
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Chem., 20 September 2022
Sec. Organic Chemistry
Volume 10 - 2022 | https://doi.org/10.3389/fchem.2022.1013977
This article is part of the Research TopicMulticomponent Reactions (MCRs) Towards Scaffolds with Versatile ApplicationsView all 6 articles
A convenient approach for the construction of pharmaceutically valuable 3-trifluoromethyl-1,2,4-triazoles has been developed, which employs the readily available trifluoroacetimidoyl chlorides, hydrazine hydrate and benzene-1,3,5-triyl triformate (TFBen) as starting materials. The multi-component reaction features broad substrate scope, high efficiency, and scalability, providing a facile and straightforward route to the biologically important 3-trifluoromethyl-1,2,4-triazole scaffolds in moderate to good yields. Considering its broad-spectrum pharmaceutical activity, the method offers the opportunity for the further study towards the toxicity risk assessment and structure-activity relationship of the pharmaceuticals containing trifluoromethyl-1,2,4-triazole cores.
1,2,4-Triazoles, especially trifluoromethyl-substituted 1,2,4-triazoles, have found extensive applications in the field of pharmaceutical, agrochemicals, biology, functional materials, and ligand chemistry (Koltin and Hitchcock, 1997; Shivarama Holla, et al., 2002; Haycock-Lewandowski, et al., 2008; Tao, et al., 2010; Zhou and Wang, 2012; Romagnoli, et al., 2014). For instance, the commercial sitagliptin is a potent inhibitor of DPP-IV and is used as a new treatment for type II diabetes (Hansen, et al., 2005). Other trifluoromethyl-1,2,4-triazole derivatives, have been applied as anticonvulsant drug, GlyT1 inhibitor, anti-HIV-1 reagent, and NKI-receptor ligand (Figure 1) (Lebsack, et al., 2004; Girardet, et al., 2006; Syvanen, et al., 2007; Sugane, et al., 2011; Sakurada, et al., 2015). It is well-known that the occurrence of trifluoromethyl group could significantly improve the physicochemical and pharmacological properties of the parent molecules due to the unique character of fluorine atom (Müller, et al., 2007; Gillis, et al., 2015; Zhou, et al., 2016; Han, et al., 2020). Therefore, the exploration of efficient and practical strategies for the preparation of trifluoromethyl-1,2,4-triazoles is highly desirable.
Traditional methods for the synthesis of trifluoromethyl-1,2,4-triazoles usually suffer from tedious reaction procedures, narrow substrate scope and lower efficiency (Buscemi, et al., 2003; Buscemi, et al., 2006; Funabiki, et al., 1999; Sibgatulin, et al., 2010). Recent years have witnessed considerable achievements about the construction of trifluoromethyl-substituted 1,2,4-triazoles (Zhang, et al., 2019), which include transition metal-catalyzed three-component reaction of aryldiazonium salts with fluorinated diazo reagents and nitriles (Peng, et al., 2020). Our groups also developed a series of convenient approaches for the assembly of this kind of important five-membered N-heterocycle by using trifluoroacetimidoyl chlorides (Hu, et al., 2019; Du, et al., 2020) and trifluoroacetimidohydrazides (Zhang, et al., 2021a; Zhang, et al., 2021b; Zhang, et al., 2021c; Lu, et al., 2022a; Zhang, et al., 2022) as versatile trifluoromethyl synthons. Compared with the in-depth study toward the synthesis of 5-trifluoromethyl-1,2,4-triazoles, the relevant reports regarding the formation of the more specific 3-trifluoromethyl-1,2,4-triazoles have been rare but still of great significance. Wu, Chen and co-workers reported a copper-mediated [3 + 2] cycloaddition of trifluoroacetimidoyl chlorides and N-isocyanoiminotriphenylphphorane (NIITP) to efficiently access 3-trifluoromethyl-1,2,4-triazoles (Figure 2A) (Yang, et al., 2021). They also utilized D-glucose (Lu, et al., 2021) and N,N-dimethylformamide (DMF) (Lu, et al., 2022b) as an inexpensive C1 source to realize [4 + 1] cyclization reaction with trifluoroacetimidohydrazides for preparing 3-trifluoromethyl-1,2,4-triazoles (Figures 2B,C). Very recently, Hu and co-workers described a tandem addition/cyclization reaction of trifluoromethyl N-acylhydrazones and cyanamide to afford polysubstituted 3-trifluoromethyl-1,2,4-triazolines, which could be oxidized to 1,2,4-triazoles with NBS (Figure 2D) (Liu, et al., 2022). Despite notable advances having been gained, other facile pathways to access the valuable trifluoromethyl-substituted N-heterocycles deserve to be further investigated.
Benzene-1,3,5-triyl triformate (TFBen) is first designed and developed by Wu and co-workers and has usually been used as a potent CO surrogate in diverse carbonylative transformations (Jiang, et al., 2016; Yang, et al., 2022). Meanwhile, TFBen is also adopted as a C1 source in the formation of a variety of heterocycles. Wu group reported a metal-free annulation of hydrazides with benzene-1,3,5-triyl triformate (TFBen) to produce 1,3,4-oxadiazoles (Yin, et al., 2018). Our group disclosed a palladium-catalyzed three-component carbonylative reaction of trifluoroacetimidohydrazides and aryl iodides for delivering 5-trifluoromethyl-1,2,4-triazoles (Tang, et al., 2021). In these reactions, TFBen provided a CO unit to form carbonyl-containing compounds and the latter underwent an intramolecular dehydration process. In continuation of our effort on the carbonylative reaction using CO surrogate for the efficient construction of trifluoromethyl-containing heterocycles (Chen, et al., 2020a; Chen, et al., 2020b; Yang, et al., 2020; Tang, et al., 2021; Wang, et al., 2021), we herein presented a multi-component annulation reaction of readily available trifluoroacetimidoyl chlorides (Tamura, et al., 1993; Chen, et al., 2020c), hydrazine hydrate and benzene-1,3,5-triyl triformate for the metal-free synthesis of 3-trifluoromethyl-1,2,4-triazoles (Figure 2E).
The study was initiated by the employment of trifluoroacetimidoyl chloride 1e as model substrate along with hydrazine hydrate and benzene-1,3,5-triyl triformate (TFBen) as starting materials (Table 1). The reaction proceeded smoothly in the presence of TsOH H2O in toluene at 100°C for 12 h, and the desired 3-trifluoromethyl-1,2,4-triazole product 3e was isolated in 53% yield (Table 1, entry 1). Other acidic additives were also examined, including TfOH, PivOH and TFA, and the results indicated that TFA promoted the reaction with highest efficiency (Table 1, entries 2–4). Then, a series of organic solvents were tried to test the solvent effect of this reaction. The multi-component reaction could occur in various solvents, but the obtained reaction yields were all inferior to that of toluene (Table 1, entries 5–9). Lowering and elevating the reaction temperature did not get the better outcome (Table 1, entries 10–11). When the reaction was conducted in the presence of 0.5 equiv. of TFA, the yield of product 2e was decreased to 50% (Table 1, entry 12). Furthermore, reducing the amount of hydrazine hydrate had a negative impact on the reaction (Table 1, entry 13). Considering TFBen could generate three times as much CO per molecule, increasing the amount of TFBen to 1.0 equiv. only gave the comparable result (Table 1, entry 14). In addition, the inert atmosphere also had a negligible effect on the reaction (Table 1, entry 15).
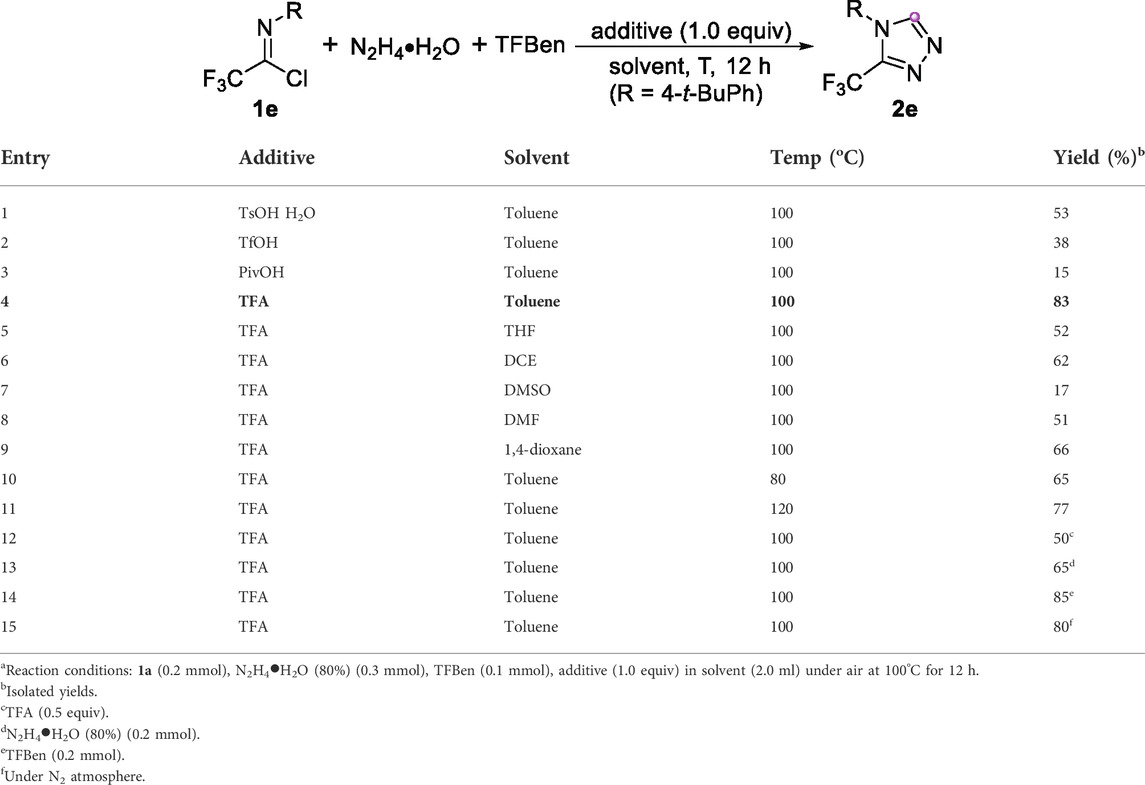
TABLE 1. Optimization of reaction Conditionsa
Having established the optimized conditions, the generality and limitation of the protocol was investigated and the result was summarized in Table 2. To our delight, the reaction exhibited good substrates compatibility, as demonstrated that diverse trifluoroacetimidoyl chlorides were smoothly tolerated in the reaction (2a-p). The reaction was not sensitive to the steric hindrance and the comparable reactivity was observed regarding the trifluoroacetimidoyl chlorides bearing ortho, meta or para substituents located at the aryl ring (2b-d). In general, the trifluoroacetimidoyl chlorides with electron-rich groups (2b-g) showed higher reactivity than that of substrates with electron-deficient groups (2h-k). The naphthalene ring could be successfully incorporated into the 1,2,4-triazole products (2l and 2m) in 75%–78% yields. In addition, other perfluoroalkyl substituted imidoyl chlorides were also amenable to the current reaction system, providing the corresponding 1,2,4-triazoles 2n-p with perfluoroalkyl group in acceptable yields.
Several control experiments were performed to have a deep understanding of the reaction mechanism (Figure 3). First, the replacement of TFBen with formaldehyde totally inhibited the reaction (Figure 3A), whereas the formic acid could participate in the reaction to give the product 2e in 40% yield (Figure 3B). Then, another commonly used CO surrogate, HCO2H/Ac2O, was applied to the reaction for producing 2e in 52% yield (Figure 3C). The above results revealed that the active carbonyl unit was released and subsequently coupled with trifluoroacetimidoyl chloride and hydrazine hydrate. The reaction of trifluoroacetimidohydrazide 1e’ with TFBen could furnish the target product 2e in high yield, suggesting the intermediacy of 1e’ (Figure 3D). When the reaction was carried out between trifluoroacetimidoyl chloride 1e and formhydrazide under the standard conditions, no desired product 2e was detected (Figure 3E), which showed the hydrazine hydrate might initially couple with 1e to form trifluoroacetimidohydrazide 1e’ and formhydrazide not acted as the reaction intermediate.
Based on the mechanistic observations and previously reported literatures (Yin, et al., 2018; Tang, et al., 2021), a plausible reaction mechanism was proposed as outlined in Figure 4. Initially, the coupling of trifluoroacetimidoyl chloride 1 and hydrazine hydrate could readily deliver trifluoroacetimidohydrazide 1’, which reacted with TFBen to give N-formyl imidohydrazide A. Then, the intramolecular nucleophilic addition occurred to lead to the five-membered heterocyclic intermediate B, followed by the dehydration process with the assistance of TFA to provide the final 3-trifluoromethyl-1,2,4-triazole products 2 and release a molecule of water.
To probe the application potential of this protocol, the reaction was performed at 5 mmol scale and the product 2e was isolated without obvious loss of efficiency (Figure 5). Due to the excellent pharmaceutical activity of the scaffold, the present method offers the opportunity for the further study towards the toxicity risk assessment and structure-activity relationship of the pharmaceuticals containing trifluoromethyl-1,2,4-triazole cores.
In conclusion, we have developed a facile and efficient strategy for the assembly of pharmaceutically valuable 3-trifluoromethyl-1,2,4-triazoles through metal-free multi-component reaction of trifluoroacetimidoyl chloride, hydrazine hydrate and TFBen. Notable features of this methodology include readily available reagents, convenient operating conditions, broad substrate scope, high efficiency, and scalability. Further studies toward the synthesis of functionalized heterocycles in a simple manner are underway.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
ZC and XW conceived and designed the experiments; BW and YS carried out the experiments and data test; YS and AC performed some synthesis and characterization; YZ and JW contributed to data analysis and discussion; ZC and X-FW wrote the paper.
This work is supported by the Open Project of Zhejiang Provincial Key Laboratory of Drug Prevention and Control Technology Research (No. 2020001).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fchem.2022.1013977/full#supplementary-material
Buscemi, S., Pace, A., Pibiri, I., Vivona, N., and Spinelli, D. (2003). Fluorinated heterocyclic compounds. An expedient route to 5-perfluoroalkyl-1, 2, 4-triazoles via an unusual hydrazinolysis of 5-perfluoroalkyl-1, 2, 4-oxadiazoles: First examples of an ANRORC-like reaction in 1, 2, 4-oxadiazole derivatives. J. Org. Chem. 68, 605–608. doi:10.1021/jo0262762
Buscemi, S., Pace, A., Piccionello, A. P., Pibiri, I., Vivona, N., Giorgi, G., et al. (2006). Five-to-Six membered ring-rearrangements in the reaction of 5-perfluoroalkyl-1, 2, 4-oxadiazoles with hydrazine and methylhydrazine. J. Org. Chem. 71, 8106–8113. doi:10.1021/jo061251e
Chen, Z., Hu, S., and Wu, X.-F. (2020c). Trifluoroacetimidoyl halides: A potent synthetic origin. Org. Chem. Front. 7, 223–254. doi:10.1039/c9qo01167f
Chen, Z., Wang, L.-C., Zhang, J., and Wu, X.-F. (2020b). Palladium-catalyzed three-component carbonylative synthesis of 2-(trifluoromethyl)quinazolin-4(3H)-ones from trifluoroacetimidoyl chlorides and amines. Org. Chem. Front. 7, 2499–2504. doi:10.1039/D0QO00819B
Chen, Z., Wang, W.-F., Yang, H., and Wu, X.-F. (2020a). Palladium-catalyzed four-component carbonylative cyclization reaction of trifluoroacetimidoyl chlorides, propargyl amines, and diaryliodonium salts: Access to trifluoromethyl-containing trisubstituted imidazoles. Org. Lett. 22, 1980–1984. doi:10.1021/acs.orglett.0c00328
Du, S., Wang, L.-C., Yang, Z., Chen, Z., and Wu, X.-F. (2020). A convenient FeCl3-mediated synthesis of 5-trifluoromethyl-1, 2, 4-triazoles from trifluoroacetimidoyl chlorides and hydrazides. Adv. Synth. Catal. 362, 5130–5134. doi:10.1002/adsc.202001015
Funabiki, K., Noma, N., Kuzuya, G., Matsui, M., and Shibata, K. (1999). A direct and general synthesis of 5-substituted 3-trifluoromethyl-1, 2, 4-triazoles via the three-component condensation reaction of ethyl trifluoroacetate, hydrazine and amidines. J. Chem. Res., 300–301. doi:10.1039/A900520J
Gillis, E. P., Eastman, K. J., Hill, M. D., Donnelly, D. J., and Meanwell, N. A. (2015). Applications of fluorine in medicinal chemistry. J. Med. Chem. 58, 8315–8359. doi:10.1021/acs.jmedchem.5b00258
Girardet, J.-L., Koh, Y.-H., De La Rosa, M., Hong, Z., and Lang, S. (2006). Preparation of S-triazolyl α-mercaptoacetanilides as inhibitors of HIV reverse transcriptase. US20060270725A1.
Han, J., Remete, A. M., Dobson, L. S., Kiss, L., Izawa, K., Moriwaki, H., et al. (2020). Next generation organofluorine containing blockbuster drugs. J. Fluor. Chem. 239, 109639. doi:10.1016/j.jfluchem.2020.109639
Hansen, K. B., Balsells, J., Dreher, S., Hsiao, Y., Kubryk, M., Palucki, M., et al. (2005). First generation process for the preparation of the DPP-IV inhibitor sitagliptin. Org. Process Res. Dev. 9, 634–639. doi:10.1021/op0500786
Haycock-Lewandowski, S. J., Wilder, A., and Åhman, J. (2008). Development of a bulk enabling route to maraviroc (UK-427, 857), a CCR-5 receptor antagonist. Org. Process Res. Dev. 12, 1094–1103. doi:10.1021/op8000614
Hu, S., Yang, Z., Chen, Z., and Wu, X.-F. (2019). Metal-free synthesis of 5-trifluoromethyl-1, 2, 4-triazoles from iodine-mediated annulation of trifluoroacetimidoyl chlorides and hydrazones. Adv. Synth. Catal. 361, 4949–4954. doi:10.1002/adsc.201900983
Jiang, L.-B., Qi, X., and Wu, X.-F. (2016). Benzene-1, 3, 5-triyl triformate (TFBen): A convenient, efficient, and non-reacting CO source in carbonylation reactions. Tetrahedron Lett. 57, 3368–3370. doi:10.1016/j.tetlet.2016.06.071
Koltin, Y., and Hitchcock, C. A. (1997). The search for new triazole antifungal agents. Curr. Opin. Chem. Biol. 1, 176–182. doi:10.1016/S1367-5931(97)80007-5
Lebsack, A. D., Gunzner, J., Wang, B., Pracitto, R., Schaffhauser, H., Santini, A., et al. (2004). Identification and synthesis of [1, 2, 4]triazolo[3, 4-a]phthalazine derivatives as high-affinity ligands to the alpha 2 delta-1 subunit of voltage gated calcium channel. Bioorg. Med. Chem. Lett. 14, 2463–2467. doi:10.1016/j.bmcl.2004.03.008
Liu, X., Liu, H., Bian, C., Wang, K. H., Wang, J., Huang, D., et al. (2022). Synthesis of 3-trifluoromethyl-1, 2, 4-triazolines and 1, 2, 4-triazoles via tandem addition/cyclization of trifluoromethyl N-acylhydrazones with cyanamide. J. Org. Chem. 87, 5882–5892. doi:10.1021/acs.joc.2c00176
Lu, S.-N., Sun, Y., Zhang, J., Chen, Z., and Wu, X.-F. (2022a). Metal-free Synthesis of 5-Trifluoromethyl-1, 2, 4-triazoles via elemental sulfur promoted oxidative cyclization of trifluoroacetimidohydrazides with benzylic and aliphatic amines. Mol. Catal. 524, 112336. doi:10.1016/j.mcat.2022.112336
Lu, S.-N., Yang, H., Zhang, J., Chen, Z., and Wu, X.-F. (2021). Oxidative cyclization of trifluoroacetimidohydrazides with D-glucose for the metal-free synthesis of 3-trifluoromethyl-1, 2, 4-triazoles. Adv. Synth. Catal. 363, 4982–4987. doi:10.1002/adsc.202100989
Lu, S.-N., Zhang, J., Li, J., Chen, Z., and Wu, X.-F. (2022b). Metal-free synthesis of 3-trifluoromethyl-1, 2, 4-triazoles via oxidative cyclization of trifluoroacetimidohydrazides with N, N-dimethylformamide as carbon synthons. Green Synth. Catal. doi:10.1016/j.gresc.2022.06.007
Müller, K., Faeh, C., and Diederich, F. (2007). Fluorine in pharmaceuticals: Looking beyond intuition. Science 317, 1881–1886. doi:10.1126/science.1131943
Peng, X., Zhang, F. G., and Ma, J. A. (2020). Cu-catalysed three-component reaction of aryldiazonium salts with fluorinated diazo reagents and nitriles: Access to difluoro- and trifluoromethylated N1-aryl-1, 2, 4-triazoles. Adv. Synth. Catal. 362, 4432–4437. doi:10.1002/adsc.202000776
Romagnoli, R., Baraldi, P. G., Salvador, M. K., Prencipe, F., Bertolasi, V., Cancellieri, M., et al. (2014). Synthesis, antimitotic and antivascular activity of 1-(3’, 4’, 5’-Trimethoxybenzoyl)-3-arylamino-5-amino-1, 2, 4-triazoles. J. Med. Chem. 57, 6795–6808. doi:10.1021/jm5008193
Sakurada, I., Hirabayashi, T., Maeda, Y., Nagasue, H., Mizuno, T., Xu, J., et al. (2015). Preparation of benzamide derivatives for use as factor IXa inhibitors. WO2015160636A1.
Shivarama Holla, B., Narayana Poojary, K., Sooryanarayana Rao, B., and Shivananda, M. K. (2002). New bis-aminomercaptotriazoles and bis-triazolothiadiazoles as possible anticancer agents. Eur. J. Med. Chem. 37, 511–517. doi:10.1016/S0223-5234(02)01358-2
Sibgatulin, D. A., Bezdudny, A. V., Mykhailiuk, P. K., Voievoda, N. M., Kondratov, I. S., Volochnyuk, D. M., et al. (2010). Novel synthetic approaches to (Trifluoromethyl)triazoles. Synthesis, 1075–1077. doi:10.1055/s-0029-1218656
Sugane, T., Tobe, T., Hamaguchi, W., Shimada, I., Maeno, K., Miyata, J., et al. (2011). Synthesis and biological evaluation of 3-Biphenyl-4-yl-4-phenyl-4H-1, 2, 4-triazoles as novel Glycine transporter 1 inhibitors. J. Med. Chem. 54, 387–391. doi:10.1021/jm101031u
Syvanen, S., Eriksson, J., Genchel, T., Lindhe, O., Antoni, G., and Langstrom, B. (2007). Synthesis of two potential NK1-receptor ligands using [1-11C]ethyl iodide and [1-11C]propyl iodide and initial PET-imaging. BMC Med. Imaging 7, 6. doi:10.1186/1471-2342-7-6
Tamura, K., Mizukami, H., Maeda, K., Watanabe, H., and Uneyama, K. (1993). One-pot synthesis of trifluoroacetimidoyl halides. J. Org. Chem. 58, 32–35. doi:10.1021/jo00053a011
Tang, J., Zhang, J., Zhang, Y., Chen, Z., and Wu, X.-F. (2021). Palladium-catalyzed carbonylative synthesis of 5-trifluoromethyl-1, 2, 4-triazoles from trifluoroacetimidohydrazides and aryl iodides. Org. Chem. Front. 8, 6089–6094. doi:10.1039/d1qo01064f
Tao, Y., Wang, Q., Ao, L., Zhong, C., Yang, C., Qin, J., et al. (2010). Highly efficient phosphorescent organic light-emitting diodes hosted by 1, 2, 4-triazole-cored triphenylamine derivatives: Relationship between structure and optoelectronic properties. J. Phys. Chem. C 114, 601–609. doi:10.1021/jp908886d
Wang, L.-C., Zhang, Y., Chen, Z., and Wu, X.-F. (2021). Palladium-catalyzed carbonylative synthesis of 2-(trifluoromethyl)quinazolin-4(3H)-ones from trifluoroacetimidoyl chlorides and nitro compounds. Adv. Synth. Catal. 363, 1417–1426. doi:10.1002/adsc.202001502
Yang, H., Lu, S.-N., Song, Y., Chen, Z., and Wu, X.-F. (2021). Copper-mediated [3 + 2] cycloaddition of trifluoroacetimidoyl chlorides and N-isocyanoiminotriphenylphosphorane for the synthesis of 3-trifluoromethyl-1, 2, 4-triazoles. Org. Chem. Front. 8, 5040–5044. doi:10.1039/d1qo00843a
Yang, H., Wang, L.-C., Wang, W.-F., Chen, Z., and Wu, X.-F. (2020). Palladium-catalyzed cascade carbonylative cyclization reaction of trifluoroacetimidoyl chlorides and 2-iodoanilines: Toward 2-(trifluoromethyl)quinazolin-4(3H)-ones synthesis. ChemistrySelect 5, 11072–11076. doi:10.1002/slct.202003269
Yang, H., Zhang, J., Chen, Z., and Wu, X. F. (2022). TFBen (Benzene-1, 3, 5-triyl triformate): A powerful and versatile CO surrogate. Chem. Rec. 22, e202100220. doi:10.1002/tcr.202100220
Yin, Z., Power, D., Wang, Z., Stewart, S., and Wu, X.-F. (2018). Synthesis of 1, 3, 4-oxadiazoles via annulation of hydrazides and benzene-1, 3, 5-triyl triformate under metal-free conditions. Synthesis 50, 3238–3242. doi:10.1055/s-0037-1609481
Zhang, F., Peng, X., and Ma, J. a. (2019). Recent advances in the synthesis of CF3-substituted triazoles and tetrazoles. Chin. J. Org. Chem. 39, 109–116. doi:10.6023/cjoc201808007
Zhang, J., Tang, J., Chen, Z., and Wu, X.-F. (2021a). Synthesis of 5-trifluoromethyl-1, 2, 4-triazoles via metal-free annulation of trifluoroacetimidohydrazides and methyl ketones. Adv. Synth. Catal. 363, 3060–3069. doi:10.1002/adsc.202100130
Zhang, J., Tang, J., Chen, Z., and Wu, X. F. (2021c). Elemental sulfur and dimethyl sulfoxide-promoted oxidative cyclization of trifluoroacetimidohydrazides with methylhetarenes for the synthesis of 3-Hetaryl-5-trifluoromethyl-1, 2, 4-triazoles. Chin. J. Chem. 39, 3443–3447. doi:10.1002/cjoc.202100579
Zhang, J., Xu, T.-H., Chen, Z., and Wu, X.-F. (2021b). Metal-free oxidative cyclization of trifluoroacetimidohydrazides with methylhetarenes: A facile access to 3-hetaryl-5-trifluoromethyl-1, 2, 4-triazoles. Org. Chem. Front. 8, 4490–4495. doi:10.1039/D1QO00790D
Zhang, Y., Yang, Z., Chen, Z., Liu, L., and Wu, X.-F. (2022). Copper-catalyzed decarbonylative cyclization of isatins and trifluoroacetimidohydrazides for the synthesis of 2-(5-trifluoromethyl-1, 2, 4-triazol-3-yl)anilines. Adv. Synth. Catal. 364, 1044–1049. doi:10.1002/adsc.202101397
Zhou, C. H., and Wang, Y. (2012). Recent researches in triazole compounds as medicinal drugs. Curr. Med. Chem. 19, 239–280. doi:10.2174/092986712803414213
Zhou, Y., Wang, J., Gu, Z., Wang, S., Zhu, W., Aceña, J. L., et al. (2016). Next generation of fluorine-containing pharmaceuticals, compounds currently in phase II–III clinical trials of major pharmaceutical companies: New structural trends and therapeutic areas. Chem. Rev. 116, 422–518. doi:10.1021/acs.chemrev.5b00392
Keywords: metal-free, multi-component reaction, trifluoromethyl-1,2,4-triazole, trifluoroacetimidoyl chloride, benzene-1,3,5-triyl triformate
Citation: Wang B, Sun Y, Cheng A, Zhu Y, Wang J, Chen Z and Wu X-F (2022) Metal-free synthesis of 3-trifluoromethyl-1,2,4-triazoles via multi-component reaction of trifluoroacetimidoyl chlorides, hydrazine hydrate and benzene-1,3,5-triyl triformate. Front. Chem. 10:1013977. doi: 10.3389/fchem.2022.1013977
Received: 08 August 2022; Accepted: 30 August 2022;
Published: 20 September 2022.
Edited by:
Ming-Yu Ngai, Stony Brook University, United StatesReviewed by:
Mohanad Mousa Kareem, University of Babylon, IraqCopyright © 2022 Wang, Sun, Cheng, Zhu, Wang, Chen and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiye Wang, d2FuZ2ppeWVAempwYy5lZHUuY24=; Zhengkai Chen, emtjaGVuQHpzdHUuZWR1LmNu; Xiao-Feng Wu, eHd1MjAyMEBkaWNwLmFjLmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.