
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Chem. , 19 October 2022
Sec. Theoretical and Computational Chemistry
Volume 10 - 2022 | https://doi.org/10.3389/fchem.2022.1048437
This article is part of the Research Topic Photocatalysis and Electrocatalysis for Energy Conversion View all 11 articles
The construction of van der Waals heterostructures offers effective boosting of the photocatalytic performance of two-dimensional materials. In this study, which uses the first-principles method, the electronic and absorptive properties of an emerging ZnO/C2N heterostructure are systematically explored to determine the structure’s photocatalytic potential. The results demonstrate that ZnO and C2N form a type-II band alignment heterostructure with a reduced band gap, and hence superior absorption in the visible region. Furthermore, the band edge positions of a ZnO/C2N heterostructure meet the requirements for spontaneous water splitting. The ZnO/C2N heterostructure is known to possess considerably improved carrier mobility, which is advantageous in the separation and migration of carriers. The Gibbs free energy calculation confirms the high catalytic activity of the ZnO/C2N heterostructure for water-splitting reactions. All the aforementioned properties, including band gap, band edge positions, and optical absorption, can be directly tuned using biaxial lateral strain. A suitable band gap, decent band edge positions, high catalytic activity, and superior carrier mobility thus identify a ZnO/C2N heterostructure as a prominent potential photocatalyst for water splitting.
The splitting of water into hydrogen (H2) and oxygen (O2) under the action of a photocatalyst has attracted extensive interest for its potential in tackling crises of energy and environmental pollution. Apart from the two basic requirements for band gap and band-edge positions, (Fujishima and Honda, 1972), excellent light absorption, low carrier recombination, and considerable carrier mobility are also necessary for a superior photocatalyst (Li et al., 2016). Extensive experimental and theoretical studies have been conducted to explore efficient novel photocatalysts.
In recent years, emerging two-dimensional (2D) materials have opened up a colorful stage for the design of new photocatalysts (Fan et al., 2021; Ren et al., 2022a; Qin et al., 2022). Notably, the naturally high surface area of 2D materials can provide more active sites for catalytic reactions (Ganguly et al., 2019). Furthermore, 2D materials shorten the migration distance of photogenerated carriers, thereby reducing the recombination of an electron–hole pair (Fan et al., 2021). A large number of 2D materials have been developed for photocatalysis, such as transition-metal dichalcogenide (Voiry et al., 2016), MXene (Cheng et al., 2019), carbonitrides (Wang et al., 2009), and others (Roger et al., 2017). In 2015, Mohammed et al. synthesized a new 2D multifunctional material C2N with a band gap of 1.96eV (Mahmood et al., 2015). The C2N monolayer possesses phonon modes close to those of graphenes (Sahin, 2015), indicating its fine thermal stability. Many facts confirm the highly tunable photocatalytic ability of monolayered C2N for water splitting (Xu et al., 2015; Ashwin Kishore and Ravindran, 2017). However, the rapid recombination of photogenerated carriers is still a serious issue for the use of the C2N monolayer in water splitting (Xu et al., 2015).
Some engineering processes have been proposed to improve the photocatalytic performance of monolayer C2N for water splitting, including doping (Du et al., 2016), defects (Zhang et al., 2018), atomic adsorption (Kishore et al., 2019; Zhang et al., 2022), and strain (Guan et al., 2015). More recently, nascent van der Waals (vdW) heterostructures (Ye et al., 2019; Zhao et al., 2021; Wang et al., 2022) have also been widely considered as a means of promoting the photocatalytic water-splitting performance of the C2N monolayer. The vdW heterostructure, composed of different 2D components, can maintain the excellent properties of those components, while some novel properties may be generated due to the interlayer coupling effects (Novoselov et al., 2016). Notably, the electron–hole pair, separated on the constituent monolayers, can substantially reduce the recombination rate of carriers, which is indeed favorable for photocatalytic water splitting (Deng et al., 2016; Novoselov et al., 2016). Kumar found that the carrier mobilities of the C2N/WS2 heterostructure with photocatalytic potential are efficiently enhanced (Kumar et al., 2018). Theoretical studies reveal that the C2N/GaTe, C2N/InTe, and C2N/InSe heterostructures are all suitable for photocatalysis, while their photocatalytic properties are sensitive to strain (Wang et al., 2019; Wang X. et al., 2020). The vdW heterostructures, such as the C2N/Janus monochalcogenides (Ma et al., 2021), CdS/C2N (Luo et al., 2017), and h-BN/C2N (Wang G. et al., 2020), are also predicted to have excellent photocatalytic performance. Recently, the novel ZnO/C2N heterostructure with a direct band gap of 2.0 eV has been reported, and its optoelectronic properties can be tuned with vertical strain and an external electric field (Song et al., 2021). Monolayered ZnO with its graphene-like structure is a 2D photocatalytic material with high carrier mobility. However, its large band gap (∼3.3 eV) (Ren et al., 2020) leads to poor absorption, limiting its photocatalytic application. Perhaps the ZnO/C2N heterostructure formed by two photocatalysts has more prominent carrier mobility and photocatalytic performance, but this remains unknown thus far.
In this work, theoretical work is conducted to comprehensively explore the electronic structure, carrier mobility, hydrogen evolution reaction (HER), and absorption properties of the ZnO/C2N heterostructure, as well as the effect of lateral strain on these properties. All the results confirm the substantial application potential of a ZnO/C2N heterostructure in photocatalysis for water splitting.
All calculations are implemented with the Vienna Ab-initio Simulation Package (VASP), based on the projected augmented wave method (PAW) (Kresse and Furthmüller, 1996a; Kresse and Furthmüller, 1996b). The generalized gradient approximation within the Perdew–Burke–Ernzerhof scheme (GGA-PBE) is used to describe the exchange-correlation functional (Perdew et al., 1996), while the DFT-D3 correction method (Grimme et al., 2010) is utilized to describe the vdW interaction between the two monolayers. The Heyd–Scuseria–Ernzerh hybrid functional (HSE06) (Heyd et al., 2003) is also adopted to determine the band gap of the ZnO/C2N heterostructure and its pristine components. The lattice constants and atomic positions of pristine ZnO and C2N monolayers are fully relaxed using the 6 × 6 × 1 and 15 × 15 × 1 G-centered Monkhorst–Pack (Monkhorst and Pack, 1976) k-mesh scheme to simplify the Brillouin zone, while a 3 × 3 × 1 k-mesh sampling is chosen for the ZnO/C2N heterostructure. All ion relaxation processes interrupt the process until the force per atom is less than 0.01 eV/Å and the energy convergence criterion of 10–5 eV is set. The plane wave cutoff of 450 eV is used throughout this work, and a 20 Å vacuum toward the z-direction is applied to shield interaction between neighboring layers. VASPKIT and VESTA are used for visualization (Momma and Izumi, 2011; Wang et al., 2021).
The geometric structures of ZnO and C2N monolayers are shown in Supplementary Figures S1A–C. Their lattice parameters are found to be 3.29 Å and 8.32 Å, respectively, which is consistent with previous reports (Mahmood et al., 2015; Lee et al., 2016). Both monolayers are direct band gap semiconductors, as the band structures are presented in Supplementary Figures S1B–D. The band gap values of ZnO and C2N determined by HSE06 are 3.28 eV and 2.47 eV, which are close to the reported results (Mahmood et al., 2015; Lee et al., 2016).
In order to minimize the strain effect, a 5 × 5 ZnO supercell and a 2 × 2 C2N supercell are used to make up the ZnO/C2N heterostructure. The ZnO/C2N heterostructure is constructed by fixing the C2N layer and shifting the ZnO layer to a high symmetry location. According to the location of the ZnO layer in the lattice, three kinds of stacking configurations (SCs) for the ZnO/C2N heterostructure are formed, as shown in Figure 1. In the interests of thermodynamic stability and for determining the most stable SC of the ZnO/C2N heterostructure, the binding energy Eb and the formation energy Ef are calculated using the Supplementary Equations S1, S2. According to the results in Supplementary Table S1, Ef and Eb are close to those of typical vdW heterostructures (Guo et al., 2017; Fan et al., 2019; Bafekry et al., 2020; Guo et al., 2020). The negative values confirm that all ZnO/C2N heterostructures can be prepared experimentally, as their stabilities are slightly different. The ZnO/C2N heterostructure in SC-Ⅲ, with an interlayer distance d of 3.14 Å, is provided with the most beneficial stability. Therefore, the ZnO/C2N heterostructure in SC-Ⅲ is the focus of the following research. Moreover, the ab initio molecular dynamic (AIMD) simulation is performed at 300 K to check the thermodynamic stability of the ZnO/C2N heterostructure. As the snapshot for the last frame shows in Supplementary Figure S3A, the ZnO/C2N heterostructure maintains good structural integrity within 6 ps, demonstrating its stability at room temperature. The time-dependent evolution of total potential energies, exhibited in Supplementary Figure S3B, also proves its thermal stability.
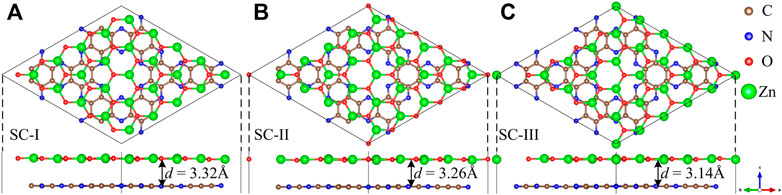
FIGURE 1. Optimized geometric structures of the (A) SC‐I, (B) SC‐II, (C) SC‐III ZnO/C2N heterostructure.
The band structures of the ZnO/C2N heterostructure within the three SCs are calculated, together with the projected density of states (PDOS). As shown in Figure 2A, the ZnO/C2N heterostructure is a direct band gap semiconductor, as both the VBM and CBM emerge at the G-point. Results of band structures also indicate that the SC has a negligible impact on electronic property. ZnO/C2N heterostructures in three SCs possess the same band gap of 0.77 eV and 1.99 eV, based on the PBE functional and the HSE06 functional, respectively. The band gaps are smaller than those of pristine monolayers, meaning that forming a heterostructure can evidently reduce the band gap and widen the range of absorption. Moreover, both the Figures 2A,B demonstrate that a type‐II heterostructure is formed when ZnO comes into contact with C2N.
Figure 3 shows the electron transfer between two component layers. Work function (Wf) is a serious parameter defining the ability of a catalyst surface to attract electrons (Vayenas et al., 1990). The values of Wf for ZnO and C2N are calculated as 4.82 eV and 5.78 eV, portending that electron flow from the ZnO layer to the C2N layer at the interface. Electron migration from ZnO to C2N is also observed from the planar-averaged charge density difference Δρ in Figure 3B, which ceases until the Fermi level is aligned, producing the positively charged ZnO layer and the negatively charged C2N layer. The visual charge density difference is also exhibited in Figure 3B, in which the yellow and cyan marked areas represent electron accumulation and electron depletion, respectively. Therefore, a potential drop of 8.32 eV is formed in Figure 3A. Finally, a built-in electric field, pointing from ZnO to C2N, is generated at the interface. The Bader charge calculation (Tang et al., 2009) also demonstrates the result of 0.203 electrons transferred from ZnO to C2N. It is worth noting that the prominent potential drop in the ZnO/C2N heterostructure can also offer a critical promotion for the separation of the photogenerated electron and hole, thereby reducing the recombination of carriers.
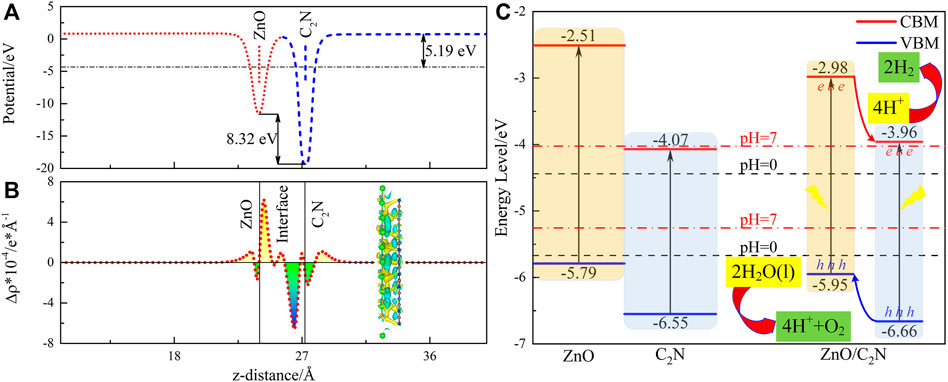
FIGURE 3. (A) Electrostatic potential, (B) planar-average and visual charge density difference Δρ, and (C) band alignment for the ZnO/C2N heterostructure.
Suitable band edge positions (EVBM and ECBM) and decent band alignment are crucial for photocatalysis (Zheng et al., 2018; Tang et al., 2020). The method proposed by Toroker et al. (2011) has been employed to evaluate the EVBM and ECBM of the ZnO/C2N heterostructure so as to explore its potential as a photocatalyst. It is obvious from Figure 3C that both the EVBM and ECBM of the two monolayers (Wang et al., 2018; Zhang X. et al., 2019) and the ZnO/C2N heterostructure, meet the redox potential requirements for a photocatalyst in an acidic environment (pH = 0). The ZnO/C2N heterostructure still has the talent of photocatalysis for water splitting in a neutral environment with pH = 7. Furthermore, a higher CBM and VBM of ZnO than those of C2N can be observed. It is thus suggested that hydrogen evolution reaction (HER) occurs on the C2N layer, while an oxygen evolution reaction (OER) happens on the ZnO layer. We then expand the type-Ⅱ mechanism in the ZnO/C2N heterostructure to boost HER and OER for water splitting. The conduction band offset (CBO) and valence band offset (VBO) are calculated as 0.98 eV and 0.69 eV, respectively. When the heterostructure is irradiated, the CBO promotes the transfer of photogenerated electrons in the CB of the ZnO layer to the CB of the C2N layer. The photogenerated holes in the C2N layer are driven by the VBO to the VB of the ZnO layer. Finally, the photogenerated electrons and holes remain in the C2N and ZnO monolayers, respectively, bringing about carrier separation spatially. Naturally, the type-II band alignment of the ZnO/C2N heterostructure is instrumental in overcoming the recombination of carriers to achieve better photocatalytic performance.
High carrier mobility μ is essential for superior photocatalysts (Guo et al., 2020; Ren et al., 2022b). The carrier mobility μ, defined as
has been evaluated based on the deformation potential theory (Bardeen and Shockley, 1950) for both the ZnO/C2N heterostructure and the two monolayers. The methods and details are mentioned in Supplementary Section S1. As for the results of the ZnO monolayer listed in Table 1, the electron mobilities in the x- and y-directions are superior to those of the hole, conforming to previous theoretical (Ren et al., 2020) and experimental (Gonzalez-Valls and Lira-Cantu, 2009; Anta et al., 2012) results. It can be observed from Table 1 that the excellent electron mobilities of the C2N monolayer are 4265 and 1944 cm2/V/s in the x- and y-directions, mainly due to the small carrier effective mass (m*) and deformation potential constant (E1). However, the hole m* of the C2N monolayer is several times that of the electron, resulting in low hole mobility (Kumar et al., 2018; Zhang X. et al., 2019).
The hole m*s of the ZnO/C2N heterostructure in the x- and y-directions are close to those of the ZnO monolayer, while the values of E1 in the aforementioned directions are comparable to those of the C2N monolayer. The in-plane stiffness (C2D) of the ZnO/C2N heterostructure increases and is about equal to the sum of two component layers. Consequently, the hole mobilities of the heterostructure in the x- and y-directions are 529.9 and 395.9 cm2/V/s, respectively, where pronounced improvements are due to the abovementioned changes of m*, E1, and C2D. It can be deduced that higher carrier mobility will induce enhanced carrier separation and migration in the ZnO/C2N heterostructure, which should illuminate its photocatalytic prospects for application in water splitting.
In order to explore the kinetic behavior of water splitting, the Gibbs free energy difference ΔG of HER and OER on the ZnO/C2N heterostructure is calculated using the method developed by Nørskov et al. (2005). The calculation details and favor absorption sites are present in the Supplementary Material. The HER is divided into the following two reactions:
H∗ is the only intermediate of HER, and it is obvious in Figure 4A that ΔG is a function of H coverage θ. When the θ equals 2/6, ΔG can be as low as 0.14 eV, which is comparable to the value of the C2N/WS2 heterostructure (Kumar et al., 2018). Therefore, the ZnO/C2N heterostructure can be used as a potential photocatalyst for HER due to the small value of ΔG (Hinnemann et al., 2005).
The OER involves the following four steps:
The performance of absorption is an important function of a photocatalyst, as it is the first step in water splitting to produce electron–hole pairs. Superior absorption with a wide range and a high coefficient is essential for a photocatalyst’s effective solar energy utilization. The optical coefficients α(ω) of the ZnO/C2N heterostructure and two components are calculated with the following equation (Gajdoš et al., 2006):
In this equation, ω1 and ω2 represent the real and imaginary parts of the dielectric function, respectively. The result, displayed in Figure 5, demonstrates the advantage of the heterostructure in absorption over ZnO and C2N. The heterostructure not only possesses a higher absorption coefficient (∼105cm−1) than ZnO and C2N but also has a wider absorption range from visible to ultraviolet light. The improvement in absorption performance can be attributed to its reduced band gap and significantly improved carrier mobility. The excellent absorption ability can generate more electron–hole pairs in the first step of water splitting, which is beneficial for the ZnO/C2N heterostructure in realizing its efficient photocatalytic performance.
The lateral strain is a common effect in heterostructures, as well as being a proven effective means of improving the photocatalytic performance of 2D material (Feng et al., 2012; Zhang J.-R. et al., 2019). We thus undertook a full investigation of the electronic and optical properties of the ZnO/C2N heterostructure with biaxial lateral strain to explore the regulatory effect of strain on photocatalytic performance. Strain ranging from −6% to +6% was applied to the ZnO/C2N heterostructure, and the band structures calculated with the HSE06 functional in Figure 6 clearly announce the structure’s identity as a direct band gap semiconductor.
Meanwhile, it can be seen from the PDOS exhibited in Supplementary Figure S13 that the strained heterostructures also belong to type-Ⅱ heterostructures, as both the VBM and CBM of the ZnO are higher than those of C2N. Moreover, it is clear in Figure 7A that compressive strain reduces the band gap, while tensile strain increases the gap. When the lattice is compressed by 6%, the band gap decreases to 1.72 eV, while the gap value increases to 2.31 eV when the heterostructure it is expanded by 6%. Within the strain range of −2% to +6%, we can also see that the band edge positions of the heterostructure still meet the requirements of photocatalysis for water splitting at the condition of pH = 0. It is very important for heterostructures to maintain their photocatalytic ability across a wide pH range (Ren et al., 2019). The band alignment shown in Supplementary Figure S15 indicates that the strained heterostructures still possess potential application for water splitting across a wide pH range. Figure 7B shows the effect of the strain on the absorption performance of the ZnO/C2N heterostructure. Compared with the freestanding ZnO/C2N heterostructure, the compressed heterostructures have higher absorption intensity and a wider absorption range, while the lattice expansion leads to improvement in the absorption performance of the heterostructures in the ultraviolet range. The significant modification of absorption is mainly a benefit of the regulation of the band gap. All the results directly confirm that the ZnO/C2N heterostructure is a promising candidate for use in the field of water splitting.
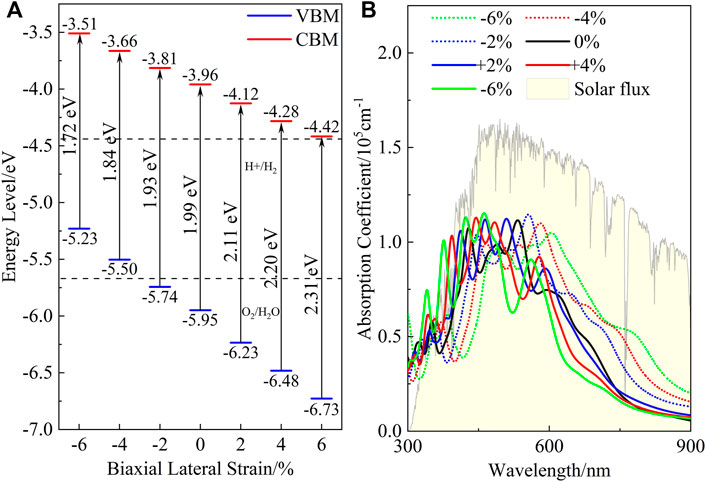
FIGURE 7. (A) Band alignment and (B) absorption spectrum of ZnO/C2N heterostructure under different strain conditions.
In this study, the electronic and absorption properties of the ZnO/C2N heterostructure are explored to reveal its potential for water splitting. The stabilized heterostructure is given a reduced band gap of 1.99 eV, while its band edge positions also meet the water-splitting requirements. The band alignment of the heterostructure belongs to type-II, which leads to the generation of a built-in electrical field between the two layers that promote carrier separation and migration. The more significant change is that the carrier mobility of the ZnO/C2N heterostructure is several times improved. The results of the Gibbs free energy calculation clearly indicate the promising catalytic ability of the ZnO/C2N heterostructure. As for the optical absorption performance, the reduced band gap and excellent carrier mobility endow the ZnO/C2N heterostructure with considerable absorption intensity and a wider absorption range. Moreover, the electronic and absorption properties of the ZnO/C2N heterostructure can be substantially tuned with biaxial lateral strain. All the results confirm that the ZnO/C2N heterostructure has potential use as a superior photocatalyst for water splitting.
The original contributions presented in the study are included in the article/Supplementary Material, and further inquiries can be directed to the corresponding author.
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
This work was financially supported by the National Natural Science Foundation of China (Grant No. 11875284), the Program for Science and Technology Innovation Talents in Universities of Henan Province (No. 21HASTIT020), and the Key Scientific Research Project of Colleges and Universities in Henan Province (No. 22A140023).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fchem.2022.1048437/full#supplementary-material
Anta, J., Guillén, E., and Tena-Zaera, R. (2012). ZnO-based dye-sensitized solar cells. J. Phys. Chem. C 116 (21), 11413–11425. doi:10.1021/jp3010025
Ashwin Kishore, M., and Ravindran, P. (2017). Tailoring the electronic band gap and band edge positions in the C2N monolayer by P and as substitution for photocatalytic water splitting. J. Phys. Chem. C 121 (40), 22216–22224. doi:10.1021/acs.jpcc.7b07776
Bafekry, A., Akgenc, B., Shayesteh, S., and Mortazavi, B. (2020). Tunable electronic and magnetic properties of graphene/carbon-nitride van der Waals heterostructures. Appl. Surf. Sci. 505, 144450. doi:10.1016/j.apsusc.2019.144450
Bardeen, J., and Shockley, W. (1950). Deformation potentials and mobilities in non-polar crystals. Phys. Rev. 80 (1), 72. doi:10.1103/PhysRev.80.72
Cheng, L., Li, X., Zhang, H., and Xiang, Q. (2019). Two-dimensional transition metal MXene-based photocatalysts for solar fuel generation. J. Phys. Chem. Lett. 10 (12), 3488–3494. doi:10.1021/acs.jpclett.9b00736
Deng, D., Novoselov, K., Fu, Q., Zheng, N., Tian, Z., and Bao, X. (2016). Catalysis with two-dimensional materials and their heterostructures. Nat. Nanotechnol. 11 (3), 218–230. doi:10.1038/nnano.2015.340
Du, J., Xia, C., Wang, T., Xiong, W., and Li, J. (2016). Modulation of the band structures and optical properties of holey C2N nanosheets by alloying with group IV and V elements. J. Mat. Chem. C 4 (39), 9294–9302. doi:10.1039/C6TC02469F
Fan, F., Wang, R., Zhang, H., and Wu, W. (2021). Emerging beyond-graphene elemental 2D materials for energy and catalysis applications. Chem. Soc. Rev. 50 (19), 10983–11031. doi:10.1039/C9CS00821G
Fan, Y., Wang, J., and Zhao, M. (2019). Spontaneous full photocatalytic water splitting on 2D MoSe2/SnSe2 and WSe2/SnSe2 vdW heterostructures. Nanoscale 11 (31), 14836–14843. doi:10.1039/C9NR03469B
Feng, J., Qian, X., Huang, C., and Li, J. (2012). Strain-engineered artificial atom as a broad-spectrum solar energy funnel. Nat. Photonics 6 (12), 866–872. doi:10.1038/nphoton.2012.285
Fujishima, A., and Honda, K. (1972). Electrochemical photolysis of water at a semiconductor electrode. Nature 238 (5358), 37–38. doi:10.1038/238037a0
Gajdoš, M., Hummer, K., Kresse, G., Furthmüller, J., and Bechstedt, F. (2006). Linear optical properties in the projector-augmented wave methodology. Phys. Rev. B 73 (4), 045112. doi:10.1103/PhysRevB.73.045112
Ganguly, P., Harb, M., Cao, Z., Cavallo, L., Breen, A., Dervin, S., et al. (2019). 2D nanomaterials for photocatalytic hydrogen production. ACS Energy Lett. 4 (7), 1687–1709. doi:10.1021/acsenergylett.9b00940
Gonzalez-Valls, I., and Lira-Cantu, M. (2009). Vertically-aligned nanostructures of ZnO for excitonic solar cells: A review. Energy Environ. Sci. 2 (1), 19–34. doi:10.1039/B811536B
Grimme, S., Antony, J., Ehrlich, S., and Krieg, H. (2010). A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 132 (15), 154104. doi:10.1063/1.3382344
Guan, S., Cheng, Y., Liu, C., Han, J., Lu, Y., Yang, S., et al. (2015). Effects of strain on electronic and optic properties of holey two-dimensional C2N crystals. Appl. Phys. Lett. 107 (23), 231904. doi:10.1063/1.4937269
Guo, H., Zhang, Z., Huang, B., Wang, X., Niu, H., Guo, Y., et al. (2020). Theoretical study on the photocatalytic properties of 2D InX (X= S, Se)/transition metal disulfide (MoS2 and WS2) van der Waals heterostructures. Nanoscale 12 (38), 20025–20032. doi:10.1039/D0NR04725B
Guo, Z., Miao, N., Zhou, J., Sa, B., and Sun, Z. (2017). Strain-mediated type-I/type-II transition in MXene/Blue phosphorene van der Waals heterostructures for flexible optical/electronic devices. J. Mat. Chem. C 5 (4), 978–984. doi:10.1039/C6TC04349F
Heyd, J., Scuseria, G., and Ernzerhof, M. (2003). Hybrid functionals based on a screened Coulomb potential. J. Chem. Phys. 118 (18), 8207–8215. doi:10.1063/1.1564060
Hinnemann, B., Moses, P., Bonde, J., Jørgensen, K., Nielsen, J., Horch, S., et al. (2005). Biomimetic hydrogen evolution: MoS2 nanoparticles as catalyst for hydrogen evolution. J. Am. Chem. Soc. 127 (15), 5308–5309. doi:10.1021/ja0504690
Kishore, M., Sjastad, A., and Ravindran, P. (2019). Influence of hydrogen and halogen adsorption on the photocatalytic water splitting activity of C2N monolayer: A first-principles study. Carbon 141, 50–58. doi:10.1016/j.carbon.2018.08.072
Kresse, G., and Furthmüller, J. (1996a). Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mat. Sci. 6 (1), 15–50. doi:10.1016/0927-0256(96)00008-0
Kresse, G., and Furthmüller, J. (1996b). Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54 (16), 11169–11186. doi:10.1103/PhysRevB.54.11169
Kumar, R., Das, D., and Singh, A. (2018). C2N/WS2 van der Waals type-II heterostructure as a promising water splitting photocatalyst. J. Catal. 359, 143–150. doi:10.1016/j.jcat.2018.01.005
Lee, J., Sorescu, D., and Deng, X. (2016). Tunable lattice constant and band gap of single-and few-layer ZnO. J. Phys. Chem. Lett. 7 (7), 1335–1340. doi:10.1021/acs.jpclett.6b00432
Li, X., Yu, J., and Jaroniec, M. (2016). Hierarchical photocatalysts. Chem. Soc. Rev. 45 (9), 2603–2636. doi:10.1039/C5CS00838G
Luo, X., Wang, G., Huang, Y., Wang, B., Yuan, H., and Chen, H. (2017). A two-dimensional layered CdS/C2N heterostructure for visible-light-driven photocatalysis. Phys. Chem. Chem. Phys. 19 (41), 28216–28224. doi:10.1039/C7CP04108J
Ma, Z., Wang, S., Li, C., and Wang, F. (2021). Strain engineering for C2N/Janus monochalcogenides van der Waals heterostructures: Potential applications for photocatalytic water splitting. Appl. Surf. Sci. 536, 147845. doi:10.1016/j.apsusc.2020.147845
Mahmood, J., Lee, E., Jung, M., Shin, D., Jeon, I., Jung, S., et al. (2015). Nitrogenated holey two-dimensional structures. Nat. Commun. 6 (1), 6486–6487. doi:10.1038/ncomms7486
Momma, K., and Izumi, F. (2011). VESTA3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 44 (6), 1272–1276. doi:10.1107/S0021889811038970
Monkhorst, H., and Pack, J. (1976). Special points for Brillouin-zone integrations. Phys. Rev. B 13 (12), 5188–5192. doi:10.1103/PhysRevB.13.5188
Nørskov, J., Bligaard, T., Logadottir, A., Kitchin, J., Chen, J., Pandelov, S., et al. (2005). Trends in the exchange current for hydrogen evolution. J. Electrochem. Soc. 152 (3), J23. doi:10.1149/1.1856988
Novoselov, K., Mishchenko, A., Carvalho, A., and Castro Neto, A. (2016). 2D materials and van der Waals heterostructures. Science 353 (6298), aac9439. doi:10.1126/science.aac9439
Perdew, J., Burke, K., and Ernzerhof, M. (1996). Generalized gradient approximation made simple. Phys. Rev. Lett. 77 (18), 3865–3868. doi:10.1103/PhysRevLett.77.3865
Qin, H., Ren, K., Zhang, G., Dai, Y., and Zhang, G. (2022). Lattice thermal conductivity of Janus MoSSe and WSSe monolayers. Phys. Chem. Chem. Phys. 24 (34), 20437–20444. doi:10.1039/D2CP01692C
Ren, K., Chen, Y., Qin, H., Feng, W., and Zhang, G. (2022a). Graphene/biphenylene heterostructure: Interfacial thermal conduction and thermal rectification. Appl. Phys. Lett. 121 (8), 082203. doi:10.1063/5.0100391
Ren, K., Ren, C., Luo, Y., Xu, Y., Yu, J., Tang, W., et al. (2019). Using van der Waals heterostructures based on two-dimensional blue phosphorus and XC (X= Ge, Si) for water-splitting photocatalysis: A first-principles study. Phys. Chem. Chem. Phys. 21 (19), 9949–9956. doi:10.1039/C8CP07680D
Ren, K., Yan, Y., Zhang, Z., Sun, M., and Schwingenschlögl, U. (2022b). A family of LixBy monolayers with a wide spectrum of potential applications. Appl. Surf. Sci. 604, 154317. doi:10.1016/j.apsusc.2022.154317
Ren, K., Yu, J., and Tang, W. (2020). Two-dimensional ZnO/BSe van der waals heterostructure used as a promising photocatalyst for water splitting: A dft study. J. Alloys Compd. 812, 152049. doi:10.1016/j.jallcom.2019.152049
Roger, I., Shipman, M., and Symes, M. (2017). Earth-abundant catalysts for electrochemical and photoelectrochemical water splitting. Nat. Rev. Chem. 1 (1), 0003–0013. doi:10.1038/s41570-016-0003
Sahin, H. (2015). Structural and phononic characteristics of nitrogenated holey graphene. Phys. Rev. B 92 (8), 085421. doi:10.1103/PhysRevB.92.085421
Song, J., Zheng, H., Liu, M., Zhang, G., Ling, D., and Wei, D. (2021). A first-principles study on the electronic and optical properties of a type-II C2N/g-ZnO van der Waals heterostructure. Phys. Chem. Chem. Phys. 23 (6), 3963–3973. doi:10.1039/D1CP00122A
Tang, W., Sanville, E., and Henkelman, G. (2009). A grid-based Bader analysis algorithm without lattice bias. J. Phys. Condens. Matter 21 (8), 084204. doi:10.1088/0953-8984/21/8/084204
Tang, Y., Liu, M., Zhou, Y., Ren, C., Zhong, X., and Wang, J. (2020). First-principles predication of facet-dependent electronic and optical properties in InSe/GaAs heterostructure with potential in solar energy utilization. J. Alloys Compd. 842, 155901. doi:10.1016/j.jallcom.2020.155901
Toroker, M., Kanan, D., Alidoust, N., Isseroff, L., Liao, P., and Carter, E. (2011). First principles scheme to evaluate band edge positions in potential transition metal oxide photocatalysts and photoelectrodes. Phys. Chem. Chem. Phys. 13 (37), 16644–16654. doi:10.1039/C1CP22128K
Vayenas, C., Bebelis, S., and Ladas, S. (1990). Dependence of catalytic rates on catalyst work function. Nature 343 (6259), 625–627. doi:10.1038/343625a0
Voiry, D., Yang, J., and Chhowalla, M. (2016). Recent strategies for improving the catalytic activity of 2D TMD nanosheets toward the hydrogen evolution reaction. Adv. Mat. 28 (29), 6197–6206. doi:10.1002/adma.201505597
Wang, B., Yuan, H., Chang, J., Chen, X., and Chen, H. (2019). Two dimensional InSe/C2N van der Waals heterojunction as enhanced visible-light-responsible photocatalyst for water splitting. Appl. Surf. Sci. 485, 375–380. doi:10.1016/j.apsusc.2019.03.344
Wang, G., Chang, J., Tang, W., Xie, W., and Ang, Y. S. (2022). 2D materials and heterostructures for photocatalytic water-splitting: A theoretical perspective. J. Phys. D. Appl. Phys. 55 (29), 293002. doi:10.1088/1361-6463/ac5771
Wang, G., Li, Z., Wu, W., Guo, H., Chen, C., Yuan, H., et al. (2020a). A two-dimensional h-BN/C2N heterostructure as a promising metal-free photocatalyst for overall water-splitting. Phys. Chem. Chem. Phys. 22 (42), 24446–24454. doi:10.1039/D0CP03925J
Wang, S., Ren, C., Tian, H., Yu, J., and Sun, M. (2018). MoS2/ZnO van der Waals heterostructure as a high-efficiency water splitting photocatalyst: A first-principles study. Phys. Chem. Chem. Phys. 20 (19), 13394–13399. doi:10.1039/C8CP00808F
Wang, V., Xu, N., Liu, J., Tang, G., and Geng, W. (2021). Vaspkit: A user-friendly interface facilitating high-throughput computing and analysis using VASP code. Comput. Phys. Commun. 267, 108033. doi:10.1016/j.cpc.2021.108033
Wang, X., Maeda, K., Thomas, A., Takanabe, K., Xin, G., Carlsson, J., et al. (2009). A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mat. 8 (1), 76–80. doi:10.1038/nmat2317
Wang, X., Wang, Y., Quhe, R., Tang, Y., Dai, X., and Tang, W. (2020b). Designing strained C2N/GaTe (InTe) heterostructures for photovoltaic and photocatalytic application. J. Alloys Compd. 816, 152559. doi:10.1016/j.jallcom.2019.152559
Xu, B., Xiang, H., Wei, Q., Liu, J., Xia, Y., Yin, J., et al. (2015). Two-dimensional graphene-like C2N: An experimentally available porous membrane for hydrogen purification. Phys. Chem. Chem. Phys. 17 (23), 15115–15118. doi:10.1039/C5CP01789K
Ye, K., Li, H., Huang, D., Xiao, S., Qiu, W., Li, M., et al. (2019). Enhancing photoelectrochemical water splitting by combining work function tuning and heterojunction engineering. Nat. Commun. 10 (1), 3687–3689. doi:10.1038/s41467-019-11586-y
Zhang, H., Zhang, X., Yang, G., and Zhou, X. (2018). Point defect effects on photoelectronic properties of the potential metal-free C2N photocatalysts: Insight from first-principles computations. J. Phys. Chem. C 122 (10), 5291–5302. doi:10.1021/acs.jpcc.7b12428
Zhang, J.-R., Deng, X., Gao, B., Chen, L., Au, C., Li, K., et al. (2019a). Theoretical study on the intrinsic properties of In2Se3/MoS2 as a photocatalyst driven by near-infrared, visible and ultraviolet light. Catal. Sci. Technol. 9 (17), 4659–4667. doi:10.1039/C9CY00997C
Zhang, J., Zhang, C., Ren, K., Lin, X., and Cui, Z. (2022). Tunable electronic and magnetic properties of Cr2Ge2Te6 monolayer by organic molecular adsorption. Nanotechnology 33 (34), 345705. doi:10.1088/1361-6528/ac715d
Zhang, X., Chen, A., Zhang, Z., Jiao, M., and Zhou, Z. (2019b). Rational design of C2N-based type-II heterojunctions for overall photocatalytic water splitting. Nanoscale Adv. 1 (1), 154–161. doi:10.1039/C8NA00084K
Zhao, D., Wang, Y., Dong, C., Huang, Y., Chen, J., Xue, F., et al. (2021). Boron-doped nitrogen-deficient carbon nitride-based Z-scheme heterostructures for photocatalytic overall water splitting. Nat. Energy 6 (4), 388–397. doi:10.1038/s41560-021-00795-9
Keywords: ZnO/C2N heterostructure, first-principles method, type-II band alignment, water splitting, carrier mobility, strain
Citation: Liu M, Tang Y, Yao H, Bai L, Song J and Ma B (2022) Theoretical study on photocatalytic performance of ZnO/C2N heterostructure towards high efficiency water splitting. Front. Chem. 10:1048437. doi: 10.3389/fchem.2022.1048437
Received: 19 September 2022; Accepted: 29 September 2022;
Published: 19 October 2022.
Edited by:
Guangzhao Wang, Yangtze Normal University, ChinaCopyright © 2022 Liu, Tang, Yao, Bai, Song and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yong Tang, MjAyMDIxMjdAaHVhbmdodWFpLmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.