- Key Laboratory of Green Chemistry and Technology of Ministry of Education, College of Chemistry, and Healthy Food Evaluation Research Center, Sichuan University, Chengdu, China
Several new chiral pillar[4]arene[1]quinone derivatives were synthesized by reacting pillar[4]arene[1]quinone (EtP4Q1), containing four 1,4-diethoxybenzene units and one benzoquinone unit, with various chiral amines via Michael addition. Due to the direct introduction of chiral substituents on the rim of pillar[n]arene and the close location of the chiral center to the rim of EtP4Q1, the newly prepared compounds showed unique chiroptical properties without complicated chiral resolution processes, and unprecedented high anisotropy factor of up to −0.018 at the charge transfer absorption band was observed. Intriguingly, the benzene sidearm attached pillar[4]arene[1]quinone derivative 1a showed solvent- and complexation-driven chirality inversion. This work provides a promising potential for absolute asymmetric synthesis of pillararene-based derivatives.
Introduction
Manipulating molecular chirality, being in the core position of contemporary chemical science (Zhang et al., 2014), has been attracting significant attention not only from the point of view of the fundamental science but also the potential applications such as chiral recognition, asymmetry catalysis, and chiral switches (Zhou and Tang, 2005; Goldup, 2016; Gao et al., 2017; Xing and Zhao, 2018; Corra et al., 2019; Yao et al., 2021b). On the other hand, supramolecular chiral photochemistry, which arises from the chiral spatial arrangement of noncovalently involved components in assemblies (Crassous, 2009), has received booming development in recent years due to their close correlation with many natural and artificial systems and a wide range of potential applications (Jung et al., 2001; Nakashima et al., 2001; Borovkov et al., 2003a; Hembury et al., 2008; Yang and Inoue, 2014; Chen et al., 2015; Liu et al., 2015; Wang X. et al., 2020). Compared with molecular chirality, the supramolecular chirality is more attractive in terms of their regulatability by the external conditions such as temperature (Yao et al., 2017; Fan et al., 2019), pH (Kanagaraj et al., 2020; Liang et al., 2020; Hao et al., 2021), redox (Xiao et al., 2020), light (De Poli et al., 2016), chemical additives (Lee et al., 2018), pressure (Yao et al., 2021a), and solvents (Borovkov et al., 2003b; Fan et al., 2019). Pillar[n]arenes (Ogoshi et al., 2008; Xue et al., 2012; Pan et al., 2015; Fan et al., 2016; Jie et al., 2018; Lv et al., 2018; Li G. et al., 2019; Xiao et al., 2019; Ji et al., 2020a; Lou and Yang, 2020; Mi et al., 2020; Liu et al., 2021; Peng et al., 2021), as a relatively new class of synthetic macrocyclic hosts with some unique properties (Guo et al., (2018); Lai et al., (2019); Liu et al., (2019); Wang et al., (2021), have proved to be an ideal platform to construct unimolecular chirality based on different external stimuli-driven. We have demonstrated that the chirality of pillar[n]arene derivatives could be manipulated by external stimuli, including temperature, redox, light, and pressure (Yao et al., 2017; Xiao et al., 2020; Yao et al., 2021a; Yao et al., 2021b). The synthetic approaches for obtaining chiral pillar[5]arenes include introducing chiral or bulky groups on the openings, fusing a side ring onto one subunit, or threading with an axle to block the interconversion between Sp and Rp conformers (Ogoshi et al., 2011; Chen et al., 2013; Strutt et al., 2014b; Shurpik et al., 2016; Li Q. et al., 2019; Ma et al., 2019; Zhang et al., 2019). However, pillar[5]arenes’ planar-chiral Sp and Rp enantiomers need to be separated by HPLC enantio-resolution of the racemic mixture to study their chiroptical properties. Synthesis of chiral pillar[5]arenes without the complicated chiral resolution processes should be more convenient and valuable for studying supramolecular chirality switching. It has been reported that 1,4-benzoquinone undergoes the Michael addition reaction with aliphatic or aromatic amines to selectively afford 2,5-bis(alkyl/arylamino)-1,4-benzoquinones (Almeida Barbosa et al., 2010; Strutt et al., 2014a; Li et al., 2018; Kiruthika et al., 2020; Li et al., 2020). In this work, we report the synthesis of several new chiral pillar[4]arene[1]quinone derivatives and their unique chiroptical properties. We report the synthesis of several new pillar[4]arene[1]quinone derivatives by attaching chiral amines onto the quinone ring of EtP4Q1. Homochiral compounds were obtained in moderate to good separate yield without complicated HPLC chiral resolution. These compounds showed unique chiroptical properties with unprecedented high anisotropy g factor of up to −0.018 at the charge transfer absorption band; moreover, the benzene sidearm attached pillar[4]arene[1]quinone derivative 1a showed solvent- and complexation-driven chirality inversion.
Experiment
Compounds
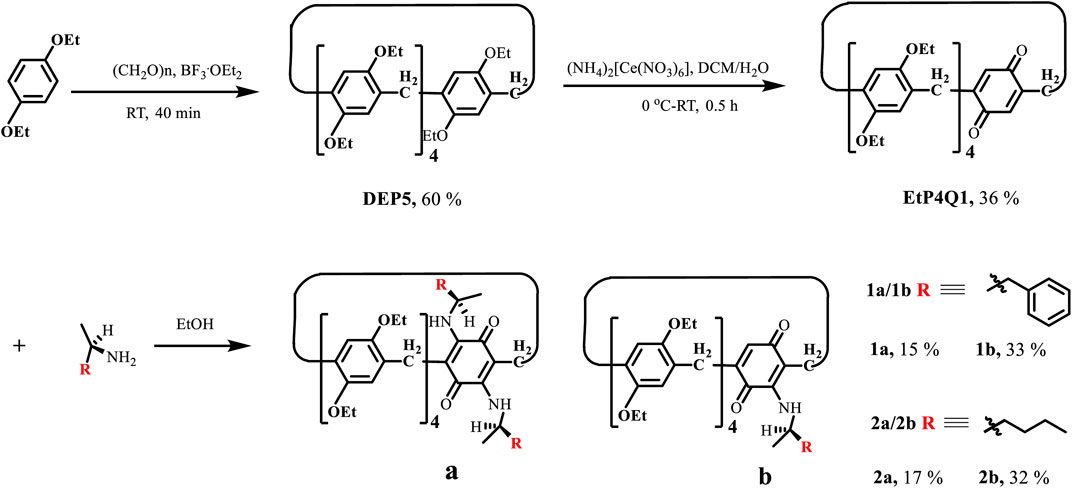
A general reaction scheme for the synthesis of chiral pillar[4]arene[1]quinone derivatives is shown in Scheme 1. Diethyl hydroquinone ether–based pillar[5]arene (DEP5) was synthesized according to the literature procedure (Ogoshi et al., 2010). Pillar[4]arene[1]quinone (EtP4Q1), in which a benzoquinone unit replaces a diethoxybenzene unit in DEP5, was synthesized by partial oxidation with ammonium cerium nitrate, following a modified version of the literature procedure (Han et al., 2012). The reactions of the achiral EtP4Q1 with chiral amines were carried out in ethanol at 75°C in an oil bath for 24 h (Almeida Barbosa et al., 2010; Li et al., 2018). After the solvent was removed under vacuum, the residue was purified by silica gel flash column chromatography using ethyl acetate/petroleum ether as the eluent to give the desired target product (see the supplementary file for detailed experimental procedures and characterizations).
Materials and Instruments
Unless otherwise noted, all reagents and materials were commercially available and used without further purification. 1H NMR was recorded in a CDCl3 solution at room temperature on Bruker AMX-400 (operating at 400 MHz for 1HNMR), and all chemical shifts are reported in ppm with TMS as the internal standard. HRMS data were measured with a Waters Q-TOF Premier instrument. UV-vis spectra were obtained on a JASCO V650 spectrometer at room temperature. Circular dichroism spectra were recorded on a JASCO J-1500 spectrometer, and the obtained data were analyzed using ORIGIN 9.0 software.
Results and Discussion
Synthesis of 1a/1b and 2a/2b
1,4-Benzoquinones were known to undergo the Michael addition reaction with organic amines to give 2,5-bis(amino)-1,4-benzoquinones (Almeida Barbosa et al., 2010). Huang and coworkers demonstrated that pillar[4]arene[1]quinone could physically adsorb organic amines in the solid-state, which underwent in situ Michael addition by elevating the temperature to realize so-called solid–vapor post-synthetic modification (Li et al., 2018). In general, pillar[n]arene derivatives have a pair of planar chiral enantiomeric conformers, which could interconvert through the “oxygen-through-the-annulus” rotation. The attachment of bulky groups on the rims of pillar[n]arene could block the interconversion and lead to a pair of separable enantiomers. The same could be realized by introducing a side ring or threading an axle. Enantiopure pillar[n]arene derivatives showed extremely strong chiroptical properties at the absorption band of hydroquinone ethers due to the inter-ring unit exciton coupling effect. Direct introduction of chiral substituents on the rim of pillar[n]arene could also lead to chiral pillar[n]arene derivatives (Ogoshi et al., 2011; Strutt et al., 2012; Chen et al., 2013; Strutt et al., 2014b; Shurpik et al., 2016; Li Q. et al., 2019; Ma et al., 2019; Zhang et al., 2019). However, the chiral substituents are far away from the aromatic rings in distances and usually show weak chiroptical induction. EtP4Q1 showed brown charge transfer absorption. The Michael addition reaction allows chiral amines to be introduced onto the quinone ring directly, and we envisioned that the chiral EtP4Q1 should offer unique chiroptical properties differing from other chiral pillar[5]arene derivatives. EtP4Q1 was reacted with chiral (R)-(+)-α-methylbenzylamine (Scheme 1) in ethanol, which led to two brown products in 15 and 33% yields, respectively, which were demonstrated to be the mono- (1a) and di-substituted (1b) products, respectively, based on the NMR and HRMS analyses. The same was true in the reaction of (R)-2-aminohexane, which gave the mono- and di-substituted products 2a and 2b, respectively, after the silica gel chromatography separation.
UV-Vis Spectral Studies
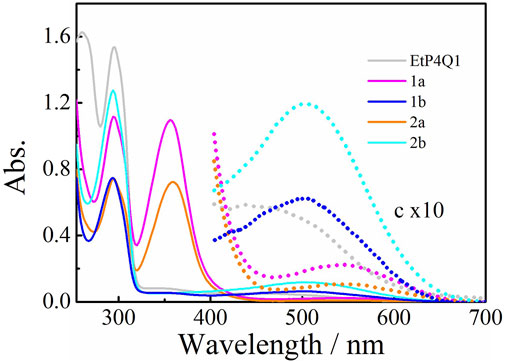
The UV-vis spectra of chiral amine-substituted pillar[4]arene[1]quinones were measured in chloroform at 25°C. EtP4Q1 showed a sharp absorption peak at 294 nm and a broad absorption at the visible range (Figure 1), assignable to the transitions of hydroquinone ether units and the intramolecular charge transfer, respectively (Mi et al., 2020; Mi et al., 2021). The UV-vis spectra of the mono-substituted pillar[4]arene[1]quinone derivatives 1b and 2b exhibited two major transitions, showing a weak broad absorption that tailed to 400–700 nm, which is assignable to a CT transition. Similar to that of EtP4Q1, the strong absorption that peaked at ca. 300 nm could be ascribed to the absorption of the hydroquinone units. Interestingly, 1a and 2a showed two intensive peaks in the UV range and a broad absorption at 450–700 nm that is bathochromic shifted with a concomitant decrease in intensity compared to those of 1b and 2b. The attenuated CT interaction of 1a and 2a could be presumably ascribed to the reduced electron withdraw property of the benzoquinone ring when substituted with two amino substituents, which weakened the intramolecular CT interactions. An independent spectral titration for the intermolecular complexation between EtP4Q1 and (R)-(+)-α-methylbenzylamine, by increasing the concentration of (R)-(+)-α-methylbenzylamine, was carried out in CHL at 25°C (ESI, Supplementary Figure S13). It turned out that the addition of (R)-(+)-α-methylbenzylamine to a solution of EtP4Q1 did not lead to new absorption in the wavelengths range of 300–400 nm and visible region in the UV-vis spectroscopy, demonstrating that the new absorptions originated from the conjugation of the chiral amine-substituent in the quinone ring.
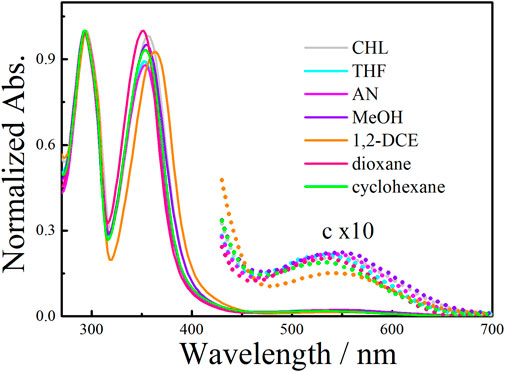
Weak ground-state intermolecular interactions, including CT, should be significantly affected by environmental factors such as solvent polarity, temperature, and so on (Saito et al., 2004). We have demonstrated that intermolecular supramolecular interactions can be effectively manipulated by adjusting the environmental effectors, including temperature or solvent (Ji et al., 2020b). As illustrated in Figure 2, the solvent-dependent UV-vis absorption spectra of 1a revealed the CT transition was not restricted to chloroform solution but rather could be observed in various solvents. Moreover, the absorption spectra of the 1a (50 μM), being measured in chloroform at various temperatures (Supplementary Figure S14), showed inconspicuous temperature-dependent behavior of the CT band, confirming that the intramolecular CT dominate in the macrocyclic structure.
Chiroptical Properties of the Macrocyclic Compounds
As mentioned above, pillar[5]arene derivatives possess a pair of enantiomeric conformers, and in general, they adapt per-Rp (Rp, Rp, Rp, Rp, and Rp) or per-Sp (Sp, Sp, Sp, Sp, and Sp) configurations to avoid inter-subunit steric repulsion. We have demonstrated that Rp and Sp conformers gave intensive positive and negative circular dichromism (CD) signals, respectively, at the extrema around 310 nm. The Rp and Sp conformers usually have an equal population (Yao et al., 2017; Xiao et al., 2020). Such conformational equilibrium could be broken by the complexation of a chiral guest to induce CD response, and thus being applied to chiral sensing (Ji et al., 2020a; Chen et al., 2020). In the chiral amine-substituted EtP4Q1 derivatives, the chiral aliphatic amine or aromatic amine is anchored on the quinone subunit, with the chiral center located close to the rim of EtP4Q1, which was expected to significantly influence the chiroptical properties. CD spectra of 1a/1b and 2a/2b were measured at 25°C in chloroform to study the chiroptical properties (Figure 3). Negative Cotton effects at around 300 nm were observed for 1a, 1b, 2a, and 2b, assignable to the π-π* transition of hydroquinone units, which indicated that the hydroquinone units arranged in Sp configurations in the presence of the chiral amine group.
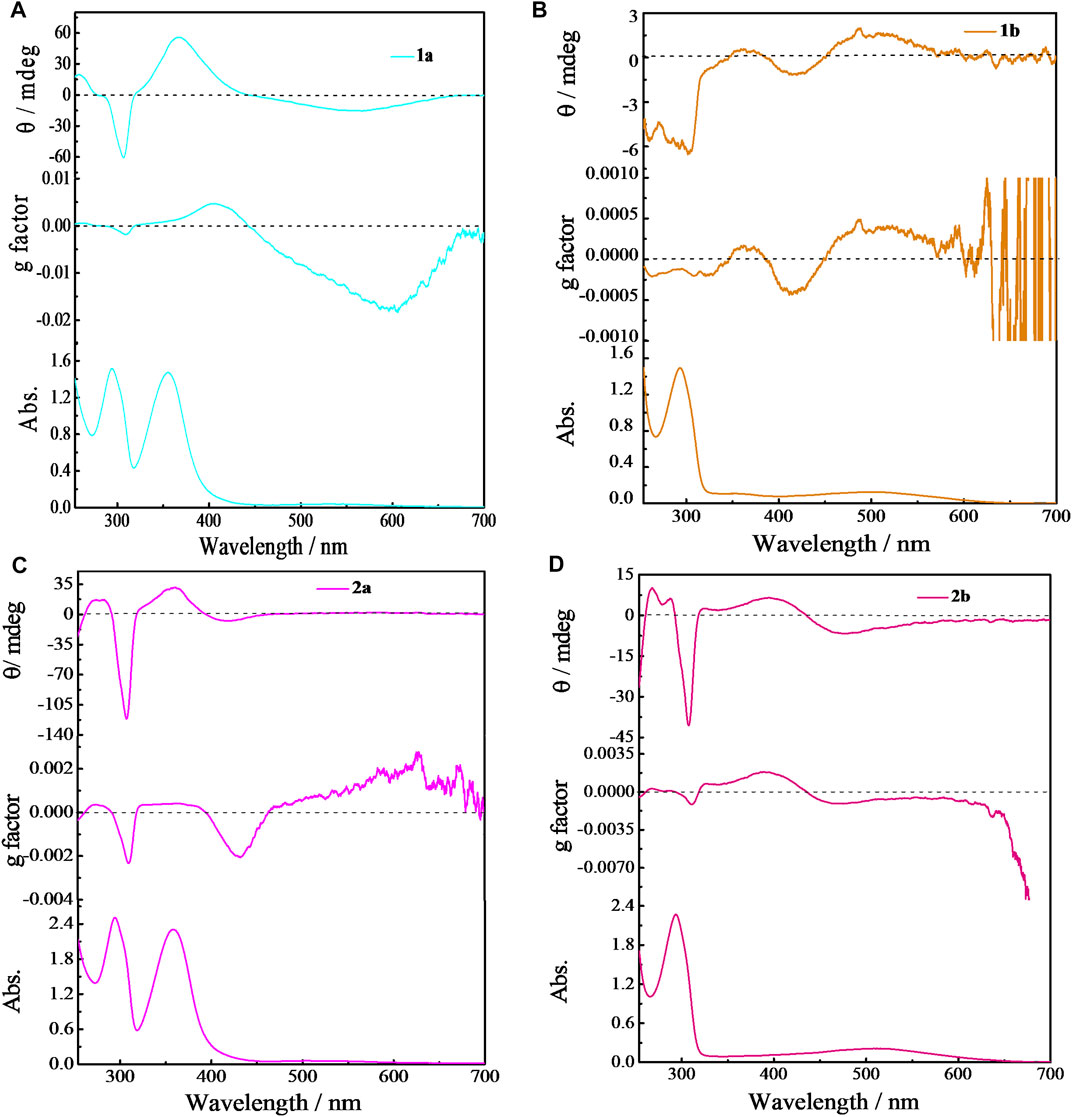
FIGURE 3. Circular dichroism and UV-vis absorption spectra of EtP4Q1-derivated compounds (100 μM) in CHL solution (A) 1a; (B) 1b; (C) 2a; (D) 2b.
Despite the CD signals at 310 nm, new Cotton effects appeared in the region of 300–400 nm of 1a and 2a, which could be ascribed to the transition of 2,5-bis(alkyl/arylamino)-1,4-benzoquinones (Martini and Nachod, 1951; Li et al., 2020). In addition, strong Cotton effects in CT transition in the wavelength region of 400–700 nm were observed (Wang H. J. et al., 2020). In particular, the g factor of up to −0.018 was observed with 1a, which, to our knowledge, is the largest g factor ever reported for CT transition (Mori and Inoue, 2005; Mori et al., 2006).
The effect of solvents on the planar chirality of 1a was investigated. We have demonstrated that negative CD extrema at ca. 310 nm corresponds to Sp configuration of pillar[n]arenes, and vice versa for the Rp configuration(Yao et al., 2017; Xiao et al., 2020). The strong CD spectra observed with these chiral EtP4Q1 derivatives suggested an unequal population of chiral conformers. We expected that variation of environmental conditions might switch the equilibrium of conformers and thus cause chiroptical change. Indeed, 1a exhibits negative CDex in most of the solvents examined, including hexane, acetonitrile, decahydronaphthalene, chloroform, methanol, and THF (Figure 4A), suggesting the Sp configuration dominate in these solvents. However, the CDex at ca. 300 nm was inverted in sign accompanying by a hypochromic shift to give positive CDex in 1,2-dichloroethane and dichloromethane, indicating inversion of planar chirality to Rp. This result revealed that the relative stability between diastereomeric conformers could be significantly changed by the solvent. The following two aspects were responsible for the chiroptical switching process. The solvation of the chiral amine will cause significant steric interaction between solvent molecules surrounding the chiral amine substituents and hydroquinone ether units to thus critically affect the chiral arrangement of hydroquinone subunits. Also, DCM and 1,2-DCE were known to complex with pillar[5]arene derivatives, which will push the sidearms of the chiral amine towards the outside of the cavity. Indeed, NMR titration experiments of compound 1a in CDCl3 upon adding different potions of 1,2-DCE showed that the proton signals of chiral amine significantly shifted downfield and the aromatic protons in pillar[5]arene become broad first and then separated into multiple peaks, when added more than 8% 1,2-DCE (Supplementary Figure S26). The chiral center that is closely located at the opening of the macrocyclic ring played an important role in the chiral inversion behavior. Solvent-dependent chiroptical changes were also observed with 1b (Supplementary Figure S20).
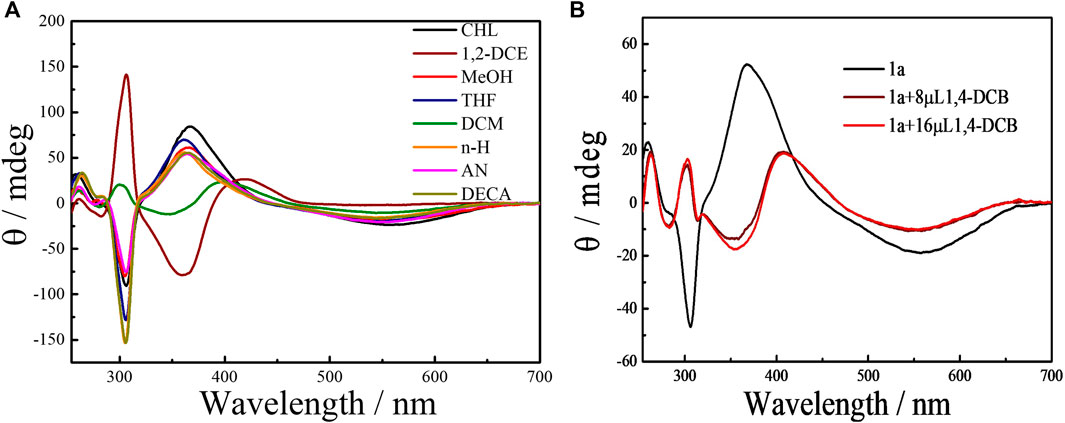
FIGURE 4. (A) CD spectra of 1a (50 μM) in various solvents at 25°C. Key: n-H, n-hexane; DECA, decalin; DCM, dichloromethane; THF, tetrahydrofuran; 1,2-DCE, 1,2-dichloroethane; CHL, chloroform; AN, acetonitrile and MeOH, methanol. (B) CD spectra of 1a (50 μM) in chloroform solvents by adding 1,4-dicyanobutane (1,4-DCB) at 25°C.
The complexation-driven chiral optical switching has also been observed with bicyclic pillar[5]arene derivatives due to the exclusion of the side ring by the complexation of a guest molecule (Yao et al., 2017; Fan et al., 2019; Xiao et al., 2020). We found that stereoinversion with a sign-switching of CDex from negative to positive was induced by the addition of 1,4-dicyanobutane (1,4-DCB), a strong P[5] cavity binder (Shu et al., 2012), to 1a in chloroform (Figure 4B; Supplementary Figures S24, S25).
This is consistent with the chiral inversion phenomenon observed in the solvents of DCM and 1,2-DCE, further suggesting that the benzene ring of the chiral amine is located toward the inside of the cavity. The bulky and rigid benzene ring should cause significant steric repulsion with the complexed 1,4-DCB, when directing inside the cavity to lead to conformational inversion. This conclusion could be supported by the fact that the originally negative CDex intensity in dichloromethane was further enhanced rather than inverted upon the gradual addition of 1,4-DCB to a solution of 1a (Supplementary Figure S23). However, for 2a and 2b, which possess aliphatic sidearms, no solvent-/complexation-driven Sp to Rp chirality switching could be observed (ESI, Supplementary Figures S21, S22). We ascribe this to the flexible aliphatic sidearm in 2a/2b, which will not bring significant steric interaction with the complexed guest/solvent molecules.
We have demonstrated that temperature variation could also cause chiroptical switching of bicyclic pillar[n]arenes due to the relatively large entropy changes between the self-included and self-excluded conformations. Variation temperature CD of 1a was measured in different solvents, which, however, showed only the intensity’s variation to a certain extent (ESI, Supplementary Figures S16–S19) while the CD sign was never inverted. Similar was true with other chiral EtP4Q1 derivatives, suggesting a small entropy difference between diastereomeric conformers.
Conclusion
In summary, we synthesized a series of new chiral amines functionalized pillar[4]arene[1]quinones, which showed unique chiroptical properties. In particular, 1a showed strong CD signals at the CT absorption band with an unprecedented high anisotropy g factor of up to −0.018. Interestingly, we found that the pillar[4]arene[1]quinone having a benzene sidearm showed solvent- and complexation-driven chirality inversion, while no chirality inversion could be observed with the analogs having aliphatic sidearm. The present results opened a new window for synthesizing pillar[n]arene-based stimuli-responsive chiral molecular devices and provide a promising potential for absolute asymmetric synthesis of pillararene-based derivatives.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding authors.
Author Contributions
CY contributed to the design of the experiment, analysis of the results, and manuscript revision. WW was responsible for advising the project and review the manuscript. ZY advised on data analysis. JY, JJ, TZ, and CL were responsible for experimental studies on synthesizing and characterizing the target compounds and prepared the manuscript. All authors contributed to the manuscript and approved the submitted version.
Funding
We acknowledge the support of this work by the National Natural Science Foundation of China (No. 92056116, 21871194, 21971169, 21572142), National Key Research and Development Program of China (No. 2017YFA0505903), Science & Technology Department of Sichuan Province (2019YJ0160, 2019YJ0090, 2017SZ0021), and Fundamental Research Funds for the Central Universities (20826041D4117); compound characterization was obtained with the support of the Comprehensive Training Platform of Specialized Laboratory, College of Chemistry and Prof. Peng Wu of Analytical & Testing Center, Sichuan University, which is greatly appreciated.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fchem.2021.713305/full#supplementary-material
References
Almeida Barbosa, L. C., Alves Pereira, U., Alvares Maltha, C. R., Ricardo Teixeira, R., Moreira Valente, V. M., Oliveira Ferreira, J. R., et al. (2010). Synthesis and Biological Evaluation of 2,5-Bis(alkylamino)-1,4-Benzoquinones. Molecules 15, 5629–5643. doi:10.3390/molecules15085629
Borovkov, V. V., Harada, T., Hembury, G. A., Inoue, Y., and Kuroda, R. (2003a). Solid-State Supramolecular Chirogenesis: High Optical Activity and Gradual Development of Zinc Octaethylporphyrin Aggregates. Angew. Chem. Int. Ed. 42, 1746–1749. doi:10.1002/anie.200250524
Borovkov, V. V., Hembury, G. A., and Inoue, Y. (2003b). The Origin of Solvent-Controlled Supramolecular Chirality Switching in a Bis(Zinc Porphyrin) System. Angew. Chem. Int. Ed. 42, 5310–5314. doi:10.1002/anie.200352493
Chen, L., Si, W., Zhang, L., Tang, G., Li, Z.-T., and Hou, J.-L. (2013). Chiral Selective Transmembrane Transport of Amino Acids through Artificial Channels. J. Am. Chem. Soc. 135, 2152–2155. doi:10.1021/ja312704e
Chen, Y., Fu, L., Sun, B., Qian, C., Wang, R., Jiang, J., et al. (2020). Competitive Selection of Conformation Chirality of Water-Soluble Pillar[5]arene Induced by Amino Acid Derivatives. Org. Lett. 22, 2266–2270. doi:10.1021/acs.orglett.0c00468
Chen, Z., Wang, Q., Wu, X., Li, Z., and Jiang, Y.-B. (2015). Optical Chirality Sensing Using Macrocycles, Synthetic and Supramolecular Oligomers/polymers, and Nanoparticle Based Sensors. Chem. Soc. Rev. 44, 4249–4263. doi:10.1039/c4cs00531g
Corra, S., de Vet, C., Groppi, J., La Rosa, M., Silvi, S., Baroncini, M., et al. (2019). Chemical On/Off Switching of Mechanically Planar Chirality and Chiral Anion Recognition in a [2]Rotaxane Molecular Shuttle. J. Am. Chem. Soc. 141, 9129–9133. doi:10.1021/jacs.9b00941
Crassous, J. (2009). Chiral Transfer in Coordination Complexes: Towards Molecular Materials. Chem. Soc. Rev. 38, 830–845. doi:10.1039/B806203J
De Poli, M., Zawodny, W., Quinonero, O., Lorch, M., Webb, S. J., and Clayden, J. (2016). Conformational Photoswitching of a Synthetic Peptide Foldamer Bound Within a Phospholipid Bilayer. Science 352, 575–580. doi:10.1126/science.aad8352
Fan, C., Wu, W., Chruma, J. J., Zhao, J., and Yang, C. (2016). Enhanced Triplet-Triplet Energy Transfer and Upconversion Fluorescence Through Host-Guest Complexation. J. Am. Chem. Soc. 138, 15405–15412. doi:10.1021/jacs.6b07946
Fan, C., Yao, J., Li, G., Guo, C., Wu, W., Su, D., et al. (2019). Precise Manipulation of Temperature‐Driven Chirality Switching of Molecular Universal Joints Through Solvent Mixing. Chem. Eur. J. 25, 12526–12537. doi:10.1002/chem.201902676
Gao, W., Zhang, Z., Li, P.-F., Tang, Y.-Y., Xiong, R.-G., Yuan, G., et al. (2017). Chiral Molecular Ferroelectrics with Polarized Optical Effect and Electroresistive Switching. ACS Nano 11, 11739–11745. doi:10.1021/acsnano.7b07090
Goldup, S. M. (2016). A Chiral Catalyst with a Ring to it. Nat. Chem 8, 404–406. doi:10.1038/nchem.2509
Guo, S., Song, Y., He, Y., Hu, X.-Y., and Wang, L. (2018). Highly Efficient Artificial Light-Harvesting Systems Constructed in Aqueous Solution Based on Supramolecular Self-Assembly. Angew. Chem. Int. Ed. 57, 3163–3167. doi:10.1002/anie.201800175
Han, C., Zhang, Z., Yu, G., and Huang, F. (2012). Syntheses of a Pillar[4]arene[1]quinone and a Difunctionalized Pillar[5]arene by Partial Oxidation. Chem. Commun. 48, 9876–9878. doi:10.1039/C2CC35498E
Hao, T., Yang, Y., Liang, W., Fan, C., Wang, X., Wu, W., et al. (2021). Trace Mild Acid-Catalysed Z → E Isomerization of Norbornene-Fused Stilbene Derivatives: Intelligent Chiral Molecular Photoswitches with Controllable Self-Recovery. Chem. Sci. 12, 2614–2622. doi:10.1039/D0SC05213B
Hembury, G. A., Borovkov, V. V., and Inoue, Y. (2008). Chirality-Sensing Supramolecular Systems. Chem. Rev. 108, 1–73. doi:10.1021/cr050005k
Ji, J., Li, Y., Xiao, C., Cheng, G., Luo, K., Gong, Q., et al. (2020a). Supramolecular Enantiomeric and Structural Differentiation of Amino Acid Derivatives with Achiral Pillar[5]arene Homologs. Chem. Commun. 56, 161–164. doi:10.1039/C9CC08541F
Ji, J., Wu, W., Wei, X., Rao, M., Zhou, D., Cheng, G., et al. (2020b). Synergetic Effects in the Enantiodifferentiating Photocyclodimerization of 2-anthracenecarboxylic Acid Mediated by β-cyclodextrin-pillar[5]arene-hybridized Hosts. Chem. Commun. 56, 6197–6200. doi:10.1039/D0CC02055A
Jie, K., Zhou, Y., Li, E., and Huang, F. (2018). Nonporous Adaptive Crystals of Pillararenes. Acc. Chem. Res. 51, 2064–2072. doi:10.1021/acs.accounts.8b00255
Jung, J. H., Kobayashi, H., Masuda, M., Shimizu, T., and Shinkai, S. (2001). Helical Ribbon Aggregate Composed of a Crown-Appended Cholesterol Derivative Which Acts as an Amphiphilic Gelator of Organic Solvents and as a Template for Chiral Silica Transcription. J. Am. Chem. Soc. 123, 8785–8789. doi:10.1021/ja010508h
Kanagaraj, K., Liang, W., Rao, M., Yao, J., Wu, W., Cheng, G., et al. (2020). pH-Controlled Chirality Inversion in Enantiodifferentiating Photocyclodimerization of 2-Antharacenecarboxylic Acid Mediated by γ-Cyclodextrin Derivatives. Org. Lett. 22, 5273–5278. doi:10.1021/acs.orglett.0c01194
Kiruthika, J., Srividhya, S., and Arunachalam, M. (2020). Anion-Responsive Pseudo[3]rotaxane from a Difunctionalized Pillar[4]arene[1]quinone and a Bis-Imidazolium Cation. Org. Lett. 22, 7831–7836. doi:10.1021/acs.orglett.0c02710
Lai, H., Zhao, T., Deng, Y., Fan, C., Wu, W., and Yang, C. (2019). Assembly-enhanced Triplet-Triplet Annihilation Upconversion in the Aggregation Formed by Schiff-Base Pt(II) Complex Grafting-Permethyl-β-CD and 9, 10-diphenylanthracence Dimer. Chin. Chem. Lett. 30, 1979–1983. doi:10.1016/j.cclet.2019.09.009
Lee, E., Ju, H., Park, I.-H., Jung, J. H., Ikeda, M., Kuwahara, S., et al. (2018). pseudo[1]Catenane-Type Pillar[5]thiacrown Whose Planar Chiral Inversion Is Triggered by Metal Cation and Controlled by Anion. J. Am. Chem. Soc. 140, 9669–9677. doi:10.1021/jacs.8b05751
Li, E., Jie, K., Fang, Y., Cai, P., and Huang, F. (2020). Transformation of Nonporous Adaptive Pillar[4]arene[1]quinone Crystals into Fluorescent Crystals Via Multi-step Solid-Vapor Postsynthetic Modification for Fluorescence Turn-On Sensing of Ethylenediamine. J. Am. Chem. Soc. 142, 15560–15568. doi:10.1021/jacs.0c07482
Li, E., Jie, K., Zhou, Y., Zhao, R., and Huang, F. (2018). Post-synthetic Modification of Nonporous Adaptive Crystals of Pillar[4]arene[1]quinone by Capturing Vaporized Amines. J. Am. Chem. Soc. 140, 15070–15079. doi:10.1021/jacs.8b10192
Li, G., Fan, C., Cheng, G., Wu, W., and Yang, C. (2019). Synthesis, Enantioseparation and Photophysical Properties of Planar-Chiral Pillar[5]arene Derivatives Bearing Fluorophore Fragments. Beilstein J. Org. Chem. 15, 1601–1611. doi:10.3762/bjoc.15.164
Li, Q., Li, X., Ning, L., Tan, C.-H., Mu, Y., and Wang, R. (2019). Hyperfast Water Transport through Biomimetic Nanochannels from Peptide-Attached (pR)-Pillar[5]arene. Small 15, 1804678. doi:10.1002/smll.201804678
Liang, H., Hua, B., Xu, F., Gan, L.-S., Shao, L., and Huang, F. (2020). Acid/Base-Tunable Unimolecular Chirality Switching of a Pillar[5]azacrown Pseudo[1]Catenane. J. Am. Chem. Soc. 142, 19772–19778. doi:10.1021/jacs.0c10570
Liu, C., Yao, J., Xiao, C., Zhao, T., Selvapalam, N., Zhou, C., et al. (2021). Electrochemiluminescent Chiral Discrimination with a Pillar[5]arene Molecular Universal Joint-Coordinated Ruthenium Complex. Org. Lett. 23, 3885–3890. doi:10.1021/acs.orglett.1c01016
Liu, M., Zhang, L., and Wang, T. (2015). Supramolecular Chirality in Self-Assembled Systems. Chem. Rev. 115, 7304–7397. doi:10.1021/cr500671p
Liu, R., Zhang, Y., Wu, W., Liang, W., Huang, Q., Yu, X., et al. (2019). Temperature-driven Braking of γ-cyclodextrin-curcubit[6]uril-cowheeled [4]rotaxanes. Chin. Chem. Lett. 30, 577–581. doi:10.1016/j.cclet.2018.12.002
Lou, X. Y., and Yang, Y. W. (2020). Pillar[ N ]arene‐Based Supramolecular Switches in Solution and on Surfaces. Adv. Mater. 32, 2003263. doi:10.1002/adma.202003263
Lv, Y., Xiao, C., and Yang, C. (2018). A Pillar[5]arene-Calix[4]pyrrole Enantioselective Receptor for Mandelate Anion Recognition. New J. Chem. 42, 19357–19359. doi:10.1039/C8NJ04802A
Ma, J., Yan, H., Quan, J., Bi, J., Tian, D., and Li, H. (2019). Enantioselective Dynamic Self-Assembly of Histidine Droplets on Pillar[5]arene-Modified Interfaces. ACS Appl. Mater. Inter. 11, 1665–1671. doi:10.1021/acsami.8b18202
Martini, C. M., and Nachod, F. C. (1951). Absorption Spectra of Some Substituted Benzoquinones. J. Am. Chem. Soc. 73, 2953–2954. doi:10.1021/ja01150a522
Mi, Y., Ma, J., Liang, W., Xiao, C., Wu, W., Zhou, D., et al. (2021). Guest-Binding-Induced Interhetero Hosts Charge Transfer Crystallization: Selective Coloration of Commonly Used Organic Solvents. J. Am. Chem. Soc. 143, 1553–1561. doi:10.1021/jacs.0c11833
Mi, Y., Yao, J., Ma, J., Dai, L., Xiao, C., Wu, W., et al. (2020). Fulleropillar[4]arene: The Synthesis and Complexation Properties. Org. Lett. 22, 2118–2123. doi:10.1021/acs.orglett.9b04607
Mori, T., and Inoue, Y. (2005). Circular Dichroism of a Chiral Tethered Donor-Acceptor System: Enhanced Anisotropy Factors in Charge-Transfer Transitions by Dimer Formation and by Confinement. Angew. Chem. Int. Ed. 44, 2582–2585. doi:10.1002/anie.200462071
Mori, T., Ko, Y. H., Kim, K., and Inoue, Y. (2006). Circular Dichroism of Intra- and Intermolecular Charge-Transfer Complexes. Enhancement of Anisotropy Factors by Dimer Formation and by Confinement. J. Org. Chem. 71, 3232–3247. doi:10.1021/jo0602672
Nakashima, H., Koe, J. R., Torimitsu, K., and Fujiki, M. (2001). Transfer and Amplification of Chiral Molecular Information to Polysilylene Aggregates. J. Am. Chem. Soc. 123, 4847–4848. doi:10.1021/ja010119n
Ogoshi, T., Kanai, S., Fujinami, S., Yamagishi, T.-a., and Nakamoto, Y. (2008). para-Bridged Symmetrical Pillar[5]arenes: Their Lewis Acid Catalyzed Synthesis and Host-Guest Property. J. Am. Chem. Soc. 130, 5022–5023. doi:10.1021/ja711260m
Ogoshi, T., Kitajima, K., Aoki, T., Fujinami, S., Yamagishi, T.-a., and Nakamoto, Y. (2010). Synthesis and Conformational Characteristics of Alkyl-Substituted Pillar[5]arenes. J. Org. Chem. 75, 3268–3273. doi:10.1021/jo100273n
Ogoshi, T., Shiga, R., Yamagishi, T.-a., and Nakamoto, Y. (2011). Planar-Chiral Pillar[5]arene: Chiral Switches Induced by Multiexternal Stimulus of Temperature, Solvents, and Addition of Achiral Guest Molecule. J. Org. Chem. 76, 618–622. doi:10.1021/jo1021508
Pan, S., Ni, M., Mu, B., Li, Q., Hu, X.-Y., Lin, C., et al. (2015). Well-Defined Pillararene-Based Azobenzene Liquid Crystalline Photoresponsive Materials and Their Thin Films with Photomodulated Surfaces. Adv. Funct. Mater. 25, 3571–3580. doi:10.1002/adfm.201500942
Peng, C., Liang, W., Ji, J., Fan, C., Kanagaraj, K., Wu, W., et al. (2021). Pyrene-tiaraed Pillar[5]arene: Strong Intramolecular Excimer Emission Applicable for Photo-Writing. Chin. Chem. Lett. 32, 345–348. doi:10.1016/j.cclet.2020.03.079
Saito, H., Mori, T., Wada, T., and Inoue, Y. (2004). Diastereoselective [2 + 2] Photocycloaddition of Stilbene to Chiral Fumarate. Direct versus Charge-Transfer Excitation. J. Am. Chem. Soc. 126, 1900–1906. doi:10.1021/ja0370140
Shu, X., Chen, S., Li, J., Chen, Z., Weng, L., Jia, X., et al. (2012). Highly Effective Binding of Neutral Dinitriles by Simple Pillar[5]arenes. Chem. Commun. 48, 2967–2969. doi:10.1039/C2CC00153E
Shurpik, D. N., Padnya, P. L., Evtugyn, V. G., Mukhametzyanov, T. A., Khannanov, A. A., Kutyreva, M. P., et al. (2016). Synthesis and Properties of Chiral Nanoparticles Based on (pS)- and (pR)-Decasubstituted Pillar[5]arenes Containing Secondary Amide Fragments. RSC Adv. 6, 9124–9131. doi:10.1039/C5RA25562G
Strutt, N. L., Fairen-Jimenez, D., Iehl, J., Lalonde, M. B., Snurr, R. Q., Farha, O. K., et al. (2012). Incorporation of an A1/A2-Difunctionalized Pillar[5]arene into a Metal-Organic Framework. J. Am. Chem. Soc. 134, 17436–17439. doi:10.1021/ja3082523
Strutt, N. L., Zhang, H., Schneebeli, S. T., and Stoddart, J. F. (2014a). Amino-Functionalized Pillar[5]arene. Chem. Eur. J. 20, 10996–11004. doi:10.1002/chem.201403235
Strutt, N. L., Zhang, H., and Stoddart, J. F. (2014b). Enantiopure Pillar[5]arene Active Domains within a Homochiral Metal-Organic Framework. Chem. Commun. 50, 7455–7458. doi:10.1039/C4CC02559H
Wang, H.-J., Zhang, H.-Y., Zhang, H.-Y., Liu, G., Dai, X., Wu, H., et al. (2020). Guest-induced Supramolecular Chirality Transfer in [2]pseudorotaxanes: Experimental and Computational Study. Org. Biomol. Chem. 18, 7649–7655. doi:10.1039/D0OB01347A
Wang, K., Jordan, J. H., Velmurugan, K., Tian, X., Zuo, M., Hu, X. Y., et al. (2021). Role of Functionalized Pillararene Architectures in Supramolecular Catalysis. Angew. Chem. Int. Ed. 60, 9205–9214. doi:10.1002/anie.202010150
Wang, X., Jia, F., Yang, L.-P., Zhou, H., and Jiang, W. (2020). Conformationally Adaptive Macrocycles with Flipping Aromatic Sidewalls. Chem. Soc. Rev. 49, 4176–4188. doi:10.1039/D0CS00341G
Xiao, C., Liang, W., Wu, W., Kanagaraj, K., Yang, Y., Wen, K., et al. (2019). Resolution and Racemization of a Planar-Chiral A1/A2-Disubstituted Pillar[5]arene. Symmetry 11, 773. doi:10.3390/sym11060773
Xiao, C., Wu, W., Liang, W., Zhou, D., Kanagaraj, K., Cheng, G., et al. (2020). Redox‐Triggered Chirality Switching and Guest‐Capture/Release with a Pillar[6]arene‐Based Molecular Universal Joint. Angew. Chem. Int. Ed. 59, 8094–8098. doi:10.1002/anie.201916285
Xing, P., and Zhao, Y. (2018). Controlling Supramolecular Chirality in Multicomponent Self-Assembled Systems. Acc. Chem. Res. 51, 2324–2334. doi:10.1021/acs.accounts.8b00312
Xue, M., Yang, Y., Chi, X., Zhang, Z., and Huang, F. (2012). Pillararenes, A New Class of Macrocycles for Supramolecular Chemistry. Acc. Chem. Res. 45, 1294–1308. doi:10.1021/ar2003418
Yang, C., and Inoue, Y. (2014). Supramolecular Photochirogenesis. Chem. Soc. Rev. 43, 4123–4143. doi:10.1039/C3CS60339C
Yao, J., Mizuno, H., Xiao, C., Wu, W., Inoue, Y., Yang, C., et al. (2021a). Pressure-driven, Solvation-Directed Planar Chirality Switching of Cyclophano-Pillar[5]arenes (Molecular Universal Joints). Chem. Sci. 12, 4361–4366. doi:10.1039/D0SC06988D
Yao, J., Wu, W., Liang, W., Feng, Y., Zhou, D., Chruma, J. J., et al. (2017). Temperature-Driven Planar Chirality Switching of a Pillar[5]arene-Based Molecular Universal Joint. Angew. Chem. Int. Ed. 56, 6869–6873. doi:10.1002/anie.201702542
Yao, J., Wu, W., Xiao, C., Su, D., Zhong, Z., Mori, T., et al. (2021b). Overtemperature-protection Intelligent Molecular Chiroptical Photoswitches. Nat. Commun. 12, 2600. doi:10.1038/s41467-021-22880-z
Zhang, J., Wang, Z., Lv, S., Zeng, X., Sun, Y., Li, H., et al. (2019). The Chiral Interfaces Fabricated by D/l-Alanine-Pillar[5]arenes for Selectively Adsorbing ctDNA. Chem. Commun. 55, 778–781. doi:10.1039/C8CC09696A
Zhang, L., Qin, L., Wang, X., Cao, H., and Liu, M. (2014). Supramolecular Chirality in Self-Assembled Soft Materials: Regulation of Chiral Nanostructures and Chiral Functions. Adv. Mater. 26, 6959–6964. doi:10.1002/adma.201305422
Keywords: pillar[4]arene[1]quinone, charge-transfer interaction, circular dichroism, anisotropy factors, chirality switching, solvent effects
Citation: Liu C, Yu Z, Yao J, Ji J, Zhao T, Wu W and Yang C (2021) Solvent-Driven Chirality Switching of a Pillar[4]arene[1]quinone Having a Chiral Amine-Substituted Quinone Subunit. Front. Chem. 9:713305. doi: 10.3389/fchem.2021.713305
Received: 22 May 2021; Accepted: 14 June 2021;
Published: 07 July 2021.
Edited by:
Tony D. James, University of Bath, United KingdomReviewed by:
Xiao-Yu Hu, Nanjing University of Aeronautics and Astronautics, ChinaPeter Cragg, University of Brighton, United Kingdom
Copyright © 2021 Liu, Yu, Yao, Ji, Zhao, Wu and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wanhua Wu, d3V3YW5odWFAc2N1LmVkdS5jbg==; Cheng Yang, eWFuZ2NoZW5neWNAc2N1LmVkdS5jbg==
 Chunhong Liu
Chunhong Liu Zhipeng Yu
Zhipeng Yu Wanhua Wu
Wanhua Wu Cheng Yang
Cheng Yang