- 1Key Laboratory of Flexible Electronics & Institute of Advanced Materials, Jiangsu National Synergistic Innovation Center for Advanced Materials, School of Energy Science and Engineering, Nanjing Tech University, Nanjing, China
- 2State Key Laboratory of Coordination Chemistry, Nanjing University, Nanjing, China
By employment of amino-functionalized dicarboxylate ligands to react with d10 metal ions, four novel metal-organic frameworks (MOFs) were obtained with the formula of {[Cd(BCPAB)(μ2-H2O)]}n (1), {[Cd(BDAB)]∙2H2O∙DMF}n (2), {[Zn(BDAB)(BPD)0.5(H2O)]∙2H2O}n (3) and {[Zn(BDAB)(DBPB)0.5(H2O)]∙2H2O}n (4) (H2BCPAB = 2,5-bis(p-carbonylphenyl)-1-aminobenzene; H2BDAB = 1,2-diamino-3,6-bis(4-carboxyphenyl)benzene); BPD = (4,4′-bipyridine); DBPB = (E,E-2,5-dimethoxy-1,4-bis-[2-pyridin-vinyl]-benzene; DMF = N,N-dimethylformamide). Complex 1 is a three-dimensional (3D) framework bearing seh-3,5-Pbca nets with point symbol of {4.62}{4.67.82}. Complex 2 exhibits a 4,4-connected new topology that has never been reported before with point symbol of {42.84}. Complex 3 and 4 are quite similar in structure and both have 3D supramolecular frameworks formed by 6-fold and 8-fold interpenetrated 2D coordination layers. The structures of these complexes were characterized by single crystal X-ray diffraction (SC-XRD), thermal gravimetric analysis (TGA) and powder X-ray diffraction (PXRD) measurements. In addition, the fluorescence properties and the sensing capability of 2–4 were investigated as well and the results indicated that complex 2 could function as sensor for Cu2+ and complex 3 could detect Cu2+ and Ag+via quenching effect.
Introduction
Metal-organic frameworks (MOFs), which are formed by coordination bonds between metal nodes and organic linkers (Tranchemontagne et al., 2009), have been one of the most rapidly developing areas of material science, not only because of the tunable porosity, controlled structure, and readily chemical functionalization of these materials, but also because of their wide potential applications such as heterogeneous catalysis, gas adsorption and storage, chemical sensing and explosive detection, drug delivery, and optoelectronics. (Barea et al., 2014; Canivet et al., 2014; Dhakshinamoorthy and Garcia, 2014; He et al., 2014; Hu et al., 2014; Liu et al., 2014; Van de Voorde et al., 2014; Silva et al., 2015; Zhu et al., 2015; Sheberla et al., 2017; Li et al., 2018; Park et al., 2018; Prasad et al., 2018; Wang et al., 2018; Cao et al., 2019; Mallick et al., 2019) For example, as a kind of new absorbent materials, quantities of MOFs have been widely investigated in the capture and separation of various gases, such as CO2, SO2, H2S, NH3, hydrocarbons and so on. (Li et al., 2009; Peng et al., 2013; Zhang et al., 2014; Trickett et al., 2017; Zárate et al., 2019a; Zárate et al., 2019b; Tchalala et al., 2019; Wang et al., 2020; Han et al., 2021) Varieties of MOFs have also been explored as luminescent materials in different fields, for example, sensing, nonlinear optical materials, OLED, and so forth. (Lustig et al., 2017; Medishetty et al., 2017; Gutiérrez et al., 2018) Although many MOFs have exhibited relatively superior performance, the majority of them do not meet the requirements of practical applications. In order to further improve the properties of MOFs, some strategies have been proposed in previous reports and the introduction of substituent groups into the organic ligands has been proved one of the most effective manners. Among various substituent groups, the influence of amino groups on the structures and properties of MOFs has been intensively studied because amino groups could coordinate with metal ions and form hydrogen bonds with guest molecules, which thus may strengthen some performance or even endow MOFs more functionalities. For instance, Hu et al. demonstrated that the supramolecular interactions of C-H···O, C···O, and O···O could distinctly enhance the adsorption capacity for CO2. (Hu et al., 2015) Dong and co-workers found that the introduction of amino groups to UiO-66 could provide sensing capability towards lysine and arginine via fluorescence turn-on effect. (Dong et al., 2020)
In consideration of the positive effect of amino groups on the properties of MOFs, we employed amino-functionalized dicarboxylate ligands to construct MOFs in this work. For this purpose, ligands 2,5-bis(p-carbonylphenyl)-1-aminobenzene (H2BCPAB) and 1,2-diamino-3,6-bis(4-carboxyphenyl)benzene) (H2BDAB) were synthesized to react with d10 metal ions Zn2+ and Cd2+ in the absence and presence of auxiliary ligands and four novel MOFs with the formula of {[Cd(BCPAB)(μ2-H2O)]}n (1), {[Cd(BDAB)]∙2H2O∙DMF}n (2), {[Zn(BDAB)(BPD)0.5(H2O)]∙2H2O}n (3) and {[Zn(BDAB)(DBPB)0.5(H2O)]∙2H2O}n (4) (BPD = (4,4′-bipyridine); DBPB = (E,E-2,5-dimethoxy-1,4-bis-[2-pyridin-vinyl]-benzene; DMF = N,N-dimethylformamide) were obtained successfully. Their structure was determined and characterized by SC-XRD, TGA, and PXRD measurements. Besides, the fluorescence properties and the sensing capability of 2–4 were investigated as well, and the sensing experiments indicated that complex 2 could function as a sensor for Cu2+ and complex 3 could detect Cu2+ and Ag+via quenching effect.
Materials and Methods
All chemicals and solvents except the organic ligands H2BCPAB, H2BDAB, and DBPB were of reagent-grade quality from commercial sources and were used without further purification. The as-synthesized complexes were characterized by thermogravimetric analysis (TGA) on a Perkin Elmer thermogravimetric analyzer Pyris 1 TGA up to 500°C using a heating rate of 10°C min−1 under a N2 atmosphere. Powder X-ray diffraction (PXRD) measurements were performed on a Bruker D8 Advance X-ray diffractometer using Cu-K α radiation (1.5418 Å), and the X-ray tube was operated at 40 kV and 40 mA. The gas sorption isotherms were measured by using a Micromeritics ASAP 2020M + C surface area analyzer. Fluorescence spectra were recorded on a PerkinElmer LS-55 fluorescence spectrophotometer. Organic ligands H2BCPAB (Scheme 1), H2BDAB (Scheme 2) and DBPB (Scheme 3) were synthesized by previously reported procedures. (Nagarkar et al., 2015; Shen et al., 2016; Dutta et al., 2018)
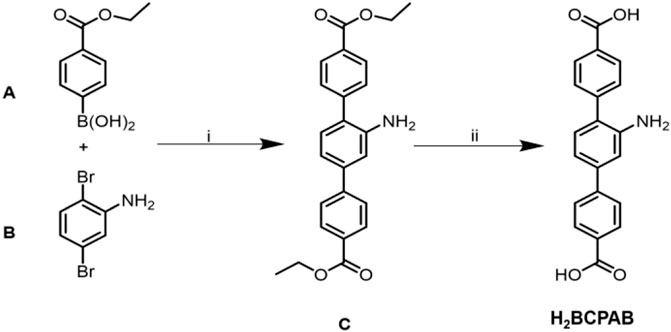
SCHEME 1. Synthesis and structures of ligand H2BCPAB. (A) Pd(PPh3)4, CsF, THF, N2; (B) KOH, THF/H2O.
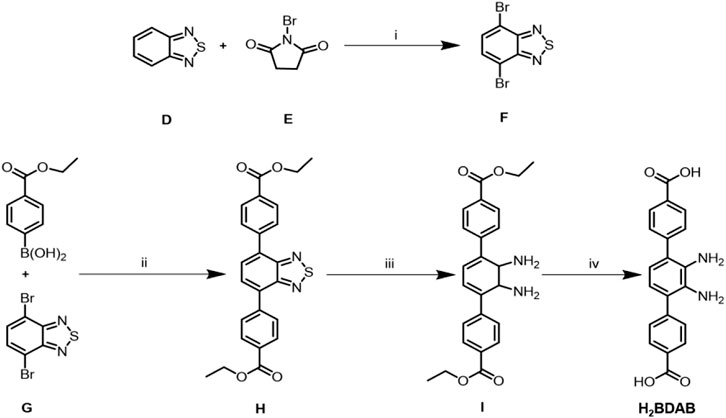
SCHEME 2. Synthesis and structures of ligand H2BDAB. (A) H2SO4 98%; (B) Pd(PPh3)4, Cs2CO3, DMF/Toluene, N2; (C) NaBH4, CoCl2˖H2O; (D) KOH, THF/H2O.
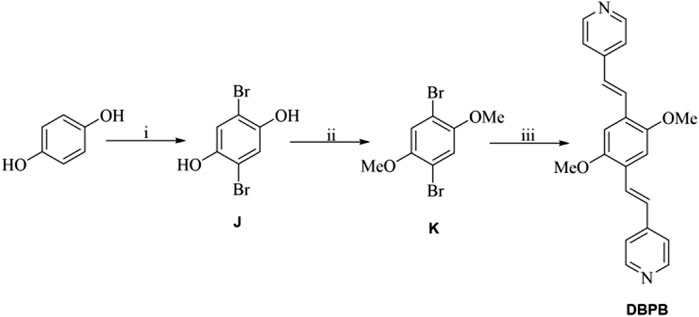
SCHEME 3. (A) Br2, AcOH; (B) K2CO3, acetone, CH3Br/(CH3)2SO4; (C) 4-vinylpyridine, Pd(PPh3)4, tris(2-methylphenyl)phosphine, Et3N, CH3CN.
Synthesis of 2,5-bis(p-ethoxycarbonylphenyl)-1-aminobenzene (C)
2,5-bis(p-ethoxycarbonylphenyl)-1-aminobenzene (C) was synthesized by previously reported procedures. To a 1,000 ml round-bottom flask was added with 2, 5-dibromoaniline (1.2 g, 5 mmol), p-ethoxycarbonylphenylboronoc acid (2.9 g, 15 mmol), Pd(PPh3)4 (0.58 g, 0.5 mmol), CsF (3.6 g, 24 mmol) and tetrahydrofuran (75 ml). The mixture solution was bubbled with N2 for more than 10 min and refluxed for 3 days. TLC (hexane : ethyl acetate = 6:1) showed that the reaction has finished. After cooling to room temperature, water was added onto the reaction mixture and then extracted with ethyl acetate (30 ml × 3). The combined organic solution was dried with anhydrous MgSO4 and concentrated in vacuum. The crude residues were purified by column flash chromatography with the eluant (hexane:ethyl acetate = 30:1) to give a light yellow solid as target product (1.35 g, yield: 67.3%).
Synthesis of 2,5-Bis (p-carbonylphenyl)-1-Aminobenzene (H2BCPAB).
Compound C (1.35 g, 3.4 mmol), KOH (6.24 g, 111 mmol), tetrahydrofuran (THF, 40 ml) and water (100 ml) were added to a 1 L round-bottom flask. The mixture solution was bubbled with N2 for more than 10 min and stirred at 50 C for 12 h. After removing THF in vacuum, the residue was added with water and then acidified with diluted HCl (1 M) until no precipitate formed. The atrovirens powder was collected by filtration as target product (0.88 g, yield: 72%). LC-MS (M + H)+found = 334.12.
Synthesis of 4,7-Dibromobenzo[c] (Tranchemontagne et al., 2009; Silva et al., 2015; Wang et al., 2018)Thiadiazole (F)
2,1,3-Benzothiadiazole (D) (0.5 g, 3.68 mmol), N-bromosuccinimide (NBS, 1.35 g, 7.61 mmol) and H2SO4 (98%, 5 ml) were added to a 25 ml round round-bottom flask. The reaction mixture was stirred at 60°C for 3 h. After cooling to room temperature, the solution was added with distilled water (25 ml) dropwise in an ice bath. The white desired solid was collected by filtration (1.06 g, yield: 97%).
Synthesis of 4,7-bis(p-ethoxycarbonylphenyl)-2,1,3-benzothiodiazole (H)
Compound F (2.49 g, 10 mmol), p-ethoxycarbonylphenylboronoc acid (5.82 g, 30 mmol), Pd(PPh3)4 (1.16 g , 1 mmol), Cs2CO3 (8.15 g , 25 mmol), N,N-dimethylformamide (DMF, 100 ml), and toluene (100 ml) were added to a 500 ml round-bottom flask. The reaction solution was bubbled with N2 for more than 10 min and refluxed at 110°C for 24 h. TLC (hexane:ethyl acetate = 8:1) showed that the reaction has finished. The reaction solution was added with water and extracted with ethyl acetate (20 ml × 3). The combined organic solution was dried with anhydrous MgSO4 and concentrated in vacuum. The crude product was purified by column flash chromatography with the eluant (hexane:ethyl acetate = 40:1) to give an orange solid as the target product (2.25 g, yield: 49%).
Synthesis of 3,6-bis(p-ethoxycarbonylphenyl)-1,2-diaminobenzene (I)
To a solution of H (1,296 mg, 3 mmol) in EtOH/THF (3:1, EtOH = ethyl alcohol) was added sodium borohydride (0.46 g, 12 mmol) and CoCl2·6H2O (29 mg, 0.12 mmol). The reaction solution was bubbled with N2 for more than 10 min and refluxed for 3 h. After removal of EtOH and THF, the residues were added with water and extracted with ethyl acetate (20 ml × 3). The combined organic phase was dried with anhydrous MgSO4 and concentrated in vacuum. The obtained solid was purified by column flash chromatography with the eluant (hexane:ethyl acetate = 20:1) to give a grey solid as the target product (0.92 mg, yield: 74.3%) . LC-MS (M + H)+found = 405.30.
Synthesis of 3,6-bis(p-carbonylphenyl)-1, 2-Diaminobenzene
Compound I (0.90 g, 2.22 mmol), KOH (4.09 g, 73 mmol), THF (20 ml), and water (60 ml) were added to a 500 ml round-bottom flask. The reaction solution was bubbled with N2 for more than 10 min and stirred at 60 C for 24 h. After removing THF in vacuum, the mixture was added with water and then acidified with diluted HCl (1 M) until no precipitate formed. The yellow powder was collected by filtration as target product (0.63 mg, 82% yield). LC-MS (M + H)+found = 349.23.
Synthesis of {[Cd(BCPAB) (μ2-H2O)]}n (1)
A mixture of H2BCPAB (6.7 mg, 0.02 mmol), Cd(NO3)2∙4H2O (31 mg, 0.1 mmol), DMA (3.5 ml), H2O (3 ml) was placed in a 25 ml glass vial and heated at 95°C for 4 days. The resultant plate crystals were washed with fresh DMA and collected. Yield: 72% (based on H2BCPAB).
Synthesis of [Cd(BDAB)]∙2H2O∙DMF}n (2)
A mixture of H2BDAB (7 mg, 0.02 mmol), Cd(NO3)2∙4H2O (31 mg, 0.1 mmol), DMF (2 ml), H2O (4 ml) was placed in a 25 ml glass vial and heated at 95°C for 4 days. The resultant plate crystals were washed with fresh DMA and collected. Yield: 93% (based on H2BDAB).
Synthesis of {[Zn(BDAB)(BPD)0.5(H2O)]∙2H2O}n (3)
A mixture of H2BDAB (7 mg, 0.02 mmol), BPD (1.5 mg, 0.01 mmol), Zn(NO3)2∙6H2O (30 mg, 0.1 mmol), DMF (3.5 ml), H2O (2.5 ml) was placed in a 25 ml glass vial and heated at 100°C for 3 days. The resultant red featheriness crystals were washed with fresh DMF and collected. Yield: 36% (based on H2BDAB).
Synthesis of {[Zn(BDAB)(DBPB)0.5(H2O)]∙2H2O}n (4)
A mixture of H2BDAB (7 mg, 0.02 mmol), DBPB (3.5 mg, 0.01 mmol), Zn(NO3)2∙6H2O (30 mg, 0.1 mmol), DMF (3.5 ml), and H2O (1.5 ml) was placed in a 25 ml glass vial and heated at 105°C for 3 days. The resultant red featheriness crystals were washed with fresh DMF and collected. Yield: 59% (based on H2BDAB).
Results and Discussion
Description of the Crystal Structure of {[Cd(BCPAB)(μ2-H2O)]}n (1)
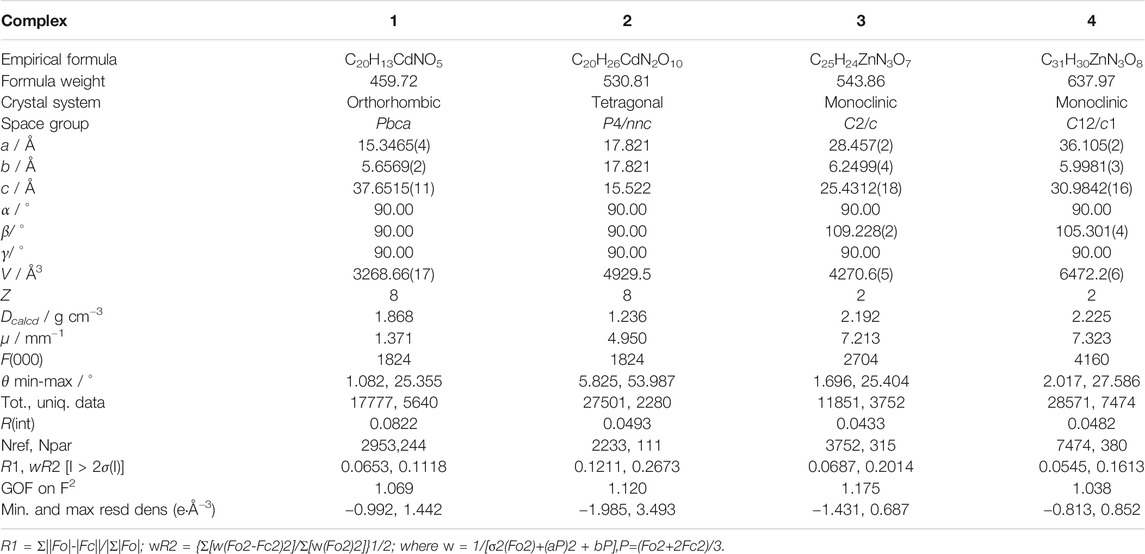
SC-XRD analysis revealed that complex 1 was crystallized in the orthorhombic system with a space group of Pbca and each asymmetric unit consisted of one Cd(II) metal center, one BCPAB2− ligand and one water molecule. As shown in Figure 1, atom Cd1 adopted a distorted pentagonal bipyramid coordination geometry to coordinate with four carboxylate oxygen atoms (O1, O2, O3#2, O4#2) from two neighboring BCPAB2− ligands, two coordinated water molecules (O5, O5#3) and one nitrogen atom (N1#1) from the amino group of BCPAB2− ligand. The Cd-O bond lengths were in the range of 2.281–2.448 Å and the Cd-N bond length was 2.425 Å, which are comparable to the previous Cd-based coordination complexes. (Spek, 1998) Further structural analysis revealed that each carboxylate group of BCPAB2− was bound to one Cd2+ ion and each Cd2+ ion coordinated with two carboxylate groups from two adjacent BCPAB2− ligands, which thus resulted in the formation of one-dimensional (1D) coordination chains (Figure 2A). Furthermore, the coordination bonds between amino groups of the BCPAB2− ligands and Cd2+ ions joined the 1D Cd2+-BCPAB2− chains together to afford two-dimensional (2D) coordination layers (Figure 2B). On the other hand, each water molecule linked two Cd2+ ions to give 1D Cd-O coordination and thus the 2D Cd2+-BCPAB2- layers were connected by the μ2-H2O molecules into the final 3D frameworks (Figure 2C; Supplementary Figure S1A). From the viewpoint of topology, the seven coordination secondary building unit and the BCPAB2− ligand can be regarded as a 5-connected and 3-connected node, respectively, and the structure of 1 could be represented as seh-3, 5-Pbca nets with point symbol of {4.62}{4.67.82} (Supplementary Figure. S1B).
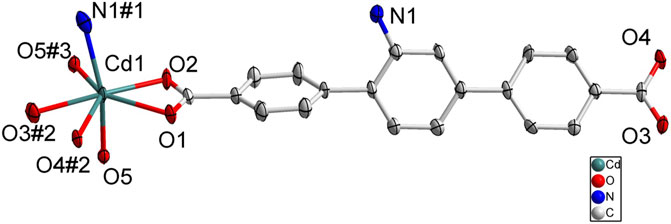
FIGURE 1. Coordination environment of Cd(II) cation in 1 with ellipsoids drawn at 50% probability level. The hydrogen atoms are omitted for clarity. Symmetry codes: #1 1-x, -1/2 + y, 3/2-z; #2 x, 3/2-y, -1/2 + z; #3 1/2-x, -1/2 + y, z.
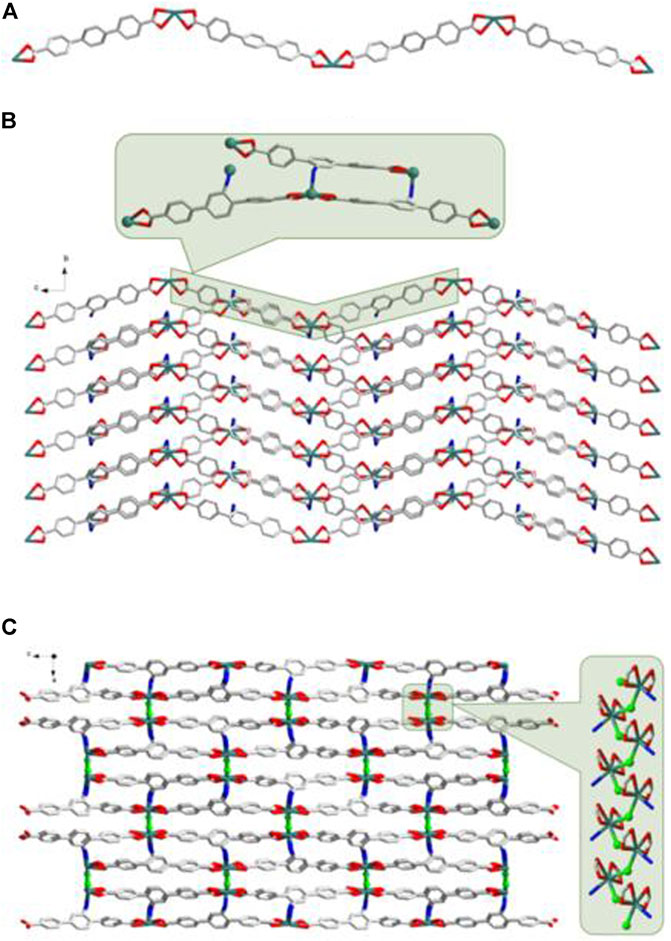
FIGURE 2. (A) The 1D Cd2+-BCPAB2- chain. Amino groups were omitted for clarity. (B) The 2D coordination layers constructed from the Cd2+-BCPAB2- chains via the bonds between amino groups and Cd2+ cations. (C) The 3D frameworks formed by the coordination between the bridged μ2-H2O molecules (green) with Cd2+ cations from different 2D Cd2+-BCPAB2- layers and the 1D chains formed by Cd2+ cations and μ2-H2O molecules. Description of the crystal structure of [Cd(BDAB)]∙2H2O∙DMF}n (2).
According to SC-XRD measurements, complex 2 was crystallized in the tetragonal P4/nnc space group and each asymmetric unit contained half one Cd2+ and half one BDAB2− ligand. As depicted in Figure 3, atom Cd1 was six-coordinated in a disordered octahedral coordination geometry with four carboxylate oxygen atoms (O1, O2, O1#1, O2#1) form two neighboring BDAB2− ligands and two amino groups (N1#2, N1#3) from another two adjacent BDAB2− ligands. Each Cd2+ ion connected two carboxylate groups from different BDAB2− ligands to form 1D helical coordination chains (Figure 4A). Further inspection into the structure found that every four helical chains could assemble into a coordination nanotubular structure with the help of the binding between Cd2+ atoms and amino groups of BDAB2− ligands on the chains (Figure 4B). The Cd2+ ions in the structure of nanotube were only coordinated with carboxylate groups or amino groups and thus they could bind to the amino groups or carboxylate groups from neighboring nanotubes, which then resulted in the formation of the final 3D coordination frameworks with 1D channels running along c-axis. From the point of topological view, because one Cd2+ cation connects four BDAB2− ligands and one BDAB2− ligand links four Cd2+ cations, the central Cd2+ cation and BDAB2− ligand can both be treated as 4-connected nodes. Thence, the network of 2 can be represented with the point symbol is {42.84} calculated by TOPOS software (Supplementary Figure S2). The total solvent cavity volume of 2 is 35.0% per unit cell calculated by PLATON. (Zou et al., 2010)
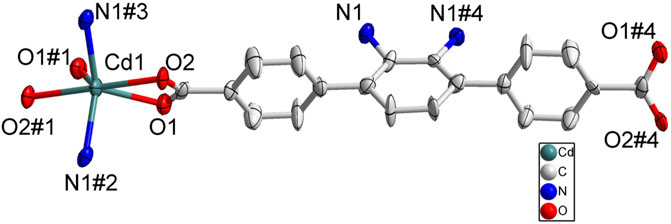
FIGURE 3. Coordination environment of Cd(II) cation in 2 with ellipsoids drawn at 50% probability level. The hydrogen atoms are omitted for clarity. Symmetry codes: #1 1/2-x, 1/2-y, -1/2-z; #2 -1/2 + x, 1/2 + y, -1/2 + z; #3 1-x, -y, -z.
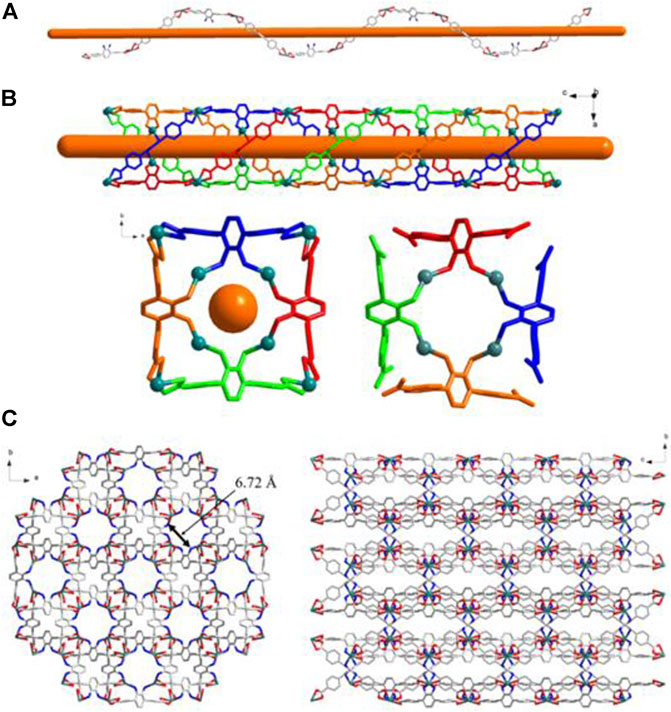
FIGURE 4. (A) The 1D helical coordination chain in 2. (B) The nanotube along b-axis and c-axis constructed from the coordination bonds between the amino groups from four different helical chains and Cd2+ cations. (C) Views of Twenty-membered-ring. (c) 3D frameworks of 2 along c and a axis.
Description of the Crystal Structure of {[Zn(BDAB)(BPD)0.5(H2O)]∙2H2O}n (3) and {[Zn(BDAB)(DBPB)0.5(H2O)]∙2H2O}n (4)
According to the results of SC-XRD measurements, complexes 3 and 4 were both crystallized in the monoclinic C2/c space group and shared a similar framework structure. Each asymmetric unit of 3 consisted of one Zn2+ cation, one BDAB2− ligand, half of one BPD molecule, and one coordinated water molecule. As illustrated in Figure 5, atom Zn1 in 3 adopted a slightly disordered tetrahedral coordination geometry surrounded by two carboxylate oxygen atoms (O2, O4#1) from two adjacent BDAB2− ligands, one nitrogen atom (N3) from the BPD ligand and one coordinated water molecule (O5). The connection between Zn2+ cations and the carboxylate groups of BDAB2− ligands afforded 1D coordination chains (Figure 6A), which were further assembled by the coordination between Zn2+ cations and the nitrogen atoms of BPD ligands to give 2D coordination networks (Figure 6B). On closer inspection, due to the existence of the large pores in the 2D networks, it could be found that six adjacent 2D networks could interlace with each other to give 6-fold interpenetrated 2D supramolecular layers via the π…π, C-H…π interactions (Figures 6C,D; Supplementary Figure S3A). Furthermore, the interpenetrated layers were joined together to generate the final 3D supramolecular architecture by the noncovalent interactions including hydrogen bonds, π…π and C-H…π interactions (Figure 6E; Supplementary Figure S3B).
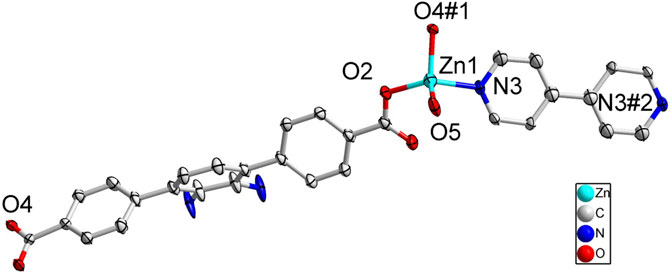
FIGURE 5. Coordination environment of Zn(II) cation in 3 with ellipsoids drawn at 50% probability level. The hydrogen atoms are omitted for clarity. Symmetry codes: #1 1/2 + x, 1/2-y, 1/2 + z; #2 3/2-x, 7/2-y, 2-y.
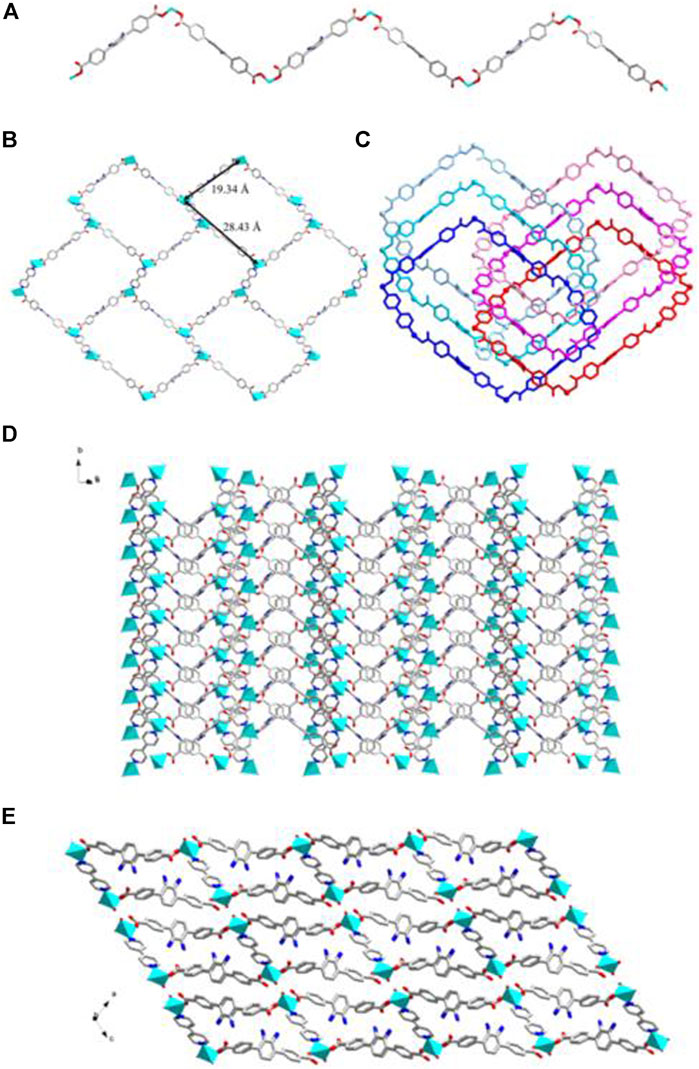
FIGURE 6. (A) The 1D BDAB2--Zn2+ chains in 3. (B) The 2D BDAB2--Zn2+-BPD coordination networks. (C) The interlaced mode of the adjacent BDAB2--Zn2+-BPD networks. (D) The 2D interpenetrated BDAB2--Zn2+-BPD layers. (E) The final 3D supramolecular architecture of 3.
Although a more complicated pyridine ligand DBPB was used instead of BPD to prepare complex 4, the structure of 4 was almost identical to that of 3 and shared the same topological structure with complex 3 (Supplementary Figure S4). Each asymmetric unit of 4 also consisted of one Zn2+ cation, one BDAB2− ligand, half of one DBPB molecule, and one coordinated water molecule. Similar to that of 3, the central Zn2+ cations in 4 also adopted a distorted tetrahedral geometry (Figure 7A) and connected the organic ligands BDAB2− and DBPB to generate 2D coordination networks (Figure 7B). But differently, due to the much larger size, the pyridine ligand DBPB could allow more 2D BDAB2−-Zn2+-DBPB coordination networks to interpenetrate with each other to give an 8-fold interpenetrated 2D supramolecular layers (Figures 7C,D; Supplementary Figure S4A). These supramolecular layers further interact with each other to give the final 3D supramolecular frameworks (Figure 7E; Supplementary Figure S4B) via various noncovalent interactions including C-H…π interactions and hydrogen bonds (Supplementary Figure S4C). Furthermore, one more difference between the structure of 3 and 4 was that 1D channels along b-axis could be observed in the framework of 4 (Figure 7E) and the total solvent cavity volume is 22.5% per unit cell calculated by PLATON. (Zou et al., 2010).
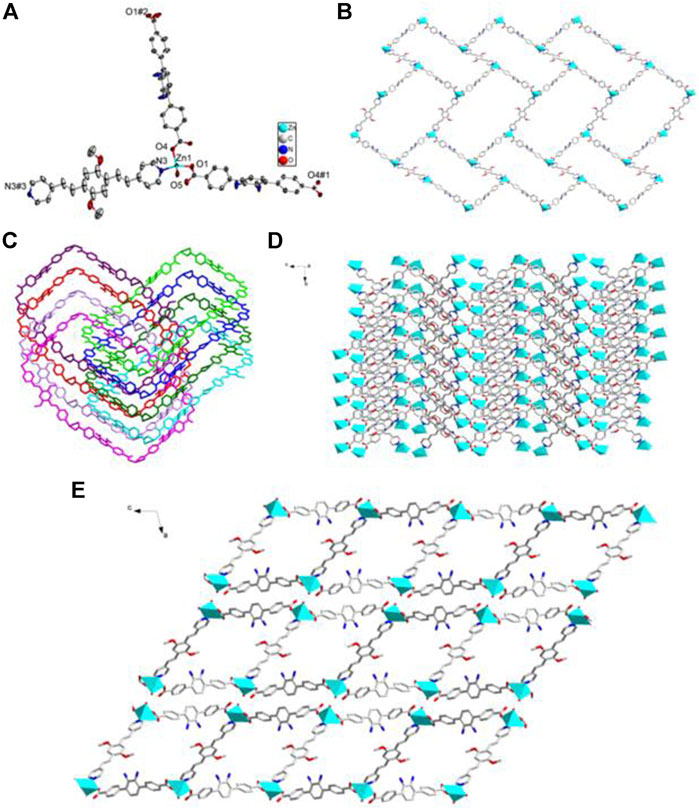
FIGURE 7. (A) Coordination environment of Zn(II) cation in 4 with ellipsoids drawn at 50% probability level. The hydrogen atoms are omitted for clarity. Symmetry codes: #1 x,-y-1,z + 1/2; #2 x,-y-1,z-1/2; #3 -x,-y+3,-z. (B) The 2D BDAB2--Zn2+-DBPB coordination networks. (C) The interlaced mode of the adjacent BDAB2--Zn2+-DBPB networks. (D) The 2D interpenetrated BDAB2--Zn2+-DBPB layers. (E) The final 3D supramolecular architecture of 4 with 1D channels along b-axis.
Powder X-ray Diffraction Results and Thermogravimetric Analyses
The PXRD experiments were carried out to confirm whether the crystal structures are truly representative of the bulk materials. The PXRD experimental and computer-simulated patterns of the corresponding complexes are shown in the ESI (Supplementary Figures S5–S8). The experimental data shows that the bulk synthesized materials are the same as the measured single crystals, suggesting the bulk-phase purity of the obtained MOFs. Furthermore, TGA experiments were also carried out in the N2 atmosphere from 30 to 500°C to examine the thermal stability of 1–4 and the results were depicted in Supplementary Figures S9–S12. Complex 1 showed a weight loss of 3.6 % from 30 to 260°C, suggesting the release of the coordinated water molecules (calcd 3.92 %) and their structure began to collapse at 400 °C. Complex 2 shows a weight loss of 19 % before 250°C, which corresponds to the release of free water and DMF molecules (calcd 19 %), and further weight loss was observed at about 380°C owing to the collapse of the framework of 2. The TGA curve of complex 3 showed that the framework structure began to decomposing at 350°C. Complex 4 displayed a weight loss of 5.4 % before 110°C corresponding to the release of free water molecules (calcd 5.6 %) and then a weight loss of 2.7 % between 110 and 180°C corresponding to the release of coordinated water molecules (calcd 2.8%). Further quick weight losses were observed at 360°C owing to the decomposition of the frameworks of 4.
Fluorescence Properties and Sensing Capacity
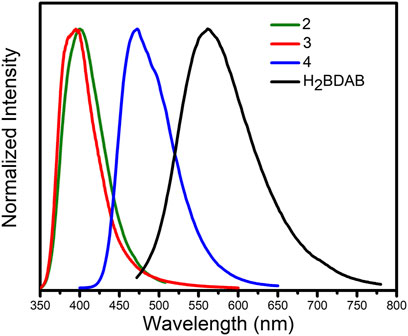
Previous studies have demonstrated that MOFs containing d10-metal ions usually exhibit outstanding fluorescence properties and could function as sensing materials for various substances. On the other hand, in consideration of the existence of channels or uncoordinated amino groups in the structure of complexes 2–4, their fluorescence properties and sensing capability were checked. Thence, the solid-state fluorescence properties of 2–4 were firstly examined at room temperature. As illustrated in Figure 8, the free ligand H2BDAB exhibited characteristic emission bands with maxima at 571 nm upon excitation at 356 nm, while the fluorescence emission maxima of 2–4 were observed at 401, 396, and 473 nm upon excitation at 330, 330, and 380 nm, respectively. Compared to the free ligand, apparent blue-shift emissions were observed for 2–4, which may be attributed to the coordination of multi-aromatic ligands to the metal centers. (Nagarkar et al., 2015) Then, the sensing capacities of 2–4 towards common metal ions were checked as well. Before the sensing experiments, the powder samples of 2–4 were fully ground and immersed in DMF to prepare stable suspension (1.0 mg ml−1), respectively. Then, the DMF solutions (50 μl, 100 mM) containing different metal ions, including K+. Mg2+, Ca2+, Co2+, Ni2+, Cu2+, Pb2+, and Ag+, were added into the DMF suspension of 2–4. The changes in the fluorescence emission intensities were recorded (Supplementary Figures S13–S15) and the results were depicted in Figure 9. It could be found that the existence of Cu2+ could cause obvious reduction in the fluorescence intensity of 2 while there was no significant change for other metal ions. As for 3, the addition of Cu2+ and Ag+ both lead to the quenching of the fluorescence quenching and the addition of other metal ions only caused slight or moderate change in the emission intensities of 3. The fluorescence emissions of 4 were either almost unchanged or enhanced and no specific response was observed for metal ions. Therefore, complex 2 may function as a fluorescent sensor for Cu2+ and complex 3 could detect Cu2+ and Ag+via fluorescence quenching effect.
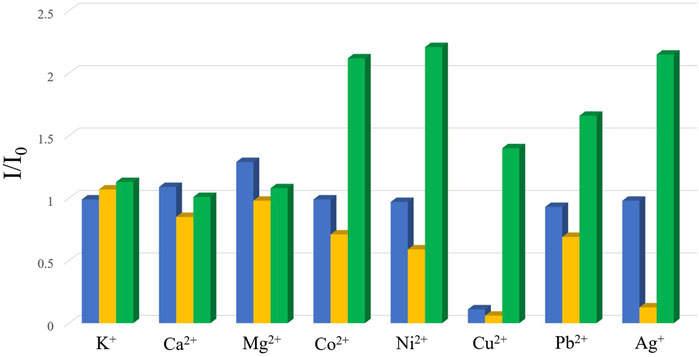
FIGURE 9. The changes in the emission intensities of 2–4 (blue: 2; yellow: 3; green: 4) upon the addition of the solutions of vairous metal ions.
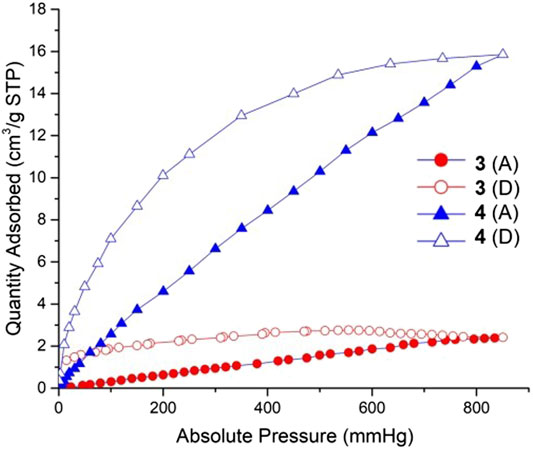
FIGURE 10. CO2 sorption isotherms for complexes 3 and 4 at 298 K. Filled symbols: adsorption; empty symbols: desorption. Red points and lines: complex 3; Blue points and lines: complex 4.
Carbon Dioxide Adsorption Properties
Coordination polymers 3 and 4 can maintain the stability of the frameworks after 10 h heating and activation. We using CO2 as the adsorptive gas to measure the sorption properties of these two complexes. The CO2 sorption isotherms of these two complexes measured at 298 K are shown in Figure 7. The gas sorption isotherms indicated a CO2 uptake of 2.4 cm3/g for 3, 15.9 cm3/g for 4. Complex 4 exhibits much more adsorption than complex 3 due to its high porosity: 1,455.9 Å3 and the accessible volumes 22.5% for 4. It is speculated that the reason for the low porosity of 3 is that the length of the co-ligand in 3 is relatively short, and the double interspersed would serve to occupy more of the free void space within the porous structure, so that the carbon dioxide molecules cannot enter the hole.
Conclusion
In summary, amino-functionalized dicarboxylate ligands H2BCPAB and H2BDAB were employed to react with d10 metal ions Cd2+ and Zn2+ to generate four novel MOFs with the formula of {[Cd(BCPAB)(μ2-H2O)]}n (1), {[Cd(BDAB)]∙2H2O∙DMF}n (2), {[Zn(BDAB)(BPD)0.5(H2O)]∙2H2O}n (3) and {[Zn(BDAB)(DBPB)0.5(H2O)]∙2H2O}n (4) in the absence and presence of auxiliary pyridyl ligands. Complexes 1 and 2 are 3D frameworks with point symbol of {4.62}{4.67.82} and {42.84}, respectively. Complexes 3 and 4 are generally isostructural and have the similar 3D supramolecular frameworks constructed from 6-fold to 8-fold 2D interpenetrated coordination layers. The fluorescence properties of 2–4 were studied and their capacity as fluorescent sensors for metal ions were explored as well. In addition, the adsorption properties of 3 and 4 for CO2 were investigated. The sensing experiments suggested that complex 2 could detect Cu2+ and complex 3 could act as a sensor for Cu2+ and Ag+via quenching effect.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author Contributions
KS and ZW designed experiments;WX and JW carried out experiments; XH and HZ analyzed experimental results; KS wrote the manuscript
Funding
This work was supported by Nanjing Tech University research start-up fund (No. 3827401787 and 3983500195).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fchem.2021.708314/full#supplementary-material
References
Barea, E., Montoro, C., and Navarro, J. A. R. (2014). Toxic Gas Removal - Metal-Organic Frameworks for the Capture and Degradation of Toxic Gases and Vapours. Chem. Soc. Rev. 43 (16), 5419–5430. doi:10.1039/c3cs60475f
Canivet, J., Fateeva, A., Guo, Y., Coasne, B., and Farrusseng, D. (2014). Water Adsorption in MOFs: Fundamentals and Applications. Chem. Soc. Rev. 43 (16), 5594–5617. doi:10.1039/c4cs00078a
Cao, C.-C., Chen, C.-X., Wei, Z.-W., Qiu, Q.-F., Zhu, N.-X., Xiong, Y.-Y., et al. (2019). Catalysis through Dynamic Spacer Installation of Multivariate Functionalities in Metal-Organic Frameworks. J. Am. Chem. Soc. 141 (6), 2589–2593. doi:10.1021/jacs.8b12372
Dhakshinamoorthy, A., and Garcia, H. (2014). Metal-organic Frameworks as Solid Catalysts for the Synthesis of Nitrogen-Containing Heterocycles. Chem. Soc. Rev. 43 (16), 5750–5765. doi:10.1039/c3cs60442j
Dong, J., Zhang, X.-D., Xie, X.-F., Guo, F., and Sun, W.-Y. (2020). Amino Group Dependent Sensing Properties of Metal-Organic Frameworks: Selective Turn-On Fluorescence Detection of Lysine and Arginine. RSC Adv. 10, 37449–37455. doi:10.1039/d0ra06879a
Dutta, G., Jana, A. K., Singh, D. K., Eswaramoorthy, M., and Natarajan, S. (2018). Encapsulation of Silver Nanoparticles in an Amine-Functionalized Porphyrin Metal-Organic Framework and its Use as a Heterogeneous Catalyst for CO2 Fixation under Atmospheric Pressure. Chem. Asian J. 13, 2677–2684. doi:10.1002/asia.201800815
Gutiérrez, M., Martin, C., Kennes, K., Hofkens, J., Van der Auweraer, M., Sánchez, F., et al. (2018). New OLEDs Based on Zirconium Metal-Organic Framework. Adv. Opt. Mater. 6, 1701060. doi:10.1002/adom.201701060
Han, X., Lu, W., Chen, Y., da Silva, I., Li, J., Lin, L., et al. (2021). High Ammonia Adsorption in MFM-300 Materials: Dynamics and Charge Transfer in Host-Guest Binding. J. Am. Chem. Soc. 143 (8), 3153–3161. doi:10.1021/jacs.0c11930
He, Y., Zhou, W., Qian, G., and Chen, B. (2014). Methane Storage in Metal-Organic Frameworks. Chem. Soc. Rev. 43 (16), 5657–5678. doi:10.1039/c4cs00032c
Hu, Z., Deibert, B. J., and Li, J. (2014). Luminescent Metal-Organic Frameworks for Chemical Sensing and Explosive Detection. Chem. Soc. Rev. 43 (16), 5815–5840. doi:10.1039/c4cs00010b
Hu, X.-L., Gong, Q.-H., Zhong, R.-L., Wang, X.-L., Qin, C., Wang, H., et al. (2015). Evidence of Amine-CO2Interactions in Two Pillared-Layer MOFs Probed by X-ray Crystallography. Chem. Eur. J. 21, 7238–7244. doi:10.1002/chem.201406495
Li, J.-R., Kuppler, R. J., and Zhou, H.-C. (2009). Selective Gas Adsorption and Separation in Metal-Organic Frameworks. Chem. Soc. Rev. 38, 1477–1504. doi:10.1039/b802426j
Li, L., Lin, R.-B., Krishna, R., Li, H., Xiang, S., Wu, H., et al. (2018). Ethane/ethylene Separation in a Metal-Organic Framework with Iron-Peroxo Sites. Science 362, 443–446. doi:10.1126/science.aat0586
Liu, J., Chen, L., Cui, H., Zhang, J., Zhang, L., and Su, C.-Y. (2014). Applications of Metal-Organic Frameworks in Heterogeneous Supramolecular Catalysis. Chem. Soc. Rev. 43 (16), 6011–6061. doi:10.1039/c4cs00094c
Lustig, W. P., Mukherjee, S., Rudd, N. D., Desai, A. V., Li, J., and Ghosh, S. K. (2017). Metal-organic Frameworks: Functional Luminescent and Photonic Materials for Sensing Applications. Chem. Soc. Rev. 46, 3242–3285. doi:10.1039/c6cs00930a
Mallick, A., El-Zohry, A. M., Shekhah, O., Yin, J., Jia, J., Aggarwal, H., et al. (2019). Unprecedented Ultralow Detection Limit of Amines Using a Thiadiazole-Functionalized Zr(IV)-Based Metal-Organic Framework. J. Am. Chem. Soc. 141 (18), 7245–7249. doi:10.1021/jacs.9b01839
Medishetty, R., Zaręba, J. K., Mayer, D., Samoć, M., and Fischer, R. A. (2017). Nonlinear Optical Properties, Upconversion and Lasing in Metal-Organic Frameworks. Chem. Soc. Rev. 46, 4976–5004. doi:10.1039/c7cs00162b
Nagarkar, S. S., Desai, A. V., Samanta, P., and Ghosh, S. K. (2015). Aqueous Phase Selective Detection of 2,4,6-trinitrophenol Using a Fluorescent Metal-Organic Framework with a Pendant Recognition Site. Dalton Trans. 44, 15175–15180. doi:10.1039/c5dt00397k
Park, J., Xu, M., Li, F., and Zhou, H.-C. (2018). 3D Long-Range Triplet Migration in a Water-Stable Metal-Organic Framework for Upconversion-Based Ultralow-Power In Vivo Imaging. J. Am. Chem. Soc. 140 (16), 5493–5499. doi:10.1021/jacs.8b01613
Peng, Y., Krungleviciute, V., Eryazici, I., Hupp, J. T., Farha, O. K., and Yildirim, T. (2013). Methane Storage in Metal-Organic Frameworks: Current Records, Surprise Findings, and Challenges. J. Am. Chem. Soc. 135 (32), 11887–11894. doi:10.1021/ja4045289
Prasad, R. R. R., Dawson, D. M., Cox, P. A., Ashbrook, S. E., Wright, P. A., Clarke, M. L., et al. (2018). A Bifunctional MOF Catalyst Containing Metal-Phosphine and Lewis Acidic Active Sites. Chem. Eur. J. 24 (57), 15309–15318. doi:10.1002/chem.201803094
Sheberla, D., Bachman, J. C., Elias, J. S., Sun, C.-J., Shao-Horn, Y., and Dincă, M. (2017). Conductive MOF Electrodes for Stable Supercapacitors with High Areal Capacitance. Nat. Mater. 16 (2), 220–224. doi:10.1038/nmat4766
Shen, K., Qin, L., and Zheng, H.-G. (2016). Diverse Structures of Metal-Organic Frameworks via a Side Chain Adjustment: Interpenetration and Gas Adsorption. Dalton Trans. 45, 16205–16210. doi:10.1039/c6dt03086f
Silva, P., Vilela, S. M. F., Tomé, J. P. C., and Almeida Paz, F. A. (2015). Multifunctional Metal-Organic Frameworks: from Academia to Industrial Applications. Chem. Soc. Rev. 44 (19), 6774–6803. doi:10.1039/c5cs00307e
Spek, A. L. (1998). Implemented as the PLATON Procedure, a Multipurpose Crystallographic Tool. Utrecht, Netherlands: Utrecht University.
Tchalala, M. R., Bhatt, P. M., Chappanda, K. N., Tavares, S. R., Adil, K., Belmabkhout, Y., et al. (2019). Fluorinated MOF Platform for Selective Removal and Sensing of SO2 from Flue Gas and Air. Nat. Commun. 10, 1328. doi:10.1038/s41467-019-09157-2
Tomar, K., Rajak, R., Sanda, S., and Konar, S. (2015). Synthesis and Characterization of Polyhedral-Based Metal-Organic Frameworks Using a Flexible Bipyrazole Ligand: Topological Analysis and Sorption Property Studies. Cryst. Growth Des. 15, 2732–2741. doi:10.1021/acs.cgd.5b00056
Tranchemontagne, D. J., Mendoza-Cortés, J. L., O’Keeffe, M., and Yaghi, O. M. (2009). Secondary Building Units, Nets and Bonding in the Chemistry of Metal-Organic Frameworks. Chem. Soc. Rev. 38 (5), 1257–1283. doi:10.1039/b817735j
Trickett, C. A., Helal, A., Al-Maythalony, B. A., Yamani, Z. H., Cordova, K. E., and Yaghi, O. M. (2017). The Chemistry of Metal-Organic Frameworks for CO2 Capture, Regeneration and Conversion. Nat. Rev. Mater. 2, 17045. doi:10.1038/natrevmats.2017.45
Van de Voorde, B., Bueken, B., Denayer, J., and De Vos, D. (2014). Adsorptive Separation on Metal-Organic Frameworks in the Liquid Phase. Chem. Soc. Rev. 43 (16), 5766–5788. doi:10.1039/c4cs00006d
Wang, Z., Jingjing, Q., Wang, X., Zhang, Z., Chen, Y., Huang, X., et al. (2018). Two-dimensional Light-Emitting Materials: Preparation, Properties and Applications. Chem. Soc. Rev. 47 (16), 6128–6174. doi:10.1039/c8cs00332g
Wang, H., Liu, Y., and Li, J. (2020). Designer Metal-Organic Frameworks for Size‐Exclusion‐Based Hydrocarbon Separations: Progress and Challenges. Adv. Mater. 32 (44), 2002603. doi:10.1002/adma.202002603
Zárate, J. A., Sánchez-González, E., Williams, D. R., González-Zamora, E., Martis, V., Martínez, A., et al. (2019). High and Energy-Efficient Reversible SO2 Uptake by a Robust Sc(iii)-Based MOF. J. Mater. Chem. A. 7, 15580–15584. doi:10.1039/c9ta02585e
Zárate, J. A., Sánchez-González, E., Jurado-Vázquez, T., Gutiérrez-Alejandre, A., González-Zamora, E., Castillo, I., et al. (2019). Outstanding Reversible H2S Capture by an Al(iii)-Based MOF. Chem. Commun. 55, 3049–3052. doi:10.1039/c8cc09379b
Zhang, Z., Yao, Z.-Z., Xiang, S., and Chen, B. (2014). Perspective of Microporous Metal-Organic Frameworks for CO2 capture and Separation. Energy Environ. Sci. 7, 2868–2899. doi:10.1039/c4ee00143e
Zhu, G., Sun, Q., Kawazoe, Y., and Jena, P. (2015). Porphyrin-based Porous Sheet: Optoelectronic Properties and Hydrogen Storage. Int. J. Hydrogen Energ. 40 (9), 3689–3696. doi:10.1016/j.ijhydene.2015.01.069
Zou, J.-P., Peng, Q., Wen, Z., Zeng, G.-S., Xing, Q.-J., and Guo, G.-C. (2010). Two Novel Metal−Organic Frameworks (MOFs) with (3,6)-Connected Net Topologies: Syntheses, Crystal Structures, Third-Order Nonlinear Optical and Luminescent Properties. Cryst. Growth Des. 10, 2613–2619. doi:10.1021/cg100104t
Keywords: metal-organic frameworks, d10 -metal ions, amino groups, fluorescence, detection
Citation: Xie W, Wu J, Hang X, Zhang H, shen K and Wang Z (2021) Four Novel d10 Metal-Organic Frameworks Incorporating Amino-Functionalized Carboxylate Ligands: Synthesis, Structures, and Fluorescence Properties. Front. Chem. 9:708314. doi: 10.3389/fchem.2021.708314
Received: 11 May 2021; Accepted: 18 June 2021;
Published: 30 August 2021.
Edited by:
Tao Yu, Northwestern Polytechnical University, ChinaReviewed by:
Zhang-Wen Wei, Sun Yat-Sen University, ChinaLiangliang Zhang, Northwestern Polytechnical University, China
Copyright © 2021 Xie, Wu, Hang, Zhang, shen and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kang shen, aWFta2FuZ3NoZW5Abmp0ZWNoLmVkdS5jbg==; Zhoulu Wang, NzM2NDgxMzUxQHFxLmNvbQ==
 Wang Xie1
Wang Xie1 Kang shen
Kang shen