu10091321
Cagel, M., Tesan, F. C., Bernabeu, E., Salgueiro, M. J., Zubillaga, M. B., Moretton, M. A., et al. (2017). Polymeric Mixed Micelles as Nanomedicines: Achievements and Perspectives. Eur. J. Pharmaceutics Biopharmaceutics 113, 211–228. doi:10.1016/j.ejpb.2016.12.019
Chang, C. Y., Wang, M. C., Miyagawa, T., Chen, Z. Y., Lin, F. H., Chen, K. H., et al. (2017). Preparation of Arginine-Glycine-Aspartic Acid-Modified Biopolymeric Nanoparticles Containing Epigalloccatechin-3-Gallate for Targeting Vascular Endothelial Cells to Inhibit Corneal Neovascularization. Int. J. Nanomedicine 12, 279–294. doi:10.2147/IJN.S114754
Chang, J., Zhang, Y., Li, Y., Lu, K., Shen, Y., Guo, Y., et al. (2018). NrF2/ARE and NF-Κb Pathway Regulation May Be the Mechanism for Lutein Inhibition of Human Breast Cancer Cell. Future Oncol. 14 (8), 719–726. doi:10.2217/fon-2017-0584
Chaplot, S. P., and Rupenthal, I. D. (2014). Dendrimers for Gene Delivery - a Potential Approach for Ocular Therapy? J. Pharm. Pharmacol. 66 (4), 542–556. doi:10.1111/jphp.12104
Chebil, L., Humeau, C., Anthoni, J., Dehez, F., Engasser, J.-M., and Ghoul, M. (2007). Solubility of Flavonoids in Organic Solvents. J. Chem. Eng. Data 52 (5), 1552–1556. doi:10.1021/je7001094
Cheng, Y.-H., Ko, Y.-C., Chang, Y.-F., Huang, S.-H., and Liu, C. J.-l. (2019). Thermosensitive Chitosan-Gelatin-Based Hydrogel Containing Curcumin-Loaded Nanoparticles and Latanoprost as a Dual-Drug Delivery System for Glaucoma Treatment. Exp. Eye Res. 179, 179–187. doi:10.1016/j.exer.2018.11.017
Cheruvu, N. P. S., Amrite, A. C., and Kompella, U. B. (2008). Effect of Eye Pigmentation on Transscleral Drug Delivery. Invest. Ophthalmol. Vis. Sci. 49 (1), 333–341. doi:10.1167/iovs.07-0214
Choi, Y. H. (2016). Berberine Hydrochloride Protects C2C12 Myoblast Cells against Oxidative Stress-Induced Damage via Induction of Nrf-2-Mediated HO-1 Expression. Dru. Dev. Res. 77 (6), 310–318. doi:10.1002/ddr.21325
Chuah, L. H., Billa, N., Roberts, C. J., Burley, J. C., and Manickam, S. (2013). Curcumin-containing Chitosan Nanoparticles as a Potential Mucoadhesive Delivery System to the colon. Pharm. Dev. Technol. 18 (3), 591–599. doi:10.3109/10837450.2011.640688
Chuang, Y.-L., Fang, H.-W., Ajitsaria, A., Chen, K.-H., Su, C.-Y., Liu, G.-S., et al. (2019). Development of Kaempferol-Loaded Gelatin Nanoparticles for the Treatment of Corneal Neovascularization in Mice. Pharmaceutics 11 (12), 635. doi:10.3390/pharmaceutics11120635
Cote, B., Carlson, L. J., Rao, D. A., and Alani, A. W. G. (2015). Combinatorial Resveratrol and Quercetin Polymeric Micelles Mitigate Doxorubicin Induced Cardiotoxicity In Vitro and In Vivo. J. Controlled Release 213, 128–133. doi:10.1016/j.jconrel.2015.06.040
Crooks, R. M., Zhao, M., Sun, L., Chechik, V., and Yeung, L. K. (2001). Dendrimer-encapsulated Metal Nanoparticles: Synthesis, Characterization, and Applications to Catalysis. Acc. Chem. Res. 34 (3), 181–190. doi:10.1021/ar000110a
Darakhshan, S., Bidmeshki Pour, A., Hosseinzadeh Colagar, A., and Sisakhtnezhad, S. (2015). Thymoquinone and its Therapeutic Potentials. Pharmacol. Res. 95-96, 138–158. doi:10.1016/j.phrs.2015.03.011
Deepak, A., Goyal, A. K., and Rath, G. (2018). Nanofiber in Transmucosal Drug Delivery. J. Drug Deliv. Sci. Technology 43, 379–387. doi:10.1016/j.jddst.2017.11.008
Dong, Y., Wan, G., Yan, P., Qian, C., Li, F., and Peng, G. (2019). Fabrication of Resveratrol Coated Gold Nanoparticles and Investigation of Their Effect on Diabetic Retinopathy in Streptozotocin Induced Diabetic Rats. J. Photochem. Photobiol. B: Biol. 195, 51–57. doi:10.1016/j.jphotobiol.2019.04.012
Du, W., An, Y., He, X., and Zhang, D. (2018). Protection of Kaempferol on Oxidative Stress-Induced Retinal Pigment Epithelial Cell Damage. Oxidative Med. Cell Longevity 2018. doi:10.1155/2018/1610751
Duan, Y., Cai, X., Du, H., and Zhai, G. (2015). Novel In Situ Gel Systems Based on P123/TPGS Mixed Micelles and Gellan Gum for Ophthalmic Delivery of Curcumin. Colloids Surf. B: Biointerfaces 128, 322–330. doi:10.1016/j.colsurfb.2015.02.007
Dudhipala, N. (2019). A Comprehensive Review on Solid Lipid Nanoparticles as Delivery Vehicle for Enhanced Pharmacokinetic and Pharmacodynamic Activity of Poorly Soluble Drugs. PCI- Approved-IJPSN 12 (2), 4421–4440. doi:10.37285/ijpsn.2019.12.2.1
Ebrahimnejad, P., Dinarvand, R., Jafari, M. R., Tabasi, S. A., and Atyabi, F. (2011). Characterization, Blood Profile and Biodistribution Properties of Surface Modified PLGA Nanoparticles of SN-38. Int. J. Pharm. 406 (1-2), 122–127. doi:10.1016/j.ijpharm.2010.12.022
Ebrahimnejad, P., Dinarvand, R., Sajadi, S. A., Atyabi, F., Ramezani, F., and Jaafari, M. R. (2009). Preparation and Characterization of Poly Lactide-Co-Glycolide Nanoparticles of SN-38. PDA J. Pharm. Sci. Technol. 63 (6), 512–520.
Ebrahimnejad, P., Dinarv, P., and Sajadi, A. (2009). Development and Validation of an Ion-Pair HPLC Chromatography for Simultaneous Determination of Lactone and Carboxylate Forms of SN-38 in Nanoparticles. J. Food Drug Anal. 17 (4). doi:10.38212/2224-6614.2602
ER, A. c. d., Rajendran, N. K., Jeyaraj, M., Ramu, A., and Rajan, M. (2021). Retinal Photoreceptors Targeting SA-G-AA Coated Multilamellar Liposomes Carrier System for Cytotoxicity and Cellular Uptake Evaluation. J. Liposome Res. 31 (2), 303–16. doi:10.1080/08982104.2020.1768111
Fahmy, H. M., Saad, E. A. E.-M. S., Sabra, N. M., El-Gohary, A. A., Mohamed, F. F., and Gaber, M. H. (2018). Treatment Merits of Latanoprost/Thymoquinone - Encapsulated Liposome for Glaucomatus Rabbits. Int. J. Pharmaceutics 548 (1), 597–608. doi:10.1016/j.ijpharm.2018.07.012
Fakhravar, Z., Ebrahimnejad, P., Daraee, H., and Akbarzadeh, A. (2016). Nanoliposomes: Synthesis Methods and Applications in Cosmetics. J. Cosmet. Laser Ther. 18 (3), 174–181. doi:10.3109/14764172.2015.1039040
Fangueiro, J. F., Calpena, A. C., Clares, B., Andreani, T., Egea, M. A., Veiga, F. J., et al. (2016). Biopharmaceutical Evaluation of Epigallocatechin Gallate-Loaded Cationic Lipid Nanoparticles (EGCG-LNs): In Vivo, In Vitro and Ex Vivo Studies. Int. J. Pharm. 502 (1-2), 161–169. doi:10.1016/j.ijpharm.2016.02.039
Fangueiro, J. F., Andreani, T., Fernandes, L., Garcia, M. L., Egea, M. A., Silva, A. M., et al. (2014). Physicochemical Characterization of Epigallocatechin Gallate Lipid Nanoparticles (EGCG-LNs) for Ocular Instillation. Colloids Surf. B: Biointerfaces 123, 452–460. doi:10.1016/j.colsurfb.2014.09.042
Faridi Esfanjani, A., Assadpour, E., and Jafari, S. M. (2018). Improving the Bioavailability of Phenolic Compounds by Loading Them within Lipid-Based Nanocarriers. Trends Food Sci. Technology 76, 56–66. doi:10.1016/j.tifs.2018.04.002
Fathi, H., Sheima, R., Danial, H., and Hooman, Y. (2017). Green Synthesis of Silver Nanoparticles Using Mentha Aquatic L Extract as the Reducing Agent. J. Kerman Univ. Med. Sci. 24 (1), 28–37.
Forouzideh, N., Nadri, S., Fattahi, A., Abdolahinia, E. D., Habibizadeh, M., Rostamizadeh, K., et al. (2020). Epigallocatechin Gallate Loaded Electrospun Silk Fibroin Scaffold with Anti-angiogenic Properties for Corneal Tissue Engineering. J. Drug Deliv. Sci. Technology 56, 101498. doi:10.1016/j.jddst.2020.101498
Gan, L., Wang, J., Jiang, M., Bartlett, H., Ouyang, D., Eperjesi, F., et al. (2013). Recent Advances in Topical Ophthalmic Drug Delivery with Lipid-Based Nanocarriers. Drug Discov. Today 18 (5-6), 290–297. doi:10.1016/j.drudis.2012.10.005
Gaudana, R., Ananthula, H. K., Parenky, A., and Mitra, A. K. (2010). Ocular Drug Delivery. Aaps J. 12 (3), 348–360. doi:10.1208/s12248-010-9183-3
Gelb, M. B., Punia, A., Sellers, S., Kadakia, P., Ormes, J. D., Khawaja, N. N., et al. (2022). Effect of Drug Incorporation and Polymer Properties on the Characteristics of Electrospun Nanofibers for Drug Delivery. J. Drug Deliv. Sci. Technology 68, 103112. doi:10.1016/j.jddst.2022.103112
Ghate, D., and Edelhauser, H. F. (2006). Ocular Drug Delivery. Expert Opin. Drug Deliv. 3 (2), 275–287. doi:10.1517/17425247.3.2.275
Gorantla, S., Rapalli, V. K., Waghule, T., Singh, P. P., Dubey, S. K., Saha, R. N., et al. (2020). Nanocarriers for Ocular Drug Delivery: Current Status and Translational Opportunity. RSC Adv. 10 (46), 27835–27855. doi:10.1039/d0ra04971a
Goyal, R., Macri, L. K., Kaplan, H. M., and Kohn, J. (2016). Nanoparticles and Nanofibers for Topical Drug Delivery. J. Controlled Release 240, 77–92. doi:10.1016/j.jconrel.2015.10.049
Guo, Y., Sun, Q., Wu, F. G., Dai, Y., and Chen, X. (2021). Polyphenol‐Containing Nanoparticles: Synthesis, Properties, and Therapeutic Delivery. Adv. Mater. 33 (22), 2007356. doi:10.1002/adma.202007356
Gupta, S. C., Patchva, S., Koh, W., and Aggarwal, B. B. (2012). Discovery of Curcumin, a Component of golden Spice, and its Miraculous Biological Activities. Clin. Exp. Pharmacol. Physiol. 39 (3), 283–299. doi:10.1111/j.1440-1681.2011.05648.x
Hippalgaonkar, K., Adelli, G. R., Hippalgaonkar, K., Repka, M. A., and Majumdar, S. (2013). Indomethacin-loaded Solid Lipid Nanoparticles for Ocular Delivery: Development, Characterization, and In Vitro Evaluation. J. Ocul. Pharmacol. Ther. 29 (2), 216–228. doi:10.1089/jop.2012.0069
Hironaka, K., Inokuchi, Y., Tozuka, Y., Shimazawa, M., Hara, H., and Takeuchi, H. (2009). Design and Evaluation of a Liposomal Delivery System Targeting the Posterior Segment of the Eye. J. controlled release 136 (3), 247–253. doi:10.1016/j.jconrel.2009.02.020
Hornung, A., Poettler, M., Friedrich, R., Zaloga, J., Unterweger, H., Lyer, S., et al. (2015). Treatment Efficiency of Free and Nanoparticle-Loaded Mitoxantrone for Magnetic Drug Targeting in Multicellular Tumor Spheroids. Molecules 20 (10), 18016–18030. doi:10.3390/molecules201018016
Huang, H.-Y., Wang, M.-C., Chen, Z.-Y., Chiu, W.-Y., Chen, K.-H., Lin, I.-C., et al. (2018). Gelatin–epigallocatechin Gallate Nanoparticles with Hyaluronic Acid Decoration as Eye Drops Can Treat Rabbit Dry-Eye Syndrome Effectively via Inflammatory Relief. Ijn 13, 7251–7273. doi:10.2147/ijn.s173198
Huang, P., Xu, Y., Wei, R., Li, H., Tang, Y., Liu, J., et al. (2011). Efficacy of Tetrandrine on Lowering Intraocular Pressure in Animal Model with Ocular Hypertension. J. Glaucoma 20 (3), 183–188. doi:10.1097/ijg.0b013e3181d7882a
Hung, T.-W., Chen, P.-N., Wu, H.-C., Wu, S.-W., Tsai, P.-Y., Hsieh, Y.-S., et al. (2017). Kaempferol Inhibits the Invasion and Migration of Renal Cancer Cells through the Downregulation of AKT and FAK Pathways. Int. J. Med. Sci. 14 (10), 984–993. doi:10.7150/ijms.20336
Ibišević, M., Smajlović, A., and Arsić, I. (2019). Optimization of High Pressure Homogenization in the Production of Liposomal Dispersions. Technologica Acta Scientific/professional J. Chem. Technol. 12 (2), 7–10.
Ibrahim, A. E., Shafaa, M. W., Khedr, M. H., and Rashed, R. F. (2019). Comparative Study between Lutein and its Liposomal Form on Cisplatin-Induced Retinal Injury in Rabbits. Cutan. Ocul. Toxicol. 38 (3), 279–285. doi:10.1080/15569527.2019.1608227
Ingle, A. P., Paralikar, P., Grupenmacher, A., Padovani, F. H., Ferrer, M. T., Rai, M., et al. (2017). “Nanotechnological Interventions for Drug Delivery in Eye Diseases,” in Nanotechnology Applied to Pharmaceutical Technology (Springer), 279–306. doi:10.1007/978-3-319-70299-5_12
Jafari, M., Heidari, D., and Ebrahimnejad, P. (2016). Synthesizing and Characterizing Functionalized Short Multiwall Carbon Nanotubes with Folate, Magnetite and Polyethylene Glycol as Multi- Targeted Nanocarrier of Anti-cancer Drugs. Iran J. Pharm. Res. 15 (2), 449–456.
Jain, N., Verma, A., and Jain, N. (2020). Formulation and Investigation of Pilocarpine Hydrochloride Niosomal Gels for the Treatment of Glaucoma: Intraocular Pressure Measurement in white Albino Rabbits. Drug Deliv. 27 (1), 888–899. doi:10.1080/10717544.2020.1775726
Jo, D. H., Yun, J.-H., Cho, C. S., Kim, J. H., Kim, J. H., and Cho, C.-H. (2019). Interaction between Microglia and Retinal Pigment Epithelial Cells Determines the Integrity of Outer Blood-Retinal Barrier in Diabetic Retinopathy. Glia 67 (2), 321–331. doi:10.1002/glia.23542
Joseph, R. R., and Venkatraman, S. S. (2017). Drug Delivery to the Eye: what Benefits Do Nanocarriers Offer? Nanomedicine 12 (6), 683–702. doi:10.2217/nnm-2016-0379
Kalam, M. A., Sultana, Y., Ali, A., Aqil, M., Mishra, A. K., and Chuttani, K. (2010). Preparation, Characterization, and Evaluation of Gatifloxacin Loaded Solid Lipid Nanoparticles as Colloidal Ocular Drug Delivery System. J. Drug Target. 18 (3), 191–204. doi:10.3109/10611860903338462
Kamaleddin, M. A. (2017). Nano-ophthalmology: Applications and Considerations. Nanomedicine: Nanotechnology, Biol. Med. 13 (4), 1459–1472. doi:10.1016/j.nano.2017.02.007
Khalili, S., and Ebrahimnezhad, P. (2017). Survival of Lactobacillus Acidophilus as Probiotic Bacteria Using Chitosan Nanoparticles. Int. J. Eng. 30 (4), 456–463.
Khan, A. W., Kotta, S., Ansari, S. H., Sharma, R. K., and Ali, J. (2015). Enhanced Dissolution and Bioavailability of Grapefruit Flavonoid Naringenin by Solid Dispersion Utilizing Fourth Generation Carrier. Drug Dev. Ind. Pharm. 41 (5), 772–779. doi:10.3109/03639045.2014.902466
Khan, M. S., Vishakante, G. D., and Bathool, A. (2013). Development and Characterization of Pilocarpine Loaded Eudragit Nanosuspensions for Ocular Drug Delivery. J. Biomed. nanotechnology 9 (1), 124–131. doi:10.1166/jbn.2013.1475
Khiev, D., Mohamed, Z. A., Vichare, R., Paulson, R., Bhatia, S., Mohapatra, S., et al. (2021). Emerging Nano-Formulations and Nanomedicines Applications for Ocular Drug Delivery. Nanomaterials 11 (1), 173. doi:10.3390/nano11010173
Kim, D., Maharjan, P., Jin, M., Park, T., Maharjan, A., Amatya, R., et al. (2019). Potential Albumin-Based Antioxidant Nanoformulations for Ocular protection against Oxidative Stress. Pharmaceutics 11 (7), 297. doi:10.3390/pharmaceutics11070297
Kim, H., Choi, J.-S., Kim, K. S., Yang, J.-A., Joo, C.-K., and Hahn, S. K. (2012). Flt1 Peptide-Hyaluronate Conjugate Micelle-like Nanoparticles Encapsulating Genistein for the Treatment of Ocular Neovascularization. Acta Biomater. 8 (11), 3932–3940. doi:10.1016/j.actbio.2012.07.016
Kim, J., Han, S.-Y., and Min, H. (2020). Ginseng for an Eye: Effects of Ginseng on Ocular Diseases. J. Ginseng Res. 44 (1), 1–7. doi:10.1016/j.jgr.2018.11.006
Kim, S.-J., Kim, M.-C., Lee, B.-J., Park, D.-H., Hong, S.-H., and Um, J.-Y. (2010). Anti-Inflammatory Activity of Chrysophanol through the Suppression of NF-kB/caspase-1 Activation In Vitro and In Vivo. Molecules 15 (9), 6436–6451. doi:10.3390/molecules15096436
Kompella, U. B., Amrite, A. C., Pacha Ravi, R., and Durazo, S. A. (2013). Nanomedicines for Back of the Eye Drug Delivery, Gene Delivery, and Imaging. Prog. Retin. Eye Res. 36, 172–198. doi:10.1016/j.preteyeres.2013.04.001
Kompella, U. B., Kadam, R. S., and Lee, V. H. (2010). Recent Advances in Ophthalmic Drug Delivery. Ther. Deliv. 1 (3), 435–456. doi:10.4155/tde.10.40
Koushan, K., Rusovici, R., Li, W., Ferguson, L., and Chalam, K. (2013). The Role of Lutein in Eye-Related Disease. Nutrients 5 (5), 1823–1839. doi:10.3390/nu5051823
Lai, S., Wei, Y., Wu, Q., Zhou, K., Liu, T., Zhang, Y., et al. (2019). Liposomes for Effective Drug Delivery to the Ocular Posterior Chamber. J. Nanobiotechnology 17 (1), 64–12. doi:10.1186/s12951-019-0498-7
Lakhani, P., Patil, A., and Majumdar, S. (2018). Recent Advances in Topical Nano Drug-Delivery Systems for the Anterior Ocular Segment. Ther. Deliv. 9 (2), 137–153. doi:10.4155/tde-2017-0088
Lakhani, P., Patil, A., Taskar, P., Ashour, E., and Majumdar, S. (2018). Curcumin-loaded Nanostructured Lipid Carriers for Ocular Drug Delivery: Design Optimization and Characterization. J. Drug Deliv. Sci. Technology 47, 159–166. doi:10.1016/j.jddst.2018.07.010
Lee, C.-H., Li, Y.-J., Huang, C.-C., and Lai, J.-Y. (2017). Poly(ε-caprolactone) Nanocapsule Carriers with Sustained Drug Release: Single Dose for Long-Term Glaucoma Treatment. Nanoscale 9 (32), 11754–11764. doi:10.1039/c7nr03221h
Lee, H., Shim, W., Kim, C. E., Choi, S. Y., Lee, H., and Yang, J. (2017). Therapeutic Efficacy of Nanocomplex of Poly(Ethylene Glycol) and Catechin for Dry Eye Disease in a Mouse Model. Invest. Ophthalmol. Vis. Sci. 58 (3), 1682–1691. doi:10.1167/iovs.16-20843
Lee, S.-M., Yang, H., Tartar, D. M., Gao, B., Luo, X., Ye, S. Q., et al. (2011). Prevention and Treatment of Diabetes with Resveratrol in a Non-obese Mouse Model of Type 1 Diabetes. Diabetologia 54 (5), 1136–1146. doi:10.1007/s00125-011-2064-1
Leonardi, A., Bucolo, C., Romano, G. L., Platania, C. B., Drago, F., Puglisi, G., et al. (2014). Influence of Different Surfactants on the Technological Properties and In Vivo Ocular Tolerability of Lipid Nanoparticles. Int. J. Pharm. 470 (1-2), 133–140. doi:10.1016/j.ijpharm.2014.04.061
Li, C., Chen, R., Xu, M., Qiao, J., Yan, L., and Guo, X. D. (2018). Hyaluronic Acid Modified MPEG-B-PAE Block Copolymer Aqueous Micelles for Efficient Ophthalmic Drug Delivery of Hydrophobic Genistein. Drug Deliv. 25 (1), 1258–1265. doi:10.1080/10717544.2018.1474972
Li, J., Guo, X., Liu, Z., Okeke, C. I., Li, N., Zhao, H., et al. (2014). Preparation and Evaluation of Charged Solid Lipid Nanoparticles of Tetrandrine for Ocular Drug Delivery System: Pharmacokinetics, Cytotoxicity and Cellular Uptake Studies. Drug Dev. Ind. Pharm. 40 (7), 980–7. doi:10.3109/03639045.2013.795582
Li, J., Jin, X., Yang, Y., Zhang, L., Liu, R., Li, Z., et al. (2020). Trimethyl Chitosan Nanoparticles for Ocular Baicalein Delivery: Preparation, Optimization, In Vitro Evaluation, In Vivo Pharmacokinetic Study and Molecular Dynamics Simulation. Int. J. Biol. Macromolecules 156. doi:10.1016/j.ijbiomac.2020.04.115
Li, J., Liu, D., Tan, G., Zhao, Z., Yang, X., and Pan, W. (2016). A Comparative Study on the Efficiency of Chitosan-N-Acetylcysteine, Chitosan Oligosaccharides or Carboxymethyl Chitosan Surface Modified Nanostructured Lipid Carrier for Ophthalmic Delivery of Curcumin. Carbohydr. Polym. 146, 435–444. doi:10.1016/j.carbpol.2016.03.079
Li, M., Xin, M., Guo, C., Lin, G., and Wu, X. (2017). New Nanomicelle Curcumin Formulation for Ocular Delivery: Improved Stability, Solubility, and Ocular Anti-inflammatory Treatment. Drug Dev. Ind. Pharm. 43 (11), 1846–1857. doi:10.1080/03639045.2017.1349787
Li, M., Zhang, L., Li, R., and Yan, M. (2020). New Resveratrol Micelle Formulation for Ocular Delivery: Characterization and In Vitro/In Vivo Evaluation. Drug Development Ind. Pharm. 46 (12), 1960–1970. doi:10.1080/03639045.2020.1828909
Li, Y.-J., Luo, L.-J., Harroun, S. G., Wei, S.-C., Unnikrishnan, B., Chang, H.-T., et al. (2019). Synergistically Dual-Functional Nano Eye-Drops for Simultaneous Anti-inflammatory and Anti-oxidative Treatment of Dry Eye Disease. Nanoscale 11 (12), 5580–5594. doi:10.1039/c9nr00376b
Liang, K., Chung, J. E., Gao, S. J., Yongvongsoontorn, N., and Kurisawa, M. (2018). Highly Augmented Drug Loading and Stability of Micellar Nanocomplexes Composed of Doxorubicin and Poly(ethylene Glycol)-Green Tea Catechin Conjugate for Cancer Therapy. Adv. Mater. 30 (14), 1706963. doi:10.1002/adma.201706963
Lim, C., Kim, D.-w., Sim, T., Hoang, N. H., Lee, J. W., Lee, E. S., et al. (2016). Preparation and Characterization of a Lutein Loading Nanoemulsion System for Ophthalmic Eye Drops. J. Drug Deliv. Sci. Technology 36, 168–174. doi:10.1016/j.jddst.2016.10.009
Lin, F., Zhang, C., Chen, X., Song, E., Sun, S., Chen, M., et al. (2015). Chrysophanol Affords Neuroprotection against Microglial Activation and Free Radical-Mediated Oxidative Damage in BV2 Murine Microglia. Int. J. Clin. Exp. Med. 8 (3), 3447–3455.
Lin, J. B., Tsubota, K., and Apte, R. S. (2016). A Glimpse at the Aging Eye. Npj Aging Mech. Dis. 2, 16003. doi:10.1038/npjamd.2016.3
Lingayat, V. J., Zarekar, N. S., and Shendge, R. S. (2017). Solid Lipid Nanoparticles: a Review. Nanoscience Nanotechnology Res. 2, 67–72.
Liu, C.-H., Huang, Y.-C., Jhang, J.-W., Liu, Y.-H., and Wu, W.-C. (2015). Quercetin Delivery to Porcine Cornea and Sclera by Solid Lipid Nanoparticles and Nanoemulsion. RSC Adv. 5 (122), 100923–100933. doi:10.1039/c5ra17423f
Liu, C.-H., Lee, G.-W., Wu, W.-C., and Wang, C.-C. (2020). Encapsulating Curcumin in Ethylene Diamine-β-Cyclodextrin Nanoparticle Improves Topical Cornea Delivery. Colloids Surf. B: Biointerfaces 186, 110726. doi:10.1016/j.colsurfb.2019.110726
Liu, C. H., Chiu, H. C., Wu, W. C., Sahoo, S. L., and Hsu, C. Y. (2014). Novel Lutein Loaded Lipid Nanoparticles on Porcine Corneal Distribution. J. Ophthalmol. 2014, 304694. doi:10.1155/2014/304694
Liu, J. L., Zhang, W. J., Li, X. D., Yang, N., Pan, W. S., Kong, J., et al. (2016). Sustained-release Genistein from Nanostructured Lipid Carrier Suppresses Human Lens Epithelial Cell Growth. Int. J. Ophthalmol. 9 (5), 643–649. doi:10.18240/ijo.2016.05.01
Liu, R., Liu, Z., Zhang, C., and Zhang, B. (2012). Nanostructured Lipid Carriers as Novel Ophthalmic Delivery System for Mangiferin: Improving In Vivo Ocular Bioavailability. J. Pharm. Sci. 101 (10), 3833–3844. doi:10.1002/jps.23251
Liu, R., Sun, L., Fang, S., Wang, S., Chen, J., Xiao, X., et al. (2016). Thermosensitive In Situ Nanogel as Ophthalmic Delivery System of Curcumin: Development, Characterization, In Vitro Permeation and In Vivo Pharmacokinetic Studies. Pharm. Dev. Technol. 21 (5), 576–582. doi:10.3109/10837450.2015.1026607
Liu, R., Wang, S., Fang, S., and Wang, J. (2016). Liquid Crystalline Nanoparticles as an Ophthalmic Delivery System for Tetrandrine: Development, Characterization, and In Vitro and In Vivo Evaluation. Nanoscale Res. Lett. 11 (1), 1–12. doi:10.1186/s11671-016-1471-0
Liu, Z., Zhang, X., Li, J., Liu, R., Shu, L., and Jin, J. (2009). Effects of Labrasol on the Corneal Drug Delivery of Baicalin. Drug Deliv. 16 (7), 399–404. doi:10.1080/10717540903126165
Liu, Z., Zhang, X., Wu, H., Li, J., Shu, L., Liu, R., et al. (2011). Preparation and Evaluation of Solid Lipid Nanoparticles of Baicalin for Ocular Drug Delivery System In Vitro and In Vivo. Drug Dev. Ind. Pharm. 37 (4), 475–481. doi:10.3109/03639045.2010.522193
Loftsson, T., and Stefánsson, E. (2017). Cyclodextrins and Topical Drug Delivery to the Anterior and Posterior Segments of the Eye. Int. J. Pharmaceutics 531 (2), 413–423. doi:10.1016/j.ijpharm.2017.04.010
López-Cano, J. J., González-Cela-Casamayor, M. A., Andrés-Guerrero, V., and Herrero-Vanrell, R. (2021). Liposomes as Vehicles for Topical Ophthalmic Drug Delivery and Ocular Surface protection. Expert Opin. Drug Deliv. 18 (7), 819–847. doi:10.1080/17425247.2021.1872542
Lou, J., Hu, W., Tian, R., Zhang, H., Jia, Y., Zhang, J., et al. (2014). Optimization and Evaluation of a Thermoresponsive Ophthalmic In Situ Gel Containing Curcumin-Loaded Albumin Nanoparticles. Int. J. Nanomedicine 9, 2517–2525. doi:10.2147/IJN.S60270
Luo, L. J., and Lai, J. Y. (2017). Epigallocatechin Gallate-Loaded Gelatin-G-Poly(N-Isopropylacrylamide) as a New Ophthalmic Pharmaceutical Formulation for Topical Use in the Treatment of Dry Eye Syndrome. Sci. Rep. 7 (1), 1–14. doi:10.1038/s41598-017-09913-8
Ma, L., Liu, Y.-L., Ma, Z.-Z., Dou, H.-L., Xu, J.-H., Wang, J.-C., et al. (2009). Targeted Treatment of Choroidal Neovascularization Using Integrin-Mediated Sterically Stabilized Liposomes Loaded with Combretastatin A4. J. Ocul. Pharmacol. Ther. 25 (3), 195–200. doi:10.1089/jop.2008.0119
Madni, A., Rahem, M. A., Tahir, N., Sarfraz, M., Jabar, A., Rehman, M., et al. (2017). Non-invasive Strategies for Targeting the Posterior Segment of Eye. Int. J. Pharm. 530 (1-2), 326–345. doi:10.1016/j.ijpharm.2017.07.065
Maharjan, P., Jin, M., Kim, D., Yang, J., Maharjan, A., Shin, M. C., et al. (2019). Evaluation of Epithelial Transport and Oxidative Stress protection of Nanoengineered Curcumin Derivative-Cyclodextrin Formulation for Ocular Delivery. Arch. Pharm. Res. 42 (10), 909–925. doi:10.1007/s12272-019-01154-9
Mains, J., and Wilson, C. G. (2013). The Vitreous Humor as a Barrier to Nanoparticle Distribution. J. Ocul. Pharmacol. Ther. 29 (2), 143–150. doi:10.1089/jop.2012.0138
Mandal, A., Bisht, R., Rupenthal, I. D., and Mitra, A. K. (2017). Polymeric Micelles for Ocular Drug Delivery: from Structural Frameworks to Recent Preclinical Studies. J. Controlled Release 248, 96–116. doi:10.1016/j.jconrel.2017.01.012
Mathur, S., and Hoskins, C. (2017). Drug Development: Lessons from Nature. Biomed. Rep. 6 (6), 612–614. doi:10.3892/br.2017.909
Maulvi, F. A., Desai, D. T., Shetty, K. H., Shah, D. O., and Willcox, M. D. P. (2021). Advances and Challenges in the Nanoparticles-Laden Contact Lenses for Ocular Drug Delivery. Int. J. Pharmaceutics 608, 121090. doi:10.1016/j.ijpharm.2021.121090
Mehnert, W., and Mäder, K. (2012). Solid Lipid Nanoparticles. Adv. Drug Deliv. Rev. 64, 83–101. doi:10.1016/j.addr.2012.09.021
Mignani, S., El Kazzouli, S., Bousmina, M., and Majoral, J.-P. (2013). Expand Classical Drug Administration Ways by Emerging Routes Using Dendrimer Drug Delivery Systems: a Concise Overview. Adv. Drug Deliv. Rev. 65 (10), 1316–1330. doi:10.1016/j.addr.2013.01.001
Mir, M., and Ebrahimnejad, P. (2014). Preparation and Characterization of Bifunctional Nanoparticles of Vitamin E TPGS-Emulsified PLGA-PEG-FOL Containing Deferasirox. Nanoscience & Nanotechnology-Asia 4 (2), 80–87.
Mukherjee, S., Ray, S., and Thakur, R. (2009). Solid Lipid Nanoparticles: a Modern Formulation Approach in Drug Delivery System. Indian J. Pharm. Sci. 71 (4), 349. doi:10.4103/0250-474x.57282
Müller, R. H., Mäder, K., and Gohla, S. (2000). Solid Lipid Nanoparticles (SLN) for Controlled Drug Delivery–A Review of the State of the Art. Eur. J. pharmaceutics biopharmaceutics 50 (1), 161–177.
Nair, K. L., Vidyanand, S., James, J., and Kumar, G. S. V. (2012). Pilocarpine-loaded poly(DL-Lactic-Co-Glycolic Acid) Nanoparticles as Potential Candidates for Controlled Drug Delivery with Enhanced Ocular Pharmacological Response. J. Appl. Polym. Sci. 124 (3), 2030–2036. doi:10.1002/app.35229
Naseri, N., Valizadeh, H., and Zakeri-Milani, P. (2015). Solid Lipid Nanoparticles and Nanostructured Lipid Carriers: Structure, Preparation and Application. Adv. Pharm. Bull. 5 (3), 305–313. doi:10.15171/apb.2015.043
Natesan, S., Pandian, S., Ponnusamy, C., Palanichamy, R., Muthusamy, S., and Kandasamy, R. (2017). Co-encapsulated Resveratrol and Quercetin in Chitosan and Peg Modified Chitosan Nanoparticles: for Efficient Intra Ocular Pressure Reduction. Int. J. Biol. macromolecules 104, 1837–1845. doi:10.1016/j.ijbiomac.2017.04.117
Nejima, R., Miyata, K., Tanabe, T., Okamoto, F., Hiraoka, T., Kiuchi, T., et al. (2005). Corneal Barrier Function, Tear Film Stability, and Corneal Sensation after Photorefractive Keratectomy and Laser In Situ Keratomileusis. Am. J. Ophthalmol. 139 (1), 64–71. doi:10.1016/j.ajo.2004.08.039
Noureddin, S. A., El-Shishtawy, R. M., and Al-Footy, K. O. (2019). Curcumin Analogues and Their Hybrid Molecules as Multifunctional Drugs. Eur. J. Med. Chem. 182, 111631. doi:10.1016/j.ejmech.2019.111631
Ogunjimi, A. T., Melo, S. M. G., Vargas-Rechia, C. G., Emery, F. S., and Lopez, R. F. V. (2017). Hydrophilic Polymeric Nanoparticles Prepared from Delonix Galactomannan with Low Cytotoxicity for Ocular Drug Delivery. Carbohydr. Polym. 157, 1065–1075. doi:10.1016/j.carbpol.2016.10.076
Omerović, N., and Vranić, E. (2019). Application of Nanoparticles in Ocular Drug Delivery Systems. Health Technology 10, 1–18. doi:10.1007/s12553-019-00381-w
Oude Blenke, E., Mastrobattista, E., and Schiffelers, R. M. (2013). Strategies for Triggered Drug Release from Tumor Targeted Liposomes. Expert Opin. Drug Deliv. 10 (10), 1399–1410. doi:10.1517/17425247.2013.805742
Pandian, S., Jeevanesan, V., Ponnusamy, C., and Natesan, S. (2016). RES‐loaded Pegylated CS NPs: for Efficient Ocular Delivery. IET nanobiotechnol. 11 (1), 32–39. doi:10.1049/iet-nbt.2016.0069
Paolicelli, P., Prego, C., Sanchez, A., and Alonso, M. J. (2010). Surface-modified PLGA-Based Nanoparticles that Can Efficiently Associate and Deliver Virus-like Particles. Nanomedicine 5 (6), 843–853. doi:10.2217/nnm.10.69
Patra, J. K., Das, G., Fraceto, L. F., Campos, E. V. R., Rodriguez-Torres, M. d. P., Acosta-Torres, L. S., et al. (2018). Nano Based Drug Delivery Systems: Recent Developments and Future Prospects. J. Nanobiotechnol 16 (1), 71. doi:10.1186/s12951-018-0392-8
Patri, A. K., Majoros, I. J., and Baker, J. R. (2002). Dendritic Polymer Macromolecular Carriers for Drug Delivery. Curr. Opin. Chem. Biol. 6 (4), 466–471. doi:10.1016/s1367-5931(02)00347-2
Pearce, W., Hsu, J., and Yeh, S. (2015). Advances in Drug Delivery to the Posterior Segment. Curr. Opin. Ophthalmol. 26 (3), 233–239. doi:10.1097/icu.0000000000000143
Pooja, D., Kadari, A., Kulhari, H., and Sistla, R. (2018). “Lipid-based Nanomedicines,” in Lipid Nanocarriers for Drug Targeting (Elsevier), 509–528. doi:10.1016/b978-0-12-813687-4.00013-x
Prausnitz, M. R., and Noonan, J. S. (1998). Permeability of Cornea, Sclera, and Conjunctiva: a Literature Analysis for Drug Delivery to the Eye. J. Pharm. Sci. 87 (12), 1479–1488. doi:10.1021/js9802594
Priwitaningrum, D. L., Blondé, J.-B. G., Sridhar, A., van Baarlen, J., Hennink, W. E., Storm, G., et al. (2016). Tumor Stroma-Containing 3D Spheroid Arrays: A Tool to Study Nanoparticle Penetration. J. controlled release 244, 257–268. doi:10.1016/j.jconrel.2016.09.004
Prow, T. W. (2010). Toxicity of Nanomaterials to the Eye. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology 2 (4), 317–33. doi:10.1002/wnan.65
Radomska-Leśniewska, D. M., Osiecka-Iwan, A., Hyc, A., Góźdź, A., Dąbrowska, A. M., and Skopiński, P. (2019). Therapeutic Potential of Curcumin in Eye Diseases. Cent. Eur. J. Immunol. 44 (2), 181–189. doi:10.5114/ceji.2019.87070
Raghava, S., Hammond, M., and Kompella, U. B. (2004). Periocular Routes for Retinal Drug Delivery. Expert Opin. Drug Deliv. 1 (1), 99–114. doi:10.1517/17425247.1.1.99
Rajpoot, K. (2019). Solid Lipid Nanoparticles: a Promising Nanomaterial in Drug Delivery. Cpd 25 (37), 3943–3959. doi:10.2174/1381612825666190903155321
Rameez, S., Bamba, I., and Palmer, A. F. (2010). Large Scale Production of Vesicles by Hollow Fiber Extrusion: a Novel Method for Generating Polymersome Encapsulated Hemoglobin Dispersions. Langmuir 26 (7), 5279–5285. doi:10.1021/la9036343
Reimondez-Troitiño, S., Csaba, N., Alonso, M. J., and de la Fuente, M. (2015). Nanotherapies for the Treatment of Ocular Diseases. Eur. J. Pharmaceutics Biopharmaceutics 95, 279–293. doi:10.1016/j.ejpb.2015.02.019
Rodríguez Villanueva, J., Navarro, M. G., and Rodríguez Villanueva, L. (2016). Dendrimers as a Promising Tool in Ocular Therapeutics: Latest Advances and Perspectives. Int. J. pharmaceutics 511 (1), 359–366. doi:10.1016/j.ijpharm.2016.07.031
Ruginǎ, D., Ghiman, R., Focșan, M., Tăbăran, F., Copaciu, F., Suciu, M., et al. (2019). Resveratrol-delivery Vehicle with Anti-VEGF Activity Carried to Human Retinal Pigmented Epithelial Cells Exposed to High-Glucose Induced Conditions. Colloids Surf. B Biointerfaces 181, 66–75. doi:10.1016/j.colsurfb.2019.04.022
Sabzevari, A., Adibkia, K., Hashemi, H., De Geest, B. G., Mohsenzadeh, N., Atyabi, F., et al. (2013). Improved Anti-inflammatory Effects in Rabbit Eye Model Using Biodegradable Poly Beta-Amino Ester Nanoparticles of Triamcinolone Acetonide. Invest. Ophthalmol. Vis. Sci. 54 (8), 5520–5526. doi:10.1167/iovs.13-12296
Sadeghi Ghadi, Z., Dinarvand, R., Asemi, N., Talebpour Amiri, F., and Ebrahimnejad, P. (2019). Preparation, Characterization and In Vivo Evaluation of Novel Hyaluronan Containing Niosomes Tailored by Box-Behnken Design to Co-encapsulate Curcumin and Quercetin. Eur. J. Pharm. Sci. 130 (2), 234–246. doi:10.1016/j.ejps.2019.01.035
Sadeghi Ghadi, Z., and Ebrahimnejad, P. (2019). Curcumin Entrapped Hyaluronan Containing Niosomes: Preparation, Characterisation and In Vitro/In Vivo Evaluation. J. microencapsulation 36 (2), 169–179. doi:10.1080/02652048.2019.1617360
Sadeghi-Ghadi, Z., Vaezi, A., Ahangarkani, F., Ilkit, M., Ebrahimnejad, P., and Badali, H. (2020). Potent In Vitro Activity of Curcumin and Quercetin Co-encapsulated in Nanovesicles without Hyaluronan against Aspergillus and Candida Isolates. J. de Mycologie Médicale 30, 101014. doi:10.1016/j.mycmed.2020.101014
Sahoo, N. G., Kakran, M., Shaal, L. A., Li, L., Müller, R. H., Pal, M., et al. (2011). Preparation and Characterization of Quercetin Nanocrystals. J. Pharm. Sci. 100 (6), 2379–2390. doi:10.1002/jps.22446
Sai, N., Dong, X., and You, L. (2020). A Novel Gel-Forming Solution Based on PEG-DSPE/Solutol HS 15 Mixed Micelles and Gellan Gum for Ophthalmic Delivery of Curcumin. Molecules 25 (1), 81. doi:10.3390/molecules25010081
Salehi, B., Fokou, P., Sharifi-Rad, M., Zucca, P., Pezzani, R., Martins, N., et al. (2019). The Therapeutic Potential of Naringenin: a Review of Clinical Trials. Pharmaceuticals 12 (1), 11. doi:10.3390/ph12010011
Salimi, A. (2018). Liposomes as a Novel Drug Delivery System: Fundamental and Pharmaceutical Application. Asian J. Pharmaceutics (Ajp) Free full text articles Asian J Pharm 12 (01).
Sánchez-López, E., Espina, M., Doktorovova, S., and Souto, E. B. (2017). Lipid Nanoparticles (SLN, NLC): Overcoming the Anatomical and Physiological Barriers of the Eye–Part II-Ocular Drug-Loaded Lipid Nanoparticles. Eur. J. Pharmaceutics Biopharmaceutics 110, 58–69. doi:10.1016/j.ejpb.2016.10.013
Sharifi, F., Nazir, I., Asim, M. H., Jahangiri, M., Ebrahimnejad, P., Matuszczak, B., et al. (2019). Zeta Potential Changing Self-Emulsifying Drug Delivery Systems Utilizing a Novel Janus-Headed Surfactant: A Promising Strategy for Enhanced Mucus Permeation. J. Mol. Liquids 291 (15), 111285–111295. doi:10.1016/j.molliq.2019.111285
Sharma, D., Ali, A. A. E., and Trivedi, L. R. (2018). An Updated Review On:Liposomes as Drug Delivery System. Pt 6 (2), 50–62. doi:10.29161/pt.v6.i2.2018.50
Sharma, R. A., Gescher, A. J., and Steward, W. P. (2005). Curcumin: the story So Far. Eur. J. Cancer 41 (13), 1955–1968. doi:10.1016/j.ejca.2005.05.009
Shen, H.-H., Chan, E. C., Lee, J. H., Bee, Y.-S., Lin, T.-W., Dusting, G. J., et al. (2015). Nanocarriers for Treatment of Ocular Neovascularization in the Back of the Eye: New Vehicles for Ophthalmic Drug Delivery. Nanomedicine 10 (13), 2093–2107. doi:10.2217/nnm.15.47
Shi, N.-Q., and Qi, X.-R. (2018). Preparation of Drug Liposomes by Reverse-phase Evaporation. Lipos.-Based Drug Deliv. Syst., 1–10. doi:10.1007/978-3-662-49231-4_3-1
Shim, W., Kim, C. E., Lee, M., Lee, S. H., Park, J., Do, M., et al. (2019). Catechin Solubilization by Spontaneous Hydrogen Bonding with Poly(ethylene Glycol) for Dry Eye Therapeutics. J. Controlled Release 307, 413–422. doi:10.1016/j.jconrel.2019.04.016
Shimamura, T., Zhao, W.-H., and Hu, Z.-Q. (2007). Mechanism of Action and Potential for Use of tea Catechin as an Antiinfective Agent. Aiamc 6 (1), 57–62. doi:10.2174/187152107779314124
Shishodia, S., Sethi, G., and Aggarwal, B. B. (2005). Curcumin: Getting Back to the Roots. Ann. New York Acad. Sci. 1056 (1), 206–217. doi:10.1196/annals.1352.010
Silva, A. C., González-Mira, E., García, M. L., Egea, M. A., Fonseca, J., Silva, R., et al. (2011). Preparation, Characterization and Biocompatibility Studies on Risperidone-Loaded Solid Lipid Nanoparticles (SLN): High Pressure Homogenization versus Ultrasound. Colloids Surf. B: Biointerfaces 86 (1), 158–165. doi:10.1016/j.colsurfb.2011.03.035
Silva, A., Martins-Gomes, C., Coutinho, T., Fangueiro, J., Sanchez-Lopez, E., Pashirova, T., et al. (2019). Soft Cationic Nanoparticles for Drug Delivery: Production and Cytotoxicity of Solid Lipid Nanoparticles (SLNs). Appl. Sci. 9 (20), 4438. doi:10.3390/app9204438
Silva, B., São Braz, B., Delgado, E., and Gonçalves, L. (2021). Colloidal Nanosystems with Mucoadhesive Properties Designed for Ocular Topical Delivery. Int. J. Pharmaceutics 606, 120873. doi:10.1016/j.ijpharm.2021.120873
Singh, B. N., Shankar, S., and Srivastava, R. K. (2011). Green tea Catechin, Epigallocatechin-3-Gallate (EGCG): Mechanisms, Perspectives and Clinical Applications. Biochem. Pharmacol. 82 (12), 1807–1821. doi:10.1016/j.bcp.2011.07.093
Sowndhararajan, K., Deepa, P., Kim, M., Park, S., and Kim, S. (2018). Neuroprotective and Cognitive Enhancement Potentials of Baicalin: a Review. Brain Sci. 8 (6), 104. doi:10.3390/brainsci8060104
Spizzirri, U. G., Iemma, F., Puoci, F., Cirillo, G., Curcio, M., Parisi, O. I., et al. (2009). Synthesis of Antioxidant Polymers by Grafting of Gallic Acid and Catechin on Gelatin. Biomacromolecules 10 (7), 1923–1930. doi:10.1021/bm900325t
Sulaiman, R. S., Basavarajappa, H. D., and Corson, T. W. (2014). Natural Product Inhibitors of Ocular Angiogenesis. Exp. Eye Res. 129, 161–171. doi:10.1016/j.exer.2014.10.002
Sultana, Y., Maurya, D. P., Iqbal, Z., and Aqil, M. (2011). Nanotechnology in Ocular Delivery: Current and Future Directions. Drugs47 (6), 441–455. doi:10.1358/dot.2011.47.6.1549023
Suri, R., Beg, S., and Kohli, K. (2020). Target Strategies for Drug Delivery Bypassing Ocular Barriers. J. Drug Deliv. Sci. Technology 55, 101389. doi:10.1016/j.jddst.2019.101389
Svitova, T. F., and Lin, M. C. (2010). Tear Lipids Interfacial Rheology: Effect of Lysozyme and Lens Care Solutions. Optom. Vis. Sci. official Publ. Am. Acad. Optom. 87 (1), 10–20. doi:10.1097/opx.0b013e3181c07908
Tahara, K., Karasawa, K., Onodera, R., and Takeuchi, H. (2017). Feasibility of Drug Delivery to the Eye's Posterior Segment by Topical Instillation of PLGA Nanoparticles. Asian J. Pharm. Sci. 12 (4), 394–399. doi:10.1016/j.ajps.2017.03.002
Tamilvanan, S., and Kumar, B. A. (2011). Influence of Acetazolamide Loading on the (In Vitro) Performances of Non-phospholipid-based Cationic Nanosized Emulsion in Comparison with Phospholipid-Based Anionic and Neutral-Charged Nanosized Emulsions. Drug Dev. Ind. Pharm. 37 (9), 1003–1015. doi:10.3109/03639045.2011.555407
Tian, B.-C., Zhang, W.-J., Xu, H.-M., Hao, M.-X., Liu, Y.-B., Yang, X.-G., et al. (2013). Further Investigation of Nanostructured Lipid Carriers as an Ocular Delivery System: In Vivo Transcorneal Mechanism and In Vitro Release Study. Colloids Surf. B: Biointerfaces 102, 251–256. doi:10.1016/j.colsurfb.2012.08.021
Tian, B., Luo, Q., Song, S., Liu, D., Pan, H., Zhang, W., et al. (2012). Novel Surface-Modified Nanostructured Lipid Carriers with Partially Deacetylated Water-Soluble Chitosan for Efficient Ocular Delivery. J. Pharm. Sci. 101 (3), 1040–1049. doi:10.1002/jps.22813
Toragall, V., Jayapala, N., and Vallikannan, B. (2020). Chitosan-oleic Acid-Sodium Alginate a Hybrid Nanocarrier as an Efficient Delivery System for Enhancement of Lutein Stability and Bioavailability. Int. J. Biol. macromolecules 150, 578–594. doi:10.1016/j.ijbiomac.2020.02.104
Üner, B., Özdemir, S., Özsoy, Y., and Taş, C. (2021). Development of Solid Lipid Nanoparticles and Nanostructured Lipid Carriers of Loteprednol Etabonate: Physicochemical Characterization and Ex Vivo Permeation Studies. doi:10.21203/rs.3.rs-1145116/v1
Üstündağ Okur, N., Gökçe, E. H., and Homan Gökçe, E. (2015). Lipid Nanoparticles for Ocular Drug Delivery. Int. J. Ophthalmic Res. 1 (3), 77–82. doi:10.17554/j.issn.2409-5680.2015.01.29
Vafaei, S. Y., Dinarvand, R., Esmaeili, M., Mahjub, R., and Toliyat, T. (2015). Controlled-release Drug Delivery System Based on Fluocinolone Acetonide-Cyclodextrin Inclusion Complex Incorporated in Multivesicular Liposomes. Pharm. Dev. Technol. 20 (7), 775–781. doi:10.3109/10837450.2014.920358
Vandamme, T. F., and Brobeck, L. (2005). Poly(amidoamine) Dendrimers as Ophthalmic Vehicles for Ocular Delivery of Pilocarpine Nitrate and Tropicamide. J. controlled release 102 (1), 23–38. doi:10.1016/j.jconrel.2004.09.015
Varela-Fernández, R., Díaz-Tomé, V., Luaces-Rodríguez, A., and Conde-Penedo, A. (2020). Drug Delivery to the Posterior Segment of the Eye: Biopharmaceutic and Pharmacokinetic Considerations. Pharmaceutics 12 (3), 269. doi:10.3390/pharmaceutics12030269
Varshochian, R., Riazi-Esfahani, M., Jeddi-Tehrani, M., Mahmoudi, A.-R., Aghazadeh, S., Mahbod, M., et al. (2015). Albuminated PLGA Nanoparticles Containing Bevacizumab Intended for Ocular Neovascularization Treatment. J. Biomed. Mater. Res. 103 (10), 3148–3156. doi:10.1002/jbm.a.35446
Venkatraman, S., Natrajan, J. V., Howden, T., and Boey, F.Inventors; Nanyang Technological University, Singapore Health Services Pte Ltd, assignee (2018). Stable Liposomal Formulations for Ocular Drug Delivery. United States: Patent US 9,956,195.
Wadhwa, S., Paliwal, R., Paliwal, S., and Vyas, S. (2009). Nanocarriers in Ocular Drug Delivery: an Update Review. Cpd 15 (23), 2724–2750. doi:10.2174/138161209788923886
Wallace, T. C., Blumberg, J. B., Johnson, E. J., and Shao, A. (2015). Dietary Bioactives: Establishing a Scientific Framework for Recommended Intakes. Adv. Nutr. 6 (1), 1–4. doi:10.3945/an.114.007294
Wang, R., Gao, Y., Liu, A., and Zhai, G. (2021). A Review of Nanocarrier-Mediated Drug Delivery Systems for Posterior Segment Eye Disease: Challenges Analysis and Recent Advances. J. Drug Target. 29 (7), 687–702. doi:10.1080/1061186x.2021.1878366
Wen, S.-Q., Jeyakkumar, P., Avula, S. R., Zhang, L., and Zhou, C.-H. (2016). Discovery of Novel Berberine Imidazoles as Safe Antimicrobial Agents by Down Regulating ROS Generation. Bioorg. Med. Chem. Lett. 26 (12), 2768–2773. doi:10.1016/j.bmcl.2016.04.070
Wilczewska, A. Z., Niemirowicz, K., Markiewicz, K. H., and Car, H. (2012). Nanoparticles as Drug Delivery Systems. Pharmacol. Rep. 64 (5), 1020–1037. doi:10.1016/s1734-1140(12)70901-5
William, B., Noémie, P., Brigitte, E., and Géraldine, P. (2020). Supercritical Fluid Methods: An Alternative to Conventional Methods to Prepare Liposomes. Chem. Eng. J. 383, 123106. doi:10.1016/j.cej.2019.123106
Wu, H., Liu, Z., Peng, J., Li, L., Li, N., Li, J., et al. (2011). Design and Evaluation of Baicalin-Containing In Situ pH-Triggered Gelling System for Sustained Ophthalmic Drug Delivery. Int. J. Pharm. 410 (1-2), 31–40. doi:10.1016/j.ijpharm.2011.03.007
Wu, Y., Yang, W., Wang, C., Hu, J., and Fu, S. (2005). Chitosan Nanoparticles as a Novel Delivery System for Ammonium Glycyrrhizinate. Int. J. Pharm. 295 (1-2), 235–245. doi:10.1016/j.ijpharm.2005.01.042
Xiao, F., Cui, H., and Zhong, X. (2018). Beneficial Effect of Daidzin in Dry Eye Rat Model through the Suppression of Inflammation and Oxidative Stress in the Cornea. Saudi J. Biol. Sci. 25 (4), 832–837. doi:10.1016/j.sjbs.2016.11.016
Yallapu, M. M., Jaggi, M., and Chauhan, S. C. (2010). β-Cyclodextrin-curcumin Self-Assembly Enhances Curcumin Delivery in Prostate Cancer Cells. Colloids Surf. B: Biointerfaces 79 (1), 113–125. doi:10.1016/j.colsurfb.2010.03.039
Yang, H., Tyagi, P., Kadam, R. S., Holden, C. A., and Kompella, U. B. (2012). Hybrid Dendrimer Hydrogel/PLGA Nanoparticle Platform Sustains Drug Delivery for One Week and Antiglaucoma Effects for Four Days Following One-Time Topical Administration. ACS nano 6 (9), 7595–7606. doi:10.1021/nn301873v
Yang, L., Yin, T., Liu, Y., Sun, J., Zhou, Y., and Liu, J. (2016). Gold Nanoparticle-Capped Mesoporous Silica-Based H2O2-Responsive Controlled Release System for Alzheimer's Disease Treatment. Acta Biomater. 46, 177–190. doi:10.1016/j.actbio.2016.09.010
Yavuz, B., Pehlivan, S. B., Vural, İ., and Ünlü, N. (2015). In Vitro/In Vivo Evaluation of Dexamethasone-PAMAM Dendrimer Complexes for Retinal Drug Delivery. J. Pharm. Sci. 104 (11), 3814–3823. doi:10.1002/jps.24588
Yee, E. M. H., Brandl, M. B., Pasquier, E., Cirillo, G., Kimpton, K., Kavallaris, M., et al. (2017). Dextran-catechin Inhibits Angiogenesis by Disrupting Copper Homeostasis in Endothelial Cells. Sci. Rep. 7 (1), 1–10. doi:10.1038/s41598-017-07452-w
Yellepeddi, V. K., and Palakurthi, S. (2016). Recent Advances in Topical Ocular Drug Delivery. J. Ocul. Pharmacol. Ther. 32 (2), 67–82. doi:10.1089/jop.2015.0047
Yu, Y., Feng, R., Li, J., Wang, Y., Song, Y., Tan, G., et al. (2019). A Hybrid Genipin-Crosslinked Dual-Sensitive Hydrogel/nanostructured Lipid Carrier Ocular Drug Delivery Platform. Asian J. Pharm. Sci. 14 (4), 423–434. doi:10.1016/j.ajps.2018.08.002
Yu, Y., Feng, R., Yu, S., Li, J., Wang, Y., Song, Y., et al. (2018). Nanostructured Lipid Carrier-Based pH and Temperature Dual-Responsive Hydrogel Composed of Carboxymethyl Chitosan and Poloxamer for Drug Delivery. Int. J. Biol. macromolecules 114, 462–469. doi:10.1016/j.ijbiomac.2018.03.117
Yu, Y., Xu, S., Yu, S., Li, J., Tan, G., Li, S., et al. (2020). A Hybrid Genipin-Cross-Linked Hydrogel/Nanostructured Lipid Carrier for Ocular Drug Delivery: Cellular, Ex Vivo, and In Vivo Evaluation. ACS Biomater. Sci. Eng. 6 (3), 1543–52. doi:10.1021/acsbiomaterials.9b01800
Zahir‐Jouzdani, F., Khonsari, F., Soleimani, M., Mahbod, M., Arefian, E., Heydari, M., et al. (2019). Nanostructured Lipid Carriers Containing Rapamycin for Prevention of Corneal Fibroblasts Proliferation and Haze Propagation after Burn Injuries: In Vitro and In Vivo. J. Cell. Physiol. 234 (4), 4702–4712. doi:10.1002/jcp.27243
Zhang, H. (2017). “Thin-film Hydration Followed by Extrusion Method for Liposome Preparation,” in Liposomes (Springer), 17–22. doi:10.1007/978-1-4939-6591-5_2
Zhang, P., Liu, X., Hu, W., Bai, Y., and Zhang, L. (2016). Preparation and Evaluation of Naringenin-Loaded Sulfobutylether-β-Cyclodextrin/chitosan Nanoparticles for Ocular Drug Delivery. Carbohydr. Polym. 149, 224–230. doi:10.1016/j.carbpol.2016.04.115
Zhang, W., Li, X., Ye, T., Chen, F., Yu, S., Chen, J., et al. (2014). Nanostructured Lipid Carrier Surface Modified with Eudragit RS 100 and its Potential Ophthalmic Functions. Int. J. Nanomedicine 9, 4305–4315. doi:10.2147/IJN.S63414
Zhang, W., Liu, J., Zhang, Q., Li, X., Yu, S., Yang, X., et al. (2014). Enhanced Cellular Uptake and Anti-proliferating Effect of Chitosan Hydrochlorides Modified Genistein Loaded NLC on Human Lens Epithelial Cells. Int. J. Pharm. 471 (1-2), 118–126. doi:10.1016/j.ijpharm.2014.05.030
Zhang, W., Li, X., Ye, T., Chen, F., Sun, X., Kong, J., et al. (2013). Design, Characterization, and In Vitro Cellular Inhibition and Uptake of Optimized Genistein-Loaded NLC for the Prevention of Posterior Capsular Opacification Using Response Surface Methodology. Int. J. pharmaceutics 454 (1), 354–366. doi:10.1016/j.ijpharm.2013.07.032
Zhao, R., Li, J., Wang, J., Yin, Z., Zhu, Y., and Liu, W. (2017). Development of Timolol-Loaded Galactosylated Chitosan Nanoparticles and Evaluation of Their Potential for Ocular Drug Delivery. AAPS PharmSciTech 18 (4), 997–1008. doi:10.1208/s12249-016-0669-x
Zhuang, P., Shen, Y., Lin, B. Q., Zhang, W. Y., and Chiou, G. C. (2011). Effect of Quercetin on Formation of Choroidal Neovascularization (CNV) in Age-Related Macular Degeneration(AMD). Eye Sci. 26 (1), 23–29. doi:10.3969/j.issn.1000-4432.2011.01.006
Keywords: nanotechnology, nanoparticles, natural products, ocular drug delivery, eye
Citation: Razavi MS, Ebrahimnejad P, Fatahi Y, D’Emanuele A and Dinarvand R (2022) Recent Developments of Nanostructures for the Ocular Delivery of Natural Compounds. Front. Chem. 10:850757. doi: 10.3389/fchem.2022.850757
Received: 08 January 2022; Accepted: 11 March 2022;
Published: 13 April 2022.
Edited by:
Shengpeng Wang, University of Macau, ChinaCopyright © 2022 Razavi, Ebrahimnejad, Fatahi, D’Emanuele and Dinarvand. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rassoul Dinarvand, ZGluYXJ2YW5kQHR1bXMuYWMuaXI=; Pedram Ebrahimnejad, cGVicmFoaW1uZWphZEBtYXp1bXMuYWMuaXI=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.