
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Chem., 10 October 2022
Sec. Medicinal and Pharmaceutical Chemistry
Volume 10 - 2022 | https://doi.org/10.3389/fchem.2022.1019663
This article is part of the Research TopicAdvances in Drug discovery and Quality EvaluationView all 10 articles
Clinically, periodontitis is a chronic nonspecific inflammation that leads to damaged teeth and their supporting gum tissues. Although many studies on periodontitis have been conducted, therapy with natural products is still rare. Silibinin has been proven to have anti-inflammatory and antioxidant activities. However, the effects of silibinin on lipopolyssacharide (LPS)-induced inflammation in periodontal ligaments (PDLs) have not yet been investigated. In this study, the PDLs were treated with silibinin (10, 20, and 40 μM) in the presence of LPS. The results showed that silibinin treatment reduced the levels of NO, PGE2, IL-6, TNF-α, MMP-1, and MMP-3 and enhanced the activities of superoxide dismutase (SOD) and glutathione (GSH). Moreover, silibinin treatment downregulated RANKL levels and upregulated OPG and ALP levels. In summary, silibinin protected PDLs against LPS-induced inflammation, oxidative stress, and osteogenic differentiation.
Periodontitis is a chronic nonspecific inflammation caused by periodontal pathogenic bacteria (Seo et al., 2004; Nagatomo et al., 2006; Yamamoto et al., 2006). In the early stages of periodontitis, only the gums are inflamed, and bleed (Choi et al., 2012; Jun et al., 2012). However, with continuous stimulation of pathogenic microorganisms and their metabolites, the periodontal tissue produces immune responses, resulting in the secretion of a large number of inflammatory factors (Kim et al., 2009; Lee et al., 2012; Lei et al., 2014). These factors damage the periodontal supporting tissue, loosening the teeth, ultimately leading to tooth loss. The periodontal ligament (PDL) is an important periodontal tissue that connects the alveolar bone and root (Grzesik and Narayanan, 2002; Choi et al., 2012; Shin et al., 2015). PDL cells, the base units of PDLs, maintain periodontal health by secreting various inflammatory factors and osteoblast/osteoclast regulators (Abiko et al., 1998; Miura et al., 2000; Gyawali and Bhattarai, 2017).
Periodontitis is mainly caused by the imbalance between host’s defense and accumulating bacteria (Slots et al., 1986; Birkedal-Hansen, 1993). Lipopolysaccharides (LPS) are bacterial membrane proteins that are present in most subgingival Gram-negative organisms (Aznar et al., 1990; Nair et al., 1996). LPS is a stimulant that induces vascular dilatation and edema of periodontal tissues. In addition, sustained LPS stimulation damages periodontal tissue by producing harmful pro-inflammatory mediators, including IL-1β, IL-6, and TNF-α (Gowen et al., 1983; Boyce et al., 1989; Milica et al., 2017). Moreover, LPS stimulation increases the receptor activator of the nuclear factor kappa-B (NF-κB) ligand (RANKL) and reduces osteoprotegerin (OPG). These mediators further stimulate periodontitis (Belibasakis et al., 2007). Thence, clearing inflammation had been recognized as an effective method for improving disease.
Phytoconstituents have been used as beneficial and therapeutic agents since ancient times owing to their low toxicity and biological benefits. Some of them have beneficial therapeutic effects in the treatment of periodontitis. Silibinin (SB) is an important polyphenol found in Silybum marianum L. (Kim et al., 2003; Esmaeil et al., 2017; Amato et al., 2019) (Figure 1). Natural products and their derivatives play increasing roles in disease prevention (Cheng et al., 2022; Zhang et al., 2022). SB has been confirmed to have stimulating health benefits and shows promising biological activities, including anti-inflammatory, antioxidant, anti-tumor, and anti-fibrotic effects (Raina et al., 2013; Federico et al., 2017; Zheng et al., 2017). As a reliever of inflammation, SB reportedly ameliorates silica-induced pulmonary fibrosis by reducing the pro-inflammatory mediators (IL-1β, IL-6, and TNF-α) and collagen deposition (Ali et al., 2021). SB is effective against LPS-induced inflammation in PBMCs in horses (Gugliandolo et al., 2020). SB also ameliorates hepatotoxicity by inhibiting inflammation and oxidative stress (Saxena et al., 2022). Moreover, SB can enhance anti-inflammatory activity when combined with thymol (Chen et al., 2020), while it is also used as a beneficial dietary supplement to maintain body health and treat liver disorders.
The aforementioned evidence suggested that SB has good anti-inflammatory activity. Similarly, many studies have shown that periodontitis can be improved by inhibiting inflammatory responses. We designed and evaluated the anti-inflammatory effects of SB on LPS-induced hPDLCs.
To evaluate the cytotoxicity of SB on hPDLs, we exposed hPDLs to various concentrations of SB (10, 20, and 40 μM) for 24 h and tested cell viability using the MTT method. Based on the MTT assay results (Figure 2), SB was found to have no effect on the cell viability, indicating non-cytotoxicity to hPDLs at the tested concentrations (10–40 μM).
NO and PGE2 are two inflammatory mediators produced by the induction of iNOS and COX-2, respectively (Jeong et al., 2009; Jeong et al., 2011). They can effectively influence inflammation and are classical markers of inflammation. Inhibition of NO and PGE2 is considered an effective strategy for the treatment of inflammation. The effects of SB on NO and PGE2 levels were assayed in LPS-induced hPDLs. From Figure 3A, it could be seen that LPS treatment significantly increased the NO level to 23.37 ± 3.04 μM compared to the control group. However, the elevated LPS-induced NO levels decreased by treatment with SB in a dose-dependent manner. The NO level reduced to 10.75 ± 0.96 μM, when treated with SB at 40 μM. Similarly, SB (40 μM) treatment inhibited the abnormally elevated PGE2 level induced by LPS stimulation to 64.12 ± 3.43 ng/ml (Figure 3B).
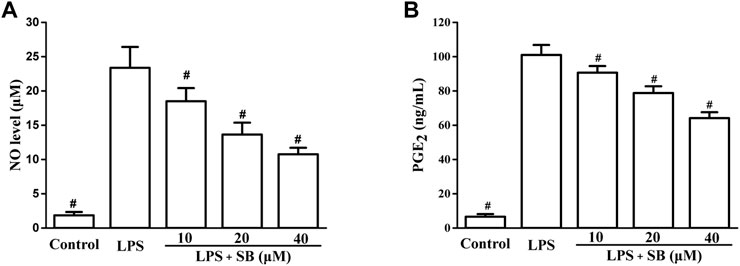
FIGURE 3. SB treatment reduced LPS-induced NO (A) and PGE2 (B) in hPDLs. #p < 0.05, compared to the LPS group.
Next, the effects of SB on LPS-induced IL-6 and TNF-α levels were examined by ELISA. It is well known that the overexpression of pro-inflammatory cytokines is closely related to various inflammatory processes (Lee et al., 2020; Tan et al., 2021). The release of pro-inflammatory cytokines results in the elimination of foreign pathogens. Therefore, reduction in pro-inflammatory cytokines is very important for the treatment of inflammation. As shown in Figure 4, LPS stimulation visibly increased IL-6 (up to 371.88 ± 21.13 pg/ml) and TNF-α (2,180.74 ± 160.30 pg/ml) levels compared to the control group. SB pre-treatment could significantly decrease the IL-6 level to 255.26 ± 10.39 pg/ml at 40 μM compared to the LPS-induced group (Figure 4A). Moreover, pre-treatment with 40 μM SB also reduced the TNF-α level to 1,419.61 ± 59.69 pg/ml (Figure 4B).
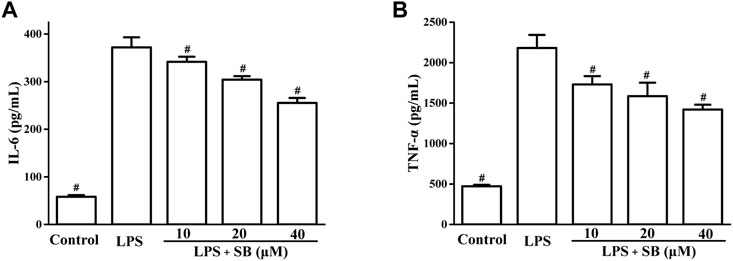
FIGURE 4. SB treatment inhibited LPS-induced IL-6 (A) and TNF-α (B) in hPDLs. #p < 0.05, compared to the LPS group.
Matrix metalloproteases (MMPs) are the major proteases of ECM metabolism and are involved in the destruction of periodontal tissues (Hosokawa et al., 2021). MMP-1 progresses and damages periodontal soft tissues by degrading type 1 collagen of periodontal tissues. MMP-3 is also reported to be involved in soft tissue destruction through the activation of pro-MMP-1. Hence, regulation of MMP-1 and MMP-3 leads to the improvement of periodontitis. SB treatment decreased LPS-induced MMP-1 and MMP-3 production in a dose-dependent manner (Figure 5). SB (40 μM) treatment reduced the MMP-1 and MMP-3 levels to 16.71 ± 1.12 and 40.72 ± 2.72 ng/ml, respectively, compared to the LPS group (30.09 ± 1.76 and 89.41 ± 4.23 ng/mL pg/ml, respectively).
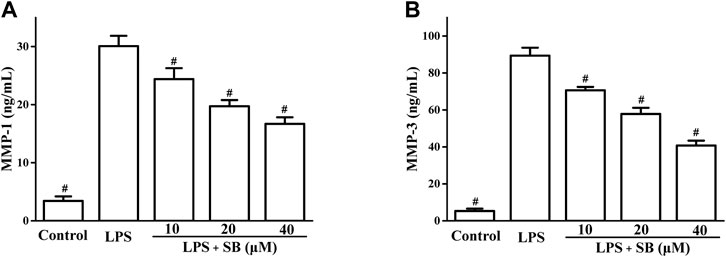
FIGURE 5. SB treatment inhibited LPS-induced MMP-1 (A) and MMP-3 (B) in hPDLs. #p < 0.05, compared to the LPS group.
It has been revealed that the inflammatory response involves cross-talk with oxidative stress in the defense against pathogenic microorganisms (Chang et al., 2014; Wang et al., 2019). The effects of SB on superoxide dismutase (SOD) and glutathione (GSH) levels, which are important indicators of oxidative stress, were assayed. The results in Figure 6 showed that LPS stimulation could obviously reduce SB on SOD levels in hPDLs, which could be increased by SB treatment (Figure 5A). Similarly, treatment with SB (Figure 6A) significantly increased GSH reduction following LPS stimulation (Figure 6B).
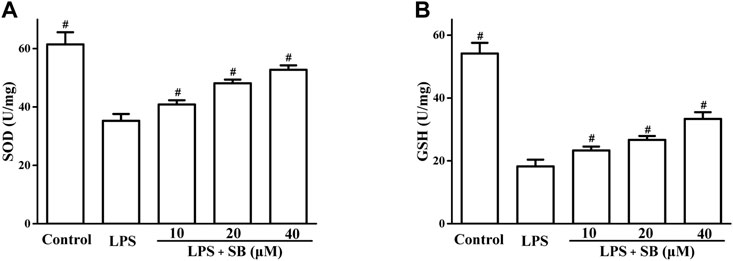
FIGURE 6. SB treatment regulated LPS-induced SOD (A) and GSH (B) in hPDLs. #p < 0.05, compared to the LPS group.
RANKL and OPG have been reported to play important roles in bone resorption. RANKL regulates osteoclast differentiation (Shu et al., 2008). OPG is a decoy receptor that binds to RANKL to regulate its activity (Bae et al., 2018). We evaluated the effects of SB on LPS-induced RANKL and OPG expressions. As shown in Figure 7A, SB treatment clearly downregulated the unusually high RANKL expression induced by LPS. However, treatment with SB enhanced the unusually low OPG levels induced by LPS (Figure 7B).
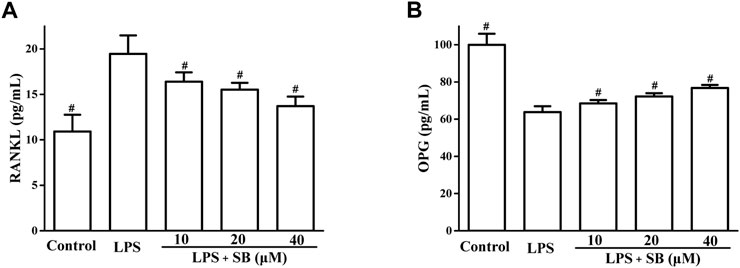
FIGURE 7. SB treatment regulated LPS-induced SOD (A) and GSH (B) in hPDLs. #p < 0.05, compared to the LPS group.
Alkaline phosphatase (ALP) is an important marker of osteoblast differentiation and plays a key role in connective tissue calcification and mineral deposits (Li and Peng, 2019). Studies have shown that LPS can inhibit ALP activity, cell metabolism, and viability in osteoblasts. Our results (Figure 8) showed that LPS treatment significantly inhibited ALP activity compared with the control group. However, the reduced ALP activity induced by LPS treatment was effectively reversed by treatment with SB.
We treated PDLs with silibinin (10, 20, and 40 μM) in the presence of LPS to investigate the protective effects of silibinin against periodontitis. Our findings revealed that silibinin treatment reduced the levels of NO, PGE2, IL-6, TNF-α, MMP-1, and MMP-3 and enhanced the activities of SOD and GSH. Moreover, silibinin treatment downregulated RANKL levels and upregulated OPG and ALP levels. Our results indicate that silibinin could affect inflammation, oxidative stress, and osteogenic differentiation capacity against LPS (Figure 9) and could be used as an effective agent for the treatment of periodontitis.
hPDLCs were prepared using previously reported methods (Blufstein et al., 2021) and cultured in α-MEM with 10% FBS, 100 U/mL penicillin, and 100 μg/ml. The cells were divided into five groups: control group (no agent), LPS group (treatment with 1 μg/ml LPS), and three SB groups (treatment with 10, 20, and 40 μM SB, before 1 μg/ml LPS treatment).
The cytotoxicity of SB on hPDLCs was assayed using the MTT assay. hPDLCs were seeded into 96-well plates for 24 h and then treated with SB (10, 20, and 40 μM) for another 24 h. The MTT reagent (0.5 mg/ml) was added to each well and incubated for 4 h. DMSO was used to dissolve the resulting crystals, followed by absorbance measurement at 570 nm.
The hPDLCs were treated with SB (10, 20, and 40 μM) for 2 h, followed by exposure to LPS (1 μg/ml) for 24 h. The NO level in the supernatant was then determined using the Griess reagent. An equal volume of the Griess reagent was added to the culture supernatant and incubated for 10 min. The absorbance was then measured at 540 nm.
After hPDLCs were treated for 24 h, the culture supernatant was harvested. PGE2 levels in each group were measured using an EIA kit according to the manufacturer’s instructions.
After hPDLCs were treated for 24 h, IL-6 and TNF-α levels were measured in the harvested culture supernatant using the corresponding IL-6, TNF-α, MMP-1, MMP-3, or OPG ELISA assay kits.
After hPDLCs were treated for 24 h, SOD and GSH levels were measured in the harvested cells using the corresponding commercial kits.
After hPDLCs were treated for 24 h, the cells were harvested and lysed and RANKL levels were measured using RANKL ELISA kits.
After hPDLCs were treated for 7 days, the harvested cells were lysed using 1% Triton X-100. After centrifugation, ALP activity of the supernatant was detected the ALP activity using an ALP assay kit.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
DM carried out the experiments, YW collected the data, and TL supervised the work.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fchem.2022.1019663/full#supplementary-material
Abiko, Y., Shimizu, N., Yamaguchi, M., Suzuki, H., and Takiguchi, H. (1998). Effect of aging on functional changes of periodontal tissue cells. Ann. Periodontol. 3, 350–369. doi:10.1902/annals.1998.3.1.350
Ali, S. A., Saifi, M. A., Godugu, C., and Talla, V. (2021). Silibinin alleviates silica-induced pulmonary fibrosis: Potential role in modulating inflammation and epithelial-mesenchymal transition. Phytotherapy Res. 35, 5290–5304. doi:10.1002/ptr.7210
Amato, A., Terzo, S., and Mule, F. (2019). Natural compounds as beneficial antioxidant agents in neurodegenerative disorders: A focus on alzheimer’s disease. Antioxidants 8, 608. doi:10.3390/antiox8120608
Aznar, C., Fitting, C., and Cavaillon, J. M. (1990). Lipopolysaccharide-induced production of cytokines by bone marrow-derived macrophages: Dissociation between intracellular interleukin 1 production and interleukin 1 release. Cytokine 2, 259–265. doi:10.1016/1043-4666(90)90026-p
Bae, W. J., Park, J. S., Kang, S. K., Kwon, I. K., and Kim, E. C. (2018). Effects of melatonin and its underlying mechanism on ethanol-stimulated senescence and osteoclastic differentiation in human periodontal ligament cells and cementoblasts. Int. J. Mol. Sci. 19, 1742. doi:10.3390/ijms19061742
Belibasakis, G. N., Bostanci, N., Hashim, A., Johansson, A., Aduse-Opoku, J., Curtis, M. A., et al. (2007). Regulation of RANKL and OPG gene expression in human gingival fibroblasts and periodontal ligament cells by porphyromonas gingivalis: A putative role of the arg-gingipains. Microb. Pathog. 43, 46–53. doi:10.1016/j.micpath.2007.03.001
Birkedal-Hansen, H. (1993). Role of cytokines and inflammatory mediators in tissue destruction. J. Periodontal Res. 28, 500–510. doi:10.1111/j.1600-0765.1993.tb02113.x
Blufstein, A., Behm, C., Kubin, B., Gahn, J., Rausch-Fan, X., Moritz1, A., et al. (2021). Effect of vitamin D3 on the osteogenic differentiation of human periodontal ligament stromal cells under inflammatory conditions. J. Periodont. Res. 56, 579–588. doi:10.1111/jre.12858
Boyce, B. F., Aufdemorte, T. B., Garrett, I. R., Yates, A. J., and Mundy, G. R. (1989). Effects of interleukin-1 on bone turnover in normal mice. Endocrinology 125, 1142–1150. doi:10.1210/endo-125-3-1142
Chang, Y. C., Chang, W. C., Hung, K. H., Yang, D. M., Cheng, Y. H., Liao, Y. W., et al. (2014). The generation of induced pluripotent stem cells for macular degeneration as a drug screening platform: Identification of curcumin as a protective agent for retinal pigment epithelial cells against oxidative stress. Front. Aging Neurosci. 6, 191. doi:10.3389/fnagi.2014.00191
Chen, J., Li, D., Xie, L., Ma, Y., Wu, P., Li, C., et al. (2020). Synergistic anti-inflammatory effects of silibinin and thymol combination on LPS-induced RAW264.7 cells by inhibition of NF-κB and MAPK activation. Phytomedicine 78, 153309. doi:10.1016/j.phymed.2020.153309
Cheng, F., Duan, D. S., Jiang, L. M., Li, B. S., Wang, J. X., Zhou, Y. J., et al. (2022). Copper-catalyzed asymmetric ring-opening reaction of cyclic diaryliodonium salts with imides. Org. Lett. 24, 1394–1399. doi:10.1021/acs.orglett.2c00247
Choi, E. Y., Jin, J. Y., Lee, J. Y., Choi, J. I., Choi, I. S., and Kim, S. J. (2012). Anti-inflammatory effects and the underlying mechanisms of action of daidzein in murine macrophages stimulated with Prevotella intermedia lipopolysaccharide. J. Periodontal Res. 47, 204–211. doi:10.1111/j.1600-0765.2011.01422.x
Esmaeil, N., Anaraki, S. B., Gharagozloo, M., and Moayedi, B. (2017). Silymarin impacts on immune system as an immunomodulator: One key for many locks. Int. Immunopharmacol. 50, 194–201. doi:10.1016/j.intimp.2017.06.030
Federico, A., Dallio, M., and Loguercio, C. (2017). Silymarin/silybin and chronic liver disease: A marriage of many years. Molecules 22, 191. doi:10.3390/molecules22020191
Gowen, M., Wood, D. D., Ihrie, E. J., McGuire, M. K., and Russell, R. G. (1983). An interleukin 1 like factor stimulates bone resorption in vitro. Nature 306, 378–380. doi:10.1038/306378a0
Grzesik, W. J., and Narayanan, A. S. (2002). Cementum and periodontal wound healing and regeneration. Crit. Rev. Oral Biol. Med. 13, 474–484. doi:10.1177/154411130201300605
Gugliandolo, E., Crupi, R., Biondi, V., Licata, P., Cuzzocrea, S., and Passantino, A. (2020). Protective effect of silibinin on lipopolysaccharide-induced inflammatory responses in equine peripheral blood mononuclear cells, an in vitro study. Animals 10, 2022. doi:10.3390/ani10112022
Gyawali, R., and Bhattarai, B. (2017). Orthodontic management in aggressive periodontitis. Int. Sch. Res. Not. 2017, 8098154. doi:10.1155/2017/8098154
Hosokawa, Y., Hosokawa, I., Ozaki, K., and Matsuo, T. (2021). Nobiletin inhibits inflammatory reaction in interleukin-1β-stimulated human periodontal ligament cells. Pharmaceutics 13, 667. doi:10.3390/pharmaceutics13050667
Jeong, G. S., Lee, D. S., Li, B., Kim, J. J., Kim, E. C., and Kim, Y. C. (2011). Anti-inflammatory effects of lindenenyl acetate via heme oxygenase-1 and AMPK in human periodontal ligament cells. Eur. J. Pharmacol. 670, 295–303. doi:10.1016/j.ejphar.2011.08.008
Jeong, G. S., Lee, S. H., Jeong, S. N., Kim, Y. C., and Kim, E. C. (2009). Anti-inflammatory effects of apigenin on nicotine- and lipopolysaccharide-stimulated human periodontal ligament cells via heme oxygenase-1. Int. Immunopharmacol. 9, 1374–1380. doi:10.1016/j.intimp.2009.08.015
Jun, I. H., Lee, D. E., Yun, J. H., Cho, A. R., Kim, C. S., You, Y. J., et al. (2012). Anti-inflammatory effect of (–)-epigallocatechin- 3-gallate on Porphyromonas gingivalis lipopolysaccharide-stimulated fibroblasts and stem cells derived from human periodontal ligament. J. Periodontal Implant Sci. 42, 185–195. doi:10.5051/jpis.2012.42.6.185
Kim, N. C., Graf, T. N., Sparacino, C. M., Wani, M. C., and Wall, M. E. (2003). Complete isolation and characterization of silybins and isosilybins from milk thistle (Silybum marianum)electronic supplementary information (ESI) available: HPLC chromatograms of isolates and extracts. Org. Biomol. Chem. 1, 1684–1689. doi:10.1039/b300099k
Kim, Y. S., Pi, S. H., Lee, Y. M., Lee, S. I., and Kim, E. C. (2009). The anti-inflammatory role of heme oxygenase-1 in lipopolysaccharide and cytokine-stimulated inducible nitric oxide synthase and nitric oxide production in human periodontal ligament cells. J. Periodontol. 80, 2045–2055. doi:10.1902/jop.2009.090145
Lee, S. A., Park, B. R., Moon, S. M., Shin, S. H., Kim, J. S., Kim, D. K., et al. (2020). Cynaroside protects human periodontal ligament cells from lipopolysaccharide-induced damage and inflammation through suppression of NF-κB activation. Arch. Oral Biol. 120, 104944. doi:10.1016/j.archoralbio.2020.104944
Lee, S. I., Park, K. H., Kim, S. J., Kang, Y. G., Lee, Y. M., and Kim, E. C. (2012). Mechanical stress-activated immune response genes via Sirtuin 1 expression in human periodontal ligament cells. Clin. Exp. Immunol. 168, 113–124. doi:10.1111/j.1365-2249.2011.04549.x
Lei, M., Li, K., Li, B., Gao, L. N., Chen, F. M., and Jin, Y. (2014). Mesenchymal stem cell characteristics of dental pulp and periodontal ligament stem cells after in vivo transplantation. Biomaterials 35, 6332–6343. doi:10.1016/j.biomaterials.2014.04.071
Li, J., and Peng, Y. J. (2019). Effect of puerarin on osteogenic differentiation of human periodontal ligament stem cells. J. Int. Med. Res. 48, 030006051985164–11. doi:10.1177/0300060519851641
Milica, K., Dušanka, K., Milica, B. P., Tatjana, J. S., Marko, J., Aleksandar, P., et al. (2017). Anti-inflammatory effect of the Salvia sclarea L. ethanolic extract on lipopolysaccharide-induced periodontitis in rats. J. Ethnopharmacol. 199, 52–59. doi:10.1016/j.jep.2017.01.020
Miura, S., Yamaguchi, M., Shimizu, N., and Abiko, Y. (2000). Mechanical stress enhances expression and production of plasminogen activator in aging human periodontal ligament cells. Mech. Ageing Dev. 112, 217–231. doi:10.1016/s0047-6374(99)00095-0
Nagatomo, K., Komaki, M., Sekiya, I., Sakaguchi, Y., Noguchi, K., Oda, S., et al. (2006). Stem cell properties of human periodontal ligament cells. J. Periodontal Res. 41, 303–310. doi:10.1111/j.1600-0765.2006.00870.x
Nair, S. P., Meghji, S., Wilson, M., Reddi, K., White, P., and Henderson, B. (1996). Bacterially induced bone destruction: Mechanisms and misconceptions. Infect. Immun. 64, 2371–2380. doi:10.1128/iai.64.7.2371-2380.1996
Raina, K., Agarwal, C., and Agarwal, R. (2013). Effect of silibinin in human colorectal cancer cells: Targeting the activation of NF-κB signaling. Mol. Carcinog. 52, 195–206. doi:10.1002/mc.21843
Saxena, N., Dhaked, R. K., and Nagar, D. P. (2022). Silibinin ameliorates abrin induced hepatotoxicity by attenuating oxidative stress, inflammation and inhibiting Fas pathway. Environ. Toxicol. Pharmacol. 93, 103868. doi:10.1016/j.etap.2022.103868
Seo, B. M., Miura, M., Gronthos, S., Bartold, P. M., Batouli, S., Brahim, J., et al. (2004). Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 364, 149–155. doi:10.1016/s0140-6736(04)16627-0
Shin, S. Y., Kim, Y. S., Lee, S. Y., Bae, W. J., Park, Y. D., Hyun, Y. C., et al. (2015). Expression of phospholipase D in periodontitis and its Role in the Inflammatory and osteoclastic response by nicotine and Lipopolysaccharide-stimulated human periodontal ligament cells. J. Periodontol. 86, 1405–1416. doi:10.1902/jop.2015.150123
Shu, L., Guan, S. M., Fu, S. M., Guo, T., Cao, M., and Ding, Y. (2008). Estrogen modulates cytokine expression in human periodontal ligament cells. J. Dent. Res. 87, 142–147. doi:10.1177/154405910808700214
Slots, J., Bragd, L., Wikstrom, M., and Dahlen, G. (1986). The occurrence of Actinobacillus actinomycetemcomitans, Bacteroides gingivalis and Bacteroides intermedius in destructive periodontal disease in adults. J. Clin. Periodontol. 13, 570–577. doi:10.1111/j.1600-051x.1986.tb00849.x
Tan, L., Cao, Z., Chen, H., Xie, Y., Yu, L., Fu, C., et al. (2021). Curcumin reduces apoptosis and promotes osteogenesis of human periodontal ligament stem cells under oxidative stress in vitro and. Life Sci. 270, 119125. doi:10.1016/j.lfs.2021.119125
Wang, Y., Zhang, Y., Yang, L., Yuan, J., Jia, J., and Yang, S. (2019). SOD2 mediates curcumin-induced protection against oxygen-glucose deprivation/reoxygenation injury in HT22 cells. Evidence-Based Complementary Altern. Med. 2019, 1–14. doi:10.1155/2019/2160642
Yamamoto, T., Kita, M., Oseko, F., Nakamura, T., Imanishi, J., and Kanamura, N. (2006). Cytokine production in human periodontal ligament cells stimulated with Porphyromonas gingivalis. J. Periodontal Res. 41, 554–559. doi:10.1111/j.1600-0765.2006.00905.x
Zhang, X., Zheng, Y. Y., Hu, C. M., Wu, X. Z., Lin, J., Xiong, Z., et al. (2022). Synthesis and biological evaluation of coumarin derivatives containing oxime ester as α-glucosidase inhibitors. Arab. J. Chem. 15, 104072. doi:10.1016/j.arabjc.2022.104072
Keywords: silibinin, inflammation, protective effects, LPS-induced, human periodontal ligament cells
Citation: Meng D, Wang Y and Liu T (2022) Protective effects of silibinin on LPS-induced inflammation in human periodontal ligament cells. Front. Chem. 10:1019663. doi: 10.3389/fchem.2022.1019663
Received: 15 August 2022; Accepted: 07 September 2022;
Published: 10 October 2022.
Edited by:
Xuetao Xu, Wuyi University, ChinaReviewed by:
Jie Chen, Shaoguan University, ChinaCopyright © 2022 Meng, Wang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tongjun Liu, bGl1dG9uZ2p1bmpuQDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.