- 1Department of Chemistry, Loker Hydrocarbon Research Institute, University of Southern California, Los Angeles, CA, United States
- 2Bridge Institute, USC Michelson Center for Convergent Bioscience, University of Southern California, Los Angeles, CA, United States
Time-dependent monitoring of the reactive intermediates provides valuable information about the mechanism of a synthetic transformation. However, the process frequently involves intermediates with short lifetimes that significantly challenge the accessibility of the desired kinetic data. We report in situ cyclic voltammetry (CV) and nuclear magnetic resonance (NMR) spectroscopy studies of the cycloaddition reaction of organobismuth(III) compounds with organic azides under the copper(I)-catalyzed conditions. A series of bismuth(III) acetylides carrying diphenyl sulfone scaffolds have been synthesized to study the underlying electronic and steric effects of the tethered moieties capable of transannular oxygen O···Bi interactions and para-functionality of the parent phenylacetylene backbones. While belonging to the family of copper-catalyzed azide-alkyne cycloaddition reactions, the reaction yielding 5-bismuth(III)-triazolide is the sole example of a complex catalytic transformation that features activity of bismuth(III) acetylides towards organic azides under copper(I)-catalyzed conditions. Stepwise continuous monitoring of the copper(I)/copper(0) redox activity of the copper(I) catalyst by cyclic voltammetry provided novel insights into the complex catalytic cycle of the bismuth(III)-triazolide formation. From CV-derived kinetic data, reaction rate parameters of the bismuth(III) acetylides coordination to the copper(I) catalyst (KA) and equilibrium concentration of the copper species [cat]eq. are compared with the overall 5-bismuth(III)-triazolide formation rate constant kobs obtained by 1H-NMR kinetic analysis.
Introduction
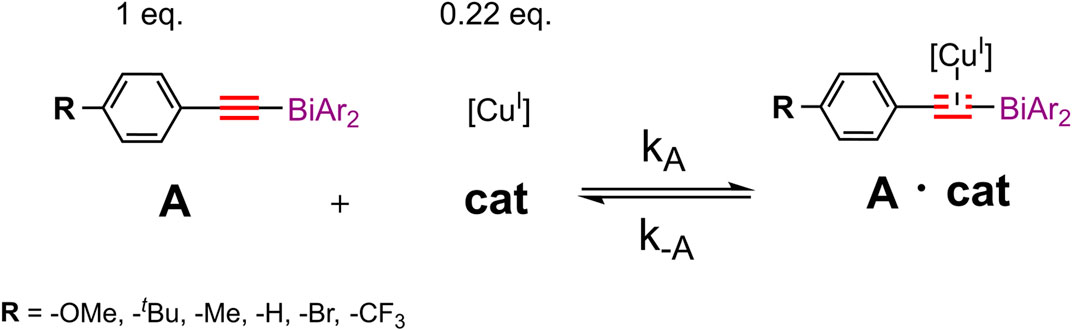
With an increased interest in the area of novel, non-toxic, and biocompatible nanomaterials, bismuth (Bi)-doped systems have become important in the area of near-infrared (NIR)-emitters and drug-delivery materials (Laguta and Razdobreev, 2018; Liu et al., 2018; Szostak et al., 2019; Orellana-Tavra et al., 2020). Bismuth has a negligibly low level of toxicity and carcinogenicity as compared to its highly-abundant neighbors in the periodic table (tin, lead, antimony, arsenic) (Spafford et al., 2008; Zhang et al., 2009; Mohan, 2010). However, biochemical and industrial applications involving bismuth-containing organic compounds remain limited (Luan et al., 2011; Ramler et al., 2019). Presently, commercially-available bismuth(III) compounds are typically inorganic salts used for instance as Lewis acid catalysts in organometallic reactions or those used for the preparation of nanoparticles (Suzuki et al., 2001; Rabin et al., 2006; Brown and Goforth, 2012; Bi et al., 2018). Recently the coordination with electron donors such as sulfur (S) and nitrogen (N) was reported to increase the thermal, air, and hydrolytic stability of bismuth in the oxidation states of (III) and (V), creating new opportunities for its applications (Raţ et al., 2013; Toma et al., 2016; Toma et al., 2017). Also, recently the bismuth(III)/(V) couple has been reported to show redox activity when participating in bimolecular interactions in solution (Inani et al., 1990). The investigation of the redox characteristics as well as the role of transition metals involved in catalytic processes through reaction kinetic profiling remains well established in organometallic chemistry (Jutand, 2008; Blackmond, 2015; Easter and Blum, 2019). Herein, we report a mechanistic study of the reactivity of para-phenyl substituted bismuth(III) acetylides in the copper(I)-catalyzed cycloaddition reactions with organic azides (Worrell et al., 2013a). Reaction progress experiments were performed using cyclic voltammetry (CV) and nuclear magnetic resonance (1H NMR) spectroscopy. To exploit bismuth’s coordination chemistry by modifying its electronic effects and geometry, we have focused our study on sulfonyl-merged bis-anionic aryl-tethered scaffolds (Ohkata et al., 1989; Suzuki et al., 2001; Nazarova and Fokin, 2018; Planas et al., 2020). By altering the electron-withdrawing nature of the sulfone moiety and the ability of the oxygen atom to intramolecularly coordinate to the bismuth center, we studied a variety of sulfone-type ligands that influence the bismuth(III) acetylides activity under copper(I)-catalyzed conditions (Figure 1).
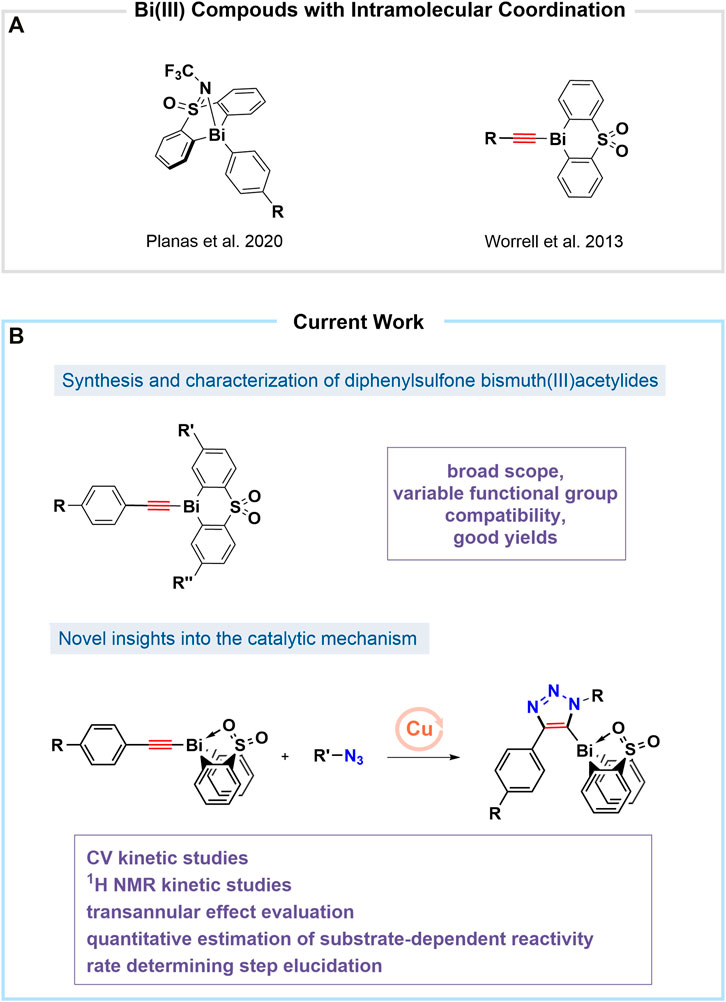
FIGURE 1. (A) Recent reports on organobismuth(III) compounds with tethered sulfonyl-linked bis-anionic ligands (Worrell et al., 2013a; Planas et al., 2020); (B) This work: 1) Synthesis of various bismuth(III) acetylides with diphenyl sulfone ligand derivatization and para-functionalization of the acetylenic unit 2) Mechanistic studies using cyclic voltammetry and NMR spectroscopy.
Materials and Methods
Cyclic Voltammetry
All electrochemical measurements were performed inside a glovebox in a nitrogen atmosphere. Anhydrous DMSO was used as the solvent. A 3-electrode glass cell consisting of a glassy carbon working electrode (BASi, MF-2012), copper foil counter and reference electrodes, and copper(I) trifluoromethanesulfonate was used for the measurements. Electrochemical data was collected on a multi-channel potentiostat (AMETEK Scientific Instruments, VersaSTAT 4). Voltammograms were recorded between −0.3 and 0.8 V vs Cu+/Cu at a scan rate of 100 mV s−1.
Cyclic Voltammetry Kinetic Studies: Bismuth(III)-Acetylide–Copper(I) Catalyst π-Complex Formation
Stock solutions of the corresponding bismuth(III) acetylides (75.0 mM), (2-azidoethyl)benzene (135 mM), and copper(I) trifluoromethanesulfonate toluene complex (16.24 mM) were prepared in anhydrous DMSO. 0.5 ml of the copper(I) trifluoromethanesulfonate toluene complex stock solution and 6.0 ml of DMSO were transferred into the 3-electrode cell and stirred (final concentration of the copper(I) trifluoro-methane-sulfonate toluene complex was 1.25 mM). Three CV scan cycles were carried out to ensure the reproducibility of the current response and the stability of the copper(I) trifluoromethanesulfonate complex. No additional supporting electrolyte was used.
The CV scans for kinetic studies were performed in the same 3-electrode cell with a glassy carbon working electrode, and copper foils as counter and reference electrodes. Bismuth(III) acetylide stock solution in anhydrous DMSO (0.5 ml) was introduced to the electrochemical cell (final concentrations were 1.16 mM of the copper(I) trifluoromethanesulfonate toluene complex and 5.35 mM of the bismuth(III) acetylide) and stirred for half a minute prior to the start of the CV scans.
Cyclic Voltammetry Kinetic Studies: Azide Insertion/Reductive Elimination Step
A solution of the (2-azidoethyl)benzene in anhydrous DMSO (0.5 ml) was introduced to an electrochemical cell after the acetylide and copper catalyst mixture attained equilibrium (General Procedure A in the Supporting Information, final concentrations were 1.08 mM of the copper(I) trifluoromethanesulfonate toluene complex, 5.0 mM of the bismuth(III) acetylide, 9.0 mM of the azide). The mixture was stirred for half a minute before the start of the electrochemistry data acquisition. Continuous electrochemical data collection between −0.3 and 0.8 V vs Cu+/Cu at a scan rate of 100 mV s−1 was performed until no further changes in the redox peaks were observed. To study the electrochemical activity on the bismuth center of the bismuth(III) acetylides, CV responses were collected between −0.3 and 1.7 V vs Cu+/Cu at a scan rate of 100 mV s−1.
1H NMR and IR Spectroscopy and X-Ray Analysis
NMR spectra were recorded on Varian Mercury 400, Varian VNMRS-500, or Varian VNMRS-600 spectrometer. Chemical shifts were referenced to residual solvent signals (Chloroform-d: δ(1H) = 7.26 ppm, δ(13C) = 77.16 ppm, DMSO-d6: δ(1H) = 2.5 ppm, δ(13C) = 39.51) as an internal reference. 19F NMR spectra were externally referenced to 80% CFCl3 in chloroform-d. The following abbreviations were used to describe NMR signal multiplicities: s = singlet, d = doublet, t = triplet, q = quartet, quin = quintet, m = multiplet, b = broad. Infrared spectra were recorded in the range 4,000–400 cm−1 on a Bruker Alpha spectrometer using a diamond ATR unit. IR intensities are described as vw (very weak), w (weak), m (medium), s (strong), vs (very strong). X-ray crystallographic analysis was performed at UCSD on a Bruker Apex II Ultra2 CCD diffractometer equipped with Mo Kα radiation. Yields refer to chromatographically and spectroscopically (1H NMR) pure materials unless otherwise is stated.
1H NMR Kinetic Studies: 5-Bismuth(III) Triazolide[X] Formation
Stock solutions for the corresponding bismuth(III) acetylides (0.100 M), (2-azidoethyl)benzene (0.688 M), 1,4-dimethoxy-benzene (internal standard) (54 mM) and copper(I) trifluoromethanesulfonate toluene complex (20 mM) were prepared in DMSO-d6. Of these stock solutions: 200 μl of the alkyne, 50 μl of the azide, and 50 μl of the reference stock solutions were transferred to an NMR tube and diluted with 450 μl of DMSO-d6 capped with a gas-tight rubber NMR septum and agitated. Lastly, 50 μl of the copper(I) trifluoro-methane-sulfonate toluene complex catalyst solution was added to the NMR tube and immediately agitated. The total volume of the reaction mixture in the NMR tube for one experiment was 800 μl.
Kinetic NMR measurements were recorded on a Varian VNMRS-600 spectrometer. The sample spinning rate was 20 Hz. 1H-NMR data were collected with the following acquisition parameters: 1 transient (scan), 5 s relaxation delay time, acquisition time of 5.824 s. Between the spectra was a 1 s pre-acquisition delay. Data was collected at 60.0°C. The temperature was calibrated against a temperature standard (ethylene glycol). Each experiment was performed twice. All reagents were dried before use. All manipulations were performed in the dry nitrogen atmosphere of a glovebox. Reaction conversions were integrated relative to an internal reference standard.
Results and Discussion
Synthesis and Characterization of Diphenyl Sulfone Bismuth(III) Acetylides
The rational design of the bismuth(III) acetylides was a crucial factor for gaining insights into the catalytic cycle. Two types of derivatization strategies were applied in the synthesis of the organobismuth acetylides: 1) diphenyl sulfone ligand derivatization with different functional groups; and 2) para-position phenylacetylene substitution with electron-deficient, neutral, and electron-rich functional groups (Suzuki et al., 2001). To study factors influencing the electronic environment of the bismuth(III) center, acetylides A[1] through A[6] were prepared using a synthetic protocol adapted from a previously reported procedure for acetylide A[3] containing a diphenyl sulfone scaffold as the tethered ligand (Figure 2B) (Suzuki et al., 1992). Acetylides A[7] through A[10] were synthesized by coupling thiols with aryl iodides and subsequent oxidation of the diphenyl sulfides (Figure 2C) (Kwong and Buchwald, 2002; Thomas et al., 2011). Thus, copper-catalyzed C(aryl)-S bond formation between aryl iodides and aryl sulfides, followed by the oxidation of the sulfide with m-CPBA, metalation with n-butyllithium, and subsequent treatment with in situ generated Bi(Br)2Ph yielded triphenyl bismuth derivatives (Figure 2A). Iodination of the triphenyl bismuth species followed by C(sp)-Bi coupling provided the bismuth(III) acetylides A[7–10]. While these substrates have not been used for the in situ kinetic studies, they were used for comparisons of the solid-state structures.
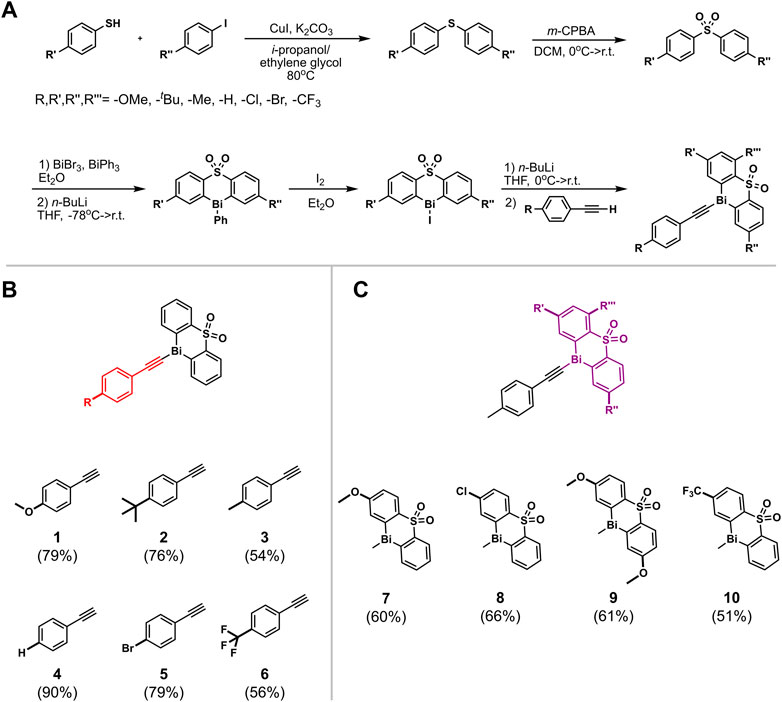
FIGURE 2. (A) Synthetic route for the synthesis of diphenyl sulfone bismuth(III) acetylides (a) CuI, K2CO3, i-propanol/ethylene glycol, 80°C; (b) m-CPBA, DCM, MgSO4 10 eq., 0°C –> r.t.; (c) 1) BiBr3, BiPh3, Et2O, 2) n-BuLi, THF, −78°C –> r. t.; (d) I2, Et2O, r. t.; (e) 1) n-BuLi, THF, −78°C –> r. t. 2) para-substituted phenylacetylene; (B) diphenyl sulfone bismuth(III) acetylides; (C) 1-ethynyl-4-methylbenzene bismuth(III) acetylides with functionalized diphenyl sulfone ligands.
Strong transannular N→Bi interactions in “butterfly-like” bisorganobismuth(III) bromides were reported as a key factor for their catalytic activity in the oxidation of thiophenols (Toma et al., 2017). To study the bismuth–oxygen intermolecular interactions, the solid-state structures of all synthesized bismuth(III) acetylides were determined by single-crystal X-ray crystallography as well as by the means of solid-state vibrational (IR) and solution phase 1H, 13C, and 19F NMR spectroscopy. The correlation between spectroscopic variables and the effects of systematic variation of substituents is an established approach to examine the electronic nature of aryl compounds (Exner and Boček, 1967; Bazan et al., 2000). A linear dependence on the substituent and its electronic origin is typically observed (Paul et al., 2002) and has been reported for other metal acetylides, such as uranium(VI) (Mullane et al., 2019) and iron(II) (Costuas et al., 2004) acetylides, and 1-alkynyl-pyridines (Yamaguchi et al., 2011). However, the vibrational analysis of the acetylenic C(sp)-C(sp) bonds for compounds A[1]-[10] revealed that the corresponding absorbance bands exhibit no linear free energy dependence relative to the Hammett constants. (Supplementary Table S3 in the Supporting Information).
Our comprehensive X-ray crystallographic characterization revealed the acetylide structure with the bismuth center in a tethered geometry is influenced not only by the para-substituents but also by the axial oxygen atom of the sulfonyl group (Figure 3). Our crystallographic analysis of the para-substituted diphenyl sulfone bismuth(III) acetylides A[1] to A[6], in particular, the distance of the transannular interactions between Bi(1) and O(1) as well as the C(sp)-C(sp) triple bond distance did not follow the expected linear trend (Hammett trend) (Ohkata et al., 1989) (Table 1). The intramolecular bismuth-oxygen(1) distance in bismuth(III) acetylides with electron-withdrawing substituents in the diphenyl sulfone scaffold, chloro- A[8] and trifluoro-methyl- A[10], is 2.878(3) Å and 2.874(2) Å that is significantly shorter than the Bi(1)-O(1) distances observed for electron-donating substituted diphenyl sulfone scaffolds, methoxy-A[7] and bismethoxy-A[9] (2.908(3) Å and 3.035(3) Å, respectively). The complete crystallographic details are given in the Supporting Information.
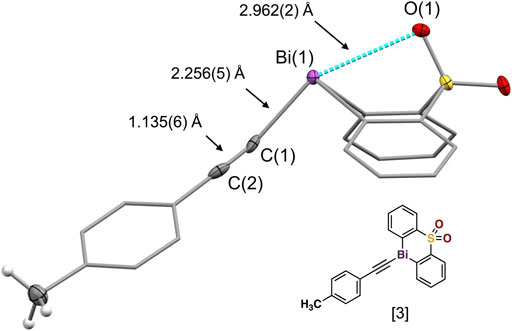
FIGURE 3. Crystal structure of the bismuth(III) acetylide [3] with labeled Bi(1)-C(1), C(1)≡C(2) and C(2)-C(sp2) bonds and transannular Bi(1)-O(1) interaction distances [Å].
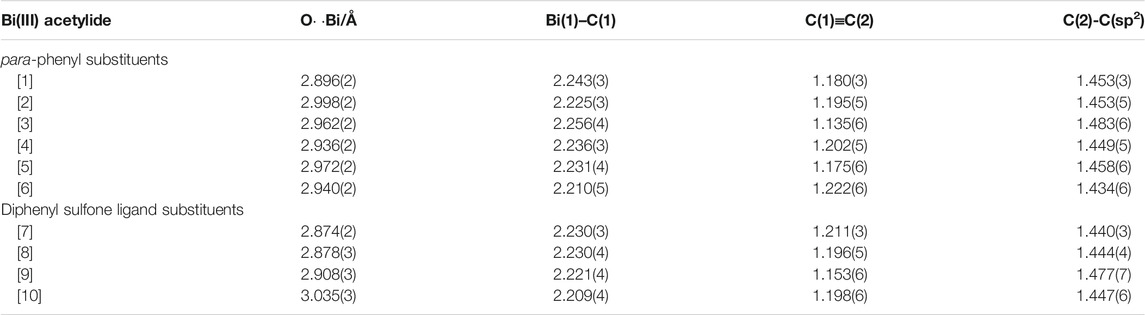
TABLE 1. Selected bond and transannular Bi(1)-O(1) interaction distances of all synthesized bismuth(III) acetylides.
Kinetic Cyclic Voltammetry for Mechanism Elucidation
The electronic functionality of triarylbismuth(V) ligands was reported to directly influence their reactivity (Barton and Finet, 1987; Abramovitch et al., 1988; Combes and Finet, 1999; Finet, 2002). With this in mind, we analyzed the reactivity trends of differently functionalized bismuth(III) acetylides towards organic azides in the copper(I) catalyzed cycloaddition reaction. Being eager to study substrate-dependent reactivity features in the mechanism, we made an effort for stepwise kinetic studies using cyclic voltammetry (CV) (Elgrishi et al., 2017; Graham, 2017; Sandford et al., 2019; Savéant and Costentin, 2019). While commonly used as an electro-analytical method, cyclic voltammetry allowed the direct investigation of the intermolecular transannular effect on bismuth(III) acetylide reactivity towards triazolide formation as well as the redox activity of the catalytic intermediates.
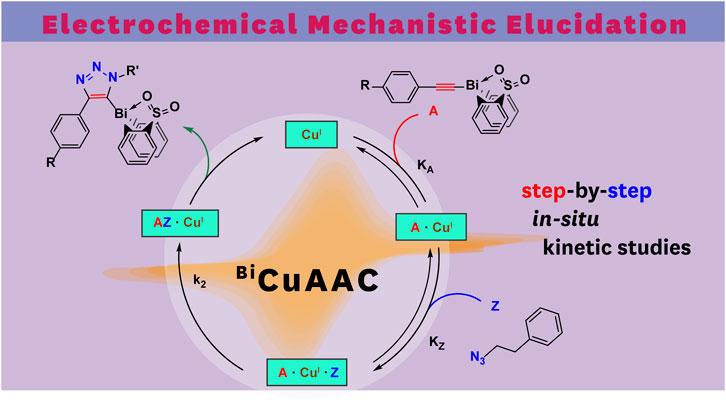
A substantial drawback of the copper(I) system is its air sensitivity; therefore, all electrochemical experiments were performed in the nitrogen atmosphere of a glovebox. Following our developed CV kinetic analysis protocol, we were able to stepwise monitor the redox activity of copper(I) after adding first the acetylide and then the organic azide reactant to access quantitative kinetic parameters of the catalytic cycle (Figure 4). Each electrochemical kinetic experiment was initiated by redox studies of a DMSO solution containing only the copper(I) trifluoromethanesulfonate toluene complex catalyst. The mechanism of the copper(I) catalyzed azide bismuth(III) acetylide cycloaddition has been postulated to initiate with a π-intermediate complex formation of copper(I) and the bismuth(III) acetylide (Worrell et al., 2013a). In a subsequent step, the azide ligation/migratory insertion occurs and reductive elimination of copper(I) yielding the 5-bismuth(III)-triazolide (Worrell et al., 2013a; Worrell et al., 2013b). The kinetics of the two independent steps: 1) π-intermediate complex of copper(I) and the bismuth(III) acetylide [A(X)·cat] formation, 2) product formation/regeneration of (CuI), were monitored by the subsequent addition of the reactants. First, a bismuth(III) acetylide was added and the kinetics of the π-complex formation was monitored by continuously recording cyclic voltammograms until no further changes in the CV responses could be detected. Subsequently, the organic azide was introduced to the mixture and the reaction progress was again monitored by continuously recording cyclic voltammograms.
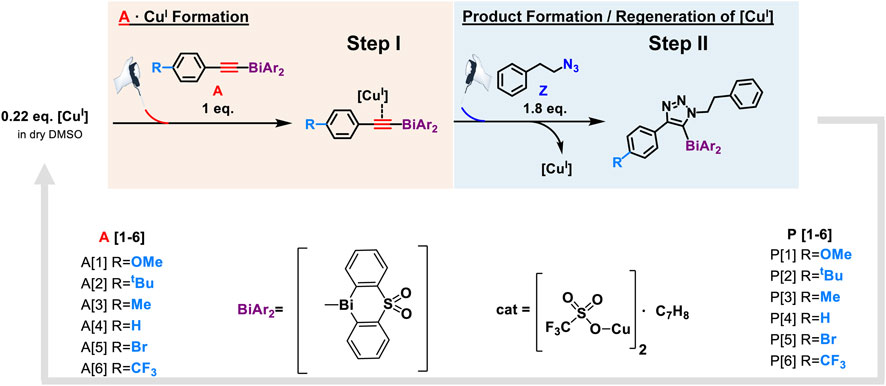
FIGURE 4. Reaction scheme of kinetic studies of copper(I)-catalyzed reaction of 5-bismuth(III) triazolides formation with cyclic voltammetry technique.
Using in situ electrochemistry as an electroanalytical tool provided vital information on the formation of intermediates, which is critical for understanding the catalytic transformation, as well as the ability to dissect the reaction rate constants and parameters from in situ experimental data (Scheme 1) (Sandford et al., 2019).
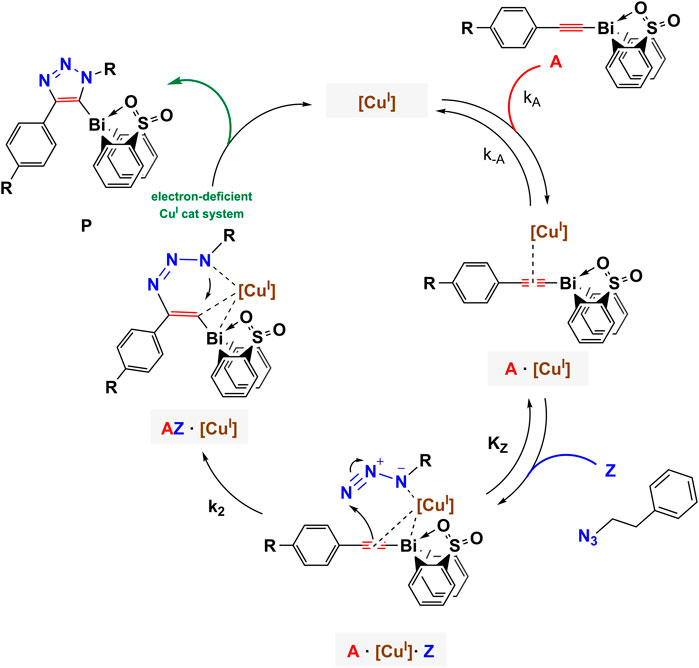
SCHEME 1. Proposed mechanistic model of azide-bismuth(III) acetylide copper(I)-catalyzed cycloaddition reaction. The electronic nature of the alkyne substrate is expected to directly influence the kinetics of the catalytic steps.
All bismuth(III) acetylides used for the kinetic studies carried the same sterically demanding diphenyl sulfone ligand. Therefore, no significant differences in the steric environment of the copper species in the copper(I)-bismuth(III) acetylide complexes are expected. As for other (metal) acetylides, coordination of the azide to copper(I) in the copper(I)-bismuth(III) acetylide complex is expected to primarily depend on the electronic nature of the copper(I) species (Partyka et al., 2009; Zhou et al., 2010; Yamada et al., 2016; Chung et al., 2018; Ma and Ding, 2020). Therefore, the formation of the first σ-bond between the β-carbon [C(2)] of the acetylide and the terminal nitrogen [N(3)] of the azide should determine the rate-limiting step of the overall transformation (Worrell et al., 2013a).
To develop a quantitative model of such a complex catalytic reaction, rate constants, and substituent effects need to be determined. To verify that the CV response is chemically reversible and thus the catalyst and the solvent are suitable for the CV timescale, each kinetic electrochemical experiment was first initiated with cyclic voltammetry studies of a solution of the copper(I) trifluoromethanesulfonate toluene complex catalyst. The cyclic voltammograms of copper(I) complex (1.25 mM, 8.12 μmol) dissolved in dry DMSO (6.5 ml) recorded at 100 mV/s revealed that the reduction of Cu(I) to Cu(0) occurred at around −0.2 V versus the Cu+/Cu0 reference electrode (Figure 5 A, blue). To collect the first set of quantitative kinetic data, we injected an almost five-fold excess of bismuth(III) acetylide, A(X), into a 1.25 mM catalyst solution. The final concentration of the bismuth(III) acetylide substrate was 5.35 mM. The formation of the [A(X)·cat] complex was monitored by continuously recording cyclic voltammograms until no further changes in the CV responses were detected.
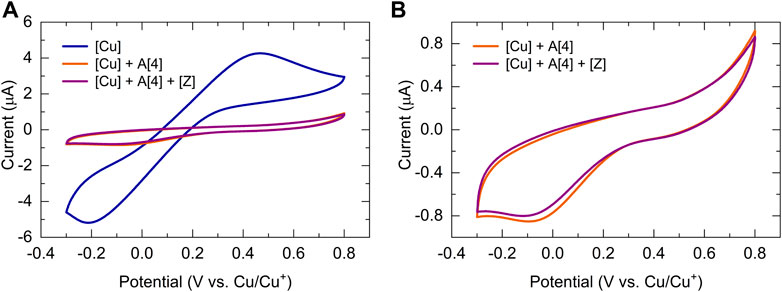
FIGURE 5. Cyclic voltammogram of bismuth(III) acetylides in the presence of the copper(I) trifluoromethanesulfonate toluene complex at 25°C in dry DMSO recorded at 100 mV/sec. Voltammograms of the (A) copper(I) trifluoromethanesulfonate catalyst (blue), after the addition of acetylide (orange), and after stepwise addition of acetylide and azide (purple); (B) copper(I) trifluoromethanesulfonate catalyst after the addition of acetylide (orange), and after stepwise addition of acetylide and azide (purple). Conditions: 3-electrode cell with glassy carbon as the working electrode, copper foils as counter, and reference electrodes.
Similar cyclic voltammetry studies of Cu(I) complexes with various triazolylamine ligands for the copper(I) catalyzed azide-alkyne cycloaddition reaction have been reported while studying reversible couple characteristics of CuI/II (Hong et al., 2008). Upon binding to the π-system of the bismuth(III) acetylide, the electrode potentials during the reduction of Cu(I) were found to slightly shift towards the higher potentials for the bismuth(III) substrates. On the contrary, the electrode potentials during the oxidation of Cu(0) to Cu(I) remained identical and stable throughout the reaction. The final CV response after the formation of the [A(X)·cat] complex results in a lowering of the Cu(I) concentration and a consequent decrease in the observed current for the reduction of Cu(I). The copper(I)-bismuth(III) acetylide complex itself is not easily reduced.
The steady decrease in the reduction current for Cu(I) after the addition of bismuth(III) acetylide is consistent with the formation of the copper(I)-bismuth(III) acetylide complex. When the redox cycle indicated the complete formation of the [A(X)·cat] complex, the azide reactant was injected and the processes on Cu(I) were monitored by continuously recording cyclic voltammograms (Figure 6B). Immediately after injection, the characteristic yellow color of the [A(X)·cat] complex began to fade and the recorded current increased due to a sudden release of free copper-catalyst. The electrochemical monitoring was continued until no further changes in current were recorded and the current stabilized at a certain value. The cyclic voltammograms of copper(I), after the formation of the copper-bismuth(III) acetylide complex, and after the azide addition/product formation are shown in Figure 5.
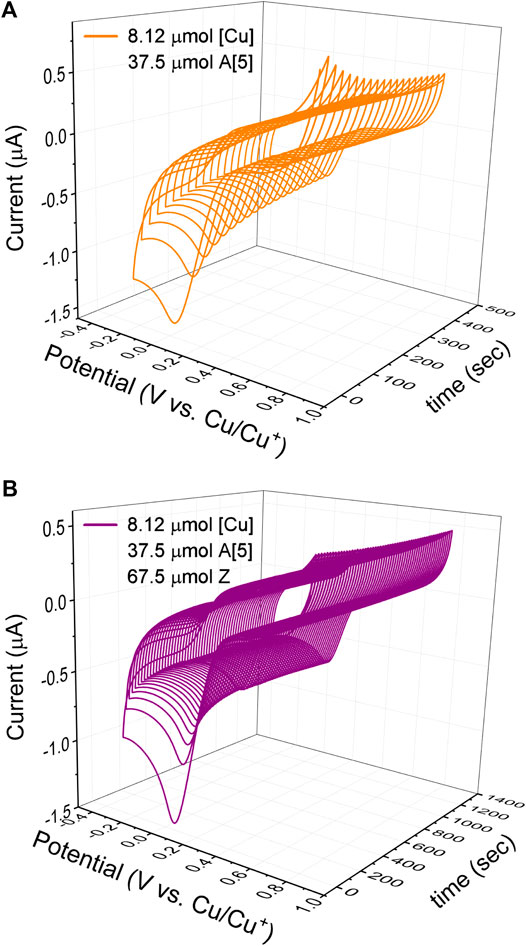
FIGURE 6. Cyclic voltammetry kinetic studies of the copper(I) catalyst in the 5-bismuth(III) triazolide formation at 25°C in dry DMSO recorded at 100 mV/sec. (A) Kinetic studies of [A [5]·cat] complex formation of the copper(I) catalyst and bismuth(III) acetylide (A [5]); (B) Kinetic studies of the azide ligation/migratory insertion and 5-bismuth(III)-triazolide, product formation. Conditions: 3-electrode cell with glassy carbon as the working electrode, copper foils as counter and reference electrodes.
Kinetic Model Parameter Estimation From Experimental Data
Cyclic voltammetry is a unique tool to extract reaction kinetic parameters, i.e. rate constants by detecting changes in peak currents or potentials versus time (Elgrishi et al., 2016; Hammerich and Speiser, 2016; Passard et al., 2016; Rountree et al., 2016; Haque et al., 2021). In situ cyclic voltammetry data was used to determine changes in the free copper(I) concentration, which allowed for the calculation of the kinetic parameters of the bismuth(III) acetylide coordination reactions during 1,2,3-triazolide P[X] formation. Since the reaction rate is a function of multiple parameters, a direct (assumption-free) mechanistic analysis would allow accurate and reliable accounting of all factors that influence the evolution of the rate-determining changes throughout the reaction (Ašperger, 2003; Peccoud et al., 2010). By direct analysis, one implies subsuming concentration variations of all involved catalytic species and reactants. Thus, kinetic profiles of concentration versus time for the stepwise experiments can be formulated in form of ordinary differential rate equations (ODEs). For the direct and reverse rate constant elucidation and their impact on the equilibrium processes, ODEs were formulated to solve the mechanistic model of the copper(I)-catalyzed formation of 5-bismuth(III) triazolides (Scheme 2). The catalytic process was investigated stepwise: first, the acetylide A[X] addition, and second, the addition of the azide Z reactant. Therefore, we were able to quantify the kinetics of the first step, the copper(I)-bismuth(III) acetylide complex formation (Figure 4, red). For simplicity, we denote the copper trifluoromethanesulfonate benzene complex catalyst (CuI) hereafter as (cat).
SCHEME 2. Reaction scheme of the first step, the π-intermediate complex formation of the copper(I) catalyst and bismuth(III) acetylide.
Considering the equilibrium process of the intermediate [A·cat] formation from [cat] and the bismuth(III) acetylide in the theoretical model (Eq. 1):
Applying the conservation law for the catalyst and bismuth(III) acetylide components yields Equations 2, 3:
where
Considering the law of conservation of mass, Equations 2, 3, the concentration of the catalyst can be expressed as Eq. 4: (Kaps and Rentrop, 1984; Butcher, 2016; Stone et al., 2018)
The integration was done using a self-developed computer program relying on the fourth-order classical Runge-Kutta method (Runge, 1895; Press and Teukolsky, 1992). The solution of Eq. 4 was found via the grid search using metrics of the LMS optimization:
where the concentration of the catalyst, [cat]exp, is derived from experimental kinetic data, and values of [cat] are obtained from Eq. 4, n is a number of the experimentally derived concentration values at n time intervals.
Based on the Randles-Sevcik equation (Hickey et al., 2019), the peak current in a cyclic voltammogram is directly proportional to a concentration of the species that participate in the electron transfer, Equation 6:
where
From the first set of kinetic data in Figure 6A for the bismuth(III) acetylide addition, we derived a linear proportionality coefficient α for each kinetic experiment of the six different acetylides while keeping the value of the initial copper(I) catalyst concentration constant as given by Equation 7:
where
The concentration of (cat) over time was obtained from
The forward reaction rate parameter
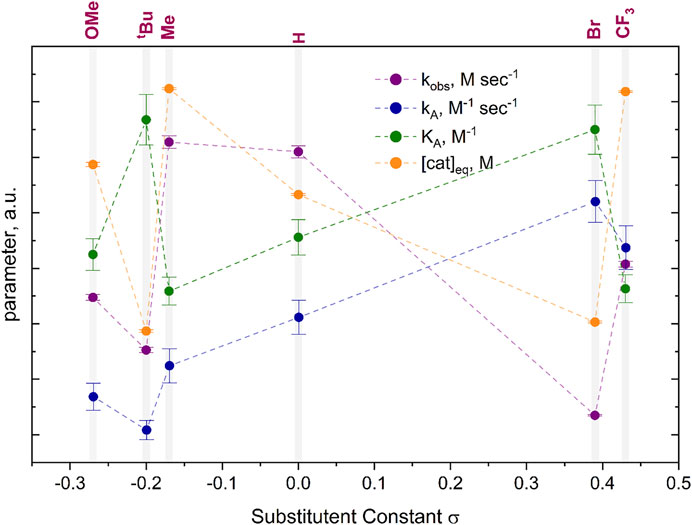
FIGURE 7. Kinetic parameters KA, kobs,
After determining the equilibrium constant,
Overall, the rate constants for the formation of the π-complex of copper(I) for all six studied bismuth(III) acetylides were found to confirm the reactivity trend determined in the 1H NMR kinetic studies. While previous studies of (metal)acetylides in the copper(I)-catalyzed cycloaddition with azides followed a linear (Hammett) reactivity trend, (Brown and Widenhoefer, 2011; Powers et al., 2015), the observation of a non-Hammett-dependent reactivity of the bismuth(III) acetylides indicates that, apart from para-phenyl substituents, other effects such as transannular electron density donation of oxygen to the bismuth center play a role in the control of the reaction kinetics. In the solid-state, the transannular Bi(1)-O(1) distance for the least reactive acetylide, the para-bromo substituted A[5], is one of the largest, whereas the more reactive substrates, A[3], A[4], and A[6], have shorter Bi(1)-O(1) bond distances. We believe that such perturbations not only affect the electron density of the C(β)-acetylene carbon but subsequently also influence the electronic environment of the copper(I) species in the (A·cat) complex. This causes different affinities of copper(I) species towards N→Cu(I) ligation (Scheme 1). This supports our previous conclusion of the overall reaction to be azide-dependent.
Increasing the potential window past the Cu(I)/Cu(0) redox pair allowed the observation of electrochemically relevant processes at the bismuth center in the bismuth(III) acetylides (Figure 8). As expected, the formation of the copper(I)–bismuth(III) acetylide complex also impacts the electronic environment of the bismuth(III) center.
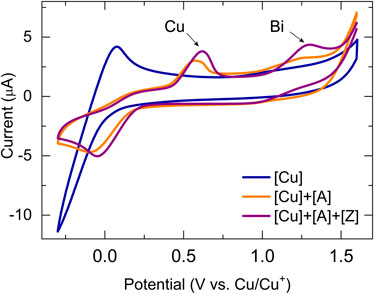
FIGURE 8. Cyclic voltammogram kinetic studies of bismuth(III) acetylide in the presence of the copper(I) trifluoromethanesulfonate toluene complex at 25°C in dry DMF at 100 mV/sec. Conditions: 3-electrode cell with glassy carbon as the working electrode, copper foils as counter, and reference electrodes.
Our detailed mechanistic modeling performed in the context of CV independent reactivity experiments revealed the electronic effects of para-phenyl functionalization together with transannular interaction determine substrates rate parameters and reactivity. Since the reaction rate is influenced by multiple parameters, a direct (assumption-free) mechanistic analysis allowed the accurate and reliable examination of all factors that influence the complex catalytic formation of 5-bismuth(III)-triazolides.
Kinetic 1H NMR Spectroscopic Experiments Supporting the CV-Derived Quantitative Mechanistic Model
To confirm the kinetic model derived from cyclic voltammetry, the reactant consumption and product formation during the catalytic transformation was continuously monitored via in situ 1H NMR spectroscopy. An NMR tube was loaded with one of the six different bismuth(III) acetylides, A[X], excess of (2-azidoethyl)benzene, and the copper(I) trifluoromethanesulfonate toluene complex [cat], according to the reaction shown in Scheme 3.
SCHEME 3. Reaction scheme of the in situ 1H NMR spectroscopy kinetic studies of copper(I)-catalyzed cycloaddition reaction of bismuth(III) acetylides with an organic azide.
1H NMR kinetic studies were performed in deuterated dimethyl sulfoxide due to its high boiling point to achieve better signal separation (Socrates, 1967; Kaufman et al., 1982; von Harbou et al., 2017). A positive side-effect of recording the spectra at elevated temperatures was the elimination of apical and equatorial ligand rotations due to Berry pseudo rotation (BPR) and Turnstile rotation (TR) processes on the NMR time scale, allowing precise peak integration. At room temperature, these processes cause a broadening of the ortho-hydrogens in the diphenyl sulfone scaffold which makes accurate signal integration difficult (see Supplementary Figure S6 in the Supporting Information) (Ugi et al., 2002). Our NMR-spectroscopic kinetic investigations match the conclusion made in the cyclic voltammetry studies, i.e. the transformation to be predominantly or exclusively azide-substrate dependent. However, the atypical reactive trend was also found in the 1H NMR kinetic study and is in good agreement with the overall trend imposed by the equilibrium concentrations
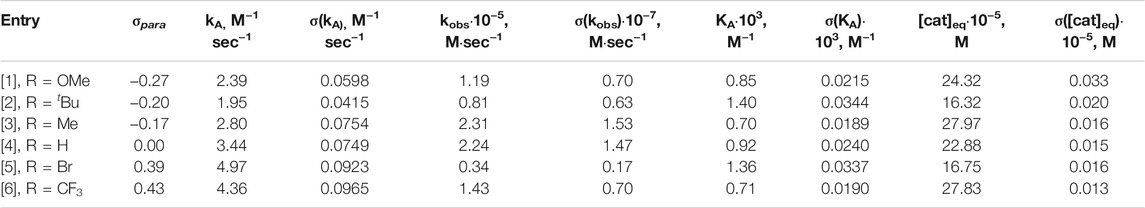
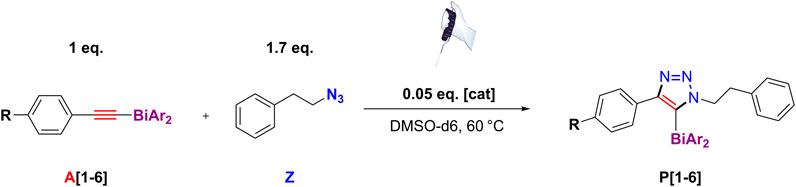
TABLE 2. Rate parameters kA, k−A, KA, [cat]eq., derived from the cyclic voltammetry kinetic studies at 25°C in dry DMSO at 100 mV/sec and kobs, obtained by 1H kinetic NMR at 60°C in dry DMSO-d6, with corresponding standard deviations.
A drastic difference in the reaction rate for the formation of P[3], P[4], and P[6] (methyl-, proto-, and trifluoromethyl-substituted) compared to the other 5-bismuth(III)-1,2,3-triazolides, P[1], P[2], and P[5], was observed (Figure 9A). The derived concentration vs times curves demonstrated either a linear or exponential dependence for reactant consumption or production formation. Exemplary, the kinetic profiles for trifluoromethyl A[6] and tert-butyl A[2] substituted bismuth(III) acetylides are shown in Figure 9B,C, respectively. This allowed the differentiation between zero-order kinetics and positive value rate orders for fast- or slow-reacting acetylides, respectively.
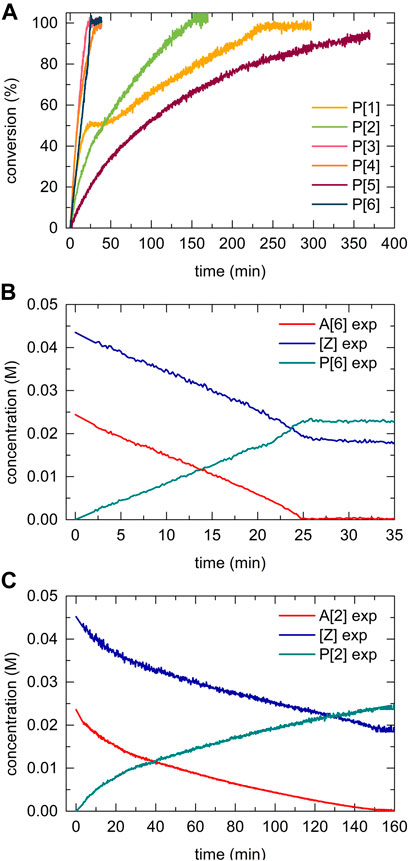
FIGURE 9. Kinetic profiles of independent experiments of copper(I)-catalyzed cycloaddition reaction of bismuth(III) acetylides with an organic azide. Conditions: A [X] = 25mM; [Z] = 43 mM [cat] = 1.25 mM; 0.8 ml volume DMSO-d6; T = 59.5°C. (A) Standard reaction conversion vs. time (C) Concentration vs. time for trifluoromethyl functionalized substrate A [6], linear dependence reveals “zero-order” kinetics; (B) Concentration vs. time for tert-butyl functionalized substrate A [2], exponential dependence reveals positive value rate-order “first-order” kinetics.
Utilizing the reaction progress kinetic analysis approach (Blackmond, 2005), various (“excess”) experiments were performed to derive the rate order and estimate whether we observe a rate-determining step shift for para-substituted acetylide substrates as previously reported for terminal alkynes in the copper(I)-catalyzed azide alkyne cycloaddition (CuAAC) reaction (Seath et al., 2017). For the para-tert-butyl substituted acetylide (2), the “excess” value was defined as the difference between the concentrations of azide and acetylide (2) reactants at the initial time, t = 0 (Figure 10A):
FIGURE 10. Kinetic profile of different-excess-experiments of the copper(I) catalyzed 5-bismuth(III) triazolide formation using A [2]. (A) Variable [A] concentration experiment, conversion vs. time. (B) rate/[Z] vs. A [2], the overlapping of the [excess] trials in the region of higher conversions signalizes a near to zero-type order in acetylide [A] substrate. Whereas, in the initial kinetic regime (at low conversions) the acetylide [A] shows non-zero rate order.
Normalized data representation for all experiments indicates that the reaction rate law exhibits non-zero bismuth(III) acetylide dependency at low conversion (beginning of the reaction) (Figure 10B). Towards the end of the reaction, zero-order or acetylide-independent but azide-dependent kinetics were found (Figure 10B). The highly reactive trifluoromethyl-para-substituted acetylide (6) proceeds with azide coordination as the turn over limiting step (zero-order in alkyne substrate) during the entire course of the reaction which results in the linear dependency of the concentration over time (Figure 9B).
Similar to the stepwise kinetic CV studies, the 1H NMR monitoring revelated that the overall reactivity is independent of the substituent constants (Hammett sigma values). However, two clusters of substrates with different reactivity trends were identified. We hypothesize that a combination of para-substituent functionality and transannular coordination of the oxygen of the diphenyl sulfone scaffold influences the electron density and reactivity of the acetylenic triple bond making it more or less prone for the coordination to copper(I) in the first step.
In addition, the 1H NMR kinetic study showed that the overall reaction rates (kobs) are directly correlating with the electronic environment of copper(I) in the (A·cat) complex as found electrochemically by the means of the equilibrium catalyst concentration [(cat)eq.]. However, the equilibrium parameter of the π-complex formation between bismuth(III) acetylide and copper(I) catalyst exhibits an inverse correlation with kobs. This indicates a slow 5-bismuth(III)-triazolide formation for cases where the coordination of the bismuth(III)-acetylide to copper(I) is fast and vice versa. Indeed, the higher is the affinity of the bismuth(III) acetylide to copper(I), the weaker the coordination of the azide during the ligation/migratory insertion event, thus exhibiting lower overall reaction rates.
Conclusion
The mechanistic elucidation of the copper(I) catalyzed cycloaddition reaction of bismuth(III) acetylides and organic azides was accomplished by kinetic in situ electrochemical (CV) as well as 1H NMR spectroscopic techniques. Novel insights into the catalytic model of the 5-bismuth(III) triazolide formation were gained. A non-Hammett-dependent reactivity trend of the bismuth(III) acetylides was observed. We attribute the unexpected non-linear reactivity trend to the more complex nature of the bismuth acetylides that is not solely influenced by Hammett substituent effects. In bismuth(III) acetylides, the reactivity of the C(sp)C(sp) triple bond is affected by various factors including transannular donation from sulfonyl oxygen to the bismuth(III) center and electron-withdrawing or -donating functional groups in para position of the phenyl ring.
The CV kinetic experiments enabled the stepwise investigation of the catalytic cycle. Electrochemical monitoring of the formation of the putative intermediate complex of the copper(I) catalyst and the bismuth(III) acetylides and the computed equilibrium catalyst concentration [(cat)eq.] were used to categorize the different acetylide substrates by reactivity. The azide ligation/migratory insertion was found to be the rate-determining step. The rate-determining step is influenced by both, the tendency of the ɑ-nitrogen (N(1) of the azide to ligate with the copper metal and the ability of the azide to form the first covalent bond between the β-carbon of the acetylide and terminal nitrogen [N(3)].
The equilibrium constant of the (A·cat) complex formation,
The experimentally derived reaction rate constants of the corresponding π-intermediate equilibrium constant (KA) and the triazolide formation (kobs) determined utilizing kinetic cyclic voltammetry and nuclear magnetic resonance studies are inversely correlated. Thus, the stronger the coordination of the copper(I) and bismuth(III) acetylide the weaker the azide ligation to copper(I), which results in slower overall reaction rates. All our findings as well as the results of the quantitative modeling support the proposed mechanistic model of the copper-mediated 5-bismuth(III)-1,2,3-triazolide formation.
Data Availability Statement
The original contributions presented in the study are included in the article Supplementary Materials.
Author Contributions
AN wrote the manuscript, synthesized all compounds, designed and performed the spectroscopic (NMR) experiments and electrochemistry (CV) experiments, performed the data analysis of NMR and CV experiments, performed all computations, and developed the kinetic model. BZ designed CV experiments together with AN and SN, performed the CV experiments together with AN. SN designed and supervised the CV kinetic experiments, assisted in data analysis, and heavily contributed to the writing of the manuscript. VF contributed to the discussion of the NMR experiments and provided extensive feedback to the manuscript.
Funding
This work was supported by the National Science Foundation (CHE-1660373).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We are grateful to the National Science Foundation (DBI- 0821671, CHE-0840366), the National Institutes of Health (1 S10 RR25432), and the University of Southern California for their sponsorship of NMR spectrometers at USC. Milan Gembicky (UCSD) and Prof. Gary J. Baliach (UCSD and United States Air Force Academy) are gratefully acknowledged for single-crystal X-ray diffraction analysis. Support by the Loker Hydrocarbon Research Institute, Bridge@USC, and Dr. Fokin’s personal funds are also gratefully acknowledged.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fchem.2022.830237/full#supplementary-material
References
Abramovitch, R. A., Barton, D. H. R., and Finet, J.-P. (1988). Newer Methods of Arylation. Tetrahedron 44 (11), 3039–3071. doi:10.1016/s0040-4020(01)85938-x
Ašperger, S. (2003). “Chemical Kinetics and Reaction Mechanisms,” in Chemical Kinetics and Inorganic Reaction Mechanisms (Boston, MA: Springer), 3–103.
Barton, D. H. R., and Finet, J.-P. (1987). Bismuth(V) Reagents in Organic Synthesis. Pure Appl. Chem. 59 (8), 937–946. doi:10.1351/pac198759080937
Bazan, G. C., Cotter, W. D., Komon, Z. J. A., Lee, R. A., and Lachicotte, R. J. (2000). Electron-Donating Properties of Boratabenzene Ligands. J. Am. Chem. Soc. 122 (7), 1371–1380. doi:10.1021/ja992413+
Bi, H., He, F., Dong, Y., Yang, D., Dai, Y., Xu, L., et al. (2018). Bismuth Nanoparticles with "Light" Property Served as a Multifunctional Probe for X-ray Computed Tomography and Fluorescence Imaging. Chem. Mater. 30 (10), 3301–3307. doi:10.1021/acs.chemmater.8b00565
Blackmond, D. G. (2015). Kinetic Profiling of Catalytic Organic Reactions as a Mechanistic Tool. J. Am. Chem. Soc. 137 (34), 10852–10866. doi:10.1021/jacs.5b05841
Blackmond, D. G. (2005). Reaction Progress Kinetic Analysis: A Powerful Methodology for Mechanistic Studies of Complex Catalytic Reactions. Angew. Chem. Int. Ed. 44 (28), 4302–4320. doi:10.1002/anie.200462544
Brown, A. L., and Goforth, A. M. (2012). pH-Dependent Synthesis and Stability of Aqueous, Elemental Bismuth Glyconanoparticle Colloids: Potentially Biocompatible X-ray Contrast Agents. Chem. Mater. 24 (9), 1599–1605. doi:10.1021/cm300083j
Brown, T. J., and Widenhoefer, R. A. (2011). Cationic Gold(I) π-Complexes of Terminal Alkynes and Their Conversion to Dinuclear σ,π-Acetylide Complexes. Organometallics 30 (21), 6003–6009. doi:10.1021/om200840g
Butcher, J. C. (2016). Numerical Methods for Ordinary Differential Equations. 3rd ed. Chichester, UK: Wiley.
Chung, R., Vo, A., Fokin, V. V., and Hein, J. E. (2018). Catalyst Activation, Chemoselectivity, and Reaction Rate Controlled by the Counterion in the Cu(I)-Catalyzed Cycloaddition between Azide and Terminal or 1-Iodoalkynes. ACS Catal. 8 (9), 7889–7897. doi:10.1021/acscatal.8b01342
Chung, R., Vo, A., and Hein, J. E. (2017). Copper-Catalyzed Hydrogen/Iodine Exchange in Terminal and 1-Iodoalkynes. ACS Catal. 7 (4), 2505–2510. doi:10.1021/acscatal.6b03515
Combes, S., and Finet, J.-P. (1999). On the Exclusion of Radical Species in the Ligand Coupling Reactions with Pentavalent Triarylbismuth Derivatives. Tetrahedron 55 (11), 3377–3386. doi:10.1016/s0040-4020(98)01148-x
Costuas, K., Paul, F., Toupet, L., Halet, J.-F., and Lapinte, C. (2004). Electron-Rich Piano-Stool Iron σ-Acetylides. Theoretical and Phenomenological Investigation of Electronic Substituent Effects in Iron(II) Acetylides. Organometallics 23 (9), 2053–2068. doi:10.1021/om030630k
Easter, Q. T., and Blum, S. A. (2019). Organic and Organometallic Chemistry at the Single-Molecule, -Particle, and -Molecular-Catalyst-Turnover Level by Fluorescence Microscopy. Acc. Chem. Res. 52 (8), 2244–2255. doi:10.1021/acs.accounts.9b00219
Elgrishi, N., McCarthy, B. D., Rountree, E. S., and Dempsey, J. L. (2016). Reaction Pathways of Hydrogen-Evolving Electrocatalysts: Electrochemical and Spectroscopic Studies of Proton-Coupled Electron Transfer Processes. ACS Catal. 6 (6), 3644–3659. doi:10.1021/acscatal.6b00778
Elgrishi, N., Rountree, K. J., McCarthy, B. D., Rountree, E. S., Eisenhart, T. T., and Dempsey, J. L. (2017). A Practical Beginner's Guide to Cyclic Voltammetry. J. Chem. Educ. 95 (2), 197–206. doi:10.1021/acs.jchemed.7b00361
Exner, O., and Boček, K. (1967). Correlation of Infra-red Frequencies and Intensities with Reactivity Data. Tetrahedron Lett. 8 (16), 1433–1436. doi:10.1016/s0040-4039(00)90976-6
Finet, J. P. (2002). Arylation Reactions with Organobismuth Reagents. Chem. Rev. 89 (7), 1487–1501. doi:10.1021/cr00097a005
Haque, M. A., Morozova, K., Lawrence, N., Ferrentino, G., and Scampicchio, M. (2021). Radical Scavenging Activity of Antioxidants by Cyclic Voltammetry. Electroanalysis 33 (1), 23–28. doi:10.1002/elan.202060245
Hickey, D. P., Sandford, C., Rhodes, Z., Gensch, T., Fries, L. R., Sigman, M. S., et al. (2019). Investigating the Role of Ligand Electronics on Stabilizing Electrocatalytically Relevant Low-Valent Co(I) Intermediates. J. Am. Chem. Soc. 141 (3), 1382–1392. doi:10.1021/jacs.8b12634
Hong, V., Udit, A. K., Evans, R. A., and Finn, M. G. (2008). Electrochemically Protected Copper(I)-Catalyzed Azide-Alkyne Cycloaddition. ChemBioChem 9 (9), 1481–1486. doi:10.1002/cbic.200700768
Inani, K. M., Gupta, Y. K., and Sharma, P. D. (1990). Kinetics and Mechanism of Silver(I) Catalyzed Oxidation of Water with Bismuth(V) in HClO4?HF Mixture. Int. J. Chem. Kinet. 22 (8), 863–869. doi:10.1002/kin.550220806
Jutand, A. (2008). Contribution of Electrochemistry to Organometallic Catalysis. Chem. Rev. 108 (7), 2300–2347. doi:10.1021/cr068072h
Kaps, P., and Rentrop, P. (1984). Application of a Variable-Order Semi-implicit Runge-Kutta Method to Chemical Models. Comput. Chem. Eng. 8 (6), 393–396. doi:10.1016/0098-1354(84)90009-7
Kaufman, D., Sterner, C., Masek, B., Svenningsen, R., and Samuelson, G. (1982). An NMR Kinetics experiment. J. Chem. Educ. 59 (10), 885. doi:10.1021/ed059p885
Kwong, F. Y., and Buchwald, S. L. (2002). A General, Efficient, and Inexpensive Catalyst System for the Coupling of Aryl Iodides and Thiols. Org. Lett. 4 (20), 3517–3520. doi:10.1021/ol0266673
Laguta, O. V., and Razdobreev, I. M. (2018). Origin of Near-Infrared Luminescence in Bismuth-Doped Silica and Germanosilicate Glass Fibers: Crystal Field Modeling. Opt. Mater. 84, 103–108. doi:10.1016/j.optmat.2018.06.047
Liu, Y., Jia, Q., and Zhou, J. (2018). Recent Advance in Near‐Infrared (NIR) Imaging Probes for Cancer Theranostics. Adv. Therap. 1 (8), 1800055. doi:10.1002/adtp.201800055
Luan, J., Zhang, L., and Hu, Z. (2011). Synthesis, Properties Characterization and Applications of Various Organobismuth Compounds. Molecules 16 (5), 4191–4230. doi:10.3390/molecules16054191
Ma, J., and Ding, S. (2020). Transition Metal‐Catalyzed Cycloaddition of Azides with Internal Alkynes. Asian J. Org. Chem. 9 (12), 1872–1888. doi:10.1002/ajoc.202000486
Mullane, K. C., Hrobárik, P., Cheisson, T., Manor, B. C., Carroll, P. J., and Schelter, E. J. (2019). 13C NMR Shifts as an Indicator of U-C Bond Covalency in Uranium(VI) Acetylide Complexes: An Experimental and Computational Study. Inorg. Chem. 58 (7), 4152–4163. doi:10.1021/acs.inorgchem.8b03175
Nazarova, A, and Fokin, V. (2018). “Mechanistic Investigations of Reactions of Bismuth(III) Acetylides with Organic Azides in the Copper(I)-Catalyzed Cycloaddition Reaction,” in Abstracts of Papers, 255th ACS National Meeting & Exposition, New Orleans, LA, United States, March 18–22, 2018.
Nazarova, A. L., and Fokin, V. V. (2017). “Mechanistic Investigation of Reactions of 1-iodoaryl Alkynes with Organic Azides in the Copper (I)-catalyzed Cycloaddition Reaction,” in Abstracts of Papers, 254th ACS National Meeting & Exposition, Washington, DC, United States, August 20–24, 2017 (Washington, DC, United States: American Chemical Society).
Nazarova, A. L., and Fokin, V. V. (2019). “High Throughput Quantitative and Qualitative Analysis of High-Density Kinetic Data Leads to Improved Understanding of Complex Catalytic Reactions,” in Abstracts of Papers, 258th ACS National Meeting & Exposition, San Diego, CA, United States, August 25–29, 2019 (San Diego, CA, United States: American Chemical Society).
Ohkata, K., Takemoto, S., Ohnishi, M., and Akiba, K.-y. (1989). Synthesis and Chemical Behaviors of 12-substituted Dibenz[c,f][1,5]azastibocine and Dibenz[c,f][1,5]azabismocine Derivatives: Evidences of 10-Pn-4 Type Hypervalent Interaction. Tetrahedron Lett. 30 (36), 4841–4844. doi:10.1016/s0040-4039(01)80523-2
Orellana-Tavra, C., Köppen, M., Li, A., Stock, N., and Fairen-Jimenez, D. (2020). Biocompatible, Crystalline, and Amorphous Bismuth-Based Metal-Organic Frameworks for Drug Delivery. ACS Appl. Mater. Inter. 12 (5), 5633–5641. doi:10.1021/acsami.9b21692
Partyka, D. V., Gao, L., Teets, T. S., Updegraff, J. B., Deligonul, N., and Gray, T. G. (2009). Copper-Catalyzed Huisgen [3 + 2] Cycloaddition of Gold(I) Alkynyls with Benzyl Azide. Syntheses, Structures, and Optical Properties. Organometallics 28 (21), 6171–6182. doi:10.1021/om9005774
Passard, G., Ullman, A. M., Brodsky, C. N., and Nocera, D. G. (2016). Oxygen Reduction Catalysis at a Dicobalt Center: The Relationship of Faradaic Efficiency to Overpotential. J. Am. Chem. Soc. 138 (9), 2925–2928. doi:10.1021/jacs.5b12828
Paul, F., Mevellec, J.-Y., and Lapinte, C. (2002). Electron-rich Fe(ii) and Fe(iii) Organoiron σ-alkynyl Complexes Bearing a Functional Aryl Group. Vibrational Spectroscopic Investigations of the Substituent Effect on the C≡C Triple Bond. J. Chem. Soc. Dalton Trans. (8), 1783. doi:10.1039/b108723c
Peccoud, J., Soliman, S., and Heiner, M. (2010). A Unique Transformation from Ordinary Differential Equations to Reaction Networks. PLoS ONE 5 (12), e14284. doi:10.1371/journal.pone.0014284
Planas, O., Wang, F., Leutzsch, M., and Cornella, J. (2020). Fluorination of Arylboronic Esters Enabled by Bismuth Redox Catalysis. Science 367 (6475), 313–317. doi:10.1126/science.aaz2258
Powers, A. R., Ghiviriga, I., Abboud, K. A., and Veige, A. S. (2015). Au-iClick Mirrors the Mechanism of Copper Catalyzed Azide-Alkyne Cycloaddition (CuAAC). Dalton Trans. 44 (33), 14747–14752. doi:10.1039/c5dt02405f
Press, W. H., and Teukolsky, S. A. (1992). Adaptive Stepsize Runge-Kutta Integration. Comput. Phys. 6 (2), 188. doi:10.1063/1.4823060
Rabin, O., Manuel Perez, J., Grimm, J., Wojtkiewicz, G., and Weissleder, R. (2006). An X-ray Computed Tomography Imaging Agent Based on Long-Circulating Bismuth Sulphide Nanoparticles. Nat. Mater 5 (2), 118–122. doi:10.1038/nmat1571
Ramler, J., Poater, J., Hirsch, F., Ritschel, B., Fischer, I., Bickelhaupt, F. M., et al. (2019). Carbon Monoxide Insertion at a Heavy P-Block Element: Unprecedented Formation of a Cationic Bismuth Carbamoyl. Chem. Sci. 10 (15), 4169–4176. doi:10.1039/c9sc00278b
Raţ, C. I., Silvestru, C., and Breunig, H. J. (2013). Hypervalent Organoantimony and -bismuth Compounds with Pendant Arm Ligands. Coord. Chem. Rev. 257 (5-6), 818–879. doi:10.1016/j.ccr.2012.07.026
Rountree, E. S., Martin, D. J., McCarthy, B. D., and Dempsey, J. L. (2016). Linear Free Energy Relationships in the Hydrogen Evolution Reaction: Kinetic Analysis of a Cobaloxime Catalyst. ACS Catal. 6 (5), 3326–3335. doi:10.1021/acscatal.6b00667
Runge, C. (1895). Ueber die numerische Auflösung von Differentialgleichungen. Math. Ann. 46 (2), 167–178. doi:10.1007/bf01446807
Sandford, C., Edwards, M. A., Klunder, K. J., Hickey, D. P., Li, M., Barman, K., et al. (2019). A Synthetic Chemist's Guide to Electroanalytical Tools for Studying Reaction Mechanisms. Chem. Sci. 10 (26), 6404–6422. doi:10.1039/c9sc01545k
Savéant, J. M., and Costentin, C. (2019). Elements of Molecular and Biomolecular Electrochemistry: An Electrochemical Approach to Electron Transfer Chemistry. 2nd ed. Hoboken, NJ: John Wiley & Sons.
Seath, C. P., Burley, G. A., and Watson, A. J. B. (2017). Determining the Origin of Rate-independent Chemoselectivity in CuAAC Reactions: An Alkyne-specific Shift in Rate-Determining Step. Angew. Chem. Int. Ed. 56 (12), 3314–3318. doi:10.1002/anie.201612288
Spafford, M. J., Christensen, J. E., Huddle, M. G., Lacey, J. R., and Mohan, R. S. (2008). Environmentally Friendly Organic Synthesis Using Bismuth Compounds. Bismuth Trifluoromethanesulfonate-Catalyzed Allylation of Dioxolanes. Aust. J. Chem. 61 (6), 419. doi:10.1071/ch08109
Stone, C. P., Alferman, A. T., and Niemeyer, K. E. (2018). Accelerating Finite-Rate Chemical Kinetics with Coprocessors: Comparing Vectorization Methods on GPUs, MICs, and CPUs. Comput. Phys. Commun. 226, 18–29. doi:10.1016/j.cpc.2018.01.015
Suzuki, H., Murafuji, T., and Azuma, N. (1992). Synthesis and reactions of some new heterocyclic bismuth-(III) and -(V) compounds. 5,10-Dihydrodibenzo[b,e]bismine and related systems. J. Chem. Soc. Perkin Trans. 1 (13), 1593. doi:10.1039/p19920001593
Suzuki, H., Komatsu, N., Ogawa, T., Murafuji, T., Ikegami, T., and Matano, Y. (2001). Organobismuth Chemistry. Amsterdam; New York: Elsevier Science.
Szostak, K., Ostaszewski, P., Pulit-Prociak, J., and Banach, M. (2019). Bismuth Oxide Nanoparticles in Drug Delivery Systems. Pharm. Chem. J. 53 (1), 48–51. doi:10.1007/s11094-019-01954-9
Thomas, J., Van Hecke, K., Robeyns, K., Van Rossom, W., Sonawane, M. P., Van Meervelt, L., et al. (2011). Homothiacalix[4]arenes: Synthetic Exploration and Solid-State Structures. Chem. Eur. J. 17 (37), 10339–10349. doi:10.1002/chem.201101690
Toma, A. M., Raţ, C. I., Pavel, O. D., Hardacre, C., Rüffer, T., Lang, H., et al. (2017). Heterocyclic Bismuth(iii) Compounds with Transannular N→Bi Interactions as Catalysts for the Oxidation of Thiophenol to Diphenyldisulfide. Catal. Sci. Technol. 7 (22), 5343–5353. doi:10.1039/c7cy00521k
Toma, A., Raţ, C. I., Silvestru, A., Rüffer, T., Lang, H., and Mehring, M. (2016). Heterocyclic Bismuth(III) Compounds with Transannular S→Bi Interactions. An Experimental and Theoretical Approach. J. Organomet. Chem. 806, 5–11. doi:10.1016/j.jorganchem.2016.01.019
Ugi, I., Marquarding, D., Klusacek, H., Gillespie, P., and Ramirez, F. (2002). Berry Pseudorotation and Turnstile Rotation. Acc. Chem. Res. 4 (8), 288–296. doi:10.1021/ar50044a004
von Harbou, E., Behrens, R., Berje, J., Brächer, A., and Hasse, H. (2017). Studying Fast Reaction Kinetics with Online NMR Spectroscopy. Chem. Ingenieur Technik 89 (4), 369–378. doi:10.1002/cite.201600068
Worrell, B. T., Ellery, S. P., and Fokin, V. V. (2013a). Copper(I)-Catalyzed Cycloaddition of Bismuth(III) Acetylides with Organic Azides: Synthesis of Stable Triazole Anion Equivalents. Angew. Chem. Int. Ed. 52 (49), 13037–13041. doi:10.1002/anie.201306192
Worrell, B. T., Malik, J. A., and Fokin, V. V. (2013b). Direct Evidence of a Dinuclear Copper Intermediate in Cu(I)-Catalyzed Azide-Alkyne Cycloadditions. Science 340 (6131), 457–460. doi:10.1126/science.1229506
Yamada, M., Matsumura, M., Uchida, Y., Kawahata, M., Murata, Y., Kakusawa, N., et al. (2016). Copper-catalyzed [3 + 2] Cycloaddition of (Phenylethynyl)di-p-tolylstibane with Organic Azides. Beilstein J. Org. Chem. 12, 1309–1313. doi:10.3762/bjoc.12.123
Yamaguchi, E., Shibahara, F., and Murai, T. (2011). 1-Alkynyl- and 1-Alkenyl-3-Arylimidazo[1,5-A]pyridines: Synthesis, Photophysical Properties, and Observation of a Linear Correlation between the Fluorescent Wavelength and Hammett Substituent Constants. J. Org. Chem. 76 (15), 6146–6158. doi:10.1021/jo200864x
Zhang, X.-W., Xia, J., Yan, H.-W., Luo, S.-L., Yin, S.-F., Au, C.-T., et al. (2009). Synthesis, Structure, and In Vitro Antiproliferative Activity of Cyclic Hypervalent Organobismuth(III) Chlorides and Their Triphenylgermylpropionate Derivatives. J. Organomet. Chem. 694 (18), 3019–3026. doi:10.1016/j.jorganchem.2009.05.003
Keywords: cyclic voltammetry, mechanistic studies, bismuth(III) acetylides, reaction kinetic analysis, copper(I)catalysis, ordinary differential equations, NMR reaction profiling, copper-mediated 1,3 cycloaddition
Citation: Nazarova AL, Zayat B, Fokin VV and Narayan SR (2022) Electrochemical Studies of the Cycloaddition Activity of Bismuth(III) Acetylides Towards Organic Azides Under Copper(I)-Catalyzed Conditions. Front. Chem. 10:830237. doi: 10.3389/fchem.2022.830237
Received: 06 December 2021; Accepted: 13 January 2022;
Published: 25 February 2022.
Edited by:
Xiaolei Wang, University of Alberta, CanadaReviewed by:
Lingjun Li, Henan Normal University, ChinaBrady Worrell, University of Denver, United States
Copyright © 2022 Nazarova, Zayat, Fokin and Narayan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Valery V. Fokin, Zm9raW5AdXNjLmVkdQ==; Sri R. Narayan, c3JuYXJheWFAdXNjLmVkdQ==
 Antonina L. Nazarova
Antonina L. Nazarova Billal Zayat
Billal Zayat Valery V. Fokin
Valery V. Fokin Sri R. Narayan
Sri R. Narayan