- State Key Laboratory Breeding Base of Green Pesticide and Agricultural Bioengineering, Key Laboratory of Green Pesticide and Agricultural Bioengineering, Ministry of Education, Guizhou University, Guiyang, China
Alginate is a water-soluble and acidic polysaccharide derived from the cell wall and intercellular substance of brown algae. It is widely distributed in brown algae, such as Laminaria, Sargassum, and Macrocystis, etc. Alginate lyase can catalytically degrade alginate in a β-eliminating manner, and its degradation product-alginate oligosaccharide (AOS) has been widely used in agriculture, medicine, cosmetics and other fields due to its wide range of biological activities. This article is mainly to make a brief introduction to the classification, source and application of alginate lyase. We hope this minireview can provide some inspirations for its development and utilization.
Introduction
Alginate is the most abundant linear polysaccharide in brown algae (about 40% of dry weight). It is composed of two uronic acid monomers, β-d-mannuronic acid and C5-epimer α-l-guluronic acid, through α/β-1,4 glycosidic bonds in different combinations of poly-guluronic acid (poly-G), poly-mannuronic acid (poly-M), and hybrid fragments of random polymerization of G and M (poly-GM). (Gacesa, 1992; Lee and Mooney, 2012; Kurakake et al., 2017).
At present, the preparation of algal oligosaccharides mainly adopts three kinds of degradation methods: chemical, physical and biological. Chemical methods include acid hydrolysis, alkaline hydrolysis and oxidative degradation, and physical methods mainly include hydrothermal method, ultrasonic method and radiation method, but these two methods have many drawbacks, which are not conducive to large-scale production. Biological methods mainly use microbial fermentation or the action of enzymes to degrade the prepared enzymes. It has the advantages of mild conditions, easy control, and strong product specificity, which has attracted people’s attention, it may be an important direction of industrial production. (Guo et al., 2016).
Introduction and application of alginate lyase
Classification of alginate lyases
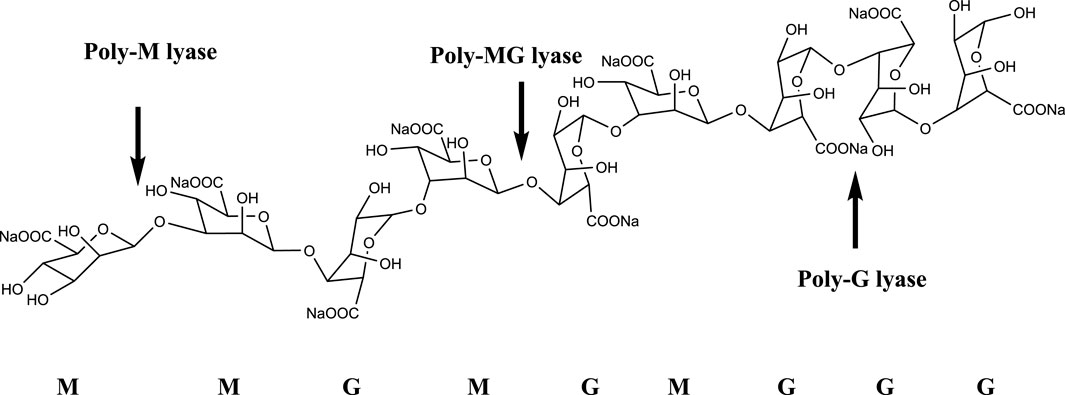
The alginate oligosaccharide produced by the enzymatic hydrolysis of alginate lyase has an unsaturated double bond between the C4 and C5 positions of the uronic acid unit at the non-reducing end, and it has a characteristic absorption peak at a specific wavelength of 235 nm. Alginate lyases can be divided into three groups based on substrate specificity, namely poly-G lyase (EC: 4.2.2.11), poly-M lyase (EC: 4.2.2.3), and displays both poly-G and poly-M lyase (Figure 1). In terms of the mode of action, alginate lyase can be divided into endonuclease and exonuclease (Wong et al., 2000). Endonuclease cleaves glycosidic bonds in algin and releases unsaturated oligosaccharides (disaccharides, trisaccharides and tetrasaccharides, etc.), while exonuclease can further degrade oligosaccharides into oligosaccharides. Monomer (Kim et al., 2012; Jagtap et al., 2014). Based on the amino acid sequence alignment, alginate lyases can be classified into different polysaccharide lyase (PL) families including PL5, PL6, PL7, PL8, PL14, PL15, PL17, PL18, PL31, PL32, PL34, PL36, PL39, and PL41 families, which are listed in the Carbohydrate-Active enzymes (CAZy) database (http://www.cazy.org/) (Barzkar et al., 2022).
Source of alginate lyase
The sources of alginate lyase are extensive, and it has been reported that the production of alginate lyase mainly comes from marine algae, marine mollusks and microorganisms (including bacteria, fungi and some viruses). Among them, there are the most reports on the source of microorganisms, including (Pseudoalteromonas sp.) (Ma et al., 2008) (Vibrio sp.), (Kawamoto et al., 2006) (Flavobacterium sp.) (An et al., 2009) (Paenibacillus sp.) (Zhu et al., 2020; Huang et al., 2022).
Application of alginate lyase
Alginate lyase is an important marine biological enzyme. AOS has various biological activities due to the differences in degradation mode, G content of degradation products (G/M ratio), molecular weight and spatial conformation. As an excellent natural antioxidant, alginate oligosaccharide has great application potential in the fields of human, animals and plants health (Yokose et al., 2009; Tondervik et al., 2014; Saberi Riseh, 2022). It can promote growth, improve stress resistance, increase yield, and inhibit fungal growth. With the implementation of the national strategy of regulating the use of chemical fertilizers and pesticides, it may become an environment-friendly bio-fertilizer and bio-pesticide in the future.
Alginate lyase is not only widely used in agriculture, but also in the fields of medicine and food. It can be used as a growth promoter for therapeutics such as antioxidants and tumor suppressors (Tusi et al., 2011; Hu et al., 2004), and can also induce cytokine production, regulate blood sugar and lipids (Iwamoto et al., 2005), which are widely used in the food and pharmaceutical industries. For example, Pseudomonas aeruginosa is one of the main pathogens of many chronic infectious diseases, such as chronic lung infection and urinary tract infection (Jain and Ohman, 2005). The study found that algin is an important component of Pseudomonas aeruginosa biofilm, and Albrecht used lyase as an adjunct therapeutic agent together with antibiotics, which made the antibiotics come into direct contact with pathogenic bacteria to achieve the therapeutic effect (Albrecht and Schiller, 2005). This shows that the enzyme has great potential in the bactericidal application of biofilm pathogens.
Due to fuel consumption, researchers have begun to pay attention to the production of biofuel. At present, although chemical catalysis has high efficiency, its high cost, complex synthesis process, and high energy consumption under synthetic conditions limit its industrial application to a certain extent (Pan et al., 2022a; Pan et al., 2022b). Seaweed is considered an ideal source for bioethanol production due to its advantages of not occupying arable land and being non-polluting. According to reports, Wargacki et al. (2012) transformed Escherichia coli to establish a system for directly fermenting brown algae to produce ethanol. However, Escherichia coli has insufficient tolerance to ethanol, making it impossible for large-scale production. Sasaki et al. (2018) developed a co-cultivation platform for bioethanol production from brown algae, consisting of engineered yeast AM1 and CDY strains, which produced 2.1 g/L of ethanol when the brown algae Ecklonia kurome was used as the sole carbon source. This research has made significant progress in the biotechnology of brown algae to bioethanol, but it is still insufficient for industrial production. Studies have shown that the synergistic effect of multiple microorganisms on the ethanol fermentation system of macroalgae will be the trend of future research.
Conclusion and outlook
Alginate lyase has attracted the attention of researchers because of its unique properties and has great potential for application in various fields. At present, the related research on alginate lyase mainly focuses on the screening of strains, the mining of genes, and the analysis of the degradation substrate and product structure. With the continuous advancement of science and technology, people have gradually deepened the research on the analysis of alginate lyase protein crystal analysis, the catalytic mechanism of the active center and the transformation strategy. This will likely improve the problems of low enzyme-producing strains, low tolerance, and unstable properties in the large-scale industrial production process, and has great application potential in agricultural protection, biofuel production, and environmental protection.
Author contributions
ZZ and AD contributed equally to this work and drafted the manuscript. YL participated in some manuscript writing and checking. TL conceptualized and directed the whole project. All of the authors contributed to scientific discussions.
Funding
We acknowledge financial support from the Program of Introducing Talents of Discipline to Universities of China (111 Program, D20023), Frontiers Science Center for Asymmetric Synthesis and Medicinal Molecules, Department of Education, Guizhou Province [Qianjiaohe KY (2020)004], and Guizhou University.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Albrecht, M.-T., and Schiller, N.-L. (2005). Alginate lyase (AlgL) activity is required for alginate biosynthesis in Pseudomonas aeruginosa. J. Bacteriol. 187 (11), 3869–3872. doi:10.1128/jb.187.11.3869-3872.2005
An, Q.-D., Zhang, G.-L., Wu, H.-T., Zhang, Z.-C., Zheng, G.-S., Luan, L., et al. (2009). Alginate-deriving oligosaccharide production by alginase from newly isolated Flavobacterium sp. LXA and its potential application in protection against pathogens. J. Appl. Microbiol. 106 (1), 161–170. doi:10.1111/j.1365-2672.2008.03988.x
Barzkar, N., Sheng, R., Sohail, M., Jahromi, S.-T., Babich, O., Sukhikh, S., et al. (2022). Alginate lyases from marine bacteria: An enzyme ocean for sustainable future. Molecules 27 (11), 3375. doi:10.3390/molecules27113375
Gacesa, P. (1992). Enzymic degradation of alginates. Int. J. Biochem. 24 (4), 545–552. doi:10.1016/0020-711X(92)90325-U
Guo, J.-J., Ma, L.-L., Shi, H.-T., Zhu, J.-B., Wu, J., Ding, Z.-W., et al. (2016). Alginate oligosaccharide prevents acute doxorubicin cardiotoxicity by suppressing oxidative stress and endoplasmic reticulum-mediated apoptosis. Mar. Drugs 14 (12), 231. doi:10.3390/md14120231
Huang, H.-Q., Zheng, Z.-G., Zou, X.-X., Wang, Z.-X., Gao, R., Zhu, J., et al. (2022). Genome analysis of a novel polysaccharide-degrading bacterium paenibacillus algicola and determination of alginate lyases. Mar. Drugs 20 (6), 388. doi:10.3390/md20060388
Hu, X.-K., Jiang, X.-L., Hwang, H.-M., Liu, S.-L., and Guan, H.-S. (2004). Antitumour activities of alginate-derived oligosaccharides and their sulphated substitution derivatives. Eur. J. Phycol. 39 (1), 67–71. doi:10.1080/09670260310001636695
Iwamoto, M., Kurachi, M., Nakashima, T., Kim, D., Yamaguchi, K., Oda, T., et al. (2005). Structure-activity relationship of alginate oligosaccharides in the induction of cytokine production from RAW264. 7 cells. FEBS Lett. 579 (20), 4423–4429. doi:10.1016/j.febslet.2005.07.007
Jagtap, S.-S., Hehemann, J.-H., Polz, M.-F., Lee, J.-K., and Zhao, H. (2014). Comparative biochemical characterization of three exolytic oligoalginate lyases from Vibrio splendidus reveals complementary substrate scope, temperature, and pH adaptations. Appl. Environ. Microbiol. 80 (14), 4207–4214. doi:10.1128/aem.01285-14
Jain, S., and Ohman, D.-E. (2005). Role of an alginate llyase for alginate tansport in mucoid Pseudomonas aeruginosa. Infect. Immun. 73 (10), 6429–6436. doi:10.1128/iai.73.10.6429-6436.2005
Kawamoto, H., Horibe, A., Miki, Y., Kimura, T., Tanaka, K., Nakagawa, T., et al. (2006). Cloning and sequencing analysis of alginate lyase genes from the marine bacterium Vibrio sp. O2. Mar. Biotechnol. 8 (5), 481–490. doi:10.1007/s10126-005-6157-z
Kim, H.-T., Chung, J.-H., Wang, D., Lee, J., Woo, H.-C., Choi, I.-G., et al. (2012). Depolymerization of alginate into a monomeric sugar acid using Alg17C, an exo-oligoalginate lyase cloned from saccharophagus degradans 2-40. Appl. Microbiol. Biotechnol. 93 (5), 2233–2239. doi:10.1007/s00253-012-3882-x
Kurakake, M., Kitagawa, Y., Okazaki, A., and Shimizu, K. (2017). Enzymatic properties of alginate lyase from paenibacillus sp. S29. Appl. Biochem. Biotechnol. 183 (4), 1455–1464. doi:10.1007/s12010-017-2513-5
Lee, K.-Y., and Mooney, D.-J. (2012). Alginate: Properties and biomedical applications. Prog. Polym. Sci. 37 (1), 106–126. doi:10.1016/j.progpolymsci.2011.06.003
Ma, L.-Y., Chi, Z.-M., Li, J., and Wu, L.-F. (2008). Overexpression of alginate lyase of Pseudoalteromonas elyakovii in Escherichia coli, purification, and characterization of the recombinant alginate lyase. World J. Microb. Biot. 24 (1), 89–96. doi:10.1007/s11274-007-9443-2
Pan, H., Xia, Q.-N., Wang, Y., Shen, Z.-F., Huang, H., Ge, Z., et al. (2022a). Recent advances in biodiesel production using functional carbon materials as acid/base catalysts. Fuel Process. Technol. 237, 107421. doi:10.1016/j.fuproc.2022.107421
Pan, H., Xia, Q.-N., Li, H., Wang, Y.-G., Shen, Z.-F., Wang, Y., et al. (2022b). Direct production of biodiesel from crude Euphorbia lathyris L. Oil catalyzed by multifunctional mesoporous composite materials. Fuel 309, 122172. doi:10.1016/j.fuel.2021.122172
Saberi Riseh, R., Gholizadeh Vazvani, M., Ebrahimi-Zarandi, M., and Skorik, Y.A. (2022). Alginate-induced disease resistance in plants. Polymers 14 (4), 661. doi:10.3390/polym14040661
Sasaki, Y., Takagi, T., Motone, K., Shibata, T., Kuroda, K., and Ueda, M. (2018). Direct bioethanol production from Brown macroalgae by Co-culture of two engineered Saccharomyces cerevisiae strains. Biosci. Biotechnol. Biochem. 82 (8), 1459–1462. doi:10.1080/09168451.2018.1467262
Tøndervik, A., Sletta, H., Klinkenberg, G., Emanuel, C., Powell, L.-C., Pritchard, M.-F., et al. (2014). Alginate oligosaccharides inhibit fungal cell growth and potentiate the activity of antifungals against Candida and Aspergillus spp. PLoS One 9 (11), e112518. doi:10.1371/journal.pone.0112518
Tusi, S.-K., Khalaj, L., Ashabi, G., Kiaei, M., and Khodagholi, F. (2011). Alginate oligosaccharide protects against endoplasmic reticulum-and mitochondrial-mediated apoptotic cell death and oxidative stress. Biomaterials 32 (23), 5438–5458. doi:10.1016/j.biomaterials.2011.04.024
Wargacki, A.-J., Leonard, E., Win, M.-N., Regitsky, D.-D., Santos, C.-N.-S., Kim, P.-B., et al. (2012). An engineered microbial platform for direct biofuel production from Brown macroalgae. Science 335 (6066), 308–313. doi:10.1126/science.1214547
Wong, T.-Y., Preston, L.-A., and Schiller, N.-L. (2000). Alginate lyase: Review of major sources and enzyme characteristics, structure-function analysis, biological roles, and applications. Annu. Rev. Microbiol. 54, 289–340. doi:10.1146/annurev.micro.54.1.289
Yokose, T., Nishikawa, T., Yamamoto, Y., Yamasaki, Y., Yamaguchi, K., and Oda, T. (2009). Growth-promoting effect of alginate oligosaccharides on a unicellular marine microalga, Nannochloropsis oculata. Biosci. Biotechnol. Biochem. 73 (2), 450–453. doi:10.1271/bbb.80692
Keywords: alginate lyase, Brown algae, AOS, agriculture, biological activity
Citation: Zheng Z, Dai A, Liu Y and Li T (2022) Sustainable alginate lyases catalyzed degradation of bio-based carbohydrates. Front. Chem. 10:1008010. doi: 10.3389/fchem.2022.1008010
Received: 31 July 2022; Accepted: 23 August 2022;
Published: 08 September 2022.
Edited by:
Yaqiong Su, Xi’an Jiaotong University, ChinaCopyright © 2022 Zheng, Dai, Liu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tingting Li, bGl0dDgyOTNAMTYzLmNvbQ==
 Zhiguo Zheng
Zhiguo Zheng Ali Dai
Ali Dai Yonggui Liu
Yonggui Liu Tingting Li
Tingting Li