
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Chem., 26 February 2021
Sec. Medicinal and Pharmaceutical Chemistry
Volume 9 - 2021 | https://doi.org/10.3389/fchem.2021.648684
This article is part of the Research TopicReactive Oxygen Species-Based Nanomaterials for Advanced Biomedical ApplicationsView all 12 articles
Cryopreservation prolongs the storage time of cells and plays an important role in modern biology, agriculture, plant science and medicine. During cryopreservation, cells may suffer many damages, such as osmotic dehydration, large ice puncture and oxidative damages from reactive oxygen species (ROS). Classic cryoprotectants (CPAs) are failing to dispose of ROS, while antioxidants can turn ROS into harmless materials and regulate oxidative stress. The combination of antioxidants and CPAs can improve the efficiency of cryopreservation while negative results may occur by misuse of antioxidants. This paper discussed the feasibility of antioxidants in cryopreservation.
Cryopreservation is a technique for preserving cells at low temperatures, which can prolong their storage time. However, organisms are easy to be damaged during freezing for the following two reasons: osmotic damage and mechanical damage. Osmotic damage is caused by the freezing of the extracellular solution, leading to increases in the concentrations of the solutes. Subsequently, the cells are damaged by osmotic dehydration. Mechanical damage refers to the puncture damage of cells by sharp ice crystals (Yang et al., 2017). Therefore, many cryoprotectants (CPAs) have been developed to reduce damages. Permeable CPAs, such as DMSO(Ock and Rho, 2011) and glycerol (Rogers et al., 2018), can enter cells to adjust osmotic pressure and reduce osmotic damage. Impermeable CPAs, such as antifreeze protein (Xiang et al., 2020) can decrease the size of extracellular ice crystals to reduce mechanical damage. The addition of CPAs can improve the efficiency of cryopreservation.
However, recent studies have shown that oxidative stress occurs in cells during cryopreservation. Oxidative stress refers to a state of imbalance between oxidation and anti-oxidation, which is caused by the massive production of reactive oxygen species (ROS) in extreme conditions such as low temperatures in cells (Evangelista-Vargas and Santiani, 2017). Cellular antioxidants, such as glutathione and thioredoxin, can resist ROS by participating the reduction process when the concentration of ROS is low (Yang et al., 2018; Alhayaza et al., 2020). However, the large amount of ROS produced during cryopreservation can cause the oxidation of proteins, lipids and nucleic acids (Chen and Li, 2020). These may cause irreversible damages to cells and even lead to apoptosis (Len et al., 2019). Classic permeable and impermeable CPAs are failing to reduce oxidative damage to cells.
Antioxidants, such as ascorbate acid (Mathew et al., 2019), glutathione (Diengdoh et al., 2019), mitoquinone (Sui et al., 2018), salidroside (Alotaibi et al., 2016), resveratrol (Longobardi et al., 2017) and so forth, can resist the oxidative stress and reduce the damages from ROS. Therefore, antioxidants and CPAs can be used together to comprehensively reduce the harm in cryopreservation. It must be noted that the misuse of antioxidants could cause negative effects. So appropriate antioxidants must be carefully selected in cryopreservation. In this paper, the source, species, properties, mechanisms and damages of ROS are introduced in detail. The results of the combination with CPAs and antioxidants are also concluded to promote the development of cryopreservation.
ROS mainly includes superoxide anion radical
In cryopreservation, the damage caused by ROS can be attributed to lipid peroxidation (Banday et al., 2017), protein oxidation (Mostek et al., 2017) and DNA damage (Ladeira et al., 2019). Lipid peroxidation (LPO) refers to the decomposition of lipids into aldehydes such as 4-hydroxynonenal (4-HNE) and malondialdehyde (MDA) under the action of ROS. The content of MDA in cells can reflect the degree of LPO (Tsikas, 2017). LPO seriously affects cells’ function due to lipid is an important part of cell membranes (Uchendu et al., 2010). Besides, MDA is highly toxic and can react with nucleic acids and proteins, further causing damages to cells (Long et al., 2009). Proteins can be converted into carbonyl proteins by ROS, and the content of carbonyl in proteins can indicate the degree of protein oxidation (Li et al., 2010). Protein oxidation can induce DNA damage, lipid damage, cell secondary damage, and lower enzyme efficiency (Davies, 2016). Furthermore, gene mutation, double/single strand breaking occur in DNA in the presence of ROS (Len et al., 2019), causing serious damage such as apoptosis (Zhao et al., 2016). Comet assay is a standard test to quantitatively detect the degree of DNA damage (Ladeira et al., 2019). All the damages caused by ROS can seriously affect the physiological function of cells and reduce the efficiency of cryopreservation.
Antioxidants are powerful substances to counter ROS. The use of specific antioxidants at appropriate concentrations can significantly reduce the damages from ROS and improve the efficiency of cryopreservation. However, the wrong use of antioxidants can result in negative results.
In cryopreservation, antioxidants can reduce oxidative stress (Mathew et al., 2019), regulate the synthesis of mitochondrial proteins (Banday et al., 2017), decrease ROS production (Zhu et al., 2019), clear intracellular ROS (Len et al., 2019), enhance the activity of antioxidant enzyme (Azadi et al., 2017), resist to LPO and DNA fragmentation (Yousefian et al., 2018). Specifically, for germ cells such as sperm, antioxidants can increase motility parameters (Toker et al., 2016), acrosomal integrity (Lone et al., 2018), mitochondrial membrane potential (Fontoura et al., 2017) and pregnancy rates (Ren et al., 2018). Therefore, the combination of antioxidants and CPAs may reduce the damages to cells caused by osmotic dehydration, large ice puncture and ROS during freezing and thawing, and improve the efficiency of cryopreservation (as shown in Table 1 and Figure 1).
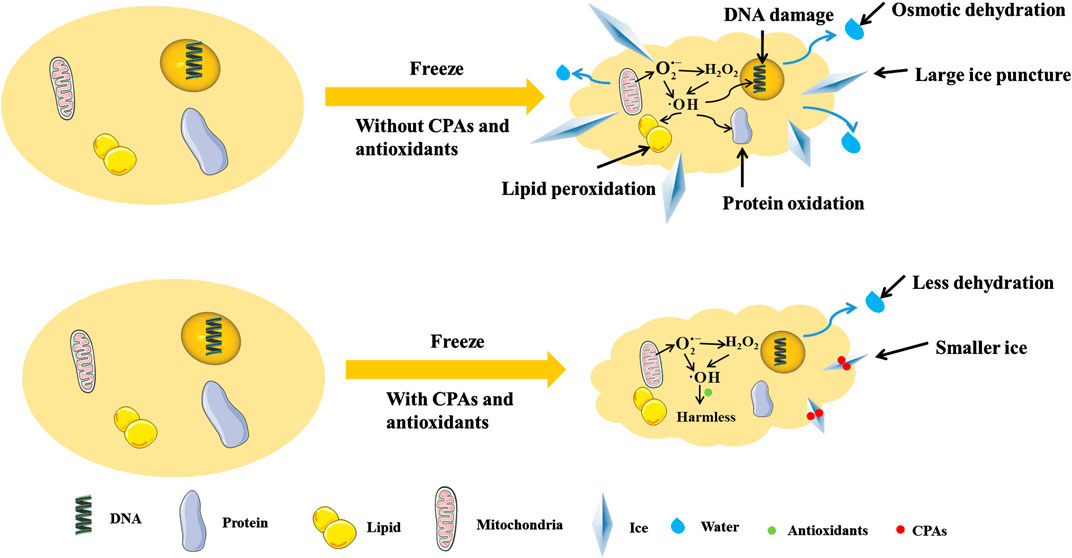
FIGURE 1. The freeze cell with/without CPAs and antioxidants. CPAs can adsorb in ice surface to inhibit ice growth, and regulate osmotic pressure to reduce dehydration. Antioxidants can reduce the production of ROS and turn ROS into harmless materials, so as to relieve the damages by ROS. The antioxidants and CPAs can use together to reduce the damages from cryopreservation comprehensively.
There are some negative effects of using antioxidants in cryopreservation. For instance, when ascorbic acid is used for cryopreservation of Aranda Broga Blue orchid, the growth regeneration percentage will be reduced from 5 to 1.7% (Khor et al., 2020). In the cryopreservation of human semen, the addition of ascorbic acid, vitamin E, and L-carnitine can adversely affect sperm motility, especially at high concentrations (Banihani and Alawneh, 2019). The reason may be that antioxidants not only reduce ROS but also have negative effects on the endogenous antifreeze mechanism of cells (Khor et al., 2020). Furthermore, the high concentrations of antioxidants transform cells from oxidative stress to reductive stress, which may also have negative effects on the structure and function of cells (Bisht and Dada, 2017). It is noticeable that the use of antioxidants in cryopreservation is not always satisfactory.
Cryopreservation is more and more widely used nowadays. Many CPAs have been developed to reduce damages during freezing and thawing. ROS produced at low temperatures can cause lipid peroxidation, protein oxidation and DNA damage, seriously affect the structure and function of cells, and even cause cell apoptosis. Traditional CPAs cannot resist ROS. Antioxidants can decrease oxidative stress, reduce the production of ROS, convert ROS into harmless substances, and increase the activity of ROS enzymes. Therefore, the use of antioxidants and CPAs in cryopreservation may increase cells’ survival rate, motility and reproductive capacity, reduce lipid peroxidation, protein oxidation and DNA damage, decrease the osmotic and mechanical damages by ice, so the efficiency of cryopreservation is increased. It must be noted that the use of antioxidants does not always have a positive effect, especially when the concentration of antioxidants is relatively high. This may be that antioxidants can destroy the natural antifreeze mechanism of cells and transform cells from oxidative stress to reductive stress. This suggests that antioxidants are a double-edged sword, and good results only occur when antioxidants are used properly.
At present, there are the following research directions of antioxidants in cryopreservation.
(1) Expanding applications. Currently, antioxidants are mainly used for the cryopreservation of cells and plant tissues. In the future, antioxidants can be used cautiously in the cryopreservation of human tissues and organs to promote the development of organ transplantation, regenerative medicine and cryomedicine.
(2) Exploring mechanisms. The microcosmic interaction between antioxidants and ROS in cells is still unclear. The study of mechanisms can guide the development and application of antioxidants.
(3) Using untapped antioxidants. Many natural and artificial antioxidants may have potential in cryopreservation and not be used yet. Using untapped antioxidants with proper CPAs may increase the efficiency of cryopreservation cheaply and effectively.
(4) Revealing effective conditions. Sometimes antioxidants may cause negative results in cryopreservation. For the development of antioxidants in cryopreservation, it is important to reveal the conditions that positive results will occur.
XL has made sustantial contributions to the conception and design of this work. YX, FL, YP, LM, QZ have took part in revising work critically for important intellectual content. ST has revised work and approved the final version to be published.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Alhayaza, R., Haque, E., Karbasiafshar, C., Sellke, F. W., and Abid, M. R. (2020). The relationship between reactive oxygen species and endothelial cell metabolism. Front. Chem. 8, 592688. doi:10.3389/fchem.2020.592688
Aliakbari, F., Sedighi Gilani, M. A., Yazdekhasti, H., Koruji, M., Asgari, H. R., Baazm, M., et al. (2017). Effects of antioxidants, catalase and α-tocopherol on cell viability and oxidative stress variables in frozen-thawed mice spermatogonial stem cells. Artif. Cell Nanomed Biotechnol. 45, 63–68. doi:10.3109/21691401.2016.1138491
Alotaibi, N. A. S., Slater, N. K. H., and Rahmoune, H. (2016). Salidroside as a novel protective agent to improve red blood cell cryopreservation. PLoS One. 11, e0162748. doi:10.1371/journal.pone.0162748
Azadi, L., Tavalaee, M., Deemeh, M. R., Arbabian, M., and Nasr-Esfahani, M. H. (2017). Effects of tempol and quercetin on human sperm function after cryopreservation. Cryo Lett. 38, 29–36.
Banday, M. N., Lone, F. A., Rasool, F., Rashid, M., and Shikari, A. (2017). Use of antioxidants reduce lipid peroxidation and improve quality of crossbred ram sperm during its cryopreservation. Cryobiology 74, 25–30. doi:10.1016/j.cryobiol.2016.12.008
Banihani, S. A., and Alawneh, R. F. (2019). Human semen samples with high antioxidant reservoir may exhibit lower post-cryopreservation recovery of sperm motility. Biomolecules 9, 111. doi:10.3390/biom9030111
Bienert, G. P., Møller, A. L., Kristiansen, K. A., Schulz, A., Møller, I. M., Schjoerring, J. K., et al. (2007). Specific aquaporins facilitate the diffusion of hydrogen peroxide across membranes. J. Biol. Chem. 282, 1183–1192. doi:10.1074/jbc.M603761200
Bisht, S., and Dada, R. (2017). Oxidative stress: major executioner in disease pathology, role in sperm DNA damage and preventive strategies. Front. Biosci. (Schol Ed.) 9, 420–447. doi:10.2741/s495
Chen, W., and Li, D. (2020). Reactive oxygen species (ROS)-Responsive nanomedicine for solving ischemia-reperfusion injury. Front. Chem. 8, 00732. doi:10.3389/fchem.2020.00732
Davies, M. J. (2016). Protein oxidation and peroxidation. Biochem. J. 473, 805–825. doi:10.1042/BJ20151227
Diengdoh, R. V., Kumaria, S., and Das, M. C. (2019). Antioxidants and improved regrowth procedure facilitated cryoconservation of Paphiopedilum insigne Wall. Ex. Lindl. - an endangered Slipper orchid. Cryobiology 87, 60–67. doi:10.1016/j.cryobiol.2019.02.003
Evangelista‐Vargas, S., and Santiani, A. (2017). Detection of intracellular reactive oxygen species (superoxide anion and hydrogen peroxide) and lipid peroxidation during cryopreservation of Alpaca spermatozoa. Reprod. Domest. Anim. 52, 819–824. doi:10.1111/rda.12984
Figueroa, E., Farias, J., Lee-Estevez, M., Valdebenito, I., Risopatrón, J., Magnotti, C., et al. (2018). Sperm cryopreservation with supplementation of α-tocopherol and ascorbic acid in freezing media increase sperm function and fertility rate in Atlantic salmon (Salmo salar). Aquaculture 493, 1–8. doi:10.1016/j.aquaculture.2018.04.046
Finkel, T., and Holbrook, N. J. (2000). Oxidants, oxidative stress and the biology of ageing. Nature 408, 239–247. doi:10.1038/35041687
Fontoura, P., Mello, M. D., Gallo-Sá, P., Erthal-Martins, M. C., Cardoso, M. C. A., and Ramos, C. (2017). Leptin improves sperm cryopreservation via antioxidant defense. J. Reprod. Infertil. 18, 172.
González-Benito, M. E., Kremer, C., Ibáñez, M. A., and Martín, C. (2016). Effect of antioxidants on the genetic stability of cryopreserved mint shoot tips by encapsulation–dehydration. Plant Cel. Tissue Organ. Cult. 127, 359–368. doi:10.1007/s11240-016-1056-8
Halliwell, B. (2006). Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiol. 141, 312–322. doi:10.1104/pp.106.077073
Huang, B., Zhang, J.-M., Chen, X.-L., Xin, X., Yin, G.-K., He, J.-J., et al. (2018). Oxidative damage and antioxidative indicators in 48 h germinated rice embryos during the vitrification-cryopreservation procedure. Plant Cel. Rep. 37, 1325–1342. doi:10.1007/s00299-018-2315-4
Jia, M. X., Jiang, X. R., Xu, J., Di, W., Shi, Y., and Liu, Y. (2018). CAT and MDH improve the germination and alleviate the oxidative stress of cryopreserved Paeonia and Magnolia pollen. Acta Physiol. Plant. 40, 37. doi:10.1007/s11738-018-2612-0
Jia, M. X., Shi, Y., Di, W., Jiang, X. R., Xu, J., and Liu, Y. (2017). ROS-induced oxidative stress is closely related to pollen deterioration following cryopreservation. Vitro Cel. Dev. Biol.-Plant 53, 433–439. doi:10.1007/s11627-017-9844-3
Khor, S. P., Yeow, L. C., Poobathy, R., Zakaria, R., Chew, B. L., and Subramaniam, S. (2020). Droplet vitrification of Aranda Broga Blue orchid: role of ascorbic acid on the antioxidant system and genetic fidelity assessments via RAPD and SCoT markers. Biotechnol. Rep. 26, e00448. doi:10.1016/j.btre.2020.e00448
Ladeira, C., Koppen, G., Scavone, F., and Giovannelli, L. (2019). The comet assay for human biomonitoring: effect of cryopreservation on DNA damage in different blood cell preparations. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 843, 11–17. doi:10.1016/j.mrgentox.2019.02.002
Lançoni, R., Celeghini, E. C. C., Alves, M. B. R., Lemes, K. M., Gonella-Diaza, A. M., Oliveira, L. Z., et al. (2018). Melatonin added to cryopreservation extenders improves the mitochondrial membrane potential of postthawed equine sperm. J. Equine Vet. Sci. 69, 78–83. doi:10.1016/j.jevs.2018.06.006
Len, J. S., Koh, W. S. D., and Tan, S.-X. (2019). The roles of reactive oxygen species and antioxidants in cryopreservation. Biosci. Rep. 39, BSR20191601. doi:10.1042/BSR20191601
Li, P., Li, Z.-H., Dzyuba, B., Hulak, M., Rodina, M., and Linhart, O. (2010). Evaluating the impacts of osmotic and oxidative stress on common carp (Cyprinus carpio, L.) sperm caused by cryopreservation techniques. Biol. Reprod. 83, 852–858. doi:10.1095/biolreprod.110.085852
Lone, S., Prasad, J., Ghosh, S., Das, G., Balamurugan, B., and Verma, M. (2018). Study on correlation of sperm quality parameters with antioxidant and oxidant status of buffalo bull semen during various stages of cryopreservation. Andrologia 50, 29430680. doi:10.1111/and.12970
Long, J., Liu, C., Sun, L., Gao, H., and Liu, J. (2009). Neuronal mitochondrial toxicity of malondialdehyde: inhibitory effects on respiratory function and enzyme activities in rat brain mitochondria. Neurochem. Res. 34, 786–794. doi:10.1007/s11064-008-9882-7
Longobardi, V., Zullo, G., Salzano, A., De Canditiis, C., Cammarano, A., De Luise, L., et al. (2017). Resveratrol prevents capacitation-like changes and improves in vitro fertilizing capability of buffalo frozen-thawed sperm. Theriogenology 88, 1–8. doi:10.1016/j.theriogenology.2016.09.046
Lu, X., Zhang, Y., Bai, H., Liu, J., Li, J., and Wu, B. (2018). Mitochondria-targeted antioxidant MitoTEMPO improves the post-thaw sperm quality. Cryobiology 80, 26–29. doi:10.1016/j.cryobiol.2017.12.009
Lv, C., Larbi, A., Wu, G., Hong, Q., and Quan, G. (2019). Improving the quality of cryopreserved goat semen with a commercial bull extender supplemented with resveratrol. Anim. Reprod. Sci. 208, 106127. doi:10.1016/j.anireprosci.2019.106127
Makashova, O. E., Babijchuk, L. O., Zubova, O. L., and Zubov, P. M. (2016). Optimization of cryopreservation technique for human cord blood nucleated cells using combination of cryoprotectant DMSO and antioxidant N-acetyl-L-cysteine. Probl. Cryobiol. Cryomedicine. 26, 295–307. doi:10.15407/cryo26.04.295
Marrocco, I., Altieri, F., and Peluso, I. (2017). Measurement and clinical significance of biomarkers of oxidative stress in humans. Oxid. Med. Cel. Longev. 2017, 6501046. doi:10.1155/2017/6501046
Mathew, L., Burritt, D. J., Mclachlan, A., and Pathirana, R. (2019). Combined pre-treatments enhance antioxidant metabolism and improve survival of cryopreserved kiwifruit shoot tips. Plant Cel Tissue Organ. Cult. 138, 193–205. doi:10.1007/s11240-019-01617-3
Mostek, A., Dietrich, M. A., Słowińska, M., and Ciereszko, A. (2017). Cryopreservation of bull semen is associated with carbonylation of sperm proteins. Theriogenology 92, 95–102. doi:10.1016/j.theriogenology.2017.01.011
Mumbengegwi, D. R., Li, Q., Li, C., Bear, C. E., and Engelhardt, J. F. (2008). Evidence for a superoxide permeability pathway in endosomal membranes. Mol. Cel. Biol. 28, 3700–3712. doi:10.1128/MCB.02038-07
Ock, S.-A., and Rho, G.-J. (2011). Effect of dimethyl sulfoxide (DMSO) on cryopreservation of porcine mesenchymal stem cells (pMSCs). Cel Transpl. 20, 1231–1239. doi:10.3727/096368910X552835
Ren, F., Feng, T., Dai, G., Wang, Y., Zhu, H., and Hu, J. (2018). Lycopene and alpha-lipoic acid improve semen antioxidant enzymes activity and cashmere goat sperm function after cryopreservation. Cryobiology 84, 27–32. doi:10.1016/j.cryobiol.2018.08.006
Ren, L., Deng, S., Chu, Y., Zhang, Y., Zhao, H., Chen, H., et al. (2020). Single-wall carbon nanotubes improve cell survival rate and reduce oxidative injury in cryopreservation of Agapanthus praecox embryogenic callus. Plant Methods. 16, 130. doi:10.1186/s13007-020-00674-6
Rienzi, L., Gracia, C., Maggiulli, R., Labarbera, A. R., Kaser, D. J., Ubaldi, F. M., et al. (2016). Oocyte, embryo and blastocyst cryopreservation in ART: systematic review and meta-analysis comparing slow-freezing versus vitrification to produce evidence for the development of global guidance. Hum. Reprod. Update. 23 (2), 139–155. doi:10.1093/humupd/dmw038
Rogers, S. C., Dosier, L. B., Mcmahon, T. J., Zhu, H., Timm, D., Zhang, H., et al. (2018). Red blood cell phenotype fidelity following glycerol cryopreservation optimized for research purposes. PLoS One. 13, 21. doi:10.1371/journal.pone.0209201
Sharma, P., Jha, A. B., Dubey, R. S., and Pessarakli, M. (2012). Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 26, 217037. doi:10.1155/2012/217037
Singh, P., Agarwal, S., Singh, H., Singh, S., Verma, P. K., Butt, M. S., et al. (2020a). Effects of Ascorbic acid as antioxidant semen additive in cryopreservation of cross-bred cattle bull semen. Int. J. Curr. Microbiol. App. Sci. 9, 3089–3099. doi:10.20546/ijcmas.2020.907.364
Singh, P., Agarwal, S., Singh, H., Verma, P. K., Pandey, A., and Kumar, S. (2020b). Antioxidant effects of Aloe vera as semen additive in cryopreservation of cattle bull semen. Int. J. Curr. Microbiol. App. Sci. 9, 1625–1635. doi:10.20546/ijcmas.2020.909.202
Sui, Y. L., Fan, Q., Wang, B., Wang, J. X., and Chang, Q. (2018). Ice-free cryopreservation of heart valve tissue: the effect of adding MitoQ to a VS83 formulation and its influence on mitochondrial dynamics. Cryobiology 81, 153–159. doi:10.1016/j.cryobiol.2018.01.008
Toker, M. B., Alcay, S., Gokce, E., and Ustuner, B. (2016). Cryopreservation of ram semen with antioxidant supplemented soybean lecithin-based extenders and impacts on incubation resilience. Cryobiology 72, 205–209. doi:10.1016/j.cryobiol.2016.05.001
Tsikas, D. (2017). Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: analytical and biological challenges. Anal. Biochem. 524, 13–30. doi:10.1016/j.ab.2016.10.021
Tvrda, E., Mackovich, A., Greifova, H., Hashim, F., and Lukac, N. (2017). Antioxidant effects of lycopene on bovine sperm survival and oxidative profile following cryopreservation. Vet. Med. 62, 429–436. doi:10.17221/86/2017-VETMED
Uchendu, E. E., Leonard, S. W., Traber, M. G., and Reed, B. M. (2010). Vitamins C and E improve regrowth and reduce lipid peroxidation of blackberry shoot tips following cryopreservation. Plant Cel. Rep. 29, 25. doi:10.1007/s00299-009-0795-y
Xiang, H., Yang, X., Ke, L., and Hu, Y. (2020). The properties, biotechnologies, and applications of antifreeze proteins. Int. J. Biol. Macromol. 153, 661–675. doi:10.1016/j.ijbiomac.2020.03.040
Yang, H., Villani, R. M., Wang, H., Simpson, M. J., Roberts, M. S., Tang, M., et al. (2018). The role of cellular reactive oxygen species in cancer chemotherapy. J. Exp. Clin. Cancer Res. 37, 266. doi:10.1186/s13046-018-0909-x
Yang, J., Pan, C., Zhang, J., Sui, X., Zhu, Y., Wen, C., et al. (2017). Exploring the potential of biocompatible osmoprotectants as high efficient cryoprotectants. ACS Appl. Mater. Inter. 9, 42516–42524. doi:10.1021/acsami.7b12189
Yousefian, I., Emamverdi, M., Karamzadeh-Dehaghani, A., Sabzian-Melei, R., Zhandi, M., and Zare-Shahneh, A. (2018). Attenuation of cryopreservation-induced oxidative stress by antioxidant: impact of Coenzyme Q10 on the quality of post-thawed buck spermatozoa. Cryobiology 81, 88–93. doi:10.1016/j.cryobiol.2018.02.005
Zhao, X., Ren, X., Zhu, R., Luo, Z., and Ren, B. (2016). Zinc oxide nanoparticles induce oxidative DNA damage and ROS-triggered mitochondria-mediated apoptosis in zebrafish embryos. Aquat. Toxicol. 180, 56–70. doi:10.1016/j.aquatox.2016.09.013
Keywords: cryopreservation, cryoprotectants, reactive oxygen species, oxidative damages, antioxidants
Citation: Liu X, Xu Y, Liu F, Pan Y, Miao L, Zhu Q and Tan S (2021) The Feasibility of Antioxidants Avoiding Oxidative Damages from Reactive Oxygen Species in Cryopreservation. Front. Chem. 9:648684. doi: 10.3389/fchem.2021.648684
Received: 01 January 2021; Accepted: 25 January 2021;
Published: 26 February 2021.
Edited by:
Kelong Ai, Central South University, ChinaReviewed by:
Weiguo Li, Harbin Institute of Technology, ChinaCopyright © 2021 Liu, Xu, Liu, Pan, Miao, Zhu and Tan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Songwen Tan, c3RhbjAzMDlAdW5pLnN5ZG5leS5lZHUuYXU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.