- Mycology Department, Consejo Nacional de Investigaciones Científicas y Tecnológicas (CONICET), Instituto de Medicina Regional, Universidad Nacional del Nordeste (UNNE), Resistencia, Argentina
In recent years, an increase in multidrug-resistant fungal strains has been observed, which, together with the limited number of clinically available antifungal agents, highlights the need for the development of new antifungal agents. Due to the proven antifungal activity of silver nanoparticles (AgNPs), there is a growing interest in their use in the treatment of fungal infections. Nanoparticles are usually synthesised through a variety of physical and chemical processes that are costly and pollute the environment. For this reason, biogenic synthesis is emerging as an environmentally friendly technology and new strategies are increasingly based on the use of biogenic AgNPs as antifungal agents for clinical use. The aim of this review is to compare the antifungal activity of different biogenic AgNPs and to summarise the current knowledge on the mechanisms of action and resistance of fungi to AgNPs. Finally, a general analysis of the toxicity of biogenic AgNPs in human and veterinary medicine is performed.
Introduction
In recent decades, fungal infections have increased and become a major public health threat. More than 300 million people suffer from severe fungal diseases and more than 2 million people die each year from mycoses, making fungal diseases one of the leading causes of death worldwide. Moreover, the problem of mycoses is exacerbated by the increase in emerging pathogenic fungi, but also by resistance to the limited antifungal drugs available, which significantly reduces the effectiveness of treatments (Denning et al., 2017; GAFFI, 2021).
From this perspective, Candida auris infections have become a global threat to human health for four main reasons: It has caused public health outbreaks; it is difficult to identify using standard laboratory methods; it has a high mortality rate; and some strains are resistant to all classes of available antifungal drugs commonly used to treat Candida infections (Du et al., 2020; CDC, 2021). On the other hand, there are certain mycoses whose treatment remains ineffective and leads to disability, including certain superficial fungal infections and implantation mycoses. This situation, which has a significant social impact, also includes the economic factor caused by the need for expensive drugs and lengthy medical care, which in many cases leads to patients not adhering to treatment (GAFFI, 2022).
The lack of antifungal drugs is mainly due to the difficulty in finding selective therapeutic targets against fungi, as they have a cellular and molecular biology very similar to that of animal cells (Konopka et al., 2019). This has prompted research into metals as alternative antifungal agents or as cofactors/adjuvants that enhance the efficacy of existing drugs (Xu et al., 2013; Mussin et al., 2019; Mani Chandrika and Sharma, 2020; Cruz-Luna et al., 2021; Lin et al., 2021).
Among the various metals, silver has a long history in medicine as an antimicrobial agent (Rai et al., 2009). Currently, silver nanoparticles (AgNPs) occupy a prominent place as potential antifungal agents for clinical use due to their broad spectrum of antimicrobial activity and their enormous number of applications in the health sciences, ranging from topical formulations to catheters impregnated with AgNPs (Rai et al., 2009; Ahamed et al., 2010; Burdușel el et al., 2018; Mosleh-Shirazi et al., 2021a).
AgNPs are particles that have all three dimensions on the nanometre scale (10–9 m). These nanoparticles can be synthesised by physical, chemical and biological methods. However, biological synthesis stands out because it is environmentally friendly, economically viable and easy to transfer to industrial production (Siddiqi et al., 2018; Mussin et al., 2021).
Biogenic synthesis of AgNP is a green synthesis method that uses organisms (plants, bacteria, fungi and algae) as a source of biomolecules that serve as reducing agents for silver ions (Ag+), coating agents and stabilisers of AgNP (Siddiqi et al., 2018; Rozhin et al., 2021).
Since natural coating agents can impart some functionality to the nanoparticles, such as antioxidant properties, anti-inflammatory properties, lower toxicity, modulation of immune response, etc., they are considered better candidates for use in human and veterinary medicine compared to nanoparticles obtained by physical and chemical methods (Vigneshwaran et al., 2007; Haggag et al., 2019; Sadeghipour et al., 2020; Mosleh-Shirazi et al., 2021b; Mujaddidi et al., 2021; Mussin et al., 2021; Rozhin et al., 2021). However, there are no organised data on the antifungal activity of biogenic AgNPs, nor on the mechanisms of action and resistance of fungi to AgNPs. For this reason, this review aims to compare the antifungal activity of different biogenic AgNPs and to summarise the current knowledge on the mechanisms of action and resistance of fungi to AgNPs. Finally, a general analysis of the toxicity of biogenic AgNPs in human and veterinary medicine is performed.
Antifungal activity of biogenic AgNPs
Thanks to their broad spectrum of antimicrobial activity and their ability to effectively inhibit biofilm formation, biogenic AgNPs have become one of the most promising options to reduce morbidity and mortality associated with fungal infections caused by resistant fungi (Ahamad et al., 2022).
The antimicrobial activity (antifungal, antibacterial, antiviral, etc.) of biogenic AgNPs is largely determined by the following factors:
The organism used for synthesis
Living organisms produce a variety of biological molecules (metabolites, proteins, lipids, etc.) that are involved in the reduction of Ag+, but also stabilise AgNPs and prevent their agglomeration. Moreover, biomolecules are the main protagonists of biological synthesis, as they determine the coating agents that can confer the desired biological properties to the synthesised AgNPs, such as: antioxidant activity, stronger antimicrobial activity, lower toxicity towards human/animal cells, modulation of the immune response and much more (Mussin et al., 2021; Spagnoletti et al., 2021).
It has even been shown that different AgNPs can be obtained from different strains of the same species under identical synthesis conditions (El-Bendary et al., 2021). Therefore, the correct identification of the species used and their preservation in a culture collection or herbarium is crucial.
The growth conditions of organism
The chemical composition of the same organism may vary according to the conditions of growth. It is therefore important that these conditions are well defined, especially if you are aiming for industrial production. In the case of plants, several points also need to be defined, such as the part of the plant to be used, the time of harvesting, post-harvest treatment, etc., (Mussin and Giusiano, 2020). All these aspects can influence the chemical composition and thus the properties of the synthesised AgNPs.
The physicochemical properties of AgNPs
The size, shape and coating agent of biogenic AgNPs determine their antifungal activity. These properties are strongly influenced by the synthesis conditions, such as: Temperature, reaction time, pH, biological molecules, molar ratio of reagents, speed and type of stirring, etc. (Song and Kim, 2009; El Badawy et al., 2010; Monteiro et al., 2012; Li et al., 2013; Agnihotri et al., 2014; Gavade et al., 2015; Ahmed et al., 2016). Therefore, standardisation of the synthesis conditions allows obtaining identical nanoparticles in each production batch.
Target organism
Due to genetic variability between species of the same genus and between strains of the same species, a given nanoparticle may exhibit different levels of antimicrobial activity (Mussin et al., 2019, 2021). Therefore, to obtain meaningful statistical values, the test must be performed against a considerable number of strains of the same species.
In addition, when assessing antimicrobial activity, it is important to note that a standardised method should be used that is widely accepted by the scientific community, as parameters such as the concentration of the inoculum, temperature and incubation time, among others, influence the assessment of antimicrobial activity. One of the most widely accepted methods is the broth microdilution method proposed by the Clinical and Laboratory Standards Institute (CLSI). This method provides a quantitative assessment of the in vitro inhibitory activity of a compound against a microorganism by determining the minimum inhibitory concentration (MIC), defined as the lowest concentration of the compound that can inhibit the growth of the microorganism, expressed in μg/ml. In addition, the method provides for the use of reference strains, quality control strains and positive inhibitory controls (antimicrobial drugs for clinical use) to ensure that the microdilution test is performed correctly and that the results are reproducible and comparable.
Table 1 summarises the most important papers in which the MIC of biogenic AgNPs against fungi of clinical importance was determined using a broth dilution method. Analysis of these studies leads us to the following conclusions:
• Fungi and plants are the main organisms that have been used for the synthesis of biogenic AgNPs to study antifungal activity.
• Comparing the works in which the same species was used to synthesise AgNPs (Marcato et al., 2012; Ishida et al., 2014; Longhi et al., 2016; Fonseca et al., 2022), we found that nanoparticles with different properties can be obtained from the same species. Therefore, it is important to consider all the above aspects about the factors that determine the antifungal activity of a biogenic AgNP, otherwise each synthesised nanoparticle must be considered as a different compound.
• Candida albicans ATCC 90028 is the most commonly used reference strain for evaluating the antifungal activity of biogenic AgNPs.
• Fluconazole and itraconazole are the main control drugs used.
• Biogenic AgNPs show a MIC range of 0.002–315.5 μg/ml against different fungal species. However, considering only the papers in which a control drug and a quality control strain were used for the CLSI broth microdilution method (Candida krusei ATCC 6258 and Candida parapsilosis ATCC 22019), the MIC range of AgNPs was 0.03–4 μg/ml against the tested fungal species. This shows, firstly, the importance of using standardised method controls and, secondly, that biogenic AgNPs have similar or even better antifungal activity than certain clinically used antifungal agents.
• Antifungal activity varies depending on the biogenic AgNP synthesised. Against the quality control strains, Candida krusei ATCC 6258 and Candida parapsilosis ATCC 22019, the biogenic AgNPs showed MIC ranges of 0.125–4 μg/ml and 0.125–6.25 μg/ml, respectively.
Mechanism of action
As mentioned earlier, the antifungal activity of biogenic AgNPs is highly dependent on the size, shape and coating agents. The great diversity of biogenic AgNPs therefore makes it difficult to decipher a single mechanism of action. For this reason, most research has focused on determining the mechanism of action of chemically synthesised AgNPs, which is attributed to the attachment of AgNPs to the surface of the fungus as a result of electrostatic attraction (Figure 1). The extracellular accumulation of AgNPs leads to a dynamic release of Ag+, which actively enter the cell and lead to an increase in the intracellular concentration and also the intracellular biosynthesis of AgNPs (Chwalibog et al., 2010; Le et al., 2012; Vazquez-Muñoz et al., 2014; Lara et al., 2015; Mussin et al., 2019). So far, no cell receptors or membrane channels have been described for the uptake of silver. However, the high-affinity copper transporter (Ctr1) has been identified as an importer of Ag+ (Ruta et al., 2018; Horstmann et al., 2019).
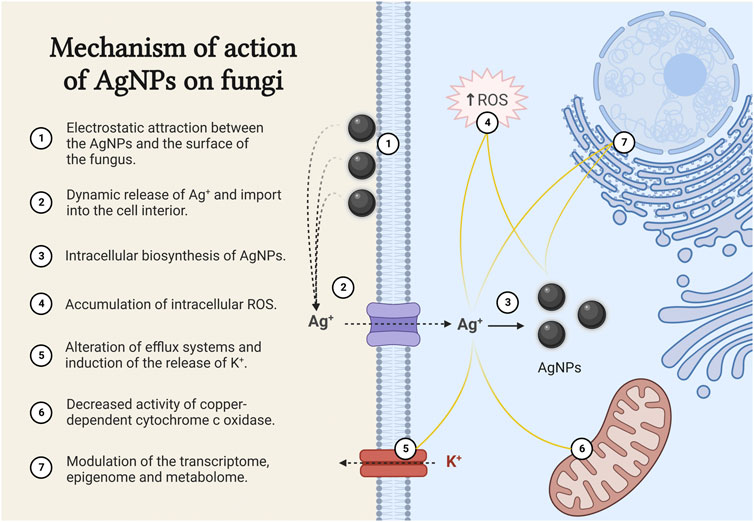
FIGURE 1. Mechanism of action of AgNPs on fungi. The figure was created with BioRender.com.
Once inside the cell, Ag+ and AgNPs act at different levels
• They lead to an accumulation of intracellular reactive oxygen species (ROS), which trigger apoptosis (Madeo et al., 1999; Hwang et al., 2012; Radhakrishnan et al., 2018).
• The intracellular accumulation of Ag+ alters efflux systems. It induces efflux of potassium ions and causes almost complete loss of intracellular potassium ions, resulting in inhibition of plasma membrane H+-ATPase activity (Vagabov et al., 2008; Cyert and Philpott, 2013).
• Ag+ enter the mitochondria via the mitochondrial copper transporter Pic2, which has a higher affinity for Ag+ than for copper ions (Cu+). This leads to a decrease in Cu+ concentration and an accumulation of Ag+ in the mitochondrial matrix, resulting in a decrease in the activity of the copper-dependent cytochrome c oxidase and consequently decreasing the rate of cellular respiration (Vest et al., 2013).
• Ag+ and AgNPs modulate the transcriptome, epigenome and metabolome and significantly alter the vital functions of fungal cells. Down-regulation of tricarboxylic acid cycle genes, genes related to redox metabolism and genes involved in ergosterol synthesis and lipid metabolism have been reported, leading to structural changes mainly at the level of biological membranes (Das and Ahmed, 2012; Babele et al., 2019; Horstmann et al., 2019; Barros et al., 2021).
Our studies suggest that AgNPs have fungicidal action against the major fungi that cause skin infections. A fungicidal agent causes death of fungal cells, while a fungistatic agent inhibits the growth or multiplication of the fungus without causing death (Mussin et al., 2021). However, the results are suggestive and further studies should be conducted.
AgNPs have also been shown to be more effective when combined with antifungal drugs. Synergistic effects have been reported with fluconazole, itraconazole, ketoconazole, clotrimazole, terbinafine, natamycin, nystatin, amphotericin B and echinocandins (Gajbhiye et al., 2009; Xu et al., 2013; Padalia et al., 2015; Patra and Baek, 2017; Mussin et al., 2019; Aabed and Mohammed, 2021; Yassin et al., 2022).
Resistance mechanisms
Since the antifungal activity of AgNPs is the result of several simultaneous processes, this has led to the assumption that fungi cannot develop resistance mechanisms to AgNPs.
Few studies have analysed the possible mechanisms of fungal resistance to silver. Terzioğlu et al. (2020) used the yeast Saccharomyces cerevisiae as a model fungal organism to investigate possible molecular mechanisms associated with resistance to silver. Their results suggest that genes involved in cell wall/membrane integrity, endocytosis and vesicular transport activities, oxidative metabolism, cellular respiration and copper homeostasis may play a role in silver resistance. In particular, the missense mutation in the RLM1 gene, which encodes a transcription factor involved in maintaining cell wall integrity and has 707 potential gene targets, may play a key role.
On the other hand, using the filamentous fungus Aspergillus nidulans, Antsotegi-Uskola et al. have suggested that the copper-transporting ATPase type PI, CrpA, may play an important role in the development of silver resistance (Antsotegi-Uskola et al., 2017).
Due to the increasing use of silver and AgNPs in many areas of human and veterinary medicine, further research is needed.
Toxicity
The toxicity of AgNPs depends on the size, shape and coating agents. For biogenic AgNPs, the coating agents play a very important role in terms of toxicity to human cells and modulation of the immune response (Mussin et al., 2021). There is evidence that biogenic AgNPs are more biocompatible than chemically synthesised AgNPs (Khan et al., 2019; Quinteros et al., 2019). However, due to the complex interactions between the different coating agents and eukaryotic cells, each biogenic AgNP should be evaluated individually to confirm its safety in humans and other animals.
The route of administration, exposure time and pharmacokinetics also influence toxicity (Stensberg et al., 2011; Aboelmaati et al., 2021; Mosleh-Shirazi et al., 2021a). Therefore, it remains to be investigated whether the gradual release of Ag+ and the broad spectrum of antimicrobial activity of AgNPs may lead to changes in the normal microbiota of humans and animals and whether this may have adverse effects over time.
Another interesting aspect of biogenic AgNPs is the reported synergistic effects with antifungals (Gajbhiye et al., 2009; Xu et al., 2013; Padalia et al., 2015; Patra and Baek, 2017; Mussin et al., 2019; Aabed and Mohammed, 2021; Yassin et al., 2022), suggesting that combined use may reduce toxicity by reducing the required dose of one or both agents.
Conclusion and future perspective
The increase in multidrug-resistant fungal pathogens and the limited number of clinically available antifungal drugs highlight the need to develop new antifungal strategies to address these problems in the face of an already complicated future.
AgNPs have been presented as a promising solution, but biological AgNPs have been shown to have several advantages over AgNPs produced by chemical and physical methods.
The antifungal activity of the different biogenic nanoparticles varies according to their physicochemical properties, which are determined by the organism used for synthesis, the growth conditions of the organism, the physicochemical properties of the AgNPs and the target organism. An important challenge for future research is therefore to standardise these conditions and determine the key biocomponents involved in the synthesis of AgNPs to produce safe and effective drugs for the treatment of fungal infections.
The wide variety of methods used to evaluate the antifungal activity of these biogenic nanoparticles highlights the need to use internationally accepted methods with appropriate controls to obtain reproducible and comparable results. Since there may be genetic variability within a species, it is important to test a considerable number of isolates of the same species to obtain meaningful results on the antifungal activity of a new agent against a particular species.
Great progress has been made in elucidating the mechanism of action of AgNPs on fungi. They have shown that they can act on multiple targets, which makes them very promising as antifungal agents for clinical use. In addition, further research is being conducted for use in healthcare settings. In the near future, these efforts will lead to a clearer picture of the antifungal potential of biogenic AgNPs and help establish them in the field of veterinary and human mycology.
The broad spectrum of antimicrobial activity and the potential synergistic effects with antifungal drugs make biogenic AgNPs viable alternatives to overcome the problematic infections caused by resistant fungi and the toxicity of currently available drugs. We anticipate that biogenic AgNPs will be used as cost-effective broad-spectrum antifungal agents. However, since toxicity and in vivo effects have not yet been sufficiently researched, we think it more likely that they will initially be used in human and veterinary medicine as antimycotics for topical application or as disinfectants for catheters, surgical materials, etc.
Author contributions
JM: conceived and designed the research, JM and GG: wrote the manuscript, analyzed and interpreted the data.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aabed, K., and Mohammed, A. E. (2021). Synergistic and antagonistic effects of biogenic silver nanoparticles in combination with antibiotics against some pathogenic microbes. Front. Bioeng. Biotechnol. 9, 652362–652414. doi:10.3389/fbioe.2021.652362
Aboelmaati, M. G., Abdel Gaber, S. A., Soliman, W. E., Elkhatib, W. F., Abdelhameed, A. M., Sahyon, H. A., et al. (2021). Biogenic and biocompatible silver nanoparticles for an apoptotic anti-ovarian activity and as polydopamine-functionalized antibiotic carrier for an augmented antibiofilm activity. Colloids Surfaces B Biointerfaces 206, 111935. doi:10.1016/j.colsurfb.2021.111935
Agnihotri, S., Mukherji, S., and Mukherji, S. (2014). Size-controlled silver nanoparticles synthesized over the range 5–100 nm using the same protocol and their antibacterial efficacy. RSC Adv. 4, 3974–3983. doi:10.1039/c3ra44507k
Ahamad, I., Bano, F., Anwer, R., Srivastava, P., Kumar, R., and Fatma, T. (2022). Antibiofilm activities of biogenic silver nanoparticles against Candida albicans. Front. Microbiol. 12, 741493–741513. doi:10.3389/fmicb.2021.741493
Ahamed, M., AlSalhi, M. S., and Siddiqui, M. K. J. (2010). Silver nanoparticle applications and human health. Clin. Chim. Acta 411, 1841–1848. doi:10.1016/j.cca.2010.08.016
Ahmad, A., Wei, Y., Syed, F., Tahir, K., Taj, R., Khan, A. U., et al. (2016). Amphotericin B-conjugated biogenic silver nanoparticles as an innovative strategy for fungal infections. Microb. Pathog. 99, 271–281. doi:10.1016/j.micpath.2016.08.031
Ahmed, S., Ahmad, M., Swami, B. L., and Ikram, S. (2016). A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: A green expertise. J. Adv. Res. 7, 17–28. doi:10.1016/j.jare.2015.02.007
Antsotegi-Uskola, M., Markina-Iñarrairaegui, A., and Ugalde, U. (2017). Copper resistance in Aspergillus nidulans relies on the PI-type ATPase CrpA, regulated by the transcription factor AceA. Front. Microbiol. 8, 912–915. doi:10.3389/fmicb.2017.00912
Babele, P. K., Singh, A. K., and Srivastava, A. (2019). Bio-inspired silver nanoparticles impose metabolic and epigenetic toxicity to Saccharomyces cerevisiae. Front. Pharmacol. 10, 1016–1115. doi:10.3389/fphar.2019.01016
Barros, D., Pradhan, A., Pascoal, C., and Cássio, F. (2021). Transcriptomics reveals the action mechanisms and cellular targets of citrate-coated silver nanoparticles in a ubiquitous aquatic fungus. Environ. Pollut. 268, 115913. doi:10.1016/j.envpol.2020.115913
Burdușel, A. C., Gherasim, O., Grumezescu, A. M., Mogoantă, L., Ficai, A., and Andronescu, E. (2018). Biomedical applications of silver nanoparticles: An up-to-date overview. Nanomaterials 8, 681–725. doi:10.3390/nano8090681
CDC (2021). Candida auris. Available at: https://www.cdc.gov/fungal/candida-auris/index.html.
Chwalibog, A., Sawosz, E., Hotowy, A., Szeliga, J., Mitura, S., Mitura, K., et al. (2010). Visualization of interaction between inorganic nanoparticles and bacteria or fungi. Int. J. Nanomedicine 5, 1085–1094. doi:10.2147/IJN.S13532
Cruz-Luna, A. R., Cruz-Martínez, H., Vásquez-López, A., and Medina, D. I. (2021). Metal nanoparticles as novel antifungal agents for sustainable agriculture: Current advances and future directions. J. Fungi (Basel). 7, 1033. doi:10.3390/jof7121033
Cyert, M. S., and Philpott, C. C. (2013). Regulation of cation balance in Saccharomyces cerevisiae. Genetics 193, 677–713. doi:10.1534/genetics.112.147207
Das, D., and Ahmed, G. (2012). Silver nanoparticles damage yeast cell wall. Int. Res. J. Biotechnol. 3, 2141–5153. Available at: http://www.interesjournals.org/IRJOB.
Denning, D. W., Perlin, D. S., Muldoon, E. G., Colombo, A. L., Chakrabarti, A., Richardson, M. D., et al. (2017). Delivering on antimicrobial resistance agenda not possible without improving fungal diagnostic capabilities. Emerg. Infect. Dis. 23, 177–183. doi:10.3201/eid2302.152042
Du, H., Bing, J., Hu, T., Ennis, C. L., Nobile, C. J., and Huang, G. (2020). Candida auris: Epidemiology, biology, antifungal resistance, and virulence. PLoS Pathog. 16, 10089211–e1009018. doi:10.1371/journal.ppat.1008921
Dutta, T., Ghosh, N. N., Das, M., Adhikary, R., Mandal, V., and Chattopadhyay, A. P. (2020). Green synthesis of antibacterial and antifungal silver nanoparticles using Citrus limetta peel extract: Experimental and theoretical studies. J. Environ. Chem. Eng. 8, 104019. doi:10.1016/j.jece.2020.104019
El Badawy, A. M., Luxton, T. P., Silva, R. G., Scheckel, K. G., Suidan, M. T., and Tolaymat, T. M. (2010). Impact of environmental conditions (pH, ionic strength, and electrolyte type) on the surface charge and aggregation of silver nanoparticles suspensions. Environ. Sci. Technol. 44, 1260–1266. doi:10.1021/es902240k
El-Bendary, M. A., Abdelraof, M., Moharam, M. E., Elmahdy, E. M., and Allam, M. A. (2021). Potential of silver nanoparticles synthesized using low active mosquitocidal Lysinibacillus sphaericus as novel antimicrobial agents. Prep. Biochem. Biotechnol. 51, 926–935. doi:10.1080/10826068.2021.1875236
Fernández, J. G., Fernández-Baldo, M. A., Berni, E., Camí, G., Durán, N., Raba, J., et al. (2016). Production of silver nanoparticles using yeasts and evaluation of their antifungal activity against phytopathogenic fungi. Process Biochem. 51, 1306–1313. doi:10.1016/j.procbio.2016.05.021
Fonseca, M. S., Rodrigues, D. M., Sokolonski, A. R., Stanisic, D., Tomé, L. M., Góes-Neto, A., et al. (2022). Activity of Fusarium oxysporum-based silver nanoparticles on Candida spp. oral isolates. Nanomaterials 12, 501. doi:10.3390/nano12030501
GAFFI (2021). Fungal disease frequency. Global action fund for fungal infections. Available at: https://gaffi.org/why/fungal-disease-frequency/.
GAFFI (2022). Treatment deficiencies. Global action fund for fungal infections. Available at: https://gaffi.org/why/treatment-deficiencies/.
Gajbhiye, M., Kesharwani, J., Ingle, A., Gade, A., and Rai, M. (2009). Fungus-mediated synthesis of silver nanoparticles and their activity against pathogenic fungi in combination with fluconazole. Nanomedicine Nanotechnol. Biol. Med. 5, 382–386. doi:10.1016/j.nano.2009.06.005
Garibo, D., Borbón-Nuñez, H. A., Díaz de León, J. N., García Mendoza, E., Estrada, I., Toledano-Magaña, Y., et al. (2020). Green synthesis of silver nanoparticles using Lysiloma acapulcensis exhibit high antimicrobial activity. Sci. Rep. 10, 12805. doi:10.1038/s41598-020-69606-7
Gautam, N., Salaria, N., Thakur, K., Kukreja, S., Yadav, N., Yadav, R., et al. (2020). Green silver nanoparticles for phytopathogen control. Proc. Natl. Acad. Sci. India Sect. B. Biol. Sci. 90, 439–446. doi:10.1007/s40011-019-01115-8
Gavade, N. L., Kadam, A. N., Suwarnkar, M. B., Ghodake, V. P., and Garadkar, K. M. (2015). Biogenic synthesis of multi-applicative silver nanoparticles by using Ziziphus Jujuba leaf extract. Spectrochimica Acta Part A Mol. Biomol. Spectrosc. 136, 953–960. doi:10.1016/j.saa.2014.09.118
Gulbagca, F., Ozdemir, S., Gulcan, M., and Sen, F. (2019). Synthesis and characterization of Rosa canina-mediated biogenic silver nanoparticles for anti-oxidant, antibacterial, antifungal, and DNA cleavage activities. Heliyon 5, e02980. doi:10.1016/j.heliyon.2019.e02980
Haggag, E. G., Elshamy, A. M., Rabeh, M. A., Gabr, N. M., Salem, M., Youssif, K. A., et al. (2019). Antiviral potential of green synthesized silver nanoparticles of Lampranthus coccineus and Malephora lutea. Int. J. Nanomedicine 14, 6217–6229. doi:10.2147/IJN.S214171
Horstmann, C., Campbell, C., Kim, D. S., and Kim, K. (2019). Transcriptome profile with 20 nm silver nanoparticles in yeast. FEMS Yeast Res. 19, 1–15. doi:10.1093/femsyr/foz003
Hwang, I. S., Lee, J., Hwang, J. H., Kim, K. J., and Lee, D. G. (2012). Silver nanoparticles induce apoptotic cell death in Candida albicans through the increase of hydroxyl radicals. FEBS J. 279, 1327–1338. doi:10.1111/j.1742-4658.2012.08527.x
Ishida, K., Cipriano, T. F., Rocha, G. M., Weissmüller, G., Gomes, F., Miranda, K., et al. (2014). Silver nanoparticle production by the fungus Fusarium oxysporum: Nanoparticle characterisation and analysis of antifungal activity against pathogenic yeasts. Mem. Inst. Oswaldo Cruz 109, 220–228. doi:10.1590/0074-0276130269
Jalal, M., Ansari, M. A., Alzohairy, M. A., Ali, S. G., Khan, H. M., Almatroudi, A., et al. (2018). Biosynthesis of silver nanoparticles from oropharyngeal candida glabrata isolates and their antimicrobial activity against clinical strains of bacteria and fungi. Nanomaterials 8, 586. doi:10.3390/nano8080586
Jamdagni, P., Khatri, P., and Rana, J. S. (2021). Biogenic synthesis of silver nanoparticles from leaf extract of Elettaria cardamomum and their antifungal activity against phytopathogens. Adv. Mater. Proc. 3, 129–135. doi:10.5185/amp.2018/977
Khan, I., Bahuguna, A., Krishnan, M., Shukla, S., Lee, H., Min, S. H., et al. (2019). The effect of biogenic manufactured silver nanoparticles on human endothelial cells and zebrafish model. Sci. Total Environ. 679, 365–377. doi:10.1016/j.scitotenv.2019.05.045
Konopka, J., Casadevall, A., Taylor, J., Heitman, J., and Cowen, L. (2019). One health: Fungal pathogens of humans, animals, and plants. Washington: American Academy of Microbiology.
Lara, H. H., Romero-Urbina, D. G., Pierce, C., Lopez-Ribot, J. L., Arellano-Jiménez, M. J., and Jose-Yacaman, M. (2015). Effect of silver nanoparticles on Candida albicans biofilms: An ultrastructural study. J. Nanobiotechnology 13, 91. doi:10.1186/s12951-015-0147-8
Le, A. T., Le, T. T., Nguyen, V. Q., Tran, H. H., Dang, D. A., Tran, Q. H., et al. (2012). Powerful colloidal silver nanoparticles for the prevention of gastrointestinal bacterial infections. Adv. Nat. Sci. Nanosci. Nanotechnol. 3, 045007. doi:10.1088/2043-6262/3/4/045007
Li, Y., Zhang, W., Niu, J., and Chen, Y. (2013). Surface-coating-dependent dissolution, aggregation, and reactive oxygen species (ROS) generation of silver nanoparticles under different irradiation conditions. Environ. Sci. Technol. 47, 10293–10301. doi:10.1021/es400945v
Lin, Y., Betts, H., Keller, S., Cariou, K., and Gasser, G. (2021). Recent developments of metal-based compounds against fungal pathogens. Chem. Soc. Rev. 50, 10346–10402. doi:10.1039/d0cs00945h
Longhi, C., Santos, J. P., Morey, A. T., Marcato, P. D., Duran, N., Pinge-Filho, P., et al. (2016). Combination of fluconazole with silver nanoparticles produced by Fusarium oxysporum improves antifungal effect against planktonic cells and biofilm of drug-resistant Candida albicans. Med. Mycol. Open. Access. 54, 428–432. doi:10.1093/mmy/myv036
Lotfy, W. A., Alkersh, B. M., Sabry, S. A., and Ghozlan, H. A. (2021). Biosynthesis of silver nanoparticles by Aspergillus terreus: Characterization, optimization, and biological activities. Front. Bioeng. Biotechnol. 9, 633468. doi:10.3389/fbioe.2021.633468
Madeo, F., Fröhlich, E., Ligr, M., Grey, M., Sigrist, S. J., Wolf, D. H., et al. (1999). Oxygen stress: A regulator of apoptosis in yeast. J. Cell Biol. 145, 757–767. doi:10.1083/jcb.145.4.757
Mani Chandrika, K. V. S., and Sharma, S. (2020). Promising antifungal agents: A minireview. Bioorg. Med. Chem. 28, 115398. doi:10.1016/j.bmc.2020.115398
Marcato, P. D., Durán, M., Huber, S., Rai, M., Melo, P. S., Alves, O. L., et al. (2012). Biogenic silver nanoparticles and its antifungal activity as a new topical transungual drug. JNanoR. 20, 99–107. doi:10.4028/www.scientific.net/jnanor.20.99
Mondal, A. H., Yadav, D., Ali, A., Khan, N., Jin, J. O., and Haq, Q. M. R. (2020). Anti-bacterial and anti-candidal activity of silver nanoparticles biosynthesized using citrobacter spp. Ms5 culture supernatant. Biomolecules 10, 944–1015. doi:10.3390/biom10060944
Monteiro, D. R., Silva, S., Negri, M., Gorup, L. F., De Camargo, E. R., Oliveira, R., et al. (2012). Silver nanoparticles: Influence of stabilizing agent and diameter on antifungal activity against Candida albicans and Candida glabrata biofilms. Lett. Appl. Microbiol. 54, 383–391. doi:10.1111/j.1472-765X.2012.03219.x
Mosleh-Shirazi, S., Abbasi, M., Shafiee, M., Kasaee, S. R., and Amani, A. M. (2021a). Renal clearable nanoparticles: An expanding horizon for improving biomedical imaging and cancer therapy. Mat. Today Commun. 26, 102064. doi:10.1016/j.mtcomm.2021.102064
Mosleh-Shirazi, S., Kouhbanani, M. A. J., Beheshtkhoo, N., Kasaee, S. R., Jangjou, A., Izadpanah, P., et al. (2021b). Biosynthesis, simulation, and characterization of Ag/AgFeO2 core–shell nanocomposites for antimicrobial applications. Appl. Phys. A 127, 857. doi:10.1007/s00339-021-05005-7
Mujaddidi, N., Nisa, S., Al Ayoubi, S., Bibi, Y., Khan, S., Sabir, M., et al. (2021). Pharmacological properties of biogenically synthesized silver nanoparticles using endophyte Bacillus cereus extract of Berberis lyceum against oxidative stress and pathogenic multidrug-resistant bacteria. Saudi J. Biol. Sci. 28, 6432–6440. doi:10.1016/j.sjbs.2021.07.009
Mussin, J., Botero, V. R., Pimentel, R. C., Rojas, F., Angiolella, L., San, E., et al. (2021). Antimicrobial and cytotoxic activity of green synthesis silver nanoparticles targeting skin and soft tissue infectious agents. Sci. Rep. 11, 14566. doi:10.1038/s41598-021-94012-y
Mussin, J. E., Roldán, M. V., Rojas, F., Sosa, M. de L. Á., Pellegri, N., and Giusiano, G. (2019). Antifungal activity of silver nanoparticles in combination with ketoconazole against Malassezia furfur. Amb. Express 9, 131–139. doi:10.1186/s13568-019-0857-7
Mussin, J., and Giusiano, G. (2020). “Ethno–Phytopharmacology: Product validation process based on traditional knowledge of medicinal plants,” in Agricultural, forestry and bioindustry biotechnology and biodiscovery. Editors P. A. Chong, and D. J. Newman (D. A. Steinmacher), 331–353. doi:10.1007/978-3-030-51358-0_17
Padalia, H., Moteriya, P., and Chanda, S. (2015). Green synthesis of silver nanoparticles from marigold flower and its synergistic antimicrobial potential. Arab. J. Chem. 8, 732–741. doi:10.1016/j.arabjc.2014.11.015
Parthiban, E., Manivannan, N., Ramanibai, R., and Mathivanan, N. (2019). Green synthesis of silver-nanoparticles from Annona reticulata leaves aqueous extract and its mosquito larvicidal and anti-microbial activity on human pathogens. Biotechnol. Rep. (Amst). 21, e00297. doi:10.1016/j.btre.2018.e00297
Patra, J. K., and Baek, K. H. (2017). Antibacterial activity and synergistic antibacterial potential of biosynthesized silver nanoparticles against foodborne pathogenic bacteria along with its anticandidal and antioxidant effects. Front. Microbiol. 8, 167–214. doi:10.3389/fmicb.2017.00167
Pereira, L., Dias, N., Carvalho, J., Fernandes, S., Santos, C., and Lima, N. (2014). Synthesis, characterization and antifungal activity of chemically and fungal-produced silver nanoparticles against Trichophyton rubrum. J. Appl. Microbiol. 117, 1601–1613. doi:10.1111/jam.12652
Qian, Y., Yu, H., He, D., Yang, H., Wang, W., Wan, X., et al. (2013). Biosynthesis of silver nanoparticles by the endophytic fungus Epicoccum nigrum and their activity against pathogenic fungi. Bioprocess Biosyst. Eng. 36, 1613–1619. doi:10.1007/s00449-013-0937-z
Quinteros, M. A., Bonilla, J. O., Alborés, S. V., Villegas, L. B., and Páez, P. L. (2019). Biogenic nanoparticles: Synthesis, stability and biocompatibility mediated by proteins of Pseudomonas aeruginosa. Colloids Surfaces B Biointerfaces 184, 110517. doi:10.1016/j.colsurfb.2019.110517
Radhakrishnan, V. S., Mudiam, M. K. R., Kumar, M., Dwivedi, S. P., Singh, S. P., and Prasad, T. (2018). Silver nanoparticles induced alterations in multiple cellular targets, which are critical for drug susceptibilities and pathogenicity in fungal pathogen (Candida albicans). Int. J. Nanomedicine 13, 2647–2663. doi:10.2147/IJN.S150648
Rai, M., Yadav, A., and Gade, A. (2009). Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 27, 76–83. doi:10.1016/j.biotechadv.2008.09.002
Różalska, B., Sadowska, B., Budzyńska, A., Bernat, P., and Różalska, S. (2018). Biogenic nanosilver synthesized in Metarhizium robertsii waste mycelium extract – as a modulator of Candida albicans morphogenesis, membrane lipidome and biofilm. PLoS One 13, 01942544–e194321. doi:10.1371/journal.pone.0194254
Rozhin, A., Batasheva, S., Kruychkova, M., Cherednichenko, Y., Rozhina, E., and Fakhrullin, R. (2021). Biogenic silver nanoparticles: Synthesis and application as antibacterial and antifungal agents. Micromachines 12, 1480. doi:10.3390/mi12121480
Ruta, L. L., Banu, M. A., Neagoe, A. D., Kissen, R., Bones, A. M., and Farcasanu, I. C. (2018). Accumulation of Ag(I) by Saccharomyces cerevisiae cells expressing plant metallothioneins. Cells 7, 266. doi:10.3390/cells7120266
Sadeghipour, Y., Alipour, M. H., Jafarbeigloo, H. R. G., Salahvarzi, A., Mirzaii, M., Amani, A. M., et al. (2020). Evaluation antibacterial activity of biosynthesized silver nanoparticles using e euphorbia pseudocactus berger extracts (Euphorbiaceae). Nanomedicine Res. J. 5, 265–275. doi:10.22034/NMRJ.2020.03.007
Salati, S., Doudi, M., and Madani, M. (2018). The biological synthesis of silver nanoparticles by mango plant extract and its anti-candida effects. J. Apple. Biotechnol. Rep. 5, 157–161. doi:10.29252/JABR.05.04.04
Salem, S. S., Ali, O. M., Reyad, A. M., Abd-Elsalam, K. A., and Hashem, A. H. (2022). Pseudomonas indica-mediated silver nanoparticles: Antifungal and antioxidant biogenic tool for suppressing mucormycosis fungi. J. Fungi (Basel). 8, 126. doi:10.3390/jof8020126
Sharifi-Rad, M., and Pohl, P. (2020). Synthesis of biogenic silver nanoparticles (Agcl-nps) using a pulicaria vulgaris gaertn. aerial part extract and their application as antibacterial, antifungal and antioxidant agents. Nanomaterials 10, 638–717. doi:10.3390/nano10040638
Siddiqi, K. S., Husen, A., and Rao, R. A. K. (2018). A review on biosynthesis of silver nanoparticles and their biocidal properties. J. Nanobiotechnology 16, 14. doi:10.1186/s12951-018-0334-5
Soares, M. R. P. S., Corrêa1, R. O., Stroppa, P. H. F., Marques, F. C., Andrade, G. F. S., Corrêa, C. C., et al. (2018). Biosynthesis of silver nanoparticles using Caesalpinia ferrea (Tul.) Martius extract: Physicochemical characterization, antifungal activity and cytotoxicity. PeerJ 6, 43611–e4416. doi:10.7717/peerj.4361
Song, J. Y., and Kim, B. S. (2009). Rapid biological synthesis of silver nanoparticles using plant leaf extracts. Bioprocess Biosyst. Eng. 32, 79–84. doi:10.1007/s00449-008-0224-6
Spagnoletti, F. N., Kronberg, F., Spedalieri, C., Munarriz, E., and Giacometti, R. (2021). Protein corona on biogenic silver nanoparticles provides higher stability and protects cells from toxicity in comparison to chemical nanoparticles. J. Environ. Manage. 297, 113434. doi:10.1016/j.jenvman.2021.113434
Stensberg, M. C., Wei, Q., McLamore, E. S., Porterfield, D. M., Wei, A., and Sepúlveda, M. S. (2011). Toxicological studies on silver nanoparticles: Challenges and opportunities in assessment, monitoring and imaging. Nanomedicine 6, 879–898. doi:10.2217/nnm.11.78
Terzioğlu, E., Alkım, C., Arslan, M., Balaban, B. G., Holyavkin, C., Kısakesen, H. İ., et al. (2020). Genomic, transcriptomic and physiological analyses of silver-resistant Saccharomyces cerevisiae obtained by evolutionary engineering. Yeast 37, 413–426. doi:10.1002/yea.3514
Vagabov, V. M., Ivanov, A. Y., Kulakovskaya, T. V., Kulakovskaya, E. V., Petrov, V. V., and Kulaev, I. S. (2008). Efflux of potassium ions from cells and spheroplasts of Saccharomyces cerevisiae yeast treated with silver and copper ions. Biochem. Mosc. 73, 1224–1227. doi:10.1134/S0006297908110084
Valsalam, S., Agastian, P., Arasu, M. V., Al-Dhabi, N. A., Ghilan, A. K. M., Kaviyarasu, K., et al. (2019). Rapid biosynthesis and characterization of silver nanoparticles from the leaf extract of Tropaeolum majus L. and its enhanced in-vitro antibacterial, antifungal, antioxidant and anticancer properties. J. Photochem. Photobiol. B Biol. 191, 65–74. doi:10.1016/j.jphotobiol.2018.12.010
Vanlalveni, C., Rajkumari, K., Biswas, A., Adhikari, P. P., Lalfakzuala, R., and Rokhum, L. (2018). Green synthesis of silver nanoparticles using nostoc linckia and its antimicrobial activity: A novel biological approach. Bionanoscience 8, 624–631. doi:10.1007/s12668-018-0520-9
Vazquez-Muñoz, R., Avalos-Borja, M., and Castro-Longoria, E. (2014). Ultrastructural analysis of candida albicans when exposed to silver nanoparticles. PLoS One 9, 1088766–e108910. doi:10.1371/journal.pone.0108876
Vest, K. E., Leary, S. C., Winge, D. R., and Cobine, P. A. (2013). Copper import into the mitochondrial matrix in Saccharomyces cerevisiae is mediated by Pic2, a mitochondrial carrier family protein. J. Biol. Chem. 288, 23884–23892. doi:10.1074/jbc.M113.470674
Vigneshwaran, N., Ashtaputre, N. M., Varadarajan, P. V., Nachane, R. P., Paralikar, K. M., and Balasubramanya, R. H. (2007). Biological synthesis of silver nanoparticles using the fungus Aspergillus flavus. Mat. Lett. 61, 1413–1418. doi:10.1016/j.matlet.2006.07.042
Wang, D., Xue, B., Wang, L., Zhang, Y., Liu, L., and Zhou, Y. (2021). Fungus-mediated green synthesis of nano-silver using Aspergillus sydowii and its antifungal/antiproliferative activities. Sci. Rep. 11, 10356–10359. doi:10.1038/s41598-021-89854-5
Wunnoo, S., Paosen, S., Lethongkam, S., Sukkurd, R., Waen-ngoen, T., Nuidate, T., et al. (2021). Biologically rapid synthesized silver nanoparticles from aqueous Eucalyptus camaldulensis leaf extract: Effects on hyphal growth, hydrolytic enzymes, and biofilm formation in Candida albicans. Biotechnol. Bioeng. 118, 1578–1592. doi:10.1002/bit.27675
Xu, Y., Gao, C., Li, X., He, Y., Zhou, L., Pang, G., et al. (2013). In vitro antifungal activity of silver nanoparticles against ocular pathogenic filamentous fungi. J. Ocul. Pharmacol. Ther. 29, 270–274. doi:10.1089/jop.2012.0155
Xue, B., He, D., Gao, S., Wang, D., Yokoyama, K., and Wang, L. (2016). Biosynthesis of silver nanoparticles by the fungus Arthroderma fulvum and its antifungal activity against genera of Candida, Aspergillus and Fusarium. Int. J. Nanomedicine 11, 1899–1906. doi:10.2147/IJN.S98339
Yassin, M. T., Mostafa, A. A.-F., Al-Askar, A. A., and Al-Otibi, F. O. (2022). Synergistic antifungal efficiency of biogenic silver nanoparticles with itraconazole against multidrug-resistant candidal strains. Crystals 12, 816. doi:10.3390/cryst12060816
Keywords: silver nanoparticles, antimicrobial, antifungal, mycoses, green synthesis, biogenic synthesis, mechanism of action, resistance mechanism
Citation: Mussin J and Giusiano G (2022) Biogenic silver nanoparticles as antifungal agents. Front. Chem. 10:1023542. doi: 10.3389/fchem.2022.1023542
Received: 19 August 2022; Accepted: 20 September 2022;
Published: 06 October 2022.
Edited by:
Syed Rashel Kabir, Rajshahi University, BangladeshReviewed by:
Asoke Chattopadhyay, University of Kalyani, IndiaAli Mohammad Amani, Shiraz University of Medical Sciences, Iran
Copyright © 2022 Mussin and Giusiano. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Javier Mussin, amF2aWVybXVzc2luQGhvdG1haWwuY29t
 Javier Mussin
Javier Mussin Gustavo Giusiano
Gustavo Giusiano