
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Chem., 12 July 2021
Sec. Medicinal and Pharmaceutical Chemistry
Volume 9 - 2021 | https://doi.org/10.3389/fchem.2021.695628
This article is part of the Research TopicFrontiers in Chemistry - Rising Stars: AsiaView all 13 articles
In this study, 17 novel pyrimidine derivatives containing an amide moiety were synthesized. Then their in vitro antifungal activities against Botryosphaeria dothidea (B. dothidea), Phomopsis sp., and Botrytis cinereal (B. cinereal) were determined. A preliminary biological test showed that compounds 5-bromo-2-fluoro-N-(2-((2-methyl-6-(trifluoromethyl)pyrimidin-4-yl)oxy)phenyl)benzamide (5f) and 5-bromo-2-fluoro-N-(3-((2-methyl-6-(trifluoromethyl)pyrimidin-4-yl)oxy)phenyl)benzamide (5o) exhibited higher antifungal activity against Phomopsis sp., with an inhibition rate of 100% compared to that of Pyrimethanil at 85.1%. In particular, compound 5o exhibited excellent antifungal activity against Phompsis sp., with the EC50 value of 10.5 μg/ml, which was even better than that of Pyrimethanil (32.1 μg/ml). As far as we know, this is the first report on the antifungal activities against B. dothidea, Phomopsis sp., and B. cinereal of this series of pyrimidine derivatives containing an amide moiety.
Plant fungal diseases pose serious threats to crop production and caused huge economic losses throughout the world (Strange and Scott, 2005). In recent years, crop cultivators continually battle with plant fungal diseases affecting crops. The available traditional fungicides used for plant fungal diseases control represent a danger to the living system by killing not only the target fungi but also affecting beneficial living systems (Patel et al., 2014). To protect crops from fungal diseases, commercial agriculture relies heavily on the inputs of chemical pesticides. The resistance of plant fungal diseases against fungicides is rapidly becoming a serious problem. Therefore, the development of novel and promising fungicides is urgently required.
Pyrimidines are important substances in the synthesis of various active molecules that are extensively used in the intermediate skeleton of agrochemicals (Chen et al., 2015; Wu et al., 2015) and have attracted more and more attention due to their extensive biological activities (Sun et al., 2006), including antiviral (Wu et al., 2016a), antibacterial (Wu et al., 2016b), antifungal (Wu et al., 2019, 2020), and insecticidal (Liu et al., 2017; Wu et al., 2020) activities. For example, Zan et al. reported pyrimidine derivatives bearing a dithioacetal moiety as effective antiviral agents for controlling the tomato chlorosis virus (ToCV) (Zan et al., 2021). Zhang et al. found a series of arylpyrazole pyrimidine ether derivatives with promising bioactivity for combating cucumber downy mildew (Zhang et al., 2019). Li and coworkers showed that pyrimidine thiourea derivatives had good herbicidal activities against Digitaria adscendens and Amaranthus retroflexus (Li et al., 2021a). In the past few years, several pyrimidine compounds have been commercialized as fungicides (such as Pyrimethanil, Fenarimol, Diflumetorim, and Mepanioyrim) for controlling plant fungal diseases, such as cucumber gray mold (Wang et al., 2021), grape downy mildew (Huang et al., 2018), kiwifruit leaf spot (Shi et al., 2021), and so on. Due to the excellent features of low toxicity, the fact that it is easily synthetized and derived, and considering pyrimidine as a parent compound, the development of promising agrochemical candidates will soon become a reality. Meanwhile, amine, a key moiety in heterocyclic chemistry, play a leading role in pesticide chemistry due to their potent bioactivities including antifungal (Cheng et al., 2020; Chen et al., 2021a), antibacterial (Chen et al., 2021a), antiviral (Tang et al., 2020), herbicidal (Sun et al., 2020), and insecticidal (Chen et al., 2021b; Li et al., 2021b) activities. In our previous work, we reported a series of novel pyrimidine derivatives containing an amine moiety (Figure 1) and found that the target compounds revealed certain antifungal, insecticidal, and antiviral activities (Wu et al., 2016a: Wu et al., 2020).
FIGURE 1. Structures of pyrimidine derivatives containing an amide moiety reported in our previous works.
This study aimed to design and synthesize a series of novel pyrimidine derivatives containing an amide moiety, and investigate their in vitro antifungal activities against Botryosphaeria dothidea (B. dothidea), Phomopsis sp., and Botrytis cinereal (B. cinereal). A biological test showed that compound 5-bromo-2-fluoro-N-(3-((2-methyl-6-(trifluoromethyl)pyrimidin-4-yl)oxy)phenyl)benzamide (5o) exhibited excellent antifungal activity against Phompsis sp., with an EC50 value of 10.5 μg/ml, which is even better than Pyrimethanil (32.1 μg/ml).
The melting points of the products were determined on an XT-4 binocular microscope (Beijing Tech Instrument Co., China) and were not corrected. 1H and 13C NMR (solvent DMSO-d6) spectra were recorded on a Bruker AVANCE HD 600 MHz Digital NMR Spectrometer (Bruker Company, Billerica, MA, United States) at room temperature using TMS as an internal standard. High-resolution mass spectrometry (HRMS) was carried out on an Agilent Technologies 6540 UHD Accurate-Mass Q-TOF LC/MS (Agilent Technologies, Palo Alto, CA, United States). All anhydrous solvents were dried and purified according to standard techniques before use. Unless otherwise noted, all common reagents and solvents were used as obtained from commercial supplies without further purifications.
As shown in Scheme 1, to a 250 ml round bottom flask, trifluoroacetoacetate (1, 0.05 mol), acetamidine hydrochloride (0.05 mol), sodium methoxide (0.075 mol), and ethanol (100 ml) were added and refluxed for 10 h. After that, the mixture was acidified with dilute HCl to pH 7. The crude products were extracted using ethyl acetate to produce intermediate 2. Then, intermediate 2 (0.05 mol), POCl3 (0.1 mol), and CH3CN (120 ml) were added to a 250 ml round bottom flask to react for 0.5 h at a reflux temperature and then diisopropylethylamine (0.06 mol) was added dropwise. After continuously refluxing for 8 h, excess POCl3 and CH3CN were distilled and then ice water (60 ml) was added. Finally, the mixture was alkalified with dilute NaOH to pH 9 and extracted using CH2Cl2 to give intermediate 3.
To a 50 ml three-necked round-bottomed flask equipped with a magnetic stirrer, the key intermediate 3 (0.01 mol) was dissolved in acetone (50 ml), Cs2CO3 (0.012 mol), and 3-aminophenol or 2-aminophenol (0.01 mol) were added. The reactions reacted for 5–6 h at room temperature, and the solvent was removed. The residue was added with water, the precipitate formed was filtered off and recrystallized from ethanol to give the intermediate 4.
To a 50 ml three-necked round-bottomed flask equipped with a magnetic stirrer, the key intermediate 4 (0.02 mol), aromatic acid (0.02 mol), and dimethylaminopyridine (0.0002 mol) dissolved in dichloromethane (10 ml), and 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (0.03 mol) were added. The reactions were reacted for 8–10 h at room temperature. The solvent was then dried under vacuum and recrystallized from ethanol to give the pure target compounds 5a–5q.
The structures were confirmed by 1H NMR, 13C NMR, and HRMS. 1H NMR, 13C NMR, and HRMS spectral data for the target compounds 5a–5q are reported in the Supplementary Material. In the 1H NMR spectra of 5a–5q, one singlet at δ 9.70–10.74 ppm were attributed to the -CONH-. A singlet at δ 6.90–7.54 ppm demonstrated the presence of the proton of the pyrimidine fragment. A singlet at δ 2.31–2.54 ppm integrating for three protons was assigned to Pyrimidine-CH3 protons. The structure of 5a–5q was also confirmed by its HRMS spectral data. In all HRMS spectrum, the molecular ion peak was noticed m/z for ([M + H]+) corresponding to all of the target molecular weight.
N-(2-((2-methyl-6-(trifluoromethyl)pyrimidin-4-yl)oxy)phenyl)benzamide (5a). White solid; yield 75%; m.p. 173–175°C; 1H NMR (DMSO-d6, 600 MHz, ppm) δ: 9.72 (s, 1H), 7.96 (d, 2H, J = 7.8 Hz), 7.76 (d, 1H, J = 7.2 Hz), 7.71 (t, 1H, J = 8.4 Hz), 7.53 (t, 2H, J = 9.0 Hz), 7.41–7.31 (m, 3H, Ph-H), 6.90 (s, 1H), 2.32 (s, 3H); 13C NMR (DMSO-d6, 150 MHz, ppm) δ: 168.66, 164.39, 162.11, 152.99 (q, J = 33.6 Hz), 144.29, 134.43, 130.75, 130.08, 129.35, 129.22, 126.84, 126.40, 125.99, 124.06, 122.24 (q, J = 272.85 Hz), 100.17, 25.75; HRMS (calcd.) for C19H14F3N3O2 (M + H)+ 374.1107, found 374.1107.
2-Chloro-N-(2-((2-methyl-6-(trifluoromethyl)pyrimidin-4-yl)oxy)phenyl)benzamide (5b). White solid; yield 49%; m.p. 157–158°C; 1H NMR (DMSO-d6, 600 MHz, ppm) δ: 9.73 (s, 1H), 7.93 (d, 1H, J = 7.8 Hz), 7.82 (d, 1H, J = 6.0 Hz), 7.66–7.63 (m, 2H), 7.49–7.46 (m, 1H), 7.43 (d, 1H, J = 7.8 Hz), 7.40–7.33 (m, 2H), 6.91 (s, 1H), 2.38 (s, 3H); 13C NMR (DMSO-d6, 150 MHz, ppm) δ: 168.75, 162.96, 162.24, 153.25 (q, J = 33.6 Hz), 144.14, 134.62, 133.51, 132.34, 131.70, 130.75, 128.70, 127.80, 127.14, 126.60, 123.97, 122.28 (q, J = 272.55 Hz), 120.46, 100.24, 25.86; HRMS (calcd.) for C19H13ClF3N3O2 (M + H)+ 408.0721, found 408.0720.
N-(2-((2-methyl-6-(trifluoromethyl)pyrimidin-4-yl)oxy)phenyl)-2-(trifluoromethyl)benzamide (5c). White solid; yield 46%; m.p. 148–150°C; 1H NMR (DMSO-d6, 600 MHz, ppm) δ: 9.77 (s, 1H), 7.94 (t, 2H, J = 6.0 Hz), 7.86–7.81 (m, 3H), 7.41–7.36 (m, 3H), 6.92 (s, 1H), 2.39 (s, 3H); 13C NMR (DMSO-d6, 150 MHz, ppm) δ: 168.75, 163.97, 162.25, 153.52 (q, J = 33.6 Hz), 144.03, 133.33, 133.21, 131.22, 130.77, 129.50, 128.33, 128.11 (q, J = 31.95 Hz), 127.52, 127.36, 126.57, 124.65 (q, J = 272.1 Hz), 123.45, 122.25 (q, J = 272.7 Hz), 120.45, 100.23, 25.85; HRMS (calcd.) for C20H13F6N3O2 (M + H)+ 442.0985, found 442.0979.
N-(2-((2-methyl-6-(trifluoromethyl)pyrimidin-4-yl)oxy)phenyl)-3-(trifluoromethyl)benzamide (5d). White solid; yield 68%; m.p. 138–140°C; 1H NMR (DMSO-d6, 600 MHz, ppm) δ: 9.81 (s, 1H), 8.30 (d, 1H, J = 7.8 Hz), 8.14 (s, 1H), 8.09 (d, 1H, J = 7.8 Hz), 7.82 (t, 1H, J = 7.8 Hz), 7.78 (d, 1H, J = 7.2 Hz), 7.48 (d, 1H, J = 8.4 Hz), 7.41–7.35 (m, 2H), 6.91 (s, 1H), 2.30 (s, 3H); 13C NMR (DMSO-d6, 150 MHz, ppm) δ: 168.66, 163.13, 162.04, 153.33 (q, J = 34.2 Hz), 144.11, 134.06, 130.82, 130.77, 130.60, 130.55, 130.16 (q, J = 31.65 Hz), 127.09, 126.44, 126.08, 126.00, 124.92 (q, J = 270.9 Hz), 124.18, 123.10 (q, J = 270.6 Hz), 100.10, 25.60; HRMS (calcd.) for C20H13F6N3O2 (M + H)+ 442.0985, found 442.0979.
2,3-Dimethoxy-N-(2-((2-methyl-6-(trifluoromethyl)pyrimidin-4-yl)oxy)phenyl)benzamide (5e). White solid; yield 50%; m.p. 130–131°C; 1H NMR (DMSO-d6, 600 MHz, ppm) δ: 7.63 (s, 1H),7.54 (d, 1H, J = 7.6 Hz), 7.46 (t, 1H, J = 7.4 Hz), 7.41 (d, 1H, J = 8.1 Hz), 7.35–7.33 (m, 2H), 7.06 (d, 1H, J = 8.1 Hz), 6.97 (t, 1H, J = 7.9 Hz), 6.92 (d, 1H, J = 7.6 Hz), 3.76 (s, 3H), 3.69 (s, 3H), 2.40 (s, 3H); 13C NMR (DMSO-d6, 150 MHz, ppm) δ: 169.52, 168.91, 162.84, 156.21 (q, J = 35.25 Hz), 152.54, 147.60, 145.80, 132.19, 131.54, 130.54, 127.24, 124.32, 124.10, 121.66 (q, J = 273.45 Hz), 115.48, 108.51, 103.05, 61.44, 56.28, 25.56; HRMS (calcd.) for C21H18F3N3O4 (M + H)+ 434.1322, found 434.1318.
3,4,5-trimethoxy-N-(2-((2-methyl-6-(trifluoromethyl)pyrimidin-4-yl)oxy)phenyl)benzamide (5g). White solid; yield 58%; m.p. 127–128°C; 1H NMR (DMSO-d6, 600 MHz, ppm) δ: 9.74 (s, 1H), 7.72 (d, 1H, J = 7.8 Hz), 7.40–7.35 (m, 3H),7.21 (s, 2H, Ph-H), 6.89 (s, 1H),3.78 (s, 6H), 3.75 (s, 3H) 2.33 (s, 3H); 13C NMR (DMSO-d6, 150 MHz, ppm) δ: 168.77, 163.92, 153.21 (q, J = 34.6 Hz), 153.18, 142.77, 130.66, 126.93, 124.22, 120.40 (q, J = 272.3 Hz),107.36, 60.67, 56.37, 25.76; HRMS (calcd.) for C22H20F3N3O5 (M + H)+ 464.1428, found 464.1420.
2-Chloro-N-(3-((2-methyl-6-(trifluoromethyl)pyrimidin-4-yl)oxy)phenyl)benzamide (5h). White solid; yield 62%; m.p.136–139°C; 1H NMR (DMSO-d6, 600 MHz, ppm) δ: 10.74 (s, 1H), 7.75 (s, 1H), 7.62–7.58 (m, 3H), 7.54–7.52 (m, 2H), 7.49–7.45 (m, 2H), 7.04 (dd, 1H, J1 = 1.3 Hz, J2 = 8.0 Hz), 2.54 (s, 3H); 13C NMR (DMSO-d6, 150 MHz, ppm) δ: 170.52, 169.76, 165.62, 156.54 (q, J = 34.65 Hz), 155.83, 152.36, 140.85, 137.16, 131.74, 130.56, 130.40, 130.18, 129.43, 127.78, 123.67 (q, J = 273.45 Hz), 117.52, 117.46, 112.91, 103.46, 25.94; HRMS (calcd.) for C19H13ClF3N3O2 (M + H)+ 408.0721, found 408.0716.
4-Fluoro-N-(3-((2-methyl-6-(trifluoromethyl)pyrimidin-4-yl)oxy)phenyl)benzamide (5i). White solid; yield 85%; m.p. 136–139°C; 1H NMR (DMSO-d6, 600 MHz, ppm) δ: 9.72 (s, 1H), 8.06–8.04 (dd, 2H, J1 = 5.4 Hz, J2 = 8.4 Hz), 7.79 (d, 1H, J = 7.2 Hz), 7.41–7.35 (m, 5H), 7.31 (t, 1H, J = 7.8 Hz), 6.91 (s, 1H), 2.37 (s, 3H); 13C NMR (DMSO-d6, 150 MHz, ppm) δ: 168.67, 167.65, 165.07, 163.46, 162.07, 153.20 (q, J = 33.75 Hz), 144.08, 133.11, 130.74, 126.87, 126.30, 125.99, 125.89, 124.18, 122.23 (q, J = 272.85 Hz), 116.48, 100.25, 25.75; HRMS (calcd.) for C19H13F4N3O2 (M + H)+ 392.1017, found 392.1013.
N-(3-((2-methyl-6-(trifluoromethyl)pyrimidin-4-yl)oxy)phenyl)-2-(trifluoromethyl)benzamide (5j). White solid; yield 73%; m.p. 140–142°C; 1H NMR (DMSO-d6, 600 MHz, ppm) δ: 10.80 (s, 1H), 7.87 (d, 1H, J = 7.7 Hz), 7.82 (t, 1H, J = 7.3 Hz), 7.75–7.72 (m, 3H), 7.58 (d, 1H, J = 7.9 Hz), 7.52 (s, 1H), 7.47 (t, 1H, J = 8.2 Hz), 7.05 (d, 1H, J = 7.7 Hz), 2.54 (s, 3H); 13C NMR (DMSO-d6, 150 MHz, ppm) δ: 170.48, 169.75, 166.26, 156.33 (q, J = 33.6 Hz), 152.36, 140.81, 136.36, 133.14, 130.55, 129.02, 126.84, 126.46 (q, J = 31.05 Hz), 123.32 (q, J = 271.8 Hz), 121.84 (q, J = 272.85 Hz), 117.50, 112.97, 103.46, 25.91; HRMS (calcd.) for C20H13F6N3O2 (M + H)+ 442.0985, found 442.0982.
N-(3-((2-methyl-6-(trifluoromethyl)pyrimidin-4-yl)oxy)phenyl)-3-(trifluoromethyl)benzamide (5k). White solid; yield 73%; m.p. 101–103°C; 1H NMR (DMSO-d6, 600 MHz, ppm) δ: 10.65 (s, 1H), 8.30 (s, 1H), 8.27 (d, 1H, J = 7.8 Hz), 7.97 (d, 1H, J = 7.7 Hz), 7.81–7.79 (m, 2H), 7.71 (d, 1H, J = 8.1 Hz), 7.50 (s, 1H), 7.48 (d, 1H, J = 8.2 Hz), 7.05 (d, 1H, J = 8.0 Hz), 2.53 (s, 3H); 13C NMR (DMSO-d6, 150 MHz, ppm) δ: 170.56, 169.79, 164.71, 156.34 (q, J = 33.6 Hz), 152.29, 140.81, 136.01, 132.35, 130.22, 130.02, 129.81 (q, J = 31.8 Hz), 128.74, 125.31 (q, J = 271.8 Hz), 124.75, 123.52, 121.82 (q, J = 273.0 Hz), 118.39, 117.52, 113.84, 103.36, 25.91; HRMS (calcd.) for C20H13F6N3O2 (M + H)+ 442.0985, found 442.0983.
N-(3-((2-methyl-6-(trifluoromethyl)pyrimidin-4-yl)oxy)phenyl)-4-(trifluoromethyl)benzamide (5l). White solid; yield 80%; m.p. 94–96°C; 1H NMR (DMSO-d6, 600 MHz, ppm) δ: 10.66 (s, 1H), 8.15 (d, 2H, J = 8.4 Hz), 7.94 (d, 2H, J = 7.8 Hz), 7.79 (t, 1H, J = 1.8 Hz), 7.69 (d, 1H, J = 9.6 Hz), 7.52 (s, 1H), 7.49 (t, 1H, J = 8.4 Hz), 7.06 (d, 2H, J = 7.8 Hz), 2.53 (s, 3H); 13C NMR (DMSO-d6, 150 MHz, ppm) δ: 170.59, 169.80, 165.13, 156.34 (q, J = 34.6 Hz), 152.31, 140.83, 138.99, 132.11 (q, J = 31.62 Hz),130.52, 129.16, 125.98, 125.31(q, J = 273.25 Hz), 120.05 (q, J = 272.51 Hz), 118.35, 117.61 103.48, 25.98; HRMS (calcd.) for C20H13F6N3O2 (M + H)+ 442.0985, found 442.0978.
2,3-Dimethoxy-N-(3-((2-methyl-6-(trifluoromethyl)pyrimidin-4-yl)oxy)phenyl)benzamide (5m). White solid; yield 52%; m.p. 108–109°C; 1H NMR (DMSO-d6, 600 MHz, ppm) δ: 10. 47 (s, 1H), 7.78 (s, 1H), 7.62 (d, 1H, J = 7.9 Hz), 7.51 (s, 1H), 7.45 (t, 1H, J = 8.2 Hz), 7.22–7.17 (m, 2H), 7.13 (dd, 1H, J1 = 1.4 Hz, J2 = 7.3 Hz), 7.02 (dd, 1H, J1 = 1.3 Hz, J2 = 8.0 Hz), 3.87 (s, 3H), 3.82 (s, 3H), 2.54 (s, 3H); 13C NMR (DMSO-d6, 150 MHz, ppm) δ: 170.55, 169.79, 165.59, 156.31 (q, J = 34.8 Hz), 153.05, 152.35, 146.32, 141.05, 131.70, 130.46, 124.72, 121.85 (q, J = 273.45 Hz), 120.45, 117.54, 117.10, 115.23, 112.91, 103.40, 61.57, 56.42, 25.92; HRMS (calcd.) for C21H18F3N3O4 (M + H)+ 434.1322, found 434.1318.
3-Bromo-4-chloro-N-(3-((2-methyl-6-(trifluoromethyl)pyrimidin-4-yl)oxy)phenyl)benzamide (5n). White solid; yield 68%; m.p. 143–145°C; 1H NMR (DMSO-d6, 600 MHz, ppm) δ: 10.56 (s, 1H), 8.35 (d, 1H, J = 1.9 Hz), 7.97 (dd, 1H, J1 = 2.1 Hz, J2 = 8.3 Hz), 7.80 (d, 1H, J = 8.4 Hz), 7.76 (s, 1H), 7.68 (d, 1H, J = 8.1 Hz), 7.54–7.52 (m, 2H, Ph-H), 7.49–7.46 (m, 2H), 7.04 (dd, 1H, J1 = 1.4 Hz, J2 = 8.0 Hz), 2.53 (s, 3H); 13C NMR (DMSO-d6, 150 MHz, ppm) δ: 170.54, 169.79, 163.71, 156.33 (q, J = 34.8 Hz), 152.27, 140.74, 137.07, 135.34, 133.25, 131.05, 130.42, 129.12, 122.11, 121.81 (q, J = 273.3 Hz), 118.31, 117.51, 113.76, 103.37, 25.92; HRMS (calcd.) for C19H12BrClF3N3O2 (M + H)+ 485.9826, found 485.9822.
5-Bromo-2-fluoro-N-(3-((2-methyl-6-(trifluoromethyl)pyrimidin-4-yl)oxy)phenyl)benzamide (5o). White solid; yield 52%; m.p.147–149 °C; 1H NMR (DMSO-d6, 600 MHz, ppm) δ: 10.74 (s, 1H), 7.89–7.87 (m, 1H), 7.79–7.77 (m, 1H, Ph-H), 7.73 (s, 1H), 7.60 (d, 1H, J = 7.9 Hz), 7.50 (s, 1H), 7.47 (t, 1H, J = 8.2 Hz), 7.38 (t, 1H, J = 9.3 Hz), 7.05 (dd, 1H, J1 = 1.4 Hz, J2 = 8.0 Hz), 2.53 (s, 3H); 13C NMR (DMSO-d6, 150 MHz, ppm) δ: 170.52, 169.76, 161.91, 159.46, 157.80, 156.32 (q, J = 34.65 Hz), 152.34, 140.55, 135.67, 132.64, 130.58, 127.15, 121.83 (q, J = 273.15 Hz), 119.24, 117.66, 116.63, 113.19, 103.42, 25.92; HRMS(calcd.) for C19H12BrF4N3O2 (M + H)+ 470.0122, found 470.0117.
2-Bromo-5-fluoro-N-(3-((2-methyl-6-(trifluoromethyl)pyrimidin-4-yl)oxy)phenyl)benzamide (5p). White solid; yield 60%; m.p.152–153 °C; 1H NMR (DMSO-d6, 600 MHz, ppm) δ: 10.78 (s, 1H), 7.78 (q, 1H, J = 5.0 Hz), 7.72 (s, 1H), 7.59–7.58 (m, 2H), 7.52 (s, 1H), 7.48 (t, 1H, J = 8.2 Hz), 7.34 (dd, 1H, J1 = 3.0 Hz, J2 = 8.6 Hz, Ph-H), 7.06 (dd, 1H, J1 = 1.4 Hz, J2 = 8.0 Hz), 2.54 (s, 3H); 13C NMR (DMSO-d6, 150 MHz, ppm) δ: 170.49, 169.75, 165.15, 162.38, 160.74, 156.31 (q, J = 34.65 Hz), 152.36, 140.82, 140.64, 135.16, 130.60, 121.84 (q, J = 273.15 Hz), 118.87, 117.59, 116.63, 114.21, 112.98, 103.49, 25.94; HRMS(calcd.) for C19H12BrF4N3O2 (M + H)+ 470.0122, found 470.0118.
3,4,5-trimethoxy-N-(3-((2-methyl-6-(trifluoromethyl)pyrimidin-4-yl)oxy)phenyl)benzamide (5q). White solid; yield 78%; m.p.116–117 °C; 1H NMR (DMSO-d6, 600 MHz, ppm) δ: 10.31 (s, 1H), 7.73 (s, 1H), 7.69 (d, 1H, J = 7.8 Hz), 7.51 (s, 1H), 7.48 (t, 1H, J = 7.8 Hz), 7.28 (s, 2H), 7.03 (d, 1H, J = 7.8 Hz), 3.87 (s, 3H), 3.74 (s, 3H), 2.51 (s, 3H); 13C NMR (DMSO-d6, 150 MHz, ppm) δ: 170.59, 169.79, 165.58, 156.31 (q, J = 34.65 Hz), 153.11, 152.27, 141.07, 140.90, 130.37, 130.23, 120.02 (q, J = 272.85 Hz), 118.41, 117.19, 113.82, 105.78, 103.35, 60.58, 56.55, 25.92; HRMS(calcd.) for C22H20F3N3O2 (M + H)+ 464.1428, found 464.1425.
The antifungal activities of all synthesized compounds at the concentration of 50 μg/ml were evaluated for their in vitro antifungal activities against the pathogenic fungi, including B. dothidea, Phompsis sp., and B. cinerea by the poison plate technique (Min et al., 2016). All the compounds were dissolved in 1 ml dimethyl sulfoxide (DMSO) before mixing with 90 ml potato dextrose agar (PDA). Mycelia dishes of approximately 5 mm diameter were cut from the culture medium and then picked up with a germfree inoculation needle and inoculated in the middle of the PDA plate aseptically. The inoculated plates were fostered at 27 ± 1 C for 3–4 days. DMSO in sterile distilled water served as a negative control, while Pyrimethanil acted as a positive control. For each treatment, three replicates were conducted. The inhibition rate I (%) was calculated by the following formula, where C (cm) represents the diameter of fungi growth on untreated PDA, and T (cm) represents the diameter of fungi on treated PDA.
The in vitro antifungal activities of the title compounds against the pathogenic fungi, including B. dothidea, Phomopsis sp., and B. cinereal at 50 μg/ml were tested and the results are shown in Table 1. Bioassay results showed that compounds 5i, 5l, 5n, and 5o had good inhibition rates on B. dothidea, with the inhibition rates of 82.1, 81.1, 84.1, and 88.5%, respectively, which were similar to Pyrimethanil (84.4%). Meanwhile, the inhibition rates of compounds 5f, 5n, 5o, and 5p against Phomopsis sp. were 100.0, 91.8, 100.0, and 93.4%, which were even better than Pyrimethanil (85.1%). In addition, some of the target compounds, for example compounds 5c (80.0%), 5i (79.6%), 5l (80.4%), 5m (83.6%), 5n (79.7%), 5o (84.7%), and 5q (80.5%), showed equally antifungal activity against B. cinerea to Pyrimethanil (82.8%).
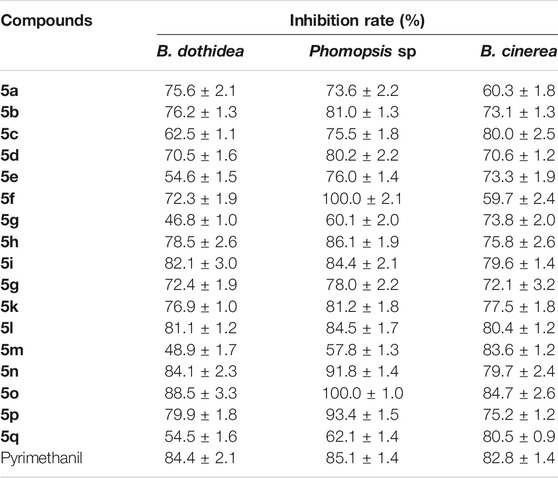
TABLE 1. | The antifungal activities of the title compounds against B. dothidea, Phomopsis sp., and B. cinereal at 50 μg/ml.
The EC50 values and antifungal diagram of the target compounds 5f, 5o, and 5p were also tested and are presented in Table 2 and Figure 2 respectively. Table 2 shows that compounds 5f, 5o, and 5p exhibited excellent antifungal activity against Phomopsis sp., with the EC50 values of 15.1, 10.5, and 19.6 μg/ml, which were superior to that of Pyrimethanil (32.1 μg/ml).
In order to design novel and more promising active small molecules of pyrimidine derivatives, SAR analysis was also performed. The chemical structure of the target compounds indicated that the position of the amine group and the position and size of the substituent group R of the target compounds significantly influence the antifungal activities against. B. dothidea, Phomopsis sp. and B. cinereal. With the amine group at 3-position of the benzene ring and F and Br atoms at 2- and 5-position, respectively, compound 5o exhibited excellent antifungal activities against. B. dothidea, Phomopsis sp. and B. cinereal, which were even better than those of Pyrimethanil. Meanwhile, in general terms, the antifungal activities of the target compounds with the amine group at 3-position of benzene ring were better than those of the corresponding target compounds with the amine group at 2-position of the benzene ring, for example, 5h > 5b and 5k > 5d.
In conclusion, a total of 17 pyrimidine derivatives containing an amide moiety were synthesized and evaluated for their in vitro fungicidal activities against B. dothidea, Phompsis sp., and B. cinerea by the poison plate technique. Bioassay results demonstrated that compound 5-bromo-2-fluoro-N-(3-((2-methyl-6-(trifluoromethyl)pyrimidin-4-yl)oxy)phenyl)benzamide (5o) exhibited excellent antifungal activity against Phompsis sp., with the EC50 value of 10.5 μg/ml, which were even better than that of Pyrimethanil. This study provided a practical tool for guiding the design and synthesis of novel and more promising active small molecules of pyrimidine derivatives for controlling Phompsis sp., This study also demonstrated that this series of pyrimidine derivatives containing an amide moiety can be used to develop potential agrochemicals. In accordance with the pesticide registration requirements in China, further field and toxicity studies of compound 5o will be undertaken in a future study.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
WW and QF contributed to the synthesis, purification, characterization of all compounds, and prepared the original manuscript. LW and CW performed the activity research. QF and LW perfected the language and assisted with the structure elucidation and manuscript revision. WW designed and supervised the research and revised the manuscript. All authors discussed, edited, and approved the final version.
This research was financially supported by the Science and Technology Fund Project of Guizhou (NO (2020)1Z023), the National Natural Science Foundation of China (No. 31701821), Guizhou Provincial Department of Education Youth Science and Technology Talents Growth Project (QJHKYZ (2018)291), the Guizhou Province Biological and Pharmaceutical engineering Research Center (No. QJHKY(2019)051), the Special Funding of Guiyang Science and Technology Bureau and Guiyang University (GYU-KY-(2021)), and Undergraduate Innovation and Entrepreneurship Training Program (NO. 2019520835).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fchem.2021.695628/full#supplementary-material.
Chen, M., Lu, D., Zhang, X., Chen, M., Dong, C., Wang, X., et al. (2021a). Synthesis and Biological Activities of Novel S-β-D-Glucopyranoside Derivatives of 1,2,4-triazole. Phosphorus, Sulfur, Silicon Relat. Elem., 1–6. doi:10.1080/10426507.2021.1901704
Chen, S., Zhang, Y., Liu, Y., and Wang, Q. (2021b). Highly Efficient Synthesis and Acaricidal and Insecticidal Activities of Novel Oxazolines with N-Heterocyclic Substituents. J. Agric. Food Chem. 69, 3601–3606. doi:10.1021/acs.jafc.0c05558
Chen, X. M., Wang, S. H., Cui, D. L., and Li, B. (2015). The Synthesis and Herbicidal Activity of 5‐(substituted-Phenyl)-4,6-Dioxo-4,5,6,7-Tetrahydropyrazolo[3,4-D]pyrimidines. J. Heterocyclic Chem. 52, 607–610. doi:10.1002/jhet.2047
Cheng, Y.-N., Jiang, Z.-H., Sun, L.-S., Su, Z.-Y., Zhang, M.-M., and Li, H.-L. (2020). Synthesis of 1, 2, 4-triazole Benzoyl Arylamine Derivatives and Their High Antifungal Activities. Eur. J. Med. Chem. 200, 112463. doi:10.1016/j.ejmech.2020.112463
Huang, Y. X., Li, M., Pan, X. J., Wu, X. M., Xiang, X. L., Li, W. Z., et al. (2018). Control Effect of Several Fungicides on Grape Downy Mildew. Agrochemicals 57, 836–839. doi:10.16820/j.cnki.1006-0413.2018.11.017
Li, H., Zhao, Y., Sun, P., Gao, L., Li, Y., Xiong, L., et al. (2021a). Synthesis and Insecticidal Evaluation of Novel Anthranilic Diamides Derivatives Containing 4‐Chlorine Substituted N ‐Pyridylpyrazole. Chin. J. Chem. 39, 75–80. doi:10.1002/cjoc.202000013
Li, J.-h., Wang, Y., Wu, Y.-p., Li, R.-h., Liang, S., Zhang, J., et al. (2021b). Synthesis, Herbicidal Activity Study and Molecular Docking of Novel Pyrimidine Thiourea. Pestic. Biochem. Physiol. 172, 104766. doi:10.1016/j.pestbp.2020.104766
Liu, X.-H., Wang, Q., Sun, Z.-H., Wedge, D. E., Becnel, J. J., Estep, A. S., et al. (2017). Synthesis and Insecticidal Activity of Novel Pyrimidine Derivatives Containing Urea Pharmacophore againstAedes Aegypti. Pest Manag. Sci. 73, 953–959. doi:10.1002/ps.4370
Min, L.-J., Shi, Y.-X., Wu, H.-K., Sun, Z.-H., Liu, X.-H., Li, B.-J., et al. (2016). Microwave-assisted Synthesis and Antifungal Activity of Some Novel Thioethers Containing 1,2,4-triazole Moiety. Appl. Sci. 5, 1211–1220. doi:10.3390/app5041211
Patel, N., Desai, P., Patel, N., Jha, A., and Gautam, H. K. (2014). Agronanotechnology for Plant Fungal Disease Management: A Review. Int. J. Curr. Microbiol. App. Sci. 3, 71–84. doi:10.5455/ijmsph.2014.110320141
Shi, J. Q., Zhang, R. Q., He, L. N., Chen, J., Hu, A. L., Yin, X. H., et al. (2021). Screening of Fungicide for Controlling Kiwifruit Leaf Spot. Agrochemicals 60, 294–296. doi:10.16820/j.cnki.1006-0413.2021.04.016
Strange, R. N., and Scott, P. R. (2005). Plant Disease: a Threat to Global Food Security. Annu. Rev. Phytopathol. 43, 83–116. doi:10.1146/annurev.phyto.43.113004.133839
Sun, F. F., Ma, N., and Li, Z. M. (2006). 2-Amino-4-(2,2,2-trifluoroethoxy) Pyrimidine. Acta Cryst. 62, 3864–3865. doi:10.1107/s1600536806029564
Sun, X., Ji, Z., Wei, S., and Ji, Z. (2020). Design, Synthesis and Herbicidal Activity of 5-Cyclopropyl-N-Phenylisoxazole-4-Carboxamides. J. Mol. Struct. 1220, 128628. doi:10.1016/j.molstruc.2020.128628
Tang, X., Zhang, C., Chen, M., Xue, Y., Liu, T., and Xue, W. (2020). Synthesis and Antiviral Activity of Novel Myricetin Derivatives Containing Ferulic Acid Amide Scaffolds. New J. Chem. 44, 2374–2379. doi:10.1039/C9NJ05867B
Wang, Y., Li, J., Zhang, X. Y., Li, Y. L., Hu, B., Sun, H., et al. (2021). Controlling Test of High Efficiency and Low Toxicity Fungicides to Botrytis Cinerea on Cucumber. Vegetables 1, 38–41.
Wu, W.-N., Gao, M.-N., Tu, H., and Ouyang, G.-P. (2016a). Synthesis and Antibacterial Activity of Novel Substituted Purine Derivatives. J. Heterocyclic Chem. 53, 2042–2048. doi:10.1002/jhet.2527
Wu, W.-N., Jiang, Y.-M., Fei, Q., and Du, H.-T. (2019). Synthesis and Fungicidal Activity of Novel 1,2,4-triazole Derivatives Containing a Pyrimidine Moiety. Phosphorus, Sulfur, Silicon Relat. Elem. 194, 1171–1175. doi:10.1080/10426507.2019.1633321
Wu, W.-N., Tai, A.-Q., Chen, Q., and Ouyang, G.-P. (2016b). Synthesis and Antiviral Bioactivity of Novel 2-substituted Methlthio-5-(4-Amino-2-Methylpyrimidin-5-Yl)-1,3,4-Thiadiazole Derivatives. J. Heterocyclic Chem. 53, 626–632. doi:10.1002/jhet.2435
Wu, W., Chen, M., Fei, Q., Ge, Y., Zhu, Y., Chen, H., et al. (2020). Synthesis and Bioactivities Study of Novel Pyridylpyrazol Amide Derivatives Containing Pyrimidine Motifs. Front. Chem. 8, 522. doi:10.3389/fchem.2020.00522
Wu, W., Chen, Q., Tai, A., Jiang, G., and Ouyang, G. (2015). Synthesis and Antiviral Activity of 2-substituted Methylthio-5-(4-Amino-2-Methylpyrimidin-5-Yl)-1,3,4-Oxadiazole Derivatives. Bioorg. Med. Chem. Lett. 25, 2243–2246. doi:10.1016/j.bmcl.2015.02.069
Zan, N., Xie, D., Li, M., Jiang, D., and Song, B. (2020). Design, Synthesis, and Anti-ToCV Activity of Novel Pyrimidine Derivatives Bearing a Dithioacetal Moiety that Targets ToCV Coat Protein. J. Agric. Food Chem. 68, 6280–6285. doi:10.1021/acs.jafc.0c00987
Keywords: pyrimidine, amide, synthesize, antifungal activity, kiwifruit soft rot disease
Citation: Wu W, Lan W, Wu C and Fei Q (2021) Synthesis and Antifungal Activity of Pyrimidine Derivatives Containing an Amide Moiety. Front. Chem. 9:695628. doi: 10.3389/fchem.2021.695628
Received: 16 April 2021; Accepted: 31 May 2021;
Published: 12 July 2021.
Edited by:
Wukun Liu, Nanjing University of Chinese Medicine, ChinaReviewed by:
Saad Shaaban, Mansoura University, EgyptCopyright © 2021 Wu, Lan, Wu and Fei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenneng Wu, d3V3ZW5uZW5nMTIzQDEyNi5jb20=; Qiang Fei, ZnFvcmdhbmljQDEyNi5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.