- 1Department of Mathematics, College of Sciences, University of Sharjah, Sharjah, United Arab Emirates
- 2School of Natural Sciences, National University of Sciences and Technology, Islamabad, Pakistan
- 3Department of Computer and Information Sciences, Northumbria University, Newcastle upon Tyne, United Kingdom
The Zagreb connection indices are the known topological descriptors of the graphs that are constructed from the connection cardinality (degree of given nodes lying at a distance 2) presented in 1972 to determine the total electron energy of the alternate hydrocarbons. For a long time, these connection indices did not receive much research attention. Ali and Trinajstić [Mol. Inform. 37, Art. No. 1800008, 2018] examined the Zagreb connection indices and found that they compared to basic Zagreb indices and that they provide a finer value for the correlation coefficient for the 13 physico-chemical characteristics of the octane isomers. This article acquires the formulae of expected values of the first Zagreb connection index of a random cyclooctatetraene chain, a random polyphenyls chain, and a random chain network with l number of octagons, hexagons, and pentagons, respectively. The article presents extreme and average values of all the above random chains concerning a set of special chains, including the meta-chain, the ortho-chain, and the para-chain.
1 Introduction
Graph theory is vital to various disciplines, including the chemical and biological sciences. One of the objectives of chemical graph theory is its primary and significant role in studying physico-chemical reactions and biological activities and pointing out the structural properties of molecular graphs, etc., Topological descriptors have played a significant role in achieving the desired properties of molecular graphs. Topological descriptors are molecular structural invariants that theoretically and mathematically explain the connectivity characteristics of nano-materials and chemical compounds. Therefore, topological indices produce sharper approaches to measuring their behavior and characteristics.
For 20 years, hydrocarbons and their derivatives have received attention from researchers because these compounds only have two members, carbon and hydrogen. We can acquire various types of hydrocarbon derivatives by replacing their molecular hydrogen atoms with different types of other atomic groups. A large number of valuable hydrocarbons are available in plants and some valuable characteristics of hydrocarbons are important to chemical raw materials and fuel.
Throughout this article, the vertex and edge sets of a graph
Suppose that
The Zagreb indices are the most notable invariants, and they have many valuable applications in chemistry. In 1972 Gutman and Trinajstić (Gutman and Trinajstić, 1972) established the first vertex degree dependent Zagreb index of a graph
Motivated by how influential they have become and the many important applications of primary Zagreb indices, Naji et al. (Soner and Naji, 2016; Gutman et al., 2017) presented the concept of Zagreb connection indices (leap Zagreb indices), constructed from the second degrees of the vertices of a graph
The chemical applications of ZC1 were presented in (8), indicating that the given index has a wide co-relation with the physical characteristics of chemical compounds, for instance, boiling point, enthalpy of evaporation, entropy, acentric factor, and standard enthalpy of vaporization. Let fl present the cardinality of the subset of vertices of
Huang et al. (2014) determined the expected values for Kirchhoff indices of random polyphenyl and spiro chains. Ma et al. (2017), Yang and Zhang. (2012), and Qi et al. (2022) independently acquired the expected value of Wiener indices of random polyphenyl chain and random spiro chain. Zhang et al. (2020) have provided expected values of the Schultz, Gutman, multiplicative degree-Kirchhoff, and additive degree-Kirchhoff indices of random polyphenylene chains. Raza and Imran. (2021) obtained expected values of modified second Zagreb, symmetric difference, inverse symmetric, and augmented Zagreb indices in random cyclooctane chains. Zhang et al. (2021) established the formulae for expected values of Sombor indices of a general random chain. Recently, many studies have explored the expected values of different topological indices. For further information, we refer readers to the following studies (Raza, 2020b; Fang et al., 2021; Raza, 2021; Jahanbanni, 2022; Raza et al., 2022).
Motivated by the above research, the present study determined the explicit formulae for expected values of the first Zagreb connection index of the random cyclooctatetraene chain, random polyphenyls chain, and random chain network with l octagons, hexagons, and pentagons, respectively. Moreover, we examined the average and extreme values of the Zagreb connection index among all the above-mentioned random chains corresponding to their set.
2 The first Zagreb connection index of random cyclooctatetraene chain
Cyclooctatetraene, having chemical formula C8H8, is an organic compound whose full name is ‘1, 3, 5, 7 − cyclooctene. Its structure is a cyclic polyolefin-like benzene, but it is not aromatic, see (Willis et al., 1952; Mathews and Lipscomb, 1959; Traetteberg et al., 1970). It has the same chemical characteristics as unsaturated hydrocarbons and is easy to construct explosive organic peroxides, (Milas and NolanPetrus, 1958; Donald and Whitehead, 1969; Garavelli et al., 2002; Schwamm et al., 2019).
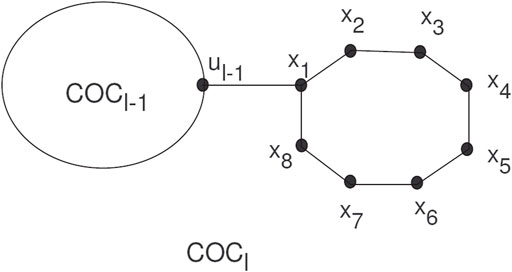
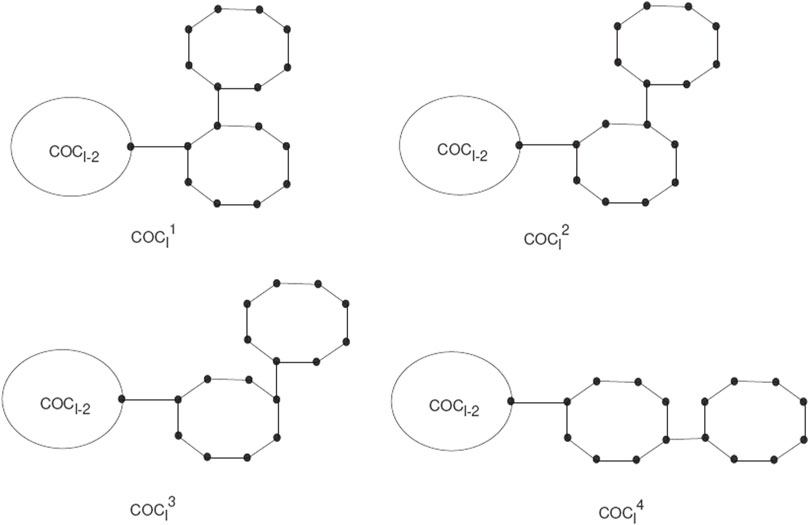
Spiro compounds are valuable types of cycloaltanes in organic chemistry. A spiro union is a join of two rings that have a common atom between both rings, and a join of a direct union among the rings is known as a free spiro union in spiro compounds. In a cyclooctatylene chain, octagons are joined by cut vertices or cut edges. A random cyclooctatetraene chain COCl, has l octagons, and can be constructed by a cyclooctatetraene chain COCl−1 with l−1 octagons attached to a new octagon Gl by a bridge (see Figure 1).
The COCl is a cyclooctatetraene chain with l ≥ 2 having G1, G2, … , Gl octagons. The new octagon can be joined by four different schemes, which give the local orderings. We use these as
A random cyclooctatetraene chain COCl(k1, k2, k3) is a cyclooctatetraene chain constructed by step-by-step attachment of new octagons. At every step p = 2, 3, … , l a random choice is constructed from one of the four possible chains:
1
2
3
4
Where all the given probabilities are constant. In this section, we will discuss the expected value for the first Zagreb connection index among random cyclooctatetraene chains with l octagons.
3 The first Zagreb connection index of a random polyphenyls chain
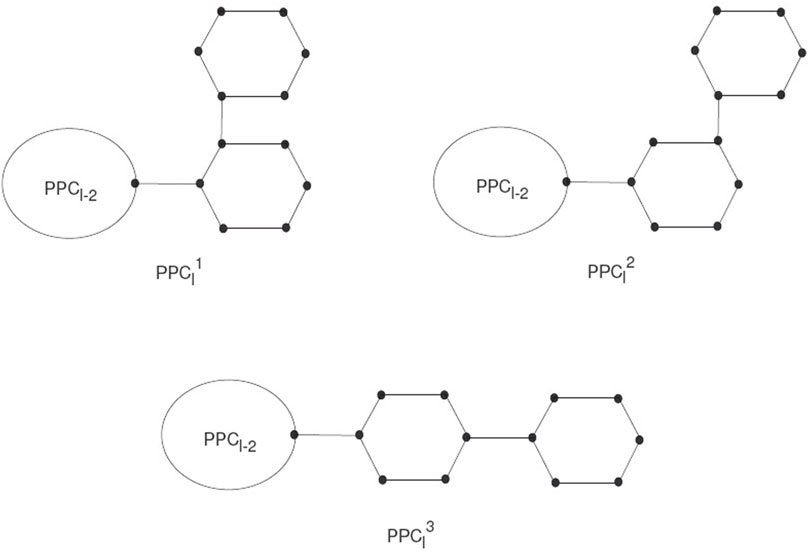
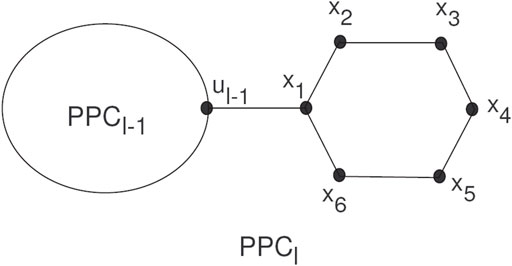
Polyphenyls showed a molecular graph corresponding to a type of macrocyclic aromatic hydrocarbons, and these molecular graphs of polyphenyls construct a polyphenyl structure. Polyphenyls and their derivatives have applications in drug synthesis, organic synthesis, heat exchangers, etc., and have received attention from chemists. A random polyphenyl chain PPCl with l hexagons can be constructed by a polyphenyl chain PPCl−1 using l−1 hexagons attached to a new hexagon Gl by a bridge (see Figure 3).
The PCCl will be a polyphenyl chain with l ≥ 2 having G1, G2, … , Gl hexagons. PPCl is the meta-chain Ml, the ortho-chain
A random polyphenyl chain PPCl(k1, k2) is a polyphenyl chain constructed by step-by-step attachment of new hexagons. At every step p = 2, 3, … , l, a random choice construct one of the three possible chains:
1
2
3
Where all the given probabilities are constant. In this section, we discuss the expected value for the first Zagreb connection index of the random polyphenyl chain with l hexagons.
4 The first Zagreb connection index of random chain network PGl
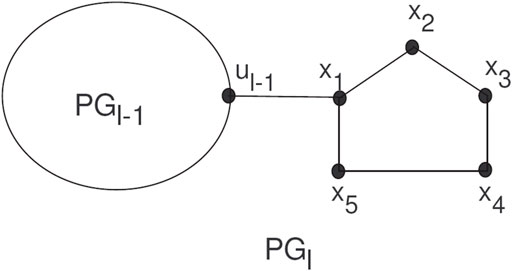
The random chain networks PGl with l pentagons can be constructed by PGl−1 having l−1 pentagons attached to a new pentagon Hl by a bridge (see Figure 5).
The PGl will be a random chain network with l ≥ 2, and H1, H2, … , Hl pentagons. For l ≥ 3, there are two ways to attach pentagons at the end and get
1
2
Where all the given probabilities are constant.
This section discusses the expected value for the first Zagreb connection index of the random chain network with l pentagons. The proof of Theorem 4.1 is the same as the proofs of Theorem 2.1 and Theorem 3.1; therefore, we omit it here.
5 The average values for the first Zagreb connection index
This section finds the average values for the first Zagreb connection index concerning the sets of all cyclooctatetraene chains with l octagons, polyphenyl chains with l hexagons, and chain networks with l pentagons. Let
The average value concerning sets
6 Conclusion
This study computed the expected values of the first Zagreb connection index in a random cyclooctatetraene chain, random polyphenyls chain, and random chain network with l, octagons, hexagons, and pentagons, respectively. It has discussed the maximum chain and the minimum chain of the COCl, PPCl, and PGl, respectively, concerning the expected values of these chains. The average values discussed in all of the above are considered random chains for unique chains.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author contributions
Investigation: ZR, SA, and YS: Writing: ZR, SA, and YS; Review: ZR, SA, and YS.
Funding
This research was funded by the University of Sharjah.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fchem.2022.1067874/full#supplementary-material
References
Akhter, S., Imran, M., Farahan, M. R., and Javaid, I. (2017). On topological properties of hexagonal and silicate networks. Hac. J. Math. Stat. 48 (3), 1–13. doi:10.15672/hjms.2017.541
Akhter, S., Imran, M., Gao, W., and Farahani, M. R. (2018). On topological indices of honeycomb networks and graphene networks. Hac. J. Math. Stat. 47 (1), 19–35. doi:10.15672/hjms.2017.464
Akhter, S., Imran, M., and Iqbal, Z. (2020). Mostar indices of SiO2 nanostructures and melem chain nanostructures. Int. J. Quantum Chem. 121 (5). doi:10.1002/qua.26520
Akhter, S., and Imran, M. (2016). On molecular topological properties of benzenoid structures. Can. J. Chem. 94 (8), 687–698. doi:10.1139/cjc-2016-0032
Akhter, S., Imran, M., and Raza, Z. (2016). On the general sum-connectivity index and general Randić index of cacti. J. Inequal. Appl. 2016 (1), 300–309. doi:10.1186/s13660-016-1250-6
Ali, A., and Trinajstić, N. (2018). A novel/old modification of the first Zagreb index. Mol. Inf. 37 (6–7), 1800008. doi:10.1002/minf.201800008
Bao, L. J., Raza, Z., and Javaid, M. (2020). Zagreb connection numbers for cellular neural networks. Discrete Dyn. Nat. Soc. 2020, 1–8. doi:10.1155/2020/8038304
Basavanagoud, B., and Chitra, E. (2018). On the leap Zagreb indices of generalized xyz-point-line transformation graphs Txyz(G), when z = 1. Int. J. Math. Comb. 2 (44–66).
Basavanagoud, B., and Jakkannavar, P. (2018). Computing the first leap Zagreb index of some nanostructures. Inter. J. Appl. Math. 6 (2–B), 141–150.
Cao, J., Ali, U., Javaid, M., and Huang, C. (2020). Zagreb connection indices of molecular graphs based on operations. Complexity 2020, 1–15. doi:10.1155/2020/7385682
Donald, H. L., and Whitehead, M. A. (1969). Molecular geometry and bond energy. III. cyclooctatetraene and related compounds. J. Am. Chem. Soc. 91 (2), 238–242. doi:10.1021/ja01030a003
Du, Z., Ali, A., and Trinajstić, N. (2019). Alkanes with the first three maximal/minimal modified first Zagreb connection indices. Mol. Inf. 38 (4), 1800116. doi:10.1002/minf.201800116
Ducoffe, G., Marinescu-Ghemeci, R., and Obreja, C. (2018). International symposium on symbolic and numeric algorithms for scientific computing (SYNASC). Timisoara, Rom. 141–148.
Ducoffe, G., Marinescu-Ghemeci, R., and Obreja, C. (2018). Proceedings of the 16th cologne-twente workshop on graphs and combinatorial optimization. Paris, Fr. CNAM 65–68.
Fang, X., You, L., and Liu, H. (2021). The expected values of Sombor indices in random hexagonal chains, phenylene chains, and Sombor indices of some chemical graphs. Int. J. Quantum. Chem. 121 (17). doi:10.1002/qua.26740
Fatima, N., Bhatti, A. A., Ali, A., and Gao, W. (2019). Zagreb connection indices of two dendrimer nanostars. Acta Chem. Iasi 27 (1), 1–14. doi:10.2478/achi-2019-0001
Garavelli, M., Bernardi, F., Cembran, A., Castaño, O., Frutos, L. M., Merchán, M., et al. (2002). Cyclooctatetraene computational photo- and thermal chemistry: A reactivity model for conjugated hydrocarbons. J. Am. Chem. Soc. 124 (46), 13770–13789. doi:10.1021/ja020741v
Gutman, I. (2013). Degree-based topological indices. Croat. Chem. Acta. 86, 351–361. doi:10.5562/cca2294
Gutman, I., Milovanović, E., and Milovanović, I. (2020). Beyond the Zagreb indices. AKCE Int. J. Graphs Comb. 17 (1), 74–85. doi:10.1016/j.akcej.2018.05.002
Gutman, I., Naji, A. M., and Soner, N. D. (2017). The first leap Zagreb index of some graph operations. Commun. Comb. Optim. 2 (2), 99–117.
Gutman, I., and Trinajstić, N. (1972). Graph theory and molecular orbitals, Total π electron energy of alternant hydrocarbons. Chem. Phys. Lett. 17 (4), 535–538. doi:10.1016/0009-2614(72)85099-1
Huang, G. H., Kuang, M. J., and Deng, H. Y. (2014). The expected values of Kirchhoff indices in the random polyphenyl and spiro chains. ARS Math. Contem. 9 (2), 197–207. doi:10.26493/1855-3974.458.7b0
Jahanbanni, A. (2022). The expected values of the first Zagreb and Randić indices in random polyphenyl chains. Polycycl. Aromat. Compd. 42 (4), 1851–1860. doi:10.1080/10406638.2020.1809472
Khalid, S., Kok, J., and Ali, A. (2018). Zagreb connection indices of TiO2 nanotubes. Chem. Bulg. J. Sci. Edu. 27 (1), 86–92.
Ma, L., Bian, H., Liu, B. J., and Yu, H. Z. (2017). The expected values of the Wiener index in the random phenylene and spiro chains. ARS Comb. 130, 267–274.
Manzoor, S., Fatima, N., Bhatti, A. A., and Ali, A. (2018). Zagreb connection indices of some nanostructures. Acta Chem. Iasi 26 (2), 169–180. doi:10.2478/achi-2018-0011
Mathews, F. S., and Lipscomb, W. N. (1959). The structure of silver cyclooctatetraene nitrate. J. Phy. Chem. 63 (6), 845–850. doi:10.1021/j150576a017
Milas, N., and NolanPetrus, J. (1958). Notes-ozonization of cyclooctatetraene. J. Org. Chem. 23 (4), 624–625. doi:10.1021/jo01098a611
Naji, A. M., Davvaz, B., and Mahde, S. S. (2020). A study on some properties of leap graphs. Commun. Comb. Optim. 5 (1), 9–17.
Naji, A. M., and Soner, N. D. (2018). The first leap Zagreb index of some graph operations. Int. J. Appl. Graph Theor. 2 (1), 7–18.
Qi, J., Fang, M., and Geng, X. (2022). The expected value for the Wiener index in the random spiro chains. Polycycl. Aromat. Compd. 1–11.
Raza, Z., and Imran, M. (2021). Expected values of some molecular descriptors in random cyclooctane chains. Symmetry 13 (11), 2197. doi:10.3390/sym13112197
Raza, Z. (2020). Leap Zagreb connection numbers for some networks models. J. Chem. 20 (6), 1407–1413. doi:10.22146/ijc.53393
Raza, Z., Naz, K., and Ahmad, S. (2022). Expected values of molecular descriptors in random polyphenyl chains. Emerg. Sci. J. 6 (1), 151–165. doi:10.28991/esj-2022-06-01-012
Raza, Z. (2020). The expected values of arithmetic bond connectivity and geometric indices in random phenylene chains. Heliyon 6 (7), e04479. doi:10.1016/j.heliyon.2020.e04479
Raza, Z. (2021). The expected values of some indices in random phenylene chains. Eur. Phys. J. Plus 136 (11–15), 91. doi:10.1140/epjp/s13360-021-01082-y
Raza, Z. (2022). Zagreb connection indices for some Benzenoid systems. Polycycl. Aromat. Compd. 42 (4), 1814–1827. doi:10.1080/10406638.2020.1809469
Schwamm, R. J., Anker, M. D., Lein, M., and Coles, M. P. (2019). Reduction vs. Addition: The reaction of an aluminyl anion with 1, 3, 5, 7 − cyclooctatetraene. Angew. Chem. Int. Ed. 58 (5), 1489–1493. in English) 131. doi:10.1002/anie.201811675
Shao, Z., Gutman, I., and Li, Z. (2018). Leap Zagreb indices of trees and unicyclic graphs. Commun. Comb. Optim. 3 (2), 179–194.
Shao, Z., Gutman, I., Li, Z., Wang, S., and Wu, P. (2018). Leap Zagreb indices of trees and unicyclic graphs. Commun. Comb. Optim. 3 (2), 179–194.
Soner, N. D., and Naji, A. M. (2016). The k-distance neighborhood polynomial of a graph. Int. J. Math. Comput. Sci. 3 (9), 2359–2364.
Tang, J. H., Ali, U., Javaid, M., and Shabbir, K. (2019). Zagreb connection indices of subdivision and semi-total point operations on graphs. J. Chem.
Traetteberg, M., Hagen, G., and Cyvin, S. J. (1970). IV. 1, 3, 5, 7-Cyclooctatetraene. Z. Für Naturforsch. B 25 (2), 134–138. doi:10.1515/znb-1970-0201
Willis, B. P., George, C. P., and Kenneth, S. P. (1952). The structure of cyclooctatetraene. J. Am. Chem. Soc. 74 (13), 3437–3438. doi:10.1021/ja01133a524
Yang, W., and Zhang, F. (2012). Wiener index in random polyphenyl chains. MATCH Commun. Math. Comp. Chem. 68, 371–376.
Ye, A., Qureshi, M. I., Fahad, A., Aslam, A., Jamil, M. K., Zafar, A., et al. (2019). Zagreb connection number index of nanotubes and regular hexagonal lattice. Open Chem. 17 (1), 75–80. doi:10.1515/chem-2019-0007
Zhang, L., Li, Q., Li, S., and Zhang, M. (2020). The expected values for the Schultz index, Gutman index, multiplicative degree Kirchhoff index, and additive degree Kirchhoff index of a random polyphenylene chain. Discrete Appl. Math. 282, 243–256. doi:10.1016/j.dam.2019.11.007
Keywords: average value, expected value, random cyclooctatetraene chain, random polyphenyls chain, zagreb connection indices
Citation: Raza Z, Akhter S and Shang Y (2023) Expected value of first Zagreb connection index in random cyclooctatetraene chain, random polyphenyls chain, and random chain network. Front. Chem. 10:1067874. doi: 10.3389/fchem.2022.1067874
Received: 12 October 2022; Accepted: 07 December 2022;
Published: 04 January 2023.
Edited by:
Anakuthil Anoop, Indian Institute of Technology Kharagpur, IndiaReviewed by:
Parvez Ali, Qassim University, Saudi ArabiaRogers Mathew, Indian Institute of Technology Hyderabad, India
Copyright © 2023 Raza, Akhter and Shang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zahid Raza, enJhemFAc2hhcmphaC5hYy5hZQ==; Yilun Shang, eWlsdW4uc2hhbmdAbm9ydGh1bWJyaWEuYWMudWs=
 Zahid Raza
Zahid Raza Shehnaz Akhter
Shehnaz Akhter Yilun Shang
Yilun Shang