- 1School of Microelectronics, Hubei University, Wuhan, China
- 2Hubei Yangtze Memory Laboratories, Wuhan, China
- 3College of Optical and Electronic Technology, China Jiliang University, Hangzhou, China
Among the new energy storage devices, aqueous zinc ion batteries (AZIBs) have become the current research hot spot with significant advantages of low cost, high safety, and environmental protection. However, the cycle stability of cathode materials is unsatisfactory, which leads to great obstacles in the practical application of AZIBs. In recent years, a large number of studies have been carried out systematically and deeply around the optimization strategy of cathode material stability of AZIBs. In this review, the factors of cyclic stability attenuation of cathode materials and the strategies of optimizing the stability of cathode materials for AZIBs by vacancy, doping, object modification, and combination engineering were summarized. In addition, the mechanism and applicable material system of relevant optimization strategies were put forward, and finally, the future research direction was proposed in this article.
Introduction
In response to the global climate crisis, the research of new energy storage devices has been widely focused on expanding the application of renewable energy to replace fossil energy (Tan et al., 2020a; Wang et al., 2020a; Gan et al., 2020; Cai et al., 2021a; Liu et al., 2021a; Cai et al., 2021b; Deng et al., 2021; Zhao et al., 2021). In the field of new energy storage, lithium-ion batteries have been widely used because of their high energy density and wide working voltage (Park et al., 2021; Xia et al., 2021; Feng et al., 2022). However, the scarcity of lithium resources increases the cost of lithium batteries, and the majority of the organic electrolyte used are poisonous or flammable, reducing the safety of lithium batteries (Li et al., 2021a; Du et al., 2021; Hou et al., 2021). Comparatively, zinc metal has the advantages of non-toxic, low cost, and redox potential, which is more suitable for aqueous electrolytes (Yao et al., 2021). Moreover, the high density and multi-charge of zinc render aqueous zinc ion batteries (AZIBs) with excellent energy density, which makes it have great application prospects (Gao et al., 2021). However, the low cycle stability of AZIBs is an inevitable problem. As one of the most core components, cathode materials for the improvement of AZIB performance critically depend on the optimization of stability. The storage mechanism and capacity attenuation of zinc ions in AZIBs system have not been fully clarified. Thus, the latest research progress is necessary to be summarized, which is conducive to providing the following research direction.
Herein, the primary factors causing the performance degradation of cathode materials for AZIBs are summarized, and optimization strategies for the stability of cathode materials are introduced. Finally, according to the optimization strategy introduced in the summary, some problems to be further studied will be put forward, and the subsequent optimization research of stability will be prospected.
Performance Degradation of Cathode Materials
The strong electrostatic interaction and large steric effect between divalent Zn2+ and the main structure of cathode materials in AZIBs lead to poor cyclicity and very slow intercalation kinetics. Meanwhile, the pH, additives, types, and concentrations of zinc salts in the electrolyte will also affect the energy storage characteristics of cathode materials. The attenuation of cathode material performance is mainly divided into the following situations:
Irreversible phase transition: During the charge–discharge process of the battery, Zn2+ intercalation, ion/molecule co-intercalation, and conversion reaction are likely to cause irreversible damage to the structure of cathode materials (Chen et al., 2020). For instance, ZnxMnO2 will be formed when Zn2+ is inserted into the space of MnO2 with a layered structure, while MnOOH with a tunnel structure will be formed when H+ is inserted into the material in solution (Liu et al., 2021b; Ma et al., 2021). This phase transition in varying degrees will destroy part of the original structure, resulting in the attenuation of performance. Moreover, the H+ insertion process is usually accompanied by-products [such as Zn4SO4(OH)6·5H2O] with the change of pH, which will cause the adhesion of insulation corrosion on the cathode surface and also continuously reduce the electrochemical activity of the cathode (Li et al., 2019).
Cathodic dissolution: The dissolution and diffusion of cathode materials in electrolytes are irreversible to a certain extent, which will cause the instability of the material structure. For example, the Jahn–Teller effect in high-valence manganese-based oxides induces the irreversible transformation of some Mn3+ to Mn2+ in the process of cathode discharge and then will destroy the main structure of materials (Heo et al., 2021). In addition, for most material systems such as vanadium-based compounds, Prussian blue and analogs, and their structures are not stable in electrolytes, and irreversible dissolution will occur when the cathode is discharged for a long time (Wan and Niu, 2019; Li et al., 2021b).
In conclusion, the performance degradation of cathode materials is not only due to the influence of the electrolyte environment but also related to its own structural characteristics and reaction mechanism. Moreover, according to the research reported at present, the cycle stability of cathode materials can be optimized from four aspects: introduction of vacancy, substitution/gap doping, object modification, and combination engineering.
Stability Optimizations for Cathode Materials
Introduction of Vacancy
The introduction of an appropriate amount of vacancy engineering (oxygen vacancy, metal vacancy, etc.) has been confirmed that it not only can reduce the bandgap, improve the conductivity, and promote the diffusion kinetics of H+/Zn2+ to improve the capacity but also enhance the structural stability to inhibit its dissolution, so as to improve the cycle stability (Wang et al., 2020b; Luo et al., 2020; Tan et al., 2020b; Cao et al., 2021; Tong et al., 2021; Cui et al., 2022). Zhang et al. achieved the doping of Cu2+ substituting Mn3+ by solvothermal and annealing and synthesized oxygen-containing vacancy Mn2O3 (OCu-Mn2O3) (Liu et al., 2020a). The uniform distribution of oxygen vacancies can adjust the internal electric field and crystal structure by compensating the non-zero dipole moment (in Figure 1A), thereby promoting the reaction kinetics and improving the stability of the crystal structure. Unlike the rapid decline in the capacity of Zn||Mn2O3 battery (capacity retention less than 50%), the capacity of Zn||OCu-Mn2O3 battery still retains 88% of the initial capacity after 600 cycles at 1 Ag−1. In addition, Peng et al. prepared pristine V6O13 (p-VO) via electrodeposition and the self-assembly process, and then, oxygen-deficient V6O13 cathode (Od-VO) was obtained by annealing (Liao et al., 2020). Simulated results indicated that the introduced oxygen vacancy can reduce the Gibbs desorption free energy of Od-VO, which is more conducive to the desorption of Zn2+ than p-VO (shown in Figure 1B). The prepared Od-VO cathode has displayed roughly a capacity retention rate of 95% after 200 cycles at 0.2 Ag−1, which is significantly higher than p-VO cathode (collapsed within 180 cycles). Moreover, Kim et al. synthesized in situ growth of ZnMn2O4@C with Mn deficiency (Mn-d-ZMO@C) from the ZnO-MnO@C nanocomposite by solvent dry process and annealing methods (Islam et al., 2021). As shown in Figure 1C, ZnO-MnO@C transformed into Mn-d-ZMO@C via an aging process in electrolytes, which was along with the formation of Zn4(OH)6SO4·5H2O (ZBS) on the surface. Furthermore, Mn-d-ZMO@C and by-products realized reversible conversion by reacting with Zn2+ and Mn2+, respectively. The Zn/Mn-d-ZMO@C cell still maintained 84% of the highest capacity (98 mAh g−1) after 2000 cycles at 3 Ag−1. Thus, it can be seen that some vacancy optimization strategies reported recently have provided detailed analyses of the concentration and location distribution of introduced vacancies. However, more material systems need to be further studied to verify the universality of the optimization mechanism of this strategy.
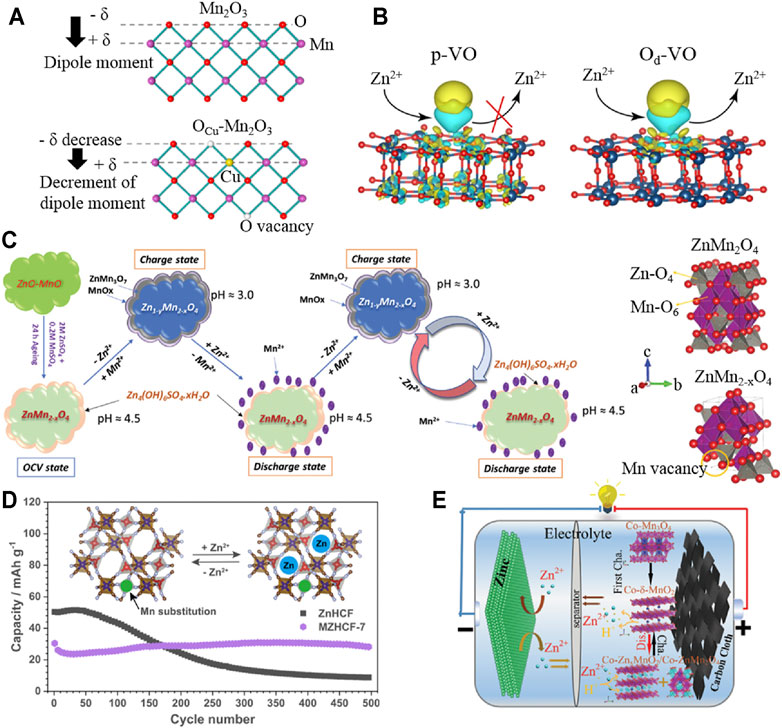
FIGURE 1. Vacancies and doping modification of cathode materials. (A) Atomic structure models of a single layer height in Mn2O3 and Ocu-Mn2O3, respectively; (B) illustrations of the Zn2+ storage/release for p-VO and Od-VO; (C) schematic illustration for the reaction mechanism of the in situ formed Zn/Mn-d-ZMO@C; (D) schematic diagram of the reaction mechanism of MZHCFs; (E) schematic illustration of Zn|| Co-Mn3O4/CAN battery. Reproduced with permission (Liu et al., 2020a; Liao et al., 2020; Islam et al., 2021; Ji et al., 2021; Ni et al., 2021).
Substitution/Gap Doping
As reported earlier, the vacancy defects caused by doping modification have been confirmed stabilizing the crystal structure of cathode materials. Besides, the substitution doping of multivalent metal ions can effectively reduce the formation energy of cathode materials, which can effectively inhibit the collapse of crystal structure (Kim et al., 2021; Li et al., 2020). Ni et al. synthesized Mn-substituted zinc hexacyanoferrate materials (MZHCFs) using a simple precipitation method (Ni et al., 2021). The substitution of Mn ions in the N-bonded sites can restrain the cubic-rhombohedral phase transition and the dissolution of active materials in electrolytes, resulting in improving the structural stability. As shown in Figure 1D, the MZHCF (MZHCF-7) with Mn content of 7% retained 94% of the initial capacity (far more than 17% of ZnHCF) after 500 cycles at 0.25 Ag−1, displaying a significant synergistic optimization effect. In addition, the gap doping of heteroatoms (especially metals with similar ion radius) has been proved to effectively stabilize the phase transition structure and inhibit the dissolution of materials, which contributes to improving the reversibility of cathodic electrochemical reaction (Xu et al., 2021a; Chen et al., 2021). Moreover, Wang et al. obtained multivalent cobalt (Co2+, Co3+)-doped Mn3O4 nanosheets (Co-Mn3O4/CNA) based on carbon nanosheets array by electrodeposition on the basis of Co-MOF precursors prepared in water bath and annealing (Ji et al., 2021). Doped Co2+ in the interlayer of initial phase change products δ-MnO2 can play a supporting role due to strong adsorption energy (in Figure 1E). Meanwhile, doped Co4+ in the [MnO6] octahedral structure can improve the conductivity of Mn4+ and maintain a high specific capacity, which is owing to its low energy bandgap. In the subsequent charge–discharge process, cobalt with different valence states not only plays a supporting role in the phase change products but also can effectively inhibit the Jahn–Teller effect and promote the diffusion of ions. The prepared Co-Mn3O4/CNA cathode can still maintain 80% of the initial capacity after 1,100 cycles at 2 Ag−1. Nevertheless, the current research on doping modification has not further analyzed the influence of doping position and the proportion of different doping components on the stability of optimized materials. Furthermore, the similarities and differences of optimization mechanisms from different doping elements still need to be further discussed.
Object Modification
The stability optimization strategy of cathode materials also includes object modification methods such as intercalation and surface coating. Moreover, object modification has been proved to effectively promote the reversibility of the reaction process and inhibit the dissolution of structures (Zhang et al., 2021). For layered cathode materials, the insertion of highly stable objects can promote the interlayer reversible transfer of Zn2+ (Liu et al., 2020b; He et al., 2021a; He et al., 2021b; Li et al., 2021c). Li et al. synthesized MoS2/graphene nanomaterials with a sandwich interlayer structure by solution stirring in an argon atmosphere at room temperature (Li et al., 2021d). Figures 2A–C show that reduced graphene oxide (rGO) was inserted between MoS2 layers, resulting in the significant expansion of the MoS2 layer spacing and the sharp decrease in the Zn2+ migration barrier. In addition, the stable flow structure alleviates the instability caused by interlayer stacking. The prepared cathode has a capacity retention rate of 88.2% after 1,800 cycles at 1 Ag−1, and its optimization effect is significantly outstanding compared with the previously reported transition metal sulfide cathode.
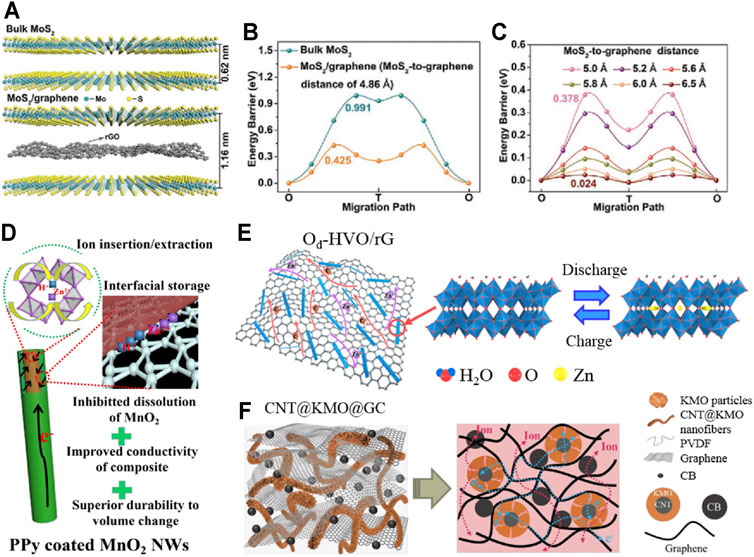
FIGURE 2. Structural modification and composite of cathode materials: (A) Crystal structures of bulk MoS2 and MoS2/graphene; (B,C) the corresponding migration energy barriers with the variation of the MoS2-to-graphene distance; (D) schematic illustration of freestanding CNT/MnO2-PPy; (E) schematic diagram of Zn2+ (de)intercalating mechanism in Od-HVO/rG; (F) illustration of electron/ion transport and ion diffusions across the electrodes of CNT@KMO@GC. Reproduced with permission (Zhang et al., 2020a; Li et al., 2021d; Huang et al., 2021; Wang et al., 2021).
In addition, the surface coating belongs to the modification of the electrode/electrolyte interface, which is an effective strategy to inhibit dissolution and phase transformation of cathode materials (Gao et al., 2020). It has been confirmed that coating materials with high stability and conductivity can effectively improve the specific capacity and cycle stability of the cathode (Bin et al., 2021; Xu et al., 2021b; Ren et al., 2021; Xing et al., 2021). Yang et al. prepared an independent flexible membrane (CNT/MnO2-PPy) composed of carbon nanotubes and polypyrrole (PPy)-coated MnO2 nanowires through typical in situ reaction self-assembly and vacuum filtration (Zhang et al., 2020a). MnO2 nanowires coated with PPy (about 5 nm in thickness) are uniformly dispersed in highly conductive and interconnected carbon nanotube networks, improving the reaction kinetics and structural stability of the cathode (in Figure 2D). After 1,000 cycles at 1 Ag−1, the optimized electrode still maintained 87.4% of the initial capacity. Nevertheless, the range of structural modification materials used at present is limited, and the related synthesis processes still do not meet the needs of economic efficiency. Then, there are still some challenges in practical application.
Combination Engineering
The adjunction of materials with a high stability structure for combination is also an exploration direction to improve the stability of cathode (Zhang et al., 2020b; Shan et al., 2021). The optimization strategy of combination engineering usually includes carbon-based materials, which can improve the electron transmission efficiency and structural stability of materials (Yang et al., 2021; Zeng et al., 2021). Hou et al. synthesized a 3D reticular graphene-based hydrated vanadium dioxide composite (Od-HVO/rG) with abundant oxygen vacancies using the solvothermal method (Huang et al., 2021). The research confirmed that oxygen vacancy defects can provide more active sites and promote the reversibility of the reaction, while the highly conductive and robust rG sponge can promote electron migration and reduce the accumulation of Od-HVO to improve the conductivity and structural stability, as shown in Figure 2E. Compared with HVO (capacity retention of 86.5%) and Od-HVO (capacity retention of 93.6%), the Od-HVO/rG cathode expressed scarcely any attenuation after 750 cycles at 5 Ag−1. Moreover, Li et al. obtained a cathode material (CNT@KMO@GC) composed of graphene (G), carbon black (CB), and K-sodium manganite (KxMnO2·yH2O, KMO) based on core–shell carbon nanotube (CNT) by hydrothermal and solution treatment (Wang et al., 2021). In Figure 2F, KMO provides the main charge storage due to the interlayer intercalation of K+ and H2O; CNT provides a conductive framework for the loaded KMO owing to high conductivity and structural stability; G and CB provide the conductive network to reduce the accumulation of active substances. The prepared cathode has a capacity retention rate of 65.2% after 10,000 cycles at 5 Ag−1, which is significantly higher than KMO (39.1% of the initial capacity) and CNT@KMO (51.5% of initial capacity). However, the influence of the composite ratio on stability optimization has not been deeply analyzed, and the composite research of non-carbon matrix materials needs to be further explored. Chen et al. revealed the performance attenuation mechanism of MnO2-based AZIBs by contrasting with different polymorphs and found that the low manganese dissolution of R-MnO2 inhibits the degradation of performance (Liao et al., 2022). Therefore, the reasonable composite design of MnO2 polymorphs with high initial capacity and R-MnO2 may have certain advantages in capacity and stability compared with single crystal form, which provides a direction for the next optimization.
Summary and Perspectives
In summary, the progress of cathode stability optimization for aqueous zinc ion batteries has been reviewed; the main of which can be divided into four aspects, including the introduction of vacancy, substitution/gap doping, object modification, and combination engineering. Thus, cathode stability optimization strategies can be designed from three aspects: inhibiting material dissolution, improving reaction reversibility, and enhancing structural stability.
However, there are several aspects to be further researched in the aforementioned optimization schemes of cathode materials. For quantitative analysis, most of the doping and composite research studies lack exploring the relationship between concentration/location and the optimization degree of stability. For universality analysis, material systems introduced into optimization research are still limited. For practical application, some synthetic processes, such as surface coating, still need to meet the demands of the economy, efficiency, and safety. In addition, the realization of the most stable cathode performance needs to eliminate the factors that reduce the reversibility according to the reaction mechanism of materials, such as inhibiting the irreversible dissolution of materials and the formation of inert by-products. Therefore, these fields to be explored can be the focuses of stability optimization in the future.
Author Contributions
YG was responsible for text editing and article composing. JL and JjZ were responsible for the review. HW and JnZ were responsible for article review and revision.
Funding
This work was financially supported by the National Natural Science Foundation of China (No. 52002122), the Science and Technology Department of Hubei Province (No. 2019AAA038), the application Fundamental Research Project of Wuhan Science and Technology Bureau (No. 2019010701011396), and the Project funded by China Postdoctoral Science Foundation (No. 2021M690947).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Bin, D., Wang, Y., Tamirat, A. G., Zhu, P., Yang, B., Wang, J., et al. (2021). Stable High-Voltage Aqueous Zinc Battery Based on Carbon-Coated NaVPO4F Cathode. ACS Sustain. Chem. Eng. 9, 3223–3231. doi:10.1021/acssuschemeng.0c08651
Cai, P., Momen, R., Li, M., Tian, Y., Yang, L., Zou, K., et al. (2021). Functional Carbon Materials Processed by NH3 Plasma for Advanced Full-Carbon Sodium-Ion Capacitors. Chem. Eng. J. 420, 129647. doi:10.1016/j.cej.2021.129647
Cai, P., Zou, K., Deng, X., Wang, B., Zheng, M., Li, L., et al. (2021). Comprehensive Understanding of Sodium‐Ion Capacitors: Definition, Mechanisms, Configurations, Materials, Key Technologies, and Future Developments. Adv. Energ. Mater. 11, 2003804. doi:10.1002/aenm.202003804
Cao, J., Zhang, D., Yue, Y., Wang, X., Pakornchote, T., Bovornratanaraks, T., et al. (2021). Oxygen Defect Enriched (NH4)2V10O25·8H2O Nanosheets for superior Aqueous Zinc‐ion Batteries. Nano Energy 84, 105876. doi:10.1016/j.nanoen.2021.105876
Chen, C., Shi, M., Zhao, Y., Yang, C., Zhao, L., and Yan, C. (2021). Al-intercalated MnO2 Cathode with Reversible Phase Transition for Aqueous Zn-Ion Batteries. Chem. Eng. J. 422, 130375. doi:10.1016/j.cej.2021.130375
Chen, Q., Jin, J., Kou, Z., Liao, C., Liu, Z., Zhou, L., et al. (2020). Zn 2+ Pre‐Intercalation Stabilizes the Tunnel Structure of MnO 2 Nanowires and Enables Zinc‐Ion Hybrid Supercapacitor of Battery‐Level Energy Density. Small 16, 2000091. doi:10.1002/smll.202000091
Cui, F., Wang, D., Hu, F., Yu, X., Guan, C., Song, G., et al. (2022). Deficiency and Surface Engineering Boosting Electronic and Ionic Kinetics in NH4V4O10 for High-Performance Aqueous Zinc-Ion Battery. Energ. Storage Mater. 44, 197–205. doi:10.1016/j.ensm.2021.10.001
Deng, X., Zou, K., Momen, R., Cai, P., Chen, J., Hou, H., et al. (2021). High Content Anion (S/Se/P) Doping Assisted by Defect Engineering with Fast Charge Transfer Kinetics for High-Performance Sodium Ion Capacitors. Sci. Bull. 66, 1858–1868. doi:10.1016/j.scib.2021.04.042
Du, K. D., Ang, E. H., Wu, X. L., and Liu, Y. (2021). Progresses in Sustainable Recycling Technology of Spent Lithium‐Ion Batteries. Energ. Environ. Mater. doi:10.1002/eem2.12271
Feng, X. Y., Wu, H. H., Gao, B., Świętosławski, M., He, X., and Zhang, Q. B. (2022). Lithiophilic N-Doped Carbon Bowls Induced Li Deposition in Layered Graphene Film for Advanced Lithium Metal Batteries. Nano Res. 15, 352–360.
Gan, Y., Wang, C., Chen, X., Liang, P., Wan, H., Liu, X., et al. (2020). High Conductivity Ni12P5 Nanowires as High-Rate Electrode Material for Battery-Supercapacitor Hybrid Devices. Chem. Eng. J. 392, 123661. doi:10.1016/j.cej.2019.123661
Gao, J., Xie, X., Liang, S., Lu, B., and Zhou, J. (2021). Inorganic Colloidal Electrolyte for Highly Robust Zinc-Ion Batteries. Nano-micro Lett. 13, 1–12. doi:10.1007/s40820-021-00595-6
Gao, Q.-L., Li, D.-S., Liu, X.-M., Wang, Y.-F., Liu, W.-L., Ren, M.-M., et al. (2020). Biomass-derived Mesoporous Carbons Materials Coated by α-Mn3O4 with Ultrafast Zinc-Ion Diffusion Ability as Cathode for Aqueous Zinc Ion Batteries. Electrochimica Acta 335, 135642. doi:10.1016/j.electacta.2020.135642
He, D., Peng, Y., Ding, Y., Xu, X., Huang, Y., Li, Z., et al. (2021). Suppressing the Skeleton Decomposition in Ti-Doped NH4V4O10 for Durable Aqueous Zinc Ion Battery. J. Power Sourc. 484, 229284. doi:10.1016/j.jpowsour.2020.229284
He, T., Weng, S., Ye, Y., Cheng, J., Wang, X., Wang, X., et al. (2021). Cation- Deficient Zn0.3(NH4)0.3V4O100.91H2O for Rechargeable Aqueous Zinc Battery with superior Low- Temperature Performance. Energ. Storage Mater. 38, 389–396. doi:10.1016/j.ensm.2021.03.025
Heo, J., Chong, S., Kim, S., Kim, R., Shin, K., Kim, J., et al. (2021). Suppressing Charge Disproportionation of MnO 2 Cathodes in Rechargeable Zinc Ion Batteries via Cooperative Jahn‐Teller Distortion. Batteries & Supercaps 4, 1881–1888. doi:10.1002/batt.202100181
Hou, J., Wang, L., Feng, X., Terada, J., Lu, L., Yamazaki, S., et al. (2021). Thermal Runaway of Lithium‐ion Batteries Employing Flame‐retardant Fluorinated Electrolytes. Energy Environ. Mater. doi:10.1002/eem2.12297
Huang, S., He, S., Qin, H., and Hou, X. (2021). Oxygen Defect Hydrated Vanadium Dioxide/graphene as a superior Cathode for Aqueous Zn Batteries. ACS Appl. Mater. Inter. 13, 44379–44388. doi:10.1021/acsami.1c12653
Islam, S., Alfaruqi, M. H., Putro, D. Y., Park, S., Kim, S., Lee, S., et al. (2021). In Situ Oriented Mn Deficient ZnMn 2 O 4 @C Nanoarchitecture for Durable Rechargeable Aqueous Zinc‐Ion Batteries. Adv. Sci. 8, 2002636. doi:10.1002/advs.202002636
Ji, J., Wan, H., Zhang, B., Wang, C., Gan, Y., Tan, Q., et al. (2021). Co 2+/3+/4+ ‐Regulated Electron State of Mn‐O for Superb Aqueous Zinc‐Manganese Oxide Batteries. Adv. Energ. Mater. 11, 2003203. doi:10.1002/aenm.202003203
Kim, S., Koo, B.-R., Jo, Y.-R., An, H.-R., Lee, Y.-G., Huang, C., et al. (2021). Defect Engineering via the F-Doping of β-MnO2 Cathode to Design Hierarchical Spheres of Interlaced Nanosheets for superior High-Rate Aqueous Zinc Ion Batteries. J. Mater. Chem. A. 9, 17211–17222. doi:10.1039/d1ta04051k
Li, B., Parekh, M. H., Pol, V. G., Adams, T. E., Fleetwood, J., Jones, C. M., et al. (2021). Operando Monitoring of Electrode Temperatures during Overcharge‐Caused Thermal Runaway. Energy Technol. 9, 2100497. doi:10.1002/ente.202100497
Li, M., Mou, J., Zhong, L., Liu, T., Xu, Y., Pan, W., et al. (2021). Porous Ultrathin W-Doped VO2 Nanosheets Enable Boosted Zn2+ (De)Intercalation Kinetics in VO2 for High-Performance Aqueous Zn-Ion Batteries. ACS Sustain. Chem. Eng. 9, 14193–14201. doi:10.1021/acssuschemeng.1c04675
Li, Q. L., Zhang, Q. C., Zhou, Z. Y., Gong, W. B., Liu, C. L., Feng, Y. B., et al. (2020). Boosting Zn-Ion Storage Capability of Self-Standing Zn-Doped Co3O4 Nanowire Array as Advanced Cathodes for High-Performance Wearable Aqueous Rechargeable Co//Zn Batteries. Nano Res. 14, 1–9. doi:10.1007/s12274-020-3046-8
Li, S., Liu, Y., Zhao, X., Shen, Q., Zhao, W., Tan, Q., et al. (2021). Sandwich‐Like Heterostructures of MoS 2 /Graphene with Enlarged Interlayer Spacing and Enhanced Hydrophilicity as High‐Performance Cathodes for Aqueous Zinc‐Ion Batteries. Adv. Mater. 33, 2007480. doi:10.1002/adma.202007480
Li, Z., Ganapathy, S., Xu, Y., Zhou, Z., Sarilar, M., and Wagemaker, M. (2019). Mechanistic Insight into the Electrochemical Performance of Zn/VO 2 Batteries with an Aqueous ZnSO 4 Electrolyte. Adv. Energ. Mater. 9, 1900237. doi:10.1002/aenm.201900237
Li, Z., Liu, T., Meng, R., Gao, L., Zou, Y., Peng, P., et al. (2021). Insights into the Structure Stability of Prussian Blue for Aqueous Zinc Ion Batteries. Energ. Environ. Mater. 4, 111–116. doi:10.1002/eem2.12108
Liao, M., Wang, J., Ye, L., Sun, H., Wen, Y., Wang, C., et al. (2020). A Deep‐Cycle Aqueous Zinc‐Ion Battery Containing an Oxygen‐Deficient Vanadium Oxide Cathode. Angew. Chem. 132, 2293–2298. doi:10.1002/ange.201912203
Liao, Y., Chen, H.-C., Yang, C., Liu, R., Peng, Z., Cao, H., et al. (2022). Unveiling Performance Evolution Mechanisms of MnO2 Polymorphs for Durable Aqueous Zinc-Ion Batteries. Energ. Storage Mater. 44, 508–516. doi:10.1016/j.ensm.2021.10.039
Liu, N., Wu, X., Yin, Y., Chen, A., Zhao, C., Guo, Z., et al. (2020). Constructing the Efficient Ion Diffusion Pathway by Introducing Oxygen Defects in Mn2O3 for High-Performance Aqueous Zinc-Ion Batteries. ACS Appl. Mater. Inter. 12, 28199–28205. doi:10.1021/acsami.0c05968
Liu, S., Zhu, H., Zhang, B., Li, G., Zhu, H., Ren, Y., et al. (2020). Tuning the Kinetics of Zinc‐Ion Insertion/Extraction in V 2 O 5 by In Situ Polyaniline Intercalation Enables Improved Aqueous Zinc‐Ion Storage Performance. Adv. Mater. 32, 2001113. doi:10.1002/adma.202001113
Liu, W., Zhang, X., Huang, Y., Jiang, B., Chang, Z., Xu, C., et al. (2021). β-MnO2 with Proton Conversion Mechanism in Rechargeable Zinc Ion Battery. J. Energ. Chem. 56, 365–373. doi:10.1016/j.jechem.2020.07.027
Liu, X., Ji, T., Guo, H., Wang, H., Li, J., Liu, H., et al. (2021). Effects of Crystallinity and Defects of Layered Carbon Materials on Potassium Storage: a Review and Prediction. Electrochem. Energ. Rev. doi:10.1007/s41918-021-00114-6
Luo, H., Wang, B., Wang, C., Wu, F., Jin, F., Cong, B., et al. (2020). Synergistic Deficiency and Heterojunction Engineering Boosted VO2 Redox Kinetics for Aqueous Zinc-Ion Batteries with superior Comprehensive Performance. Energ. Storage Mater. 33, 390–398. doi:10.1016/j.ensm.2020.08.011
Ma, Y., Ma, Y., Diemant, T., Cao, K., Liu, X., Kaiser, U., et al. (2021). Unveiling the Intricate Intercalation Mechanism in Manganese Sesquioxide as Positive Electrode in Aqueous Zn‐Metal Battery. Adv. Energ. Mater. 11, 2100962. doi:10.1002/aenm.202100962
Ni, G., Xu, X., Hao, Z., Wang, W., Li, C., Yang, Y., et al. (2021). Tuning the Electrochemical Stability of Zinc Hexacyanoferrate through Manganese Substitution for Aqueous Zinc-Ion Batteries. ACS Appl. Energ. Mater. 4, 602–610. doi:10.1021/acsaem.0c02496
Park, K. Y., Zhu, Y., Torres‐Castanedo, C. G., Jung, H. J., Luu, N. S., Kahvecioglu, O., et al. (2021). Elucidating and Mitigating High‐Voltage Degradation Cascades in Cobalt‐Free LiNiO 2 Lithium‐Ion Battery Cathodes. Adv. Mater. 2021, 2106402. doi:10.1002/adma.202106402
Ren, L., Yu, G., Xu, H., Wang, W., Jiang, Y., Ji, M., et al. (2021). Doping-Induced Static Activation of MnO2 Cathodes for Aqueous Zn-Ion Batteries. ACS Sustain. Chem. Eng. 9, 12223–12232. doi:10.1021/acssuschemeng.1c03767
Shan, L., Wang, Y., Liang, S., Tang, B., Yang, Y., Wang, Z., et al. (2021). Interfacial Adsorption-Insertion Mechanism Induced by Phase Boundary toward Better Aqueous Zn‐ion Battery. InfoMat 3, 1028–1036. doi:10.1002/inf2.12223
Tan, Q., Chen, X., Wan, H., Zhang, B., Liu, X., Li, L., et al. (2020). Metal-organic Framework-Derived High Conductivity Fe3C with Porous Carbon on Graphene as Advanced Anode Materials for Aqueous Battery-Supercapacitor Hybrid Devices. J. Power Sourc. 448, 227403. doi:10.1016/j.jpowsour.2019.227403
Tan, Q., Li, X., Zhang, B., Chen, X., Tian, Y., Wan, H., et al. (2020). Valence Engineering via In Situ Carbon Reduction on Octahedron Sites Mn 3 O 4 for Ultra‐Long Cycle Life Aqueous Zn‐Ion Battery. Adv. Energ. Mater. 10, 2001050. doi:10.1002/aenm.202001050
Tong, H., Li, T., Liu, J., Gong, D., Xiao, J., Shen, L., et al. (2021). Fabrication of the Oxygen Vacancy Amorphous MnO 2 /Carbon Nanotube as Cathode for Advanced Aqueous Zinc‐Ion Batteries. Energ. Technol. 9, 2000769. doi:10.1002/ente.202000769
Wan, F., and Niu, Z. (2019). Design Strategies for Vanadium‐based Aqueous Zinc‐Ion Batteries. Angew. Chem. 131, 16508–16517. doi:10.1002/ange.201903941
Wang, C., Song, Z., Wan, H., Chen, X., Tan, Q., Gan, Y., et al. (2020). Ni-Co Selenide Nanowires Supported on Conductive Wearable Textile as Cathode for Flexible Battery-Supercapacitor Hybrid Devices. Chem. Eng. J. 400, 125955. doi:10.1016/j.cej.2020.125955
Wang, G., Wang, Y., Guan, B., Liu, J., Zhang, Y., Shi, X., et al. (2021). Hierarchical K‐Birnessite‐MnO 2 Carbon Framework for High‐Energy‐Density and Durable Aqueous Zinc‐Ion Battery. Small 17, 2104557. doi:10.1002/smll.202104557
Wang, J., Wang, J.-G., Qin, X., Wang, Y., You, Z., Liu, H., et al. (2020). Superfine MnO2 Nanowires with Rich Defects toward Boosted Zinc Ion Storage Performance. ACS Appl. Mater. Inter. 12, 34949–34958. doi:10.1021/acsami.0c08812
Xia, R., Zhao, K., Kuo, L. Y., Zhang, L., Cunha, D. M., Wang, Y., et al. (2021). Nickel Niobate Anodes for High Rate Lithium‐Ion Batteries. Adv. Energ. Mater. 2021, 2102972. doi:10.1002/aenm.202102972
Xing, F., Shen, X., Chen, Y., Liu, X., Chen, T., and Xu, Q. (2021). A Carbon-Coated Spinel Zinc Cobaltate Doped with Manganese and Nickel as a Cathode Material for Aqueous Zinc-Ion Batteries. Dalton Trans. 50, 5795–5806. doi:10.1039/d1dt00686j
Xu, J.-W., Gao, Q.-L., Xia, Y.-M., Lin, X.-S., Liu, W.-L., Ren, M.-M., et al. (2021). High-performance Reversible Aqueous Zinc-Ion Battery Based on Iron-Doped Alpha-Manganese Dioxide Coated by Polypyrrole. J. Colloid Interf. Sci. 598, 419–429. doi:10.1016/j.jcis.2021.04.057
Xu, J., Hu, X., Alam, M. A., Muhammad, G., Lv, Y., Wang, M., et al. (2021). Al-doped α-MnO2 Coated by Lignin for High-Performance Rechargeable Aqueous Zinc-Ion Batteries. RSC Adv. 11, 35280–35286. doi:10.1039/d1ra06808c
Yang, F., Shen, Y., Cen, Z., Wan, J., Li, S., He, G., et al. (2021). In Situ construction of Heterostructured Bimetallic Sulfide/phosphide with Rich Interfaces for High-Performance Aqueous Zn-Ion Batteries. Sci. China Mater. doi:10.1007/s40843-021-1739-0
Yao, J., Wan, H., Chen, C., Ji, J., Wang, N., Zheng, Z., et al. (2021). Oxygen-defect Enhanced Anion Adsorption Energy toward Super-rate and Durable Cathode for Ni-Zn Batteries. Nano-Micro Lett. 13, 1–14. doi:10.1007/s40820-021-00699-z
Zeng, Y. X., Wang, Y., Jin, Q., Pei, Z. H., Luan, D. Y., Zhang, X. T., et al. (2021). Rationally Designed Mn2O3-ZnMn2O4 Hollow Heterostructures from Metal-Organic Frameworks for Stable Zn-Ion Storage. Angew. Chem. Int. Edition 60, 1–7. doi:10.1002/anie.202113487
Zhang, L., Hu, J., Zhang, B., Liu, J., Wan, H., Miao, L., et al. (2021). Suppressing Cathode Dissolution via Guest Engineering for Durable Aqueous Zinc-Ion Batteries. J. Mater. Chem. A. 9, 7631–7639. doi:10.1039/d1ta00263e
Zhang, L., Miao, L., Zhang, B., Wang, J., Liu, J., Tan, Q., et al. (2020). A Durable VO2(M)/Zn Battery with Ultrahigh Rate Capability Enabled by Pseudocapacitive Proton Insertion†. J. Mater. Chem. A. 8, 1731–1740. doi:10.1039/c9ta11031c
Zhang, Y., Xu, G., Liu, X., Wei, X., Cao, J., and Yang, L. (2020). Scalable In Situ Reactive Assembly of Polypyrrole‐Coated MnO 2 Nanowire and Carbon Nanotube Composite as Freestanding Cathodes for High Performance Aqueous Zn‐Ion Batteries. ChemElectroChem 7, 2762–2770. doi:10.1002/celc.202000253
Keywords: aqueous zinc ion battery, cathode materials, cyclic stability, stability attenuation, optimization
Citation: Gan Y, Wang C, Li J, Zheng J, Wu Z, Lv L, Liang P, Wan H, Zhang J and Wang H (2022) Stability Optimization Strategies of Cathode Materials for Aqueous Zinc Ion Batteries: A Mini Review. Front. Chem. 9:828119. doi: 10.3389/fchem.2021.828119
Received: 03 December 2021; Accepted: 27 December 2021;
Published: 20 January 2022.
Edited by:
Hailong Wang, Ningxia University, ChinaCopyright © 2022 Gan, Wang, Li, Zheng, Wu, Lv, Liang, Wan, Zhang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Houzhao Wan, aG91emhhb3dAaHVidS5lZHUuY24=; Jun Zhang, Z3dlbl96aGFuZ0AxMjYuY29t
 Yi Gan
Yi Gan Cong Wang
Cong Wang Jingying Li
Jingying Li Junjie Zheng
Junjie Zheng Ziang Wu
Ziang Wu Lin Lv
Lin Lv Pei Liang
Pei Liang Houzhao Wan
Houzhao Wan Jun Zhang
Jun Zhang Hao Wang
Hao Wang