- 1Department of Chemical Science and Technologies, University of Rome Tor Vergata, Roma, Italy
- 2Department of Physics, Politecnico di Milano, Milan, Italy
- 3Department of Electronic Engineering, University of Rome Tor Vergata, Roma, Italy
- 4Department of Chemistry, Università La Sapienza, Roma, Italy
An easy and fast method to achieve chiral porphyrin films on glass is herein reported. The on-surface formation of organized supramolecular architectures with distinctive and remarkable chiroptical features strictly depends on the macrocycles used, the solvent chosen for the casting deposition, and most importantly, on the roughness of the glass slide. Dynamic light scattering studies performed on 10−4–10−6 M porphyrin solutions revealed the presence of small porphyrin aggregates, whose size and number increase depending on the initial concentration. Once transferred on surface, these protoaggregates act as nucleation seeds for the following, self-assembling into larger structures upon solvent evaporation, with a process driven by a fine balance between intermolecular and molecule–substrate interactions. The described method represents a straightforward way to fabricate porphyrin-based chiral surfaces onto a transparent and economic substrate in few minutes. The results obtained can be particularly promising for the development of sensors based on stereoselective optical active films, targeting the detection of chiral analytes of practical relevance, such as the so-called emerging pollutants released in the environment from agrochemical, food, and pharmaceutical manufacturing.
Introduction
The understanding of chirality at solid surfaces is of ever-growing importance, and an increasing number of studies have been reported over the years to shed light on the fundamentals and practices in achieving chiral surfaces with definite enantioselectivity (Gellman, 2010; Zaera, 2017; Albano et al., 2020; Xu et al., 2021). As a matter of fact, chirality can be observed or provided to achiral surfaces taking advantage of several options spanning from the addition of chiral modifiers (Zaera, 2008), the adsorbing of chiral molecules at ultra-high vacuum (Costa et al., 2015), or from liquid phase (Huang et al., 2003), or even by self-assembly of achiral molecules that form chiral domains thanks to the surface dimensional constraints (Tao and Bernasek, 2005). Specifically for the transfer of chiral molecules from solution to surface, the final chiroptical features are strictly dependent on a combination of different forces controlling the supramolecular organization in solution as well as the interaction/adsorption with the specific substrate. Over the course of our research, we found that various amphiphilic porphyrin derivatives bearing a stereogenic information on the peripheral molecular positions form chiral mesoscopic structures of tunable size in different hydro–organic media, depending on the interplaying of structural motifs and solvent bulk properties (i.e., type and relative ratio of the hydro–organic mixture used, ionic strength, and monomer concentration) (Monti et al., 2010; Caroleo et al., 2019; Stefanelli et al., 2020a). Within this set of tetrapyrroles, cationic derivatives functionalized with a peripheral (L)-proline moiety have been also exploited for the preparation of chiral films to be applied for sensor development. Two strategies have been basically reported: 1) the steering of the self-aggregation of chiral porphyrin monomers in ethanol/water mixture of proper composition to give in 1–2 days chiral assemblies that spontaneously layer on glass substrate dipped into the solution (Monti et al., 2011); 2) the use of Langmuir–Blodgett technique to assemble the porphyrin molecules onto a glass surface in chiral multilayers through air/water interfacial organization (Colozza et al., 2019). Films based on the anionic counterpart (L)ZnP(-) (Scheme 1) anchored to ZnO nanoparticles have been also chirally organized on quartz microbalance surfaces and used for the stereoselective recognition of limonene enantiomer vapors (M. Stefanelli et al., 2019). The need of realizing cost-effective and easy to handle sensor platforms prompted us to access the chiral films on common solid supports by simple operation of all the stages, starting with material preparation to film deposition. Additionally, the achievement of a convenient signal for the detection is highly desirable. With this aim in mind, we have carried out the studies herein reported, concerning the development of a straightforward method to fabricate optically active films on glass by drop-casting of (L) or (D)-prolinated porphyrin building blocks dissolved in suitable organic solvents (Scheme 1). The choice of glass as substrate for film deposition is mainly due to its optical transparency that makes the layer chirality assessment possible by circular dichroism (CD) spectroscopy on one side, along with the detection of the eventual interaction with a target analyte by means of an easily recordable optical signal change (i.e., absorption, fluorescence, and CD spectral variations upon analyte detection). We can anticipate that the chiroptical properties of the produced films are deeply influenced by the porphyrin molecular structure (i.e., the configuration of the appended proline and the presence or not of a coordinated metal ion into the core), the solvent used for the deposition, and last but not the least, the roughness of the glass substrate. These findings highlight the complex interplaying of supramolecular interactions occurring when chirality is transferred from the solution to the solid state. The easiness and the versatility of the described protocol make it a viable approach in fabricating chiral films based on porphyrins for stereoselective sensor applications.
Results and Discussion
Evaluation of the Solvent Effect on the Chirality of the Film
The challenging stage for the drop-casting production of chiral solid films is surely the identification of the proper solvent to use for the stock solution. As reported in the literature, the nature of the solvent used is extremely relevant to define the structures that the layers adopt and the overall chirality (Iavicoli et al., 2009; Chen et al., 2019), since both derive from a combination of the interaction strength of the molecules with the surface and their solubility (Zaera 2009; Mali and De Feyter, 2013). Moreover, solvent parameters like viscosity, surface tension, and vapor pressure strictly impact the film adhesion to the substrate and the evaporation rate, producing layers featuring different structural motifs and homogeneity (Resedean et al., 2020).
We carried out our investigations on the Zn porphyrin enantiomers [(D)- and (L)ZnP(-), Scheme 1], whose aggregation behavior in hydroalcoholic solution was recently studied in depth (Stefanelli et al., 2020a; Savioli et al., 2020). We have selected three solvents of different polarity, coordination abilities, and adhesion properties in which the porphyrin derivatives are soluble, namely, ethanol (EtOH), tetrahydrofuran (THF), and toluene. Films were deposited by drop-casting 10 μl from 10−4 M stock solutions of each porphyrin derivative on glass slides, followed by solvent evaporation (see the Experimental section for the detailed procedures). The layered materials were optically characterized by UV-vis and CD spectroscopies. For all the films tested, UV-vis spectra showed broad Soret bands, with peaks lying in the 435–440 nm range, red shifted if compared with the corresponding porphyrin solution (420–424 nm range), indicating that macrocycles are aggregated in J-type structures at solid state (see Supplementary Figure S1).
The assessment of the chiroptical features of the solid films have been carried out by CD spectroscopy and evidenced a remarkable influence of the solvent on the chirality at solid state.
As far as the films of (D)- or (L)ZnP(-) from THF and EtOH are concerned, negligible dichroic bands, barely observable in the 350- to 500-nm spectral range (data not shown) have been recorded, indicating an ineffective reading out of the chiral information during the self-assembly process occurring onto the glass surface in these solvents. Completely different results were obtained for the drop-casting of toluene solutions, which conversely produced layers with extensive supramolecular chirality. As shown in Figure 1A, the CD spectrum for films of (D)ZnP(-) is characterized by two sets of excitonically coupled bands at about 441, 433, and 407 nm, indicating electronic coupling between layered macrocycles that arranged in a (+) clockwise (CW) mutual conformation, as excitonic theory states (Berova et al., 2000) (Figure 1A, red trace). For the other enantiomer, a specular CD profile is obtained, featuring three bisignate negative bands with the same crossover points found for the (D) counterpart, pointing out a (−) anticlockwise (ACW) mutual orientation of porphyrin macrocycles (Figure 1A, blue trace). CD readouts (theta, expressed in mdeg), have been converted in ∆A/OD, where OD is the optical density of the sample and ∆A the difference measured in the sample absorbance between the left and right polarization of light. For this normalization purpose, both CD and optical density of films have been evaluated by performing absorbance measurements with Jasco J-1500 without moving the glass slide in order to measure these two quantities on the exact same spot. Remarkably, we found out that the normalized CD values are unexpectedly reproducible over different areas of the spot, even more so by considering that simple drop-casting method for the coating is used. This robustness results in an almost flawless specularity of CD signals obtained for films produced by toluene solutions of the two enantiomers of ZnP(-) (see Figure 1A). These spectral features were obtained independently on the batch used for deposition with the same sign and intensities, to prove a good reproducibility of the deposition method. All these findings combined definitely pointed out that the final chirality of the films is driven by the specific peripheral stereochemical information of the single unit and transferred from molecular to aggregate level upon self-assembly process.
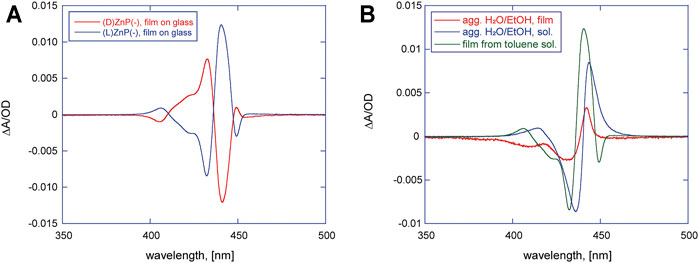
FIGURE 1. (A) Circular dichroism (CD) spectra of (D)- (red trace) and (L)ZnP(-) (blue trace) casted films on glass from 10−4 M toluene solutions. (B) (L)ZnP(-) chiral aggregates obtained in different conditions: aggregates grown from EtOH/H2O (25:75, v/v) at 5 μM concentration (blue trace) and the corresponding film on glass (red trace); chiral films on glass from 10−4 M toluene solution (green trace).
The dependence of the chiroptical features in terms of pattern and intensity on the macrocycle concentration was also investigated. Supplementary Figure S2 (Supplementary Material) shows that the CD signal magnitude of (D)ZnP(-) films on glass slides increases at higher bulk solution concentrations without any perceptible changes in the chiral features. The calculated ratios between the maximum of the CD signals and recorded OD (see the Experimental section for details) shows that this parameter is substantially constant in the investigated range of concentrations (0.013, in the 0.5–1 × 10−4 M). Similar results have been obtained for the deposition of the (L) porphyrin enantiomer in the same conditions.
Comparing the spectral features of the (L)ZnP(-) film from toluene (Figure 1B, green trace) with the ones obtained for aggregates grown in EtOH/H2O (25:75 v/v) solutions (Figure 1B, blue trace), and one deposited at solid state (Figure 1B, red trace), a certain resemblance can be observed, even if the ones obtained for films casted by toluene show a higher degree of complexity, detectable with the emerging dichroic band at 449 nm.
The close similarity between these systems allows us to invoke a like aggregation mode between porphyrin platforms during the film formation, involving the coordination of the proline carboxylate to the Zn2+ ion (Stefanelli et al., 2020b). It must be said that the aggregation conditions are rather different in the two cases. Indeed, in EtOH/H2O, the aggregation is boosted by hydrophobic effect, which can be surely excluded here in driving film formation. On the other hand, the chirality transfer during the self-assembly process on glass and the resulting nanostructured supramolecular chirality should be the result of several concomitant factors as, for example, the evaporation kinetic (Hattori et al., 2017). Moreover, it is abundantly reported that porphyrin aggregation often is based on the “sergeants-and-soldiers” principle, where a small set of specific aggregates acts as chiral seeds to catalyze the following aggregation process (Mammana et al., 2007). Based on these considerations, we decided to perform DLS studies to assess the eventual presence of protoaggregated species in the (L)ZnP(-) 10−4 M toluene bulk solutions (Table 1, entry 1). As shown in Figure 2, the sample presents aggregates with low polydispersity size distribution, centered at dh∼290 nm. Additionally, this distribution resulted to be influenced by porphyrin concentration. According to the comparative DLS analysis shown in Table 1, lowering the concentration down to 10−5 M is reflected in a lowering of the average size together with a significant increase in the size polydispersity (Table 1, entry 2). At 10−6 M, very small aggregates (∼50 nm size) were slightly detectable after high acquisition time (Table 1, entry 3). Experiment on long-term evolution of the size distribution would be desirable to understand whether such differences can be related to a change in the aggregation kinetics with porphyrin concentration. It is important to mention that toluene solutions of (D)ZnP(-) enantiomer were also checked under the same experimental conditions, showing no significant variation in the behavior of the size distribution, above reported for the (L)- counterpart.
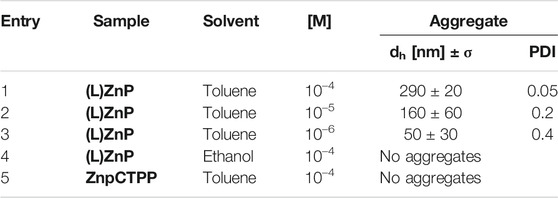
TABLE 1. Dynamic light scattering (DLS) analysis of (L)ZnP(-) and the achiral ZnpCTPP in different solvents and concentrations.
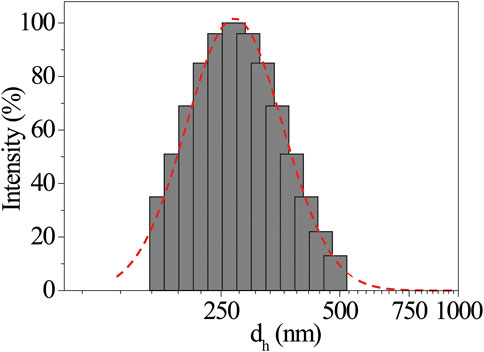
FIGURE 2. Representative dynamic light scattering (DLS) intensity size distribution of (L)ZnP(-) 10−4 M toluene solution, where the presence of self assemblies is detected. Gaussian fit in dotted line.
On the other hand, the involvement of Zn coordination on the chirality transfer from solutions to solid films is also suggested by the fact that films obtained from EtOH and THF are CD silent: In both cases, the remarkable coordination abilities toward Zn metal ions with respect to toluene make these solvents strong competitors for the aminoacidic residue. As a consequence, individual porphyrin macrocycles are well solvated, and the development of oligomeric structures is scarcely probable or driven to the formation of nucleation seeds below a critical threshold (Table 1, entry 4).
Additionally, ZnpCTPP is here utilized as a nonchiral probe since it also possesses a coordinated Zn(II) ion and a carboxylate group that should be able to drive the formation of ordered supramolecular assemblies. However, DLS studies disclose the absence of detectable aggregates in toluene at high concentration (Table 1, entry 5), proving the importance of the proline-appended group on the early aggregation stage. Furthermore, in case of films from nonchiral porphyrins, chiroptical signals should still emerge when symmetry-breaking effect of the surface, or stochastic formation of nucleation seeds with right- or left-handed orientation occur during the deposition (Chen et al., 2006). In this case, these events can be clearly ruled out by the fact that the CD signal of film from ZnpCTPP toluene solution on glass are silent.
To further investigate the network of interactions between solvent and porphyrins involved in both the aggregation process and in the effective chirality transfer to the solid state, we studied the drop-casted films of the corresponding free bases [(L)- and (D)H2P(-), Scheme 1] from THF and EtOH (toluene was excluded since these macrocycles are scarcely soluble in this solvent). Once again, films from THF did not feature any dichroic bands (data not shown). On the contrary, CD spectra of the spotted 10−4 M solutions of both enantiomers in EtOH displayed mirrored bisignated bands, although poorly reproducible in terms of intensity, sign, and spectral pattern depending on the batch solutions (data not shown). We have ascribed this evidence to the formation of small nucleation seeds of random overall symmetry, due to the lack of specific driving force such as the metal–ion coordination. The same behavior has been in fact observed upon aggregation of these species in hydroalcoholic solution (Stefanelli et al., 2020b) that results in the formation of supramolecular structures with very low g-factor.
DLS measurements support this hypothesis, since at the same concentration, (L)H2P(-) ethanol solutions showed a low tendency to form aggregates (which were rarely detectable at sizes in the range of 50–150 nm), suggesting a good solvation capacity of ethanol.
Evaluation of the Influence of the Glass Substrate on Films
It is well known that several surface properties like wettability/nature and roughness are crucial for the film formation, affecting important mechanical as well as optical features, particularly at micro/nano scale. In our studies, we have investigated the influence of the glass surface on the chiroptical properties of the formed films by drop-casting toluene (L)/(D)ZnP(-) porphyrin solutions on glass slides of different source (i.e., commercial and of low roughness). A first difference among the films formed onto these two substrates was pointed out by the corresponding UV-vis spectra (Supplementary Figure S3), which showed a Soret band more red shifted and narrower for the layers casted on the ultraflat slide, indicative of a higher specificity in forming J-aggregated structures on such a substrate. Concurrently, CD measurements showed chiroptical features exclusively in the case of this film, pointing out a remarkable influence of the substrate roughness on the final porphyrin arrangement onto the surface in a chiral fashion.
This interesting result led us to investigate more in depth the film morphology, and the results will be reported in a separate paper focused on this issue. Herein, we anticipate that the morphological investigation has highlighted a significant difference between the commercial glass and the low-roughness one. AFM images reported in the Figure 3 clearly show that the commercial glass (Figure 3A) is characterized by many hills and valleys with an overall height difference of about 75 nm. Conversely, the low-roughness glasses (Figure 3B) possess wide terraces, where the surface roughness is below 6 nm. Only in confined areas, we have observed some scratches that increase the overall roughness up to 10 nm. These evident differences between the two glass types can give a rationale of the observed porphyrin film chirality discussed above. Indeed, we can surmise that the porphyrin deposition onto a flat surface can facilitate the chiral organization of the macrocycles during the solvent evaporation. Conversely, the irregular commercial glass surface does not offer an optimal platform for a uniform film growth, which results in lack of stereospecificity.
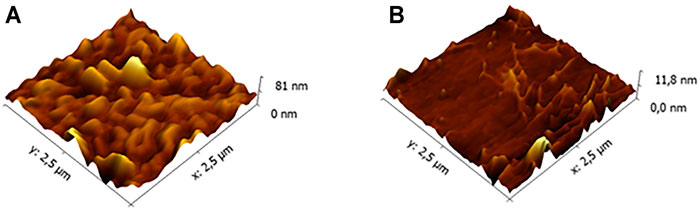
FIGURE 3. Atomic force microscopy (AFM) morphologies of (A) a commercial and (B) low-roughness glasses.
Herein, we can conclude that the film chiral arrangement onto surface is due to a fine interplay of effects, i.e., the nature of the solvent, the concentration of the porphyrin monomer, and the nature of the deposition surface.
At the end of these investigations, we also evaluated some important film properties for applicative purposes, namely, the stability over time and temperature assessed by CD spectroscopy (Supplementary Figures S4A, B, respectively). We observed that the (L)/(D) porphyrin films casted by from toluene solution undergo a small rearrangement during 24–36 h and then remain stable until 72 h after the casting. At the end of this time, ca. 25% of the CD signal is lost and is kept constant for further 7 days. The organic layers are found more sensitive to heating, preserving their optical properties when exposed to temperature up to 50°C, and deteriorating for higher temperatures. In addition, AFM investigation gave a direct visualization of the film morphological evolution as a function of time. Figure 4 summarizes and compares the (D)ZnP(-) porphyrin film after about 100 h from the sample preparation. At the beginning, the morphology shows domains characterized by dendritic structures (Figure 4A), in good agreement with analogous systems reported in literature (Iavicoli et al., 2009). The film is not stable in ambient conditions, but undergoes a new assembling where the overall film roughness is slightly reduced (Figure 4B). It is reasonable that some solvent residuals, embedded inside the film after the deposition, are removed (e.g., evaporation) with a longer time interval, and a remarkable change in the porphyrin aggregates is produced.
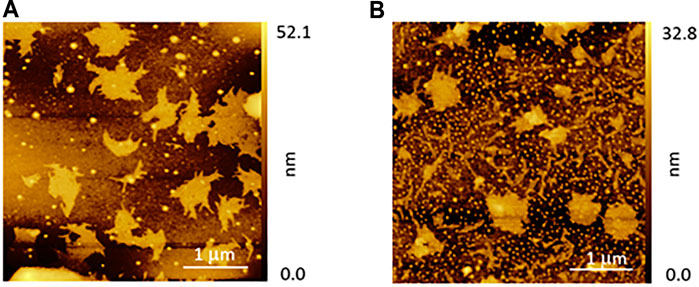
FIGURE 4. AFM morphologies of the (D)ZnP(-) film as a function of time: (A) as deposited and (B) after about 100 h with the sample taken in ambient conditions.
Conclusion and future perspectives
These studies report a convenient way to achieve chiral films by the efficient transfer of chirality from porphyrin molecular systems to film on glass by selecting a proper solvent for drop-casting process. The results obtained point out the crucial role played by nucleation seeds (small chiral porphyrin oligoaggregates), which are formed in solution. We found, in fact, that the use of oxygen donor solvents (EtOH and THF) well solvate the macrocycles either as free bases and zinc complexes, so hampering the effective supramolecular organization of the forming film in a chiral fashion. In analogy with our previous studies, the formation of chiral assemblies based on (L)/(D) prolinated porphyrins is guided by the coordination of the aminoacidic moiety to the zinc ion. For this reason, the use of toluene as solvent proves to be a good choice. Indeed, besides the noncoordinating character, toluene possesses also the highest surface tension (28.4, 26.4, and 22.1 mN/m for toluene, THF, and EtOH, respectively) and the lowest vapor pressure (29, 200, and 59 hPa at 20°C for toluene, THF, and EtOH, respectively), which favor the film adhesion to the substrate and prevent either the fast evaporation of solvent and excessive drying pattern formation, both essential conditions for a stereospecific growth of porphyrin aggregates during the self-assembly process. CD spectra evidence for films of both (L)- and (D)ZnP(-) have different patterns with respect to those observed in the case of the aggregation in solution. These results indicate the occurrence of specific interactions between the growing structures and the glass, whose roughness necessarily has to be around 1 nm for the effective chiral arrangement of macrocycles onto the surface. These findings would be of importance for the easy development of cost-effective molecular films to be potentially used in fields where chiral materials are highly desired, as asymmetric catalysis (Simonneaux et al., 2006; Mallat et al., 2007) and chiral sensors (Sannicolò et al., 2014; Shang et al., 2017; Stefanelli et al., 2019). In this regard, we can anticipate that the as-deposited films are fluorescent and highly stable when immersed in water solutions even for a prolonged period. Therefore, these features warrant for their use in optical chiral sensing measurements, which are currently ongoing in our laboratories and whose results will be reported in due course.
Experimental Section
General
Porphyrin derivatives investigated in these studies have been prepared as previously reported by our group (Stefanelli et al., 2020b) by using reagents and solvents of commercial sources (Sigma Aldrich, ChemImpex, TCI) in the highest degree of purity without further purifications. The casted films were analyzed by UV-vis spectroscopy using, according to the experimental needs, either a Varian Cary 100 or a JASCO J-1500, equipped with a thermostated cell holder set at 298 K, and purged with ultra-pure nitrogen gas. CD spectroscopic measurements were carried out by Jasco J-1500 equipped as just mentioned. The evaluation of the film optical density (OD) was performed consecutively by only changing the operative mode of J-1500 spectrophotometer without moving the sample, in order to irradiate the same spot of the porphyrin film in both absorbance and CD measurements. Different traces at different stock concentrations were taken, referenced against untreated blank glass slide. Linear dichroism contribution (LD) has been found to be <0.0004 DOD units in all the cases examined. Also, to rule out the strong contributions of birefringence effects, both front and back of the slide were measured without evidencing significative changes in the CD profiles.
Porphyrin Film Preparation
The porphyrin solutions to be casted on glass slides were prepared as follows: 10−4 M solutions of macrocycles in 4 ml of the chosen solvent (ethanol, tetrahydrofuran, or toluene) were kept under sonication for 2 min (Fisher Scientific FB15047 apparatus). The solution was then filtered first through a 0.45-μm and then a 0.22-μm FilterBio
Microscope glass slides (SuperFrost
Dynamic Light Scattering Studies
Size distribution by dynamic light scattering (DLS) was performed with Hamamatsu HC120 photometer (Brookhaven, NY, United States), equipped with a BI-200SM goniometer, a BI-9000 AT photocorrelator allowing to detect a particle size dynamic range from 5 to 5,000 nm, a solid-state laser (Suwtech Inc., SHA, CN) with a wavelength of 532 nm powered by a power supply LDC-2500 (Suwtech Inc.), and a phototube (Hamamatsu Photonics K.K., JP). The solvents used for the sample dissolution were prefiltered with a 0.22-μm hydrophobic filter (FilterBio
Atomic Force Microscopy Measurements
A commercial Keysight 5500 atomic force microscopy (AFM) equipment was employed for the sample topographic analysis. Images were collected in noncontact mode to prevent possible damages of the porphyrin aggregates during contact measures. Silicon NanoSensors tip were used with a resonance frequency in air of about 300 kHz. The apex radius is about 20 nm. After the acquisition, images were analyzed by the free Gwyddion software.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author Contributions
DM, GM, and MS contributed to the conception and design of the study. MN synthesized the title porphyrins. TT prepared the porphyrin samples and performed the drop-casting on glass. MV and GM performed the circular dichroism measurements. GB performed the AFM studies. FD and GP performed the DLS measurements. MS, GM, RP, CD, and DM wrote the manuscript. MS and RP were responsible for the financial support to the work. All authors contributed to the discussion and interpretation of the results and to the manuscript revision, and read and approved the submitted version.
Funding
This research was funded by the H2020-FETOPEN, 828779, INITIO project and by the University of Rome Tor Vergata, Italy, ASPIRE project-E84I20000220005.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fchem.2021.804893/full#supplementary-material
References
Albano, G., Pescitelli, G., and Di Bari, L. (2020). Chiroptical Properties in Thin Films of π-Conjugated Systems. Chem. Rev. 120, 10145–10243. doi:10.1021/acs.chemrev.0c00195
Berova, N., Nakanishi, K., and Woody, R. W. (2000). Circular Dichroism: Principles and Applications. 2nd Edn. Weinheim: Wiley-WCH.
Caroleo, F., Stefanelli, M., Magna, G., Venanzi, M., Paolesse, R., Sennato, S., et al. (2019). Kinetic and Spectroscopic Studies on the Chiral Self-Aggregation of Amphiphilic Zinc and Copper (L)-prolinate-tetraarylporphyrin Derivatives in Different Aqueous media. Org. Biomol. Chem. 17, 1113–1120. doi:10.1039/C8OB02689K
Chen, P., Ma, X., Duan, P., and Liu, M. (2006). Chirality Amplification of Porphyrin Assemblies Exclusively Constructed from Achiral Porphyrin Derivatives. ChemPhysChem 7, 2419–2423. doi:10.1002/cphc.200600402
Chen, K., Jiao, T., Li, J., Han, D., Wang, R., Tian, G., et al. (2019). Chiral Nanostructured Composite Films via Solvent-Tuned Self-Assembly and Their Enantioselective Performances. Langmuir 35, 3337–3345. doi:10.1021/acs.langmuir.9b00014
Colozza, N., Stefanelli, M., Venanzi, M., Paolesse, R., and Monti, D. (2019). Fabrication of Langmuir-Blodgett Chiral Films from Cationic (L)-Proline-Porphyrin Derivatives. J. Porphyrins Phthalocyanines 23, 462–468. doi:10.1142/S1088424619500305
Costa, D., Pradier, C.-M., Tielens, F., and Savio, L. (2015). Adsorption and Self-Assembly of Bio-Organic Molecules at Model Surfaces: A Route towards Increased Complexity. Surf. Sci. Rep. 70, 449–553. doi:10.1016/j.surfrep.2015.10.002
Gellman, A. J. (2010). Chiral Surfaces: Accomplishments and Challenges. ACS Nano 4, 5–10. doi:10.1021/nn901885n
Hattori, S., Vandendriessche, S., Koeckelberghs, G., Verbiest, T., and Ishii, K. (2017). Evaporation Rate-Based Selection of Supramolecular Chirality. Chem. Commun. 53, 3066–3069. doi:10.1039/C6CC09842H
Huang, J., Egan, V. M., Guo, H., Yoon, J.-Y., Briseno, A. L., Rauda, I. E., et al. (2003). Enantioselective Discrimination of D- and L-Phenylalanine by Chiral Polyaniline Thin Films. Adv. Mater. 15, 1158–1161. doi:10.1002/adma.200304835
Iavicoli, P., Simón-Sorbed, M., and Amabilino, D. B. (2009). Surface Aggregate Morphology of Chiral Porphyrins as a Function of Constitution and Amphiphilic Nature. New J. Chem. 33, 358–365. doi:10.1039/b815177f
Mali, K. S., and De Feyter, S. (2013). Principles of Molecular Assemblies Leading to Molecular Nanostructures. Phil. Trans. R. Soc. A. 371, 20120304. doi:10.1098/rsta.2012.0304
Mallat, T., Orglmeister, E., and Baiker, A. (2007). Asymmetric Catalysis at Chiral Metal Surfaces. Chem. Rev. 107, 4863–4890. doi:10.1021/cr0683663
Mammana, A., D'Urso, A., Lauceri, R., and Purrello, R. (2007). Switching off and on the Supramolecular Chiral Memory in Porphyrin Assemblies. J. Am. Chem. Soc. 129, 8062–8063. doi:10.1021/ja071447b
Monti, D., De Rossi, M., Sorrenti, A., Laguzzi, G., Gatto, E., Stefanelli, M., et al. (2010). Supramolecular Chirality in Solvent-Promoted Aggregation of Amphiphilic Porphyrin Derivatives: Kinetic Studies and Comparison between Solution Behavior and Solid-State Morphology by AFM Topography. Chem. - A Eur. J. 16, 860–870. doi:10.1002/chem.200901964
Monti, D., Stefanelli, M., Raggio, M., Colozza, N., Venanzi, M., Lettieri, R., et al. (2011). Solid State Deposition of Chiral Amphiphilic Porphyrin Derivatives on Glass Surface. J. Porphyrins Phthalocyanines 15, 1209–1219. doi:10.1142/S1088424611004117
Răsădean, D.-M., Gianga, T.-M., Jávorfi, T., Hussain, R., Siligardi, G., and Pantoș, G. D. (2020). Mapping the Chiroptical Properties of Local Domains in Thin Films of Chiral Silicon Phthalocyanines by CD Imaging. Molecules 25, 6048. doi:10.3390/molecules25246048
Sannicolò, F., Arnaboldi, S., Benincori, T., Bonometti, V., Cirilli, R., Dunsch, L., et al. (2014). Potential-Driven Chirality Manifestations and Impressive Enantioselectivity by Inherently Chiral Electroactive Organic Films. Angew. Chem. 126, 2661–2665. doi:10.1002/ange.201309585
Savioli, M., Stefanelli, M., Magna, G., Zurlo, F., Caso, M. F., Cimino, R., et al. (2020). Tunable Supramolecular Chirogenesis in the Self-Assembling of Amphiphilic Porphyrin Triggered by Chiral Amines. Ijms 21, 8557. doi:10.3390/ijms21228557
Shang, X., Song, I., Ohtsu, H., Lee, Y. H., Zhao, T., Kojima, T., et al. (2017). Supramolecular Nanostructures of Chiral Perylene Diimides with Amplified Chirality for High-Performance Chiroptical Sensing. Adv. Mater. 29, 1605828. doi:10.1002/adma.201605828
Simonneaux, G., Lemaux, P., Ferrand, Y., and Raultberthelot, J. (2006). Asymmetric Heterogeneous Catalysis by Metalloporphyrins. Coord. Chem. Rev. 250, 2212–2221. doi:10.1016/j.ccr.2006.01.014
Stefanelli, M., Magna, G., Zurlo, F., Caso, F. M., Di Bartolomeo, E., Antonaroli, S., et al. (2019). Chiral Selectivity of Porphyrin-ZnO Nanoparticle Conjugates. ACS Appl. Mater. Inter. 11, 12077–12087. doi:10.1021/acsami.8b22749
Stefanelli, M., Mandoj, F., Magna, G., Lettieri, R., Venanzi, M., Paolesse, R., et al. (2020a). The Self-Aggregation of Porphyrins with Multiple Chiral Centers in Organic/Aqueous Media: The Case of Sugar- and Steroid-Porphyrin Conjugates. Molecules 25, 4544. doi:10.3390/molecules25194544
Stefanelli, M., Savioli, M., Zurlo, F., Magna, G., Belviso, S., Marsico, G., et al. (2020b). Porphyrins through the Looking Glass: Spectroscopic and Mechanistic Insights in Supramolecular Chirogenesis of New Self-Assembled Porphyrin Derivatives. Front. Chem. 8, 587842. doi:10.3389/fchem.2020.587842
Tao, F., and Bernasek, S. L. (2005). Chirality in Supramolecular Self-Assembled Monolayers of Achiral Molecules on Graphite: Formation of Enantiomorphous Domains from Arachidic Anhydride. J. Phys. Chem. B 109, 6233–6238. doi:10.1021/jp0452397
Xu, Y., Duan, J.-J., Yi, Z.-Y., Zhang, K.-X., Chen, T., and Wang, D. (2021). Chirality of Molecular Nanostructures on Surfaces via Molecular Assembly and Reaction: Manifestation and Control. Surf. Sci. Rep. 76, 100531. doi:10.1016/j.surfrep.2021.100531
Zaera, F. (2008). Chiral Modification of Solid Surfaces: a Molecular View. J. Phys. Chem. C 112, 16196–16203. doi:10.1021/jp804588v
Zaera, F. (2009). Regio-, Stereo-, and Enantioselectivity in Hydrocarbon Conversion on Metal Surfaces. Acc. Chem. Res. 42, 1152–1160. doi:10.1021/ar900049m
Keywords: porphyrins, drop-casting, solvent effect, glass surface, self-aggregation, dynamic light scattering, supramolecular chirality
Citation: Magna G, Traini T, Naitana ML, Bussetti G, Domenici F, Paradossi G, Venanzi M, Di Natale C, Paolesse R, Monti D and Stefanelli M (2022) Seeding Chiral Ensembles of Prolinated Porphyrin Derivatives on Glass Surface: Simple and Rapid Access to Chiral Porphyrin Films. Front. Chem. 9:804893. doi: 10.3389/fchem.2021.804893
Received: 29 October 2021; Accepted: 14 December 2021;
Published: 31 January 2022.
Edited by:
Chao Deng, Soochow University, ChinaReviewed by:
Maria Castriciano, University of Messina, ItalyRoberto Purrello, University of Catania, Italy
Copyright © 2022 Magna, Traini, Naitana, Bussetti, Domenici, Paradossi, Venanzi, Di Natale, Paolesse, Monti and Stefanelli. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Manuela Stefanelli, bWFudWVsYS5zdGVmYW5lbGxpQHVuaXJvbWEyLml0
 Gabriele Magna
Gabriele Magna Tanja Traini1
Tanja Traini1 Fabio Domenici
Fabio Domenici Mariano Venanzi
Mariano Venanzi Roberto Paolesse
Roberto Paolesse Donato Monti
Donato Monti Manuela Stefanelli
Manuela Stefanelli