- 1Institute for Advanced Materials, School of Materials Science and Engineering, Jiangsu University, Zhenjiang, China
- 2Institute School of Science, Jiangsu University of Science and Technology, Zhenjiang, China
- 3Shenzhen Key Laboratory of Energy Materials for Carbon Neutrality, Institute of Technology for Carbon Neutrality/Faculty of Materials Science and Energy Engineering, Shenzhen Institute of Advanced Technology (SIAT), Chinese Academy of Sciences (CAS), Shenzhen, China
Hydrogen (H2) has been considered an ideal alternative energy source for solving energy supply security and greenhouse gas reduction. Although platinum group metal (PGM) catalysts have excellent performance in hydrogen electrocatalysis, their scarcity and high cost limit their industrial application. Therefore, it is necessary to develop low-cost and efficient non-PGM catalysts. Transition metal nitrides (TMNs) have attracted much attention because of their excellent catalytic performance in hydrogen electrochemistry, including hydrogen evolution reaction (HER)/hydrogen oxidation reaction (HOR). In this paper, we review and discuss the mechanism of HER/HOR in alkaline media. We compare and evaluate electrocatalytic performance for the HER/HOR TMN catalysts recently reported. Finally, we propose the prospects and research trends in sustainable alkaline hydrogen electrocatalysis.
Introduction
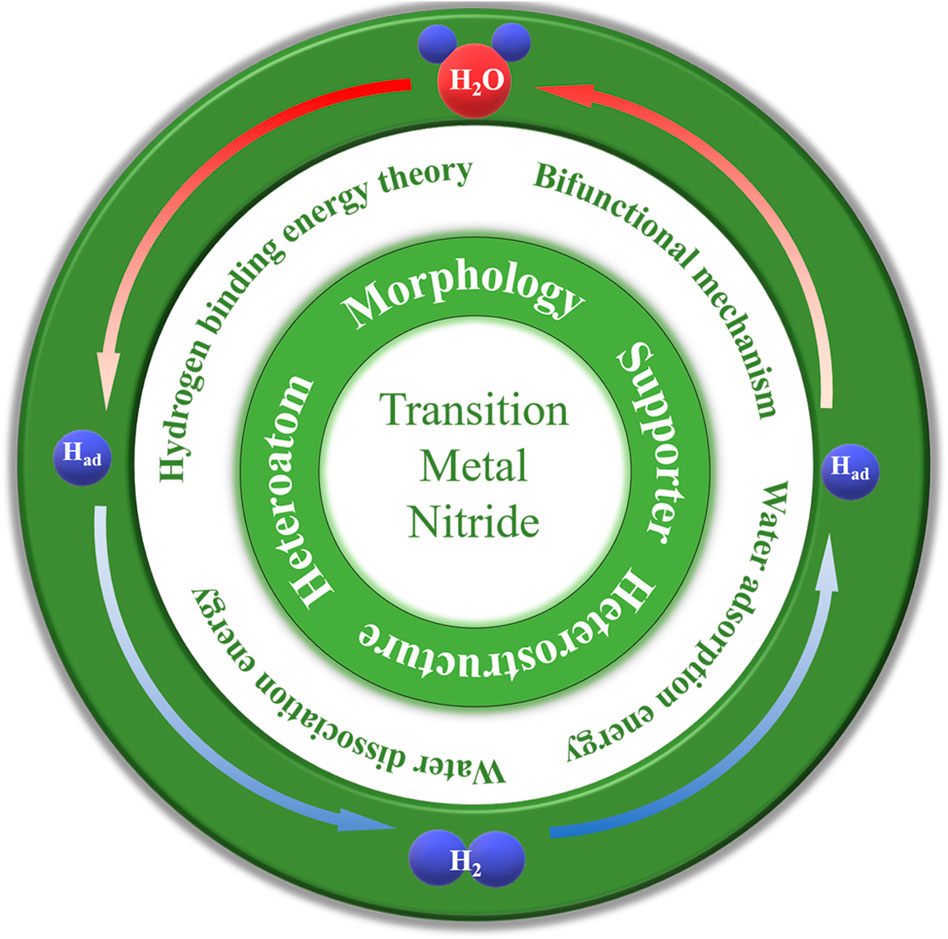
Hydrogen (H2) is considered an ideal alternative energy source when the earth's fossil fuels will be used up in the next century, owing to its high gravimetric energy density and zero carbon emissions (Jaramillo et al., 2007; Song et al., 2017). Electrocatalytic hydrogen transformation is a proven and convenient strategy for hydrogen economy (Jiao et al., 2015; Cong et al., 2018; Chu et al., 2020). High-purity H2 can be produced by hydrogen evolution reaction (HER) and oxygen evolution reaction (OER) in a water electrolyzer with the input of renewable energy resources, while the output of electricity can be achieved by fuel cells through hydrogen oxidation reaction (HOR) (Song et al., 2021). Both electrolyzers and fuel cells can be operated in acidic and alkaline solutions (Ruqia et al., 2018). Due to multiple proton-coupled electron transfer, electrocatalytic hydrogen transformation conversion in alkaline solution faces a large activation barrier, leading to sluggish kinetic activity. Efficient electrocatalysts are therefore required to reduce the energy barrier to achieve an industrially relevant requirement for the hydrogen economy. In the past few years, platinum group metal (PGM)-free catalysts have shown excellent catalytic performance in hydrogen electrochemistry. Among them, transition metal nitrides (TMNs) have attracted much attention due to excellent catalytic performance and robust stability (Figure 1). Bonding with N atoms will change the d-band structure of the transition metal and thus shrink the d-band. In addition, the active centers of N were similar to the electronic structure of Pt, so N can effectively improve the catalytic activity (Han et al., 2018; Yang et al., 2020); this results in thermal stability, chemical stability, and high electronic conductivity. However, although TMNs have developed rapidly in the past decade, they are still far from the requirements of industrial application. Therefore, it is necessary to develop novel TMNs with high activity and stability to achieve a hydrogen economy.
This article discusses the mechanism of HER/HOR in alkaline media. We analyze the catalytic performance and characteristics of the recently reported HER/HOR TMN catalysts before discussing the future development prospects and research trends of TMNs, which will benefit researchers who work in this field.
Mechanism of hydrogen evolution/oxidation reaction in alkaline solution
HER mechanism
The elementary steps of HER in alkaline media are as follows:
M is the active site on the catalyst, and M–H* is the hydrogen adsorption on the active site above the catalyst.
The HER process involves the Volmer, Heyrovsky, and Tafel steps. In the Volmer step, water is dissociated to form a reactive Had intermediate. In the Heyrovsky step, another H2O is adsorbed to M–H* and then reacts with the second electron to form H2 and OH−. In the Tafel step, two adjacent M–H* are combined to generate H2. A whole HER process will follow two pathways—Volmer–Tafel or Volmer–Heyrovsky reactions (Chen et al., 2019)—consisting of three consecutive stages—water dissociation, water adsorption, and hydrogen production.
Generally, the adsorption-free energy of M–H* (ΔGH*) is widely accepted as a key description in evaluating the kinetics of the HER catalyst. Optimum ΔGH* to zero is desirable for achieving excellent HER performance, which is regarded as a guide for developing highly efficient HER electrocatalysts. In addition, water adsorption energy (
HOR mechanism
The elementary steps of HOR in alkaline solution are shown as follows:
HOR proceeds in the reverse sequences to HER; in alkaline solution, HOR proceeds through pathways of Tafel–Volmer or Heyrovsky–Volmer. M–H* is formed through the Tafel or Heyrovsky step, and then follows the Volmer step to form H2O. In the Tafel step, H2 is dissociated in M (active site) without electron transfer to generate M–H* and is adsorbed on the surface of M. During the Heyrovsky step, H2 transfers electrons to M on the surface of the catalyst and is dissociated to generate M–H* and H2O with the coordination of adjacent OH−. Finally, M–H* binds with OH− to form H2O in the Volmer step.
Recently, Markovic et al. proposed a bifunctional mechanism (Okubo et al., 2019). They took the hydroxyl binding energy as another active descriptor and suggested that OHad is an important factor for efficient HOR under alkaline conditions. There is much discussion comparing the hydrogen binding energy (HBE) theory and the bifunctional mechanism. Although these two contradictory theories coexist, they succeed in guiding the synthesis of HOR catalysts and reflect the complexity of the HOR mechanisms in alkaline solutions.
Transition metal nitride for HER/HOR in alkaline solution
In recent decades, transition metal nitrides (TMNs) have exhibited promising HER/HOR performance under alkaline conditions. Therefore, TMNs are gradually applied to energy devices. For example, Liu et al. successfully used 1.5 V single AAA batteries to run the electrolyzer, where bifunctional NiCo nitride/NiCo2O4/GF could be acted as the anode and cathode (Liu et al., 2019) at the same time. The electrolyzer prepared by Wu et al. only needs 1.644 V to reach a large current of 1A (Wu et al., 2022). Cheng et al. and Hsieh et al. prepared Co-N-C catalyst and reached max power density of 275 and 361 mW cm−2 in AEMFCS (Cheng et al., 2022; Hsieh et al., 2022).
The reason why TMNs show outstanding potential is that N can enhance the d-electron density, resulting in contraction of the d-band of TMNs. Such a unique structure will afford TMNs an electronic structure similar to PGM metals, such as Pd and Pt. In addition, the distinguished conductivity and good corrosion resistance make TMNs high-performance. Among various TMNs, Ni3N and Co4N were synthesized by Shalom et al. and Chen et al. in 2015 (Chen et al., 2015; Shalom et al., 2015). Subsequently, great efforts have been devoted to optimizing the catalytic activities of TMNs toward HER/HOR electrocatalysts. Different design strategies were used to improve the stability of various TMN catalysts. For example, Yan et al. (Yan et al., 2021) ensured high structural stability by making TMNs contact closely with MXene and forming a strong interface junction. Yuan et al. (2020) significantly inhibited the oxidation of Co4N through a carbon shell to improve stability. Therefore, while improving catalytic performance, some design strategies can also improve the stability of TMNs.
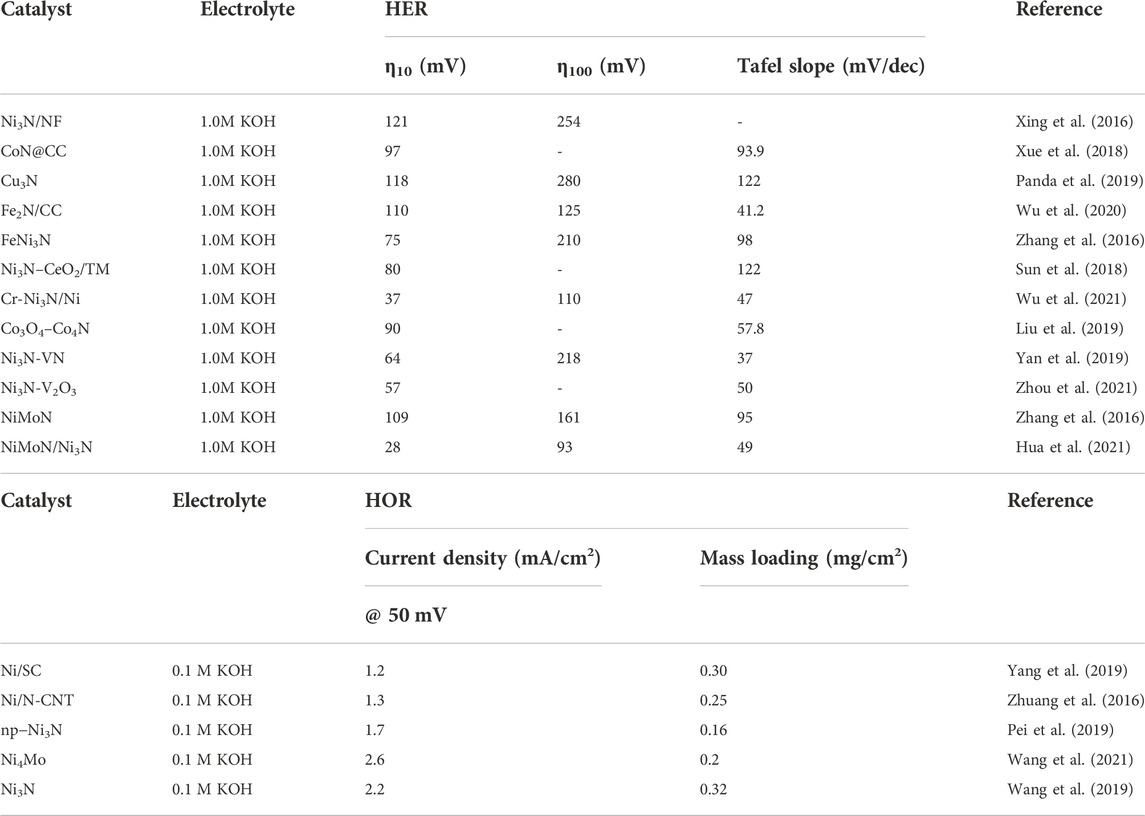
In this section, the strategies for the rational design of TMN electrocatalysts will be classified and summarized; the outstanding TMN-based electrocatalysts are compared in Table 1.
Synergistic metal-support interactive TMN catalysts
Synergistic metal-support interaction (SMSI) can be observed between metal catalysts and solid supports (Song et al., 2015; Song et al., 2020). SMSI provides possibilities for designing catalysts, modulating their d-band of catalytic sites and optimizing their binding energy of intermediates, thus resulting in accelerated reaction kinetics. Indeed, solid support is important for determining SMSI (Song et al., 2015; Song et al., 2018). An ideal solid support not only improves SMSI and re-modifies the electronic structure of metal sites but also restrains the agglomeration of metal species.
Carbon-based materials are excellent solid supports due to their large surface area, tailorable porous structure, high resistance, and convenient surface functionalization. Vulcan-XC 72R, a commercial carbon, possesses suitable surface area, pore size, and low-cost. Ni3N nanoparticles can be well-dispersed on a Vulcan-XC 72R surface and exhibit a robust HOR performance (Ni et al., 2019). In addition, a series of novel carbon-based materials have been developed. Yuan et al. (2020) designed cobalt nitrides embedded in nitrogen-doped carbon (Co4N@NC). NC acted as a conductive network to supply abundant active sites and efficiently retained the surface oxidation of Co4N. SMSI between Co4N and NC optimized DOS near the Fermi level, resulting in an enhancement of HER kinetics. Moreover, such improved HER performance has been observed in CoN/N-doped carbon nanotubes because one-dimensional nanotubes can provide ordered channels for rapid mass-diffusion and electron transfer. Porous metal–organic frameworks (MOFs) have emerged as precursors to obtaining TMN nitrides under controlled nitridation. Feng et al. (2019) synthesized MOF-74-derived NiCoN catalysts with porous rodlike structures. The abundant synergistic active sites obtained have accelerated electron transfer and modulated the chemical environment, resulting in all-pH HER performance (Feng et al., 2019). A series of nickel nitrides and cobalt nitrides derived from MOFs were reported (Hu et al., 2020; Wang et al., 2022). Jia et al. (2022) reported the construction of Ni3N/MOF-74, which shows a remarkably HER performance with an overpotential of 73 mV to afford 10 mA cm−2 in a 1.0 M KOH solution (Jia et al., 2022).
Transition metal carbides/nitrides (MXenes) as new two-dimensional materials are considered promising candidates for solid supports (Zhang et al., 2021). MXenes discovered by Gogotsi and colleagues in 2011 possess a larger specific surface area, high conductivity, good hydrophilicity, robust stability, and various functional groups (Naguib et al., 2011). Very recently, the effect of MXenes’ support on HER performance has been explored. CoNx/MXenes have been reported by Li et al. (Li et al., 2021): the construction of CoNx and MXenes can expose rich active sites, optimize electronic structure, and promote intermediate adsorption and desorption, thus improving HER kinetics. The DFT results prove furthermore that more electrons accumulate at the surface of the Co atom, arising from the strong charge transfer caused by the interface coupling between CoNx and MXenes. These improve mass/charge transport and promote water adsorption/activation in the Volmer step in HER. These studies have proven that MXenes are a promising support for exploring and developing cost-effective catalysts with high activities.
Hetero-interfacial TMN catalysts
Hetero-interface engineering is another promising strategy for developing high-performance PGM-free electrocatalysts. The interface effects will reconstruct active sites by modifying the electrochemical environment between two different active components. The reconstructed active sites at the heterogeneous interfaces can show higher activity than individual components. Up to now, the modifications of TMN-based interface catalysts, such as transition metal nitrides/transition metal (TMNs/TM), transition metal nitrides/transition metal nitrides (TMNs/TMNs), and transition metal nitrides/metal oxides (TMNs/MOs), have been reported.
It is well-known that the construction of TMNs/TM can efficiently improve conductivity and optimize the binding behaviors of catalytic sites toward reactants, intermediates, and products. In 2018, Ni3N/Ni interfacial catalysts were synthesized by electrodeposition combined with controlled nitridation, which is a pioneering report on a Ni3N-based electrocatalyst with bifunctional HER/HOR activity (Song et al., 2018). The construction of a Ni3N/Ni heterogeneous interface benefited the electron transfer at the interfacial sites and improved electrophilic properties. Strong electron redistribution occurred at the interface between Ni3N and Ni, which significantly promoted initial water adsorption and reduced the energy barrier of the subsequent water dissociation. Moreover, the H* species were achieved at the interfacial region. The ΔGH* of the interfacial site was as low as 0.01 eV, close to the ideal value of zero—resulting in an exceptional HER/HOR catalytic property. This group further developed a Co2N/Co interface with better robustness and Co tolerance (Song et al., 2019). Other TMNs/TM systems have been explored by Fan group (Fan et al., 2018) and Sun group (Sun et al., 2020). The former designed an atomic in-growth Co–Ni3N interface (Zhu et al., 2018). The epitaxial Co–Ni3N interfaces in nanoscale are responsible for robust stability due to small interfacial energy. In addition, the nanoconfinement effect can accelerate the electron transfer between Co and Ni3N domains at the epitaxial interface, resulting in excellent electrocatalyzing hydrogen production. Wang group further explored the chemical anchoring and electronic regulation at Ni3N/Ni. The formation of the Ni−N bond made the heterogeneous interface produce unique electronic states by changing the electrons’ state. Strong electron redistribution occurred at the interface between Ni3N and Ni, where the water formation reaction barrier could be significantly reduced, resulting in enhanced HOR performance (Wang et al., 2021).
The TMNs/TMN heterogeneous interface has attracted much attention due to its faster charge transfer and compatibility. CoN/Ni3N catalysts with a grass-like structure were synthesized via a controlled pyrolyzation (Ray et al., 2018). This generated abundant active centers with expeditious charge and mass transportation, inducing an enhanced HER property in 1.0 M KOH solution. A similar phenomenon was observed in Ni3N/Co3N (Huang et al., 2021) and Ni3N/Mo2N (Dai et al., 2022). In 2022, Song et al. reported a novel Ni3N/Co2N epitaxial interface, which exhibited superior bifunctional HER/HOR performance with respect to the state-of-the-art Pt catalyst due to an accelerating electron transfer at interface region. The bifunctional Ni3N/Co2N electrocatalysts exhibited excellent performance in a Swagelok type Ni-H2 battery with robust stability, demonstrating excellent rechargeability over 5000 cycles (Song et al., 2022).
Heterogeneous TMNs/MOs have proven to be efficient interfacial catalysts. Although MOs are not active for hydrogen electrochemistry, the addition of MOs benefits the modulation of the d-band and electronic structure of MOs, accelerating the kinetics of hydrogen electrochemistry. Sun et al. reported a Co4N/CeO2 interfacial system, where the in situ growth of CeO2 can efficiently modify the electronic structure of the nearby Co4N surface, favoring H2O dissociation and intermediated H* adsorption (Sun et al., 2020). Zhou group prepared Ni3N/V2O3 by nitriding a NiV–LDH (layered double hydroxide) precursor (Zhou et al., 2021). The introduction of V2O3 made the interface couple between Ni3N and V2O3; the interfacial interaction resulted in charge redistribution and generated more unoccupied electrons on the N and O in the interface region, which efficiently optimized the H2O adsorption/desorption ability. This hypothesis was further confirmed by DFT calculation, where Ni3N/V2O3 exhibits lower
Other TMN-based interface catalysts have also been reported. Sun et al. (2018) explored the abundant defects on a CoN/Ni2P surface (Sun et al., 2018). Hu et al. (2019)developed a series of CoN/Co2P-based interfacial catalytic sites (Guo et al., 2018; Hu et al., 2019). Ren et al designed Ni3N/Ni(OH)2 with excellent HER/HOR performance (Ren et al., 2022). The introduction of nickel hydroxide reduced the center of the Ni d-band center and thus reduced the adsorption strength. The strong electronic coupling made Ni3N negatively charged by interface engineering with seamless heterojunctions. The negatively charged Ni sites improved the bond interaction between the Ni sites and surface H* groups. Ni3N/Ni(OH)2 surface engineering promoted water dissociation and hydroxyl adsorption in the Volmer step toward HER and HOR.
As shown previously, interface engineering has become an effective strategy for improving catalyst activity by generating electron redistribution at the interface and realizing synergistic effects.
Heteroatom doping TMN catalysts
Heteroatom doping can be utilized to modify the charge redistribution of host materials. In contrast to interfacial engineering, heteroatom doping can retain the original structure and morphology of host materials. Due to different electronegativities, heteroatoms adjust the electronic structures of host catalytic sites and gear up the catalytic performance of electrocatalysts, killing “two birds with one stone” (Zhang et al., 2021).
Recently, Zhu group prepared Co doping for a Ni3N heterostructure catalyst (Zhu et al., 2018). The XRD results confirmed that the Ni3N phase did not change with the addition of Co. Epitaxial in-growth Co promotes electrons transferal from Co to Ni3N, where these added electrons can stabilize the H atom, thus reducing ΔGH* to improve HER performance. Ni doping of CoN is further demonstrated by Yu et al. (Yu et al., 2019). The enhanced performance was mainly due to CoN catalytic sites being modified by nearby Ni, leading to more active sites, improved conductivity, and fast charge transfer. In addition, Zhang et al. doped V into Ni3N/Ni to fabricate V doping Ni3N/Ni (Zhang et al., 2021). The charge redistribution is mainly confined to the N and Ni sites nearest to the doping V. The coupling of Ni and V dopant can promote water adsorption and optimize the value of ΔGH*. Zhang et al. reported a V-doped Ni3N nanosheet, denoted as V doping Ni3N NS(Zhang et al., 2021). V doping can effectively adjust the electronic structure of Ni3N, reduce the energy barrier of RDS, and promote dehydrogenation kinetics and H* adsorption/desorption. Wu et al. further proved that Cr doping can downshift the high unoccupied d-orbital of Ni3N (Wu et al., 2021). Cr doping exists as a Cr-n6 state, which can redistribute the surface electronic state of Ni3N to reduce the average d-band energy of Ni3N. The downshift of d-band energy can strengthen the orbital coupling between the unpaired electrons of O 2p and the unoccupied states of Ni 3d—beneficial to the adsorption and dissociation of water. Cr doping Ni3N can also promote the subsequent solution of H through the synergistic effect. Therefore, the doping of Cr enhanced the electron coupling with water molecules and promoted the dissociation kinetics of water molecules, thereby improving the HER performance, which is very close to the performance of Pt/C.
Heteroatom dopant can adjust electron density, modulate the orbit of catalytic sites, improve the conductivity of host materials, and retain the intrinsic component and structure of the host catalyst, thus improving the catalytic performance of TMNs. These results unambiguously show the advantage of the heteroatom dopant in improving catalytic performances.
Conclusions and perspectives
Although significant progress has been made in hydrogen electrochemistry, most of the reported HOR/HER catalysts in the laboratory still cannot meet the demands of industrial devices, especially PGM-free electrocatalysts. Herein, we envision the challenges and opportunities in this field:
1) Although DFT calculation is very helpful for identifying the factors affecting catalyst performance, the theoretical models cannot easily describe the real catalytic conditions, especially the thermodynamics of gas reactions, which may lead to deviation from the ideal liquid conditions under the applied potential. Thus, more accurate theories are needed to explain the complex reaction process and responsibility for developing novel PGM-free electrocatalysts.
2) Compared to PGM electrocatalysts, TMNs possess moderated chemical stability in hash conditions. Although many TMNs have achieved excellent catalytic performance, long-term stability at high current is the main challenge of transition metal nitrides for HER. Moreover, the performance of TMNs for alkaline HOR in the laboratory is far from practical in application. In addition, because industrial H2 supplies are mainly provided by steam reforming, excellent CO tolerance performance is highly desirable for HOR electrocatalysts. However, up to now, only a few electrocatalysts have exhibited moderated CO tolerance.
3) At present, most PGM-free HOR catalysts are evaluated in three electrode systems. However, the performance of TMN catalysts in hydrogen fuel cells is generally moderated, owing to the oxidation of active metal sites, which results in a rapid decrease in catalytic performance. In addition, the Hash-operated condition of water electrolysis and fuel cells will result in the changed surface oxidation of TMN electrocatalysts. Therefore, in the future, attention should be paid to improving the stability and antioxidants of PGM-free electrocatalysts under harsh reaction environments.
In summary, the present review introduces the basic and related theories of HER/HOR and analyzes the innovative HER/HOR catalysts reported recently. The different design strategies for adjusting the electronic configuration to optimize the thermodynamic process of hydrogen adsorption/desorption of the catalyst were compared: 1) solid support synergism; 2) hetero-interface engineering; 3) heteroatom doping. The research on catalysts based on TMNs is still very limited, and there is still much room for improvement, especially using HOR of TMNs. We believe that, with continuous research in this area, the commercial application of hydrogen energy will be greatly promoted.
Author contributions
ST and ZZ drafted the paper. ST and FS wrote the paper. JX, XY, and XS commented on the work and revised the work.
Funding
The work was supported by funds provided by the National Natural Science Foundation of China (22201294) and the Joint Interdisciplinary Research Project of SIAT (E25427).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Chen, P., Xu, K., Fang, Z., Tong, Y., Wu, J., Lu, X., et al. (2015). Metallic Co4N porous nanowire arrays activated by surface oxidation as electrocatalysts for the oxygen evolution reaction. Angew. Chem. Int. Ed. Engl. 127 (49), 14923–14927. doi:10.1002/ange.201506480
Chen, Z., Duan, X., Wei, W., Wang, S., and Ni, B. J. (2019). Recent advances in transition metal-based electrocatalysts for alkaline hydrogen evolution. J. Mat. Chem. A 7 (25), 14971–15005. doi:10.1039/C9TA03220G
Cheng, Y. W., Hsieh, T. H., Huang, Y. C., Tseng, P. H., Wang, Y. Z., Ho, K. S., et al. (2022). Calcined Co (II)-Chelated polyazomethine as cathode catalyst of anion exchange membrane fuel cells. Polym. (Basel). 14 (9), 1784. doi:10.3390/polym14091784
Chu, D., Nashalian, A., Zhou, Y., Zhao, X., Guo, D., He, R., et al. (2020). Low-cost and nature-friendly hierarchical porous carbon for enhanced capacitive electrochemical energy storage. ACS Appl. Energy Mat. 3 (8), 7246–7250. doi:10.1021/acsaem.0c01289
Cong, Y., Yi, B., and Song, Y. (2018). Hydrogen oxidation reaction in alkaline media: From mechanism to recent electrocatalysts. Nano Energy 44, 288–303. doi:10.1016/j.nanoen.2017.12.008
Dai, R., Zhang, H., Zhou, W., Zhou, Y., Ni, Z., Chen, J., et al. (2022). Interface engineering of bimetallic nitrides nanowires as a highly efficient bifunctional electrocatalyst for water splitting. J. Alloys Compd. 919, 165862. doi:10.1016/j.jallcom.2022.165862
Fan, M., Zheng, Y., Li, A., Li, K., Liu, H., and Qiao, Z. A. (2018). Janus CoN/Co cocatalyst in porous N-doped carbon: Toward enhanced catalytic activity for hydrogen evolution. Catal. Sci. Technol. 8 (14), 3695–3703. doi:10.1039/C8CY00571K
Feng, X., Wang, H., Bo, X., and Guo, L. (2019). Bimetal–organic framework-derived porous rodlike Cobalt/Nickel nitride for All-pH value electrochemical hydrogen evolution. ACS Appl. Mat. Interfaces 11 (8), 8018–8024. doi:10.1021/acsami.8b21369
Guo, Y., Yuan, P., Zhang, J., Xia, H., Cheng, F., Zhou, M., et al. (2018). Co2P–CoN double active centers confined in N-doped carbon nanotube: Heterostructural engineering for trifunctional catalysis toward HER, ORR, OER, and Zn–air batteries driven water splitting. Adv. Funct. Mat. 28 (51), 1805641. doi:10.1002/adfm.201805641
Han, N., Liu, P., Jiang, J., Ai, L., Shao, Z., and Liu, S. (2018). Recent advances in nanostructured metal nitrides for water splitting. J. Mat. Chem. A 6 (41), 19912–19933. doi:10.1039/C8TA06529B
Hsieh, T. H., Wang, Y. Z., and Ho, K. S. (2022). Cobalt-based cathode catalysts for oxygen-reduction reaction in an anion exchange membrane fuel cell. Membr. (Basel). 12 (7), 699. doi:10.3390/membranes12070699
Hu, L., Hu, Y., Liu, R., Mao, Y., Balogun, M. S. J. T., and Tong, Y. (2019). Co-based MOF-derived Co/CoN/Co2P ternary composite embedded in N-and P-doped carbon as bifunctional nanocatalysts for efficient overall water splitting. Int. J. Hydrogen Energy 44 (23), 11402–11410. doi:10.1016/j.ijhydene.2019.03.157
Hu, S., Wang, S., Feng, C., Wu, H., Zhang, J., and Mei, H. (2020). Novel MOF-derived nickel nitride as high-performance bifunctional electrocatalysts for hydrogen evolution and urea oxidation. ACS Sustain. Chem. Eng. 8 (19), 7414–7422. doi:10.1021/acssuschemeng.0c01450
Hua, W., Sun, H., Liu, H., Li, Y., and Wang, J. G. (2021). Interface engineered NiMoN/Ni3N heterostructures for enhanced alkaline hydrogen evolution reaction. Appl. Surf. Sci. 540, 148407. doi:10.1016/j.apsusc.2020.148407
Huang, C., Zhang, B., Wu, Y., Ruan, Q., Liu, L., Su, J., et al. (2021). Experimental and theoretical investigation of reconstruction and active phases on honeycombed Ni3N-Co3N/C in water splitting. Appl. Catal. B Environ. 297, 120461. doi:10.1016/j.apcatb.2021.120461
Jaramillo, T. F., Jørgensen, K. P., Bonde, J., Nielsen, J. H., Horch, S., and Chorkendorff, I. (2007). Identification of active edge sites for electrochemical H2 evolution from MoS2 nanocatalysts. Science 317 (5834), 100–102. doi:10.1126/science.1141483
Jia, H., Yao, N., Zhu, J., Liu, Y., Lao, Y., Cong, H., et al. (2022). Ni3N modified MOF heterostructure with tailored electronic struc-ture for efficient overall water splitting. Chin. J. Struc. Chem. 41, 2208031–2208036. doi:10.14102/j.cnki.0254-5861.2022-0112
Jiao, Y., Zheng, Y., Jaroniec, M., and Qiao, S. Z. (2015). Design of electrocatalysts for oxygen-and hydrogen-involving energy conversion reactions. Chem. Soc. Rev. 44 (8), 2060–2086. doi:10.1039/C4CS00470A
Li, S., Wang, Y., Wang, H., Zhang, Q., Zhang, Z., and Liu, H. (2021). Heterostructures of MXenes and CoNx-graphene as highly active electrocatalysts for hydrogen evolution reaction in alkaline media. J. Appl. Electrochem. 51 (6), 871–878. doi:10.1007/s10800-021-01542-4
Liu, B., Cheng, J., Peng, H. Q., Chen, D., Cui, X., Shen, D., et al. (2019a). In situ nitridated porous nanosheet networked Co3O4–Co4N heteronanostructures supported on hydrophilic carbon cloth for highly efficient electrochemical hydrogen evolution. J. Mat. Chem. A 7 (2), 775–782. doi:10.1039/C8TA09800J
Liu, Z., Tan, H., Liu, D., Liu, X., Xin, J., Xie, J., et al. (2019b). Promotion of overall water splitting activity over a wide pH range by interfacial electrical effects of metallic NiCo-nitrides nanoparticle/NiCo2O4 nanoflake/graphite fibers. Adv. Sci. 6 (5), 1801829. doi:10.1002/advs.201801829
Naguib, M., Kurtoglu, M., Presser, V., Lu, J., Niu, J., Heon, M., et al. (2011). Two-dimensional nanocrystals produced by exfoliation of Ti3AlC2. Adv. Mat. 23 (37), 4248–4253. doi:10.1002/adma.201102306
Ni, W., Krammer, A., Hsu, C. S., Chen, H. M., Schüler, A., and Hu, X. (2019). Ni3N as an active hydrogen oxidation reaction catalyst in alkaline medium. Angew. Chem. Int. Ed. Engl. 131 (22), 7523–7527. doi:10.1002/ange.201902751
Okubo, K., Ohyama, J., and Satsuma, A. (2019). Surface modification of Pt nanoparticles with other metals boosting the alkaline hydrogen oxidation reaction. Chem. Commun. 55 (21), 3101–3104. doi:10.1039/C9CC00582J
Panda, C., Menezes, P. W., Zheng, M., Orthmann, S., and Driess, M. (2019). In situ formation of nanostructured core–shell Cu3N–CuO to promote alkaline water electrolysis. ACS Energy Lett. 4 (3), 747–754. doi:10.1021/acsenergylett.9b00091
Pei, Z., Huang, Y., Tang, Z., Ma, L., Liu, Z., Xue, Q., et al. (2019). Enabling highly efficient, flexible and rechargeable quasi-solid-state zn-air batteries via catalyst engineering and electrolyte functionalization. Energy Storage Mat. 20, 234–242. doi:10.1016/j.ensm.2018.11.010
Ray, C., Lee, S. C., Jin, B., Kundu, A., Park, J. H., and Jun, S. C. (2018). Conceptual design of three-dimensional CoN/Ni3N-coupled nanograsses integrated on N-doped carbon to serve as efficient and robust water splitting electrocatalysts. J. Mat. Chem. A 6 (10), 4466–4476. doi:10.1039/C7TA10933D
Ren, J. T., Wang, Y. S., Song, Y. J., Chen, L., and Yuan, Z. Y. (2022). Interface engineering of in− situ formed nickel hydr (oxy) oxides on nickel nitrides to boost alkaline hydrogen electrocatalysis. Appl. Catal. B Environ. 309, 121279. doi:10.1016/j.apcatb.2022.121279
Ruqia, B., and Choi, S. I. (2018). Pt and Pt–Ni (OH) 2 electrodes for the hydrogen evolution reaction in alkaline electrolytes and their nanoscaled electrocatalysts. ChemSusChem 11 (16), 2643–2653. doi:10.1002/cssc.201800781
Shalom, M., Ressnig, D., Yang, X., Clavel, G., Fellinger, T. P., and Antonietti, M. (2015). Nickel nitride as an efficient electrocatalyst for water splitting. J. Mat. Chem. A 3 (15), 8171–8177. doi:10.1039/C5TA00078E
Song, F., Li, W., and Sun, Y. (2017). Metal–organic frameworks and their derivatives for photocatalytic water splitting. Inorganics 5 (3), 40. doi:10.3390/inorganics5030040
Song, F., Li, W., Yang, J., Han, G., Liao, P., and Sun, Y. (2018b). Interfacing nickel nitride and nickel boosts both electrocatalytic hydrogen evolution and oxidation reactions. Nat. Commun. 9 (1), 4531. doi:10.1038/s41467-018-06728-7
Song, F., Li, W., Yang, J., Han, G., Yan, T., Liu, X., et al. (2019). Interfacial sites between cobalt nitride and cobalt act as bifunctional catalysts for hydrogen electrochemistry. ACS Energy Lett. 4 (7), 1594–1601. doi:10.1021/acsenergylett.9b00738
Song, F., Zhang, T., Qian, Y., Shaw, J., Chen, S., Chen, G., et al. (2021). Multifunctional electrocatalysts of nickel boride nanoparticles for superior hydrogen oxidation and water splitting. Mat. Today Energy 22, 100846. doi:10.1016/j.mtener.2021.100846
Song, F., Zhang, T., Zhou, D., Sun, P., Lu, Z., Bian, H., et al. (2022). Charge transfer of interfacial catalysts for hydrogen energy. ACS Mat. Lett. 4 (5), 967–977. doi:10.1021/acsmaterialslett.2c00143
Song, F. Z., Yang, X., and Xu, Q. (2020). Ultrafine bimetallic Pt–Ni nanoparticles achieved by metal–organic framework templated zirconia/porous carbon/reduced graphene oxide: Remarkable catalytic activity in dehydrogenation of hydrous hydrazine. Small Methods 4 (1), 1900707. doi:10.1002/smtd.201900707
Song, F. Z., Zhu, Q. L., Tsumori, N., and Xu, Q. (2015b). Diamine-alkalized reduced graphene oxide: Immobilization of sub-2 nm palladium nanoparticles and optimization of catalytic activity for dehydrogenation of formic acid. ACS Catal. 5 (9), 5141–5144. doi:10.1021/acscatal.5b01411
Song, F. Z., Zhu, Q. L., and Xu, Q. (2015a). Monodispersed PtNi nanoparticles deposited on diamine-alkalized graphene for highly efficient dehydrogenation of hydrous hydrazine at room temperature. J. Mat. Chem. A 3 (46), 23090–23094. doi:10.1039/C5TA05664K
Song, F. Z., Zhu, Q. L., Yang, X., Zhan, W. W., Pachfule, P., Tsumori, N., et al. (2018a). Hydrogen generation: Metal-organic framework templated porous carbon-metal oxide/reduced graphene oxide as superior support of bimetallic nanoparticles for efficient hydrogen generation from formic acid (adv. Energy mater. 1/2018). Adv. Energy Mat. 8 (1), 1770139. doi:10.1002/aenm.201870006
Sun, H., Tian, C., Fan, G., Qi, J., Liu, Z., Yan, Z., et al. (2020a). Boosting activity on Co4N porous nanosheet by coupling CeO2 for efficient electrochemical overall water splitting at high current densities. Adv. Funct. Mat. 30 (32), 1910596. doi:10.1002/adfm.201910596
Sun, K., Zhang, T., Tan, L., Zhou, D., Qian, Y., Gao, X., et al. (2020b). Interface catalysts of Ni/Co2N for hydrogen electrochemistry. ACS Appl. Mat. Interfaces 12 (26), 29357–29364. doi:10.1021/acsami.0c06644
Sun, T., Zhang, S., Xu, L., Wang, D., and Li, Y. (2018a). An efficient multifunctional hybrid electrocatalyst: Ni2P nanoparticles on MOF-derived Co, N-doped porous carbon polyhedrons for oxygen reduction and water splitting. Chem. Commun. 54 (85), 12101–12104. doi:10.1039/C8CC06566G
Sun, Z., Zhang, J., Xie, J., Zheng, X., Wang, M., Li, X., et al. (2018b). High-performance alkaline hydrogen evolution electrocatalyzed by a Ni3N–CeO2 nanohybrid. Inorg. Chem. Front. 5 (12), 3042–3045. doi:10.1039/C8QI00905H
Wang, M., Yang, H., Shi, J., Chen, Y., Zhou, Y., Wang, L., et al. (2021a). Alloying nickel with molybdenum significantly accelerates alkaline hydrogen electrocatalysis. Angew. Chem. Int. Ed. Engl. 133 (11), 5835–5841. doi:10.1002/ange.202013047
Wang, T., Wang, M., Yang, H., Xu, M., Zuo, C., Feng, K., et al. (2019). Weakening hydrogen adsorption on nickel via interstitial nitrogen doping promotes bifunctional hydrogen electrocatalysis in alkaline solution. Energy Environ. Sci. 12 (12), 3522–3529. doi:10.1039/C9EE01743G
Wang, X., Li, X., Cai, T., Cui, Y., Kong, D., Xu, J., et al. (2021b). Enhancing hydrogen oxidation electrocatalysis of nickel-based catalyst by simultaneous chemical anchoring and electronic structure regulation. Chem. Eng. J. 425, 130654. doi:10.1016/j.cej.2021.130654
Wang, X., Tong, Y., Li, X., Zhao, L., Cui, Y., Wang, Y., et al. (2022). Trace nitrogen-incorporation stimulates dual active sites of nickel catalysts for efficient hydrogen oxidation electrocatalysis. Chem. Eng. J. 445, 136700. doi:10.1016/j.cej.2022.136700
Wu, T., Song, E., Zhang, S., Luo, M., Zhao, C., Zhao, W., et al. (2022). Engineering metallic heterostructure based on Ni3N and 2M-MoS2 for alkaline water electrolysis with industry-compatible current density and stability. Adv. Mat. 34 (9), 2108505. doi:10.1002/adma.202108505
Wu, Y., Cai, J., Xie, Y., Niu, S., Zang, Y., Wu, S., et al. (2020). Regulating the interfacial electronic coupling of Fe2N via orbital steering for hydrogen evolution catalysis. Adv. Mat. 32 (26), 1904346. doi:10.1002/adma.201904346
Wu, Y., Xie, Y., Niu, S., Zang, Y., Cai, J., Bian, Z., et al. (2021). Accelerating water dissociation kinetics of Ni3N by tuning interfacial orbital coupling. Nano Res. 14 (10), 3458–3465. doi:10.1007/s12274-021-3562-1
Xing, Z., Li, Q., Wang, D., Yang, X., and Sun, X. (2016). Self-supported nickel nitride as an efficient high-performance three-dimensional cathode for the alkaline hydrogen evolution reaction. Electrochim. Acta 191, 841–845. doi:10.1016/j.electacta.2015.12.174
Xue, Z., Kang, J., Guo, D., Zhu, C., Li, C., Zhang, X., et al. (2018). Self-supported cobalt nitride porous nanowire arrays as bifunctional electrocatalyst for overall water splitting. Electrochim. Acta 273, 229–238. doi:10.1016/j.electacta.2018.04.056
Yan, H., Xie, Y., Wu, A., Cai, Z., Wang, L., Tian, C., et al. (2019). Anion-modulated HER and OER activities of 3D Ni–V-based interstitial compound heterojunctions for high-efficiency and stable overall water splitting. J. Mat. Chem. A Mat. 31 (23), 1901174. doi:10.1002/adma.201901174
Yan, L., and Zhang, B. (2021). Rose-like, ruthenium-modified cobalt nitride nanoflowers grown in situ on an MXene matrix for efficient and stable water electrolysis. J. Mat. Chem. A 9 (36), 20758–20765. doi:10.1039/D1TA05243H
Yang, F., Bao, X., Zhao, Y., Wang, X., Cheng, G., and Luo, W. (2019). Enhanced HOR catalytic activity of PGM-free catalysts in alkaline media: The electronic effect induced by different heteroatom doped carbon supports. J. Mat. Chem. A Mat. 7 (18), 10936–10941. doi:10.1039/C9TA01916B
Yang, X., Li, Z., Kitta, M., Tsumori, N., Guo, W., Zhang, Z., et al. (2020). Solid-solution alloy nanoclusters of the immiscible gold-rhodium system achieved by a solid ligand-assisted approach for highly efficient catalysis. Nano Res. 13 (1), 105–111. doi:10.1007/s12274-019-2579-1
Yu, L., Song, S., McElhenny, B., Ding, F., Luo, D., Yu, Y., et al. (2019). A universal synthesis strategy to make metal nitride electrocatalysts for hydrogen evolution reaction. J. Mat. Chem. A 7 (34), 19728–19732. doi:10.1039/C9TA05455C
Yuan, W., Wang, S., Ma, Y., Qiu, Y., An, Y., and Cheng, L. (2020). Interfacial engineering of cobalt nitrides and mesoporous nitrogen-doped carbon: Toward efficient overall water-splitting activity with enhanced charge-transfer efficiency. ACS Energy Lett. 5 (3), 692–700. doi:10.1021/acsenergylett.0c00116
Zhang, B., Xiao, C., Xie, S., Liang, J., Chen, X., and Tang, Y. (2016a). Iron–nickel nitride nanostructures in situ grown on surface-redox-etching nickel foam: Efficient and ultrasustainable electrocatalysts for overall water splitting. Chem. Mat. 28 (19), 6934–6941. doi:10.1021/acs.chemmater.6b02610
Zhang, H., Wang, J., Qin, F., Liu, H., and Wang, C. (2021a). V-doped Ni3N/Ni heterostructure with engineered interfaces as a bifunctional hydrogen electrocatalyst in alkaline solution: Simultaneously improving water dissociation and hydrogen adsorption. Nano Res. 14 (10), 3489–3496. doi:10.1007/s12274-021-3559-9
Zhang, J., Liu, Y., Li, J., Jin, X., Li, Y., Qian, Q., et al. (2021b). Vanadium substitution steering reaction kinetics acceleration for Ni3N nanosheets endows exceptionally energy-saving hydrogen evolution coupled with hydrazine oxidation. ACS Appl. Mat. Interfaces 13 (3), 3881–3890. doi:10.1021/acsami.0c18684
Zhang, T., Debow, S., Song, F., Qian, Y., Creasy, W. R., DeLacy, B. G., et al. (2021c). Interface catalysis of nickel molybdenum (NiMo) alloys on two-dimensional (2D) MXene for enhanced hydrogen electrochemistry. J. Phys. Chem. Lett. 12 (46), 11361–11370. doi:10.1021/acs.jpclett.1c02676
Zhang, T., Song, F., Qian, Y., Gao, H., Shaw, J., and Rao, Y. (2021d). Elemental engineering of high-charge-density boron in nickel as multifunctional electrocatalysts for hydrogen oxidation and water splitting. ACS Appl. Energy Mat. 4 (6), 5434–5442. doi:10.1021/acsaem.0c03179
Zhang, Y., Ouyang, B., Xu, J., Chen, S., Rawat, R. S., and Fan, H. J. (2016b). 3D porous hierarchical nickel–molybdenum nitrides synthesized by RF plasma as highly active and stable hydrogen-evolution-reaction electrocatalysts. Adv. Energy Mat. 6 (11), 1600221. doi:10.1002/aenm.201600221
Zhou, P., Zhai, G., Lv, X., Liu, Y., Wang, Z., Wang, P., et al. (2021). Boosting the electrocatalytic HER performance of Ni3N-V2O3 via the interface coupling effect. Appl. Catal. B Environ. 283, 119590. doi:10.1016/j.apcatb.2020.119590
Zhu, C., Wang, A. L., Xiao, W., Chao, D., Zhang, X., Tiep, N. H., et al. (2018). In situ grown epitaxial heterojunction exhibits high-performance electrocatalytic water splitting. Adv. Mat. 30 (13), 1705516. doi:10.1002/adma.201705516
Keywords: hydrogen oxidation, hydrogen evolution, transition metal nitrides, water electrolyzer, hydrogen fuel cell
Citation: Tang S, Zhang Z, Xiang J, Yang X, Shen X and Song F (2022) Recent advances in transition metal nitrides for hydrogen electrocatalysis in alkaline media: From catalyst design to application. Front. Chem. 10:1073175. doi: 10.3389/fchem.2022.1073175
Received: 18 October 2022; Accepted: 14 November 2022;
Published: 02 December 2022.
Edited by:
Nan Jiang, Guizhou University, ChinaReviewed by:
Bo Hu, Xiamen University, ChinaTong Liu, Qingdao University of Science and Technology, China
Copyright © 2022 Tang, Zhang, Xiang, Yang, Shen and Song. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fuzhan Song, Znpzb25nQHVqcy5lZHUuY24=; Jun Xiang, anhpYW5nQGp1c3QuZWR1LmNu; Xinchun Yang, eGMueWFuZ0BzaWF0LmFjLmNu
 Siyuan Tang
Siyuan Tang Zhipeng Zhang1
Zhipeng Zhang1 Fuzhan Song
Fuzhan Song