
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Chem., 21 April 2021
Sec. Organic Chemistry
Volume 9 - 2021 | https://doi.org/10.3389/fchem.2021.642073
A correction has been applied to this article in:
Corrigendum: Coronarin K and L: Two Novel Labdane Diterpenes From Roscoea purpurea: An Ayurvedic Crude Drug
 Venugopal Singamaneni1
Venugopal Singamaneni1 Bashir Lone1,2
Bashir Lone1,2 Jasvinder Singh2,3
Jasvinder Singh2,3 Pankaj Kumar2,4
Pankaj Kumar2,4 Sumeet Gairola2,4
Sumeet Gairola2,4 Shashank Singh2,3
Shashank Singh2,3 Prasoon Gupta1,2*
Prasoon Gupta1,2*The main objective of cancer treatment with chemotherapy is to kill the cancerous cells without affecting the healthy normal cells. In the present study, bioactivity-guided purification of the n-chloroform soluble fraction from the methanol extract of Roscoea purpurea resulted in the identification of two new labdane diterpenes: coronarin K (1) and coronarin L (2), along with eight known compounds, coronarin A (3), bisdemethoxycurcumin (4), kaempferol 3-O-methyl ether (5), kaempferol (6), fenozan acid (7), 3-(3-methoxy,4-hydroxyphenyl)-2-propenoic acid ferulic acid (8), caffeic acid (9), and gallic acid (10). The structural identification of new compounds (1 and 2) were determined by detailed analysis of 1D (1H and 13C) and 2D NMR (COSY, HSQC, and HMBC) spectroscopic data. The relative configurations of 1 and 2 were determined with the help of NOESY correlations and comparison of optical rotations with known labdane diterpenes, with established stereochemistry, while structure of known compounds was established by direct comparison of their NMR data with those reported in the literature. This is the first report of isolation of this labdane diterpenes and phenolic classes of secondary metabolites in R. purpurea. In the preliminary screening, the methanol extract and its fractions were tested for the cytotoxic activity against a panel of four cancer cell lines (A549, HCT-116, Bxpc-3, and MCF-7); extract and its chloroform fraction were found to be active against the lung cancer cell line, A-549, with IC50 value <25 μg/ml. Owing to the notable cytotoxic activity of the chloroform fraction, the compounds (1–5) were screened for their cytotoxicity against all the cell lines by MTT assay. Coronarin K, 1 showed significant cytotoxic potential against lung cancer cell lines (A-549), with IC50 value of 13.49 μM, while other compounds did not show activity below 22 μM.
In our continuing research program to discover bioactive natural products from natural resources, especially from high-altitude Himalayan endangered medicinal plants, with profound biological activities, our attention was focused on the rhizomes of Roscoea purpurea, commonly known as kakoli, which belongs to the family of Zingiberaceae that is abundantly available in alpine grassland, grassy hillsides, and stony slopes of central to eastern Himalaya from Uttarakhand to North East states, up to an altitude of 3,300 m. It is an essential ingredient of an important Ayurvedic preparation, known as Astavarga, which is a group of eight plants claimed to be useful for healing weakness, body overweight, bone fractures, high fever, and diabetic situations, as well as for healing vata, pitta, and rakta doshas (Dhyani et al., 2010). The groups of Astavarga plants are considered as a very excellent Rasayana, with health-promoting and rejuvenating properties, and are recognized to support our immunity and have the capacity for cell renewal (Balkrishna et al., 2012). Astavarga plants are also known to restore human health and work as strong antioxidants in our body (Mathur, 2003; Pandey, 2005; Sharma and Balkrishna, 2005). Among the eight Astavarga plants, R. purpurea is one of the essential ingredients of several herbal formulations like tonic and Chyawanprash. Traditionally, it is used for the cure of diabetes, diarrhea, hypertension, fever, immunostimulant, inflammation, etc. In Nepal, its tubers are boiled for an edible purpose and are also used in traditional veterinary medicine (Singh and Rawat, 2011). In view of its significance in the Ayurvedic medicinal system, substantial pharmacological and phytochemical works have not been carried out. Previous phytochemical studies on R. purpurea have described the isolation of two principal groups of compounds: steroids and phenolic derivatives (Misra et al., 2015; Srivastava et al., 2015; Rawat et al., 2016). To date, only a few compounds have been identified and quantified through high-performance liquid chromatography (HPLC) analysis from tubers of this plant by Singh and co-workers, and they are presumed to be associated with its potent antioxidant activity (Gopal et al., 2014; Rawat et al., 2014). Alcoholic extracts of the plant have shown in vitro anti-cancer (Srivastava et al., 2015), antioxidant (Rawat et al., 2016), and immunomodulatory activities (Sahu et al., 2010). In our preliminary pharmacological study, cytotoxic activity was found for the methanol extract of R. purpurea against lung cancer cell line at IC50 25.71 (μg/ml). On bioassay-guided fractionation, activity was localized in a chloroform-soluble fraction. Further work led to the isolation of two new compounds, along with eight known compounds for the first time from this plant species. In the present paper, we have described isolation and structure elucidation of two new compounds from the rhizomes of R. purpurea and the anti-cancer activities of plant extract, fractions, and isolated compounds.
All NMR spectral data were obtained on a Bruker 400 MHz spectrometer. Chemical shifts (δ) were referenced to the residual solvent peak (CD3OD: 1H δ 3.30, 13C δ 49.0 ppm; CDCl3: 1H 7.26, 13C 77.0 ppm; DMSO-d6: 1H 2.50, 13C 39.5 ppm), and the reference point was TMS (δH and δC: 0 ppm). 1H-13C NMR correlations were established from gradient-enhanced inverse-detected HMBC and HSQC experiments, respectively. HPLC purification was done on a 1260 Infinity II Agilent system, equipped with a photo diode array (PDA) detector. HPLC solvents were purchased from Merck (Mumbai)—and water used for plant extractions and HPLC analysis was obtained from—A10 Milli-Q Advantage water system (Millipore, France). Column chromatography was done using silica gel (100–200 mesh). Thin layer chromatography (TLC) analyses were performed on Merck Kieselgel (Aufoilen) 60 F254 plates. Potato Dextrose Agar (PDA), 1% sodium hypochlorite, lactophenol cotton blue (LPCB), and 15% Glycerol were purchased from Difco, United States. Dulbecco's modified eagle medium (DMEM), Rosewell Park Memorial Institute Medium (RPMI-1640), Fetal bovine serum (FBS,10%), Rhodamine 123 (Rh 123), DAPI (4′,6-diamidino-2-phenylindole, dilactate), DMSO, gentamicin sulfate, trypsin, EDTA, PBS penicillin, streptomycin, paclitaxel, mitomycin, doxorubicin, gemcitabine, and all other chemicals were purchased from Sigma–Aldrich (St. Louis, MO, United States). Tris buffer, glacial acetic acid, and trichloroacetic acid (TCA) used in the study were purchased from Himedia (Mumbai), Fisher Scientific (Mumbai), and Merck Specialties Private Ltd., (Mumbai), respectively.
Rhizomes of R. purpurea Sm. (Syn: Roscoea procera Wall.) were collected in September 2013 from Dhanaulti, Mussorie in district Tehri Garhwal of Uttarakhand, India (between 2,500 and 3,200 m asl). The crude herb was authenticated by Dr. Sumeet Gairola, and the voucher specimen (CDR No. 4027) was deposited in the Crude Drug Repository of CSIR-IIIM, Jammu.
Air-dried R. purpurea rhizomes (2.0 kg) were grounded into powder and extracted thrice (soaked for 24 h each), with 3 L of methanol. The extract was filtered—concentrated under reduced pressure (using rotavapor) at 40°C to yield a crude extract (305 g). Water (800 ml) was then added to dissolve the crude extract. The same amount of petroleum (pet) ether was used for solvent–solvent fractionation, and the upper layer (petroleum extract) was removed (40.1 g). The residue was further partitioned, using chloroform (165 g) and n-Butanol (62.5 g). The chloroform fraction (150 g) was purified by column chromatography (silica gel, 100–200 mesh), and eluted with a gradient of pet. ether-ethyl acetate (100:0 to 0:100, 500 ml collected volumes of each fraction), and concentrated, giving 20 combined fractions (Fr.1–Fr.20) based on the TLC profile. Fraction 2 (3.0 g) was further chromatographed over silica gel, with n-hexane-ethyl acetate (100:0 to 80:20, 100 ml collected volumes of each fraction), to afford four subfractions (Fr. 2A-2D). White crystals were formed in Fr. 2B-yielded 1.0 g of compound 3 (1.0 g), which was detected on TLC [Pet. Ether-EtOAc (97:3)] as tan-brown spot by charing the dried TLC in the anisaldehyde reagent. Further, purification of fraction F-4 (6.45 g) on silica gel (60–120 mesh, 200 g), using a gradient of n-hexane–EtOAc (95 to 55%), afforded 25 fractions (100 ml each), and these fractions were separated into six subfractions (F-4A to F-4F) on the basis of similarities in their TLC profiles. Purification of fraction F-4B (1.2 g) on silica gel (230–400 mesh, 40 g), using n-hexane–EtOAc (94:6), afforded compound 1 (0.6 g) as a white crystals. Also, purification of fraction F-6 (1.1 g), over silica gel (60–120 mesh, 170 g), using n-hexane–EtOAc (80:20), afforded compound 2 (70 mg) as white powder. Moreover, fraction F-10 (10.2 g) was loaded on a silica gel column (100–200 mesh, 300 g) and eluted, using chloroform as a mobile phase, afforded 6 subfractions (F-10A–F-10F). Subfraction F-10C (3.2 g) contains two major compounds, which, on further purification, give rise to compound 4 (0.4 g) and compound 5 (1.01 g), respectively. Compound 6 (45 mg) was solidified as a yellow powder in fraction F-10E. Fr. 6C (1 g) was further purified by column chromatography (silica gel), eluted with ethyl acetate (1–5%) in petroleum ether, giving 10 fractions of volume 50 ml each. White crystals were formed in the last five fractions, which were purified by washing with HPLC grade hexane-yielded compound 7 (0.32 g). Fraction F-15 (3.7 g) was chromatographed on silica gel (60–120 mesh, 120 g), eluted with a gradient of chloroform and methanol 95–5%, and yielded F-15A to F-15J. Fraction F-15E was stored in a refrigerator to yield 10 (62 mg). Column chromatography of F-15F (1.1 g), using EtOAc–MeOH (97:3), as eluent over silica gel (230–400 mesh, 30 g), afforded 8 (28 mg) and 9 (42 mg), respectively. The chemical structures of all the isolated compounds are given in Figure 1.
Different human cancer cell lines—the lung (A549), the colon (HCT-116), the breast (MCF-7), and the pancreas (Bxpc-3)—were obtained from the National Cancer Institute, Frederick (NCI), United States. Bxpc-3 and HCT-116 cell lines were maintained in DMEM, and A549, MCF-7 cell lines were maintained in RPMI. FBS (10%), penicillin (100 units/ml), streptomycin (100 μg/ml), and glutamine (2 mM) were used for supplementation. Cells were grown at 37°C in 5% CO2 (incubator, Thermo Scientific, United States), with 98% humidity. The concentration of DMSO added to the test materials was maintained at <0.2%.
Colorimetric MTT assay was used to carry out cell viability studies (Kumar et al., 2013). The plant extract and isolates were screened at different concentrations with a maximum concentration of 100 μM. The cells were rinsed and incubated with 20 μl of MTT dye after treatment at different time intervals. The medium was removed, and DMSO was added to each well after incubation for the solubility of purple formazan crystals. Microplate Reader (BioTek Synergy HT) was used to measure the absorbance at 570 nm. GraphPad Prism Software (La Jolla, CA, United States) was used to determine cell viability percentage and IC50 values.
Lung cancer cell line (A-549) seeded in six-well plates (2 × 105) was treated with coronarin K 1 (7, 13, 18 μM) and incubated for 48 h. H2O2 was added to a well containing a medium for 2 h before termination. The cells were further incubated with 10 μM DCF for 30 min at 37°C in the dark. The cells were washed with PBS and analyzed with a fluorescence microscope (Olympus-1X53 magnification). The fluorescent intensity was measured at the emission and excitation wavelength of 480/530 nm (Banskota et al., 2015).
The dried and powdered rhizomes of R. purpurea were extracted with methanol and dried at 40–45°C. The obtained crude extract was sequentially fractionated with pet ether, CHCl3, and n-butanol. The chloroform soluble fraction was purified, using chromatographic techniques, which afforded two new compounds (1–2), together with 8 known compounds. The structures of both novel secondary metabolites were established, using detailed NMR spectroscopic studies, whereas the structures of known compounds were identified in comparison with the previously reported literature data. The 1H and 13C NMR spectra of compounds 1 and 2 have some common structural features of diterpenes based on the NMR data and gave a positive copper acetate test for diterpenes.
Coronarin K (1) was isolated as a white crystal. The molecular formula of coronarin K (1), C20H30O3, was established from the ESIMS of the [M-H]− ion at m/z 317.10, with six degrees of unsaturation. On analyzing the 13C, the NMR data showed the presence of three C=C bonds (δC 144.6, 130.1, 108.5, 143.4, 138.5, and 110.3), and three methyls (δC 33.6, 23.6, and 17.3), which revealed six double-bond equivalents and indicated the presence of one exomethylene and one furan ring in the molecule and suggested that 1 was labdane-type diterpene. A detailed analysis of the 1H NMR data (Table 1) showed the existence of a furan ring, presence of three olefinic proton signals at δH 6.43 (1H, s), 7.40 (1H, d, J = 1.2 Hz), and 7.39 (1H, d, J = 1.2 Hz), based on HSQC correlations to the olefinic carbon signals at δc 108.5 (C-14), 138.5 (C-15), and 143.4 (C-16), respectively. Furthermore, the presence of exocyclic methylene was revealed by the observation of 1H proton signals at δH 5.05 (1H, s) and 4.77 (1H, s), corresponding to δc 110.3 (C-17). The observed UV λmax at 238 nm was inconsistent with this assignment (Itokawa et al., 1988). HMBC correlations from the gem dimethyl signal at δH 1.02 (Me-18) and 1.22 (Me-19) to both C-4 (δC 34.5), a quaternary carbon at C-5 (δC 57.3), and to each other's carbon resonance-established methyl substitutions of C-4 (Figure 2).
The backbone of the bicyclic ring system was established, using detailed analysis of HMBC correlations from the three methyl signals: δH 1.02, 1.22, and 1.18, which obtained as a singlet in the 1H NMR spectrum and showed HMBC correlation with the H3-18 and H3-19 to C-3, 4, 5; H3-20 to C-1, 5, 8, and 10. The 1H NMR signals of the exocyclic methylene (δH 5.05, 4.77, δC 110.3) revealed strong HMBC correlations with H2-17 to C-7, 8, 9. The methylene H-9 also showed HMBC to C-5, 7, 10, 17, 20. Along with these, a correlation from H-9 to the oxymethine carbon (δC 65.0) is also revealed by HMBC. The presence of a decalin ring system was established with COSY correlations indicating a coupling from H2-6 to H-5 and H-7 and from H2-1 to H2-2 and H2-3.
All above HMBC and COSY correlations established connection of the bicyclic ring with furan ring. The other signal to be assigned was a methylene group CH2-11 (δH 1.84; δC 32.7). HMBC correlations from the H2-11 signal to C-12, 13 and H-12 to C-14 and 16 confirmed the connection of the C-9 to C-10 and C-12 to C-13 furan ring. Thus, the structure of 1 was characterized as Coronarin K, a new labdane diterpene. The relative signs of 1 were established by NOE correlations observed in the NOESY experiment (Figure 3) and observed optical rotation +23.3° (c.23, CHCl3), which is consistent with published data for a similar skeleton (Itokawa et al., 1988). NOE correlations from H3-20 to H-11, and H3-18, together with correlations from H3-19 to H-5, H-9; H-5 to H-9; H-9 to H-8; H2-17 and H-12 to H-16, established the decalin ring system. Thus, the structure of coronarin K (1) is defined as 5S*, 7S*, 9R*, 10S*, 12S.
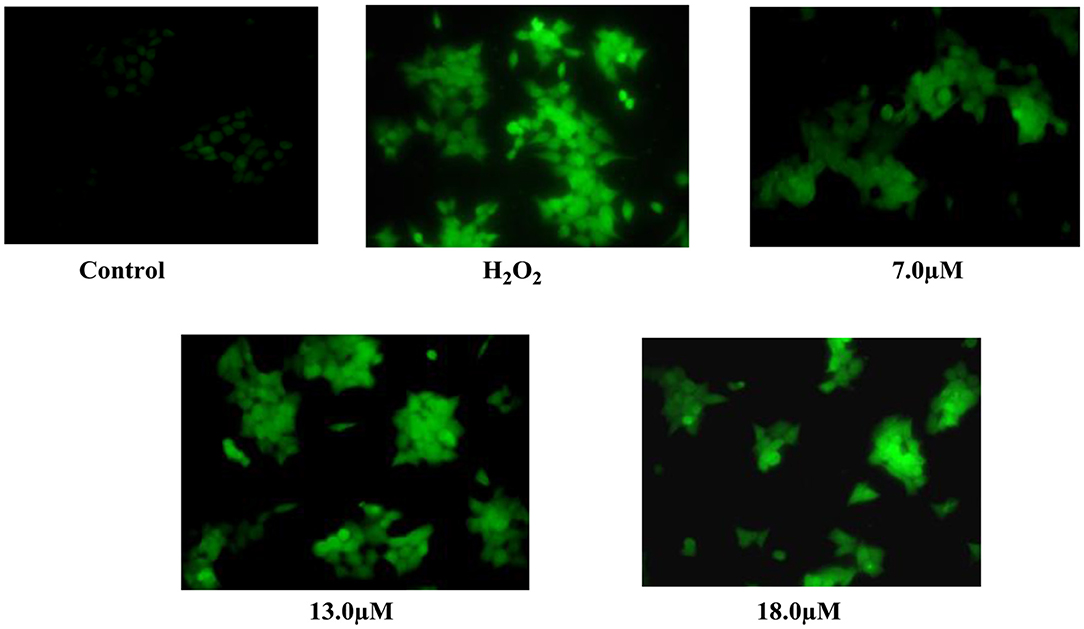
Figure 3. Effect of coronarin K (1) on cellular and nuclear morphology of A-549 in ROS production assessed from the oxidation of DCFDA by H2O2 through fluorescence microscopy at 48-h posttreatment.
Coronarin L (2) was isolated as a white solid. The molecular formula of Coronarin L (2) was established as C20H28O3, based on the ESIMS and NMR data. The cyclic lactone group in 2 was confirmed in the IR spectrum (1,750 cm−1) by the presence of C=O-stretching bands (Itokawa et al., 1988). NMR data comparison (Table 1) revealed that 2 was structurally similar to 1, except for the presence of a diene system [δH 6.88 (1H, dd, J = 15.8, 9.8 Hz Hz), δC 135.9 (C-11); δH 6.08 (1H, d, J = 15.6 Hz), δC 121.0 (C-12)] in place of oxymethine at C-12 as it was missing in 13C spectrum. Furthermore, changes in the proton and carbon chemical shifts of the furan ring suggested oxidation of the C-14–C-15 double bond to a carbonyl group. HMBC correlations from the methine signals at δH 4.87 (2H, s, H-16) to C-15 (C=O) and C-14 (δH 7.10, s), together with HMBC correlation with diene C-12, confirmed the presence of a lactone ring at C-12 adjacent to a double bond. A COSY correlation, together with the large 1H coupling constant (J = 15.8 Hz), between H-11 and H-12, established the E geometry of the C-11-C12 double bond. NOE correlations in 2 (Figure 2) and observed optical rotation +36.9° (c.20, CHCl3) were identical to that of 1; therefore, the structure of coronarin L (2) was defined as 5S*, 7S*, 9R*, 10S*.
Compound 3 was another labdane diterpene isolated as white crystals. The molecular formula was confirmed as C20H28O2 based on the significant signal observed in the HR-ESIMS spectrum at m/z 301.2167 [M+H]+. Compound 3 carries a similar chemical skeleton as compound 1 based on comparison of NMR data. After complete 1H and 13C and 2D NMR analysis, the structure was confirmed as Coronarin A, previously reported from Hedychium coronarium (Itokawa et al., 1988). The structures of the known compounds 4-10 (Figure 1) were identified as bisdemethoxycurcumin (4) (Dairam et al., 2007), kaempferol 3-O-methyl ether (5) (Hammami et al., 2004), kaempferol (6) (Gupta et al., 2007), fenozan acid (7) (Jaivel et al., 2014), 3-(3-methoxy,4-hydroxyphenyl)-2-propenoic acid (ferulic acid), (8) (Diaz et al., 2012), caffeic acid (9) (Yoshioka et al., 2004), and gallic acid (10) (Zucca et al., 2013) by direct comparison of ESIMS, 1H and 13C NMR data with the previously reported literature data. All the known compounds 4-10 were isolated for the first time from this species.
Secondary metabolites, which were isolated from the plants of the Zingiberaceae family, have wide use as a new pharmacophore in anti-cancer drug discovery. In our bio-assay-guided isolation of novel anti-cancer agents from R. purpurea, the methanol extract and respective fractions were screened for their anti-cancer activity, using the MTT assay. The methanol extract and its CHCl3 fraction showed cytotoxic activity against lung cancer cell line (A549) at IC50 27.71 and 21.35 (μg/ml), respectively. After purification of chloroform fraction, compounds 1–10 were isolated. Only compounds 1–5 were evaluated for different cancer cell lines. Among the tested compounds, Coronarin K (1) showed prominent activity against lung cancer cell line (A-549) and also showed moderate cytotoxic activity against colon cancer cell line HCT-116 (IC50 value of 26.03 μM) but had no effect on Bxpc-3 and MCF-7 in acceptable limits. Compounds 2 and 3 were ineffective against all the cell lines except pancreatic cancer cell line (Bxpc-3) at IC50 value of 26.03 μM. The remaining tested compounds displayed less activity (Table 2). In a further study, the A-549 cells were treated with compound 1 at 7.0, 13.0, 18.0 μM, and ROS generation was detected in 48 h. Hydrogen peroxide was taken as a positive control. After exposing the cells with compound 1, the fluorescence intensity of DCF was increased in a dose-dependent manner. The results showed that compound 1 exhibited prominent cytotoxicity against human lung cancer cell line (A-549), with IC50 value of 13.49 μM (Figure 3) and thereby confirmed the ROS generation is crucial to cell death.
In the present study, by the cytotoxic activity-guided isolation of R. purpurea extract, we have made an unusual finding of two new labdane diterpenes coronarin K (1) and coronarin L (2), together with the other known compounds 3–10. All the compounds have been isolated physically for the first time from this plant. Furthermore, this is the first report of the natural origin (Fenozan acid 7) of a synthetic di-tert-butyl derivative in plants. In addition to this, we have evaluated the anti-cancer activity of extract, chloroform fraction, and isolated compounds. Among all the tested compounds, only coronarin K (1) showed prominent anti-cancer activity against lung cancer cell line (A-549). Our study also suggest that the antiproliferative effect of the compounds is due to ROS generation and also led to the impaired cell migration. Finally, our study strongly suggests that screening and detailed chemical study of endangered Astavarga plants would be valuable.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
PG conceptualized and wrote the paper. VS and BL performed isolation and characterization of compounds. PK and SG helped in the collection and the identification of the plant material. JS and SS performed the anti-cancer activity. All the authors read and approved the final manuscript.
This work was supported by the Council of Scientific and Industrial Research (CSIR), New Delhi (Grant no. HCP-0010 and MLP-4011) and SERB, New Delhi (Grant no. EMR/2016/002584).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
VS (CSIR), BL (ICMR), JS (ICMR), and PK (CSIR) thankfully acknowledge the financial support in the form of research fellowships. The manuscript bears IIIM Communication No. IIIM/2022/2020.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fchem.2021.642073/full#supplementary-material
Spectroscopic data of compounds 1 and 2, including 1D (1H and 13C), 2D (1H-1H COSY, HSQC, HMBC, and NOESY), NMR and ESIMS.
Balkrishna, A., Srivastava, A., Mishra, R. K., Patel, S. P., Vashistham, R. K., Singh, A., et al. (2012). Astavarga plants – threatened medicinal herbs of the north-west himalaya. Int. J. Med. Arom. Plants. 2, 661–676.
Banskota, S., Regmi, S. C., and Kim, J. A. (2015). NOX1 to NOX2 switch deactivates AMPK and induces invasive phenotype in colon cancer cells through overexpression of MMP-7. Mol. Cancer 14:123. doi: 10.1186/s12943-015-0379-0
Dairam, A., Limson, J. L., Watkins, G. M., Antunes, E., and Daya, S. (2007). Curcuminoids, curcumin and demethoxycurcumin reduce lead-induced memory deficits in male wistar rats. J. Agric. Food Chem. 55, 1039–1044. doi: 10.1021/jf063446t
Dhyani, A., Nautiyal, B. P., and Nautiyal, M. C. (2010). Importance of astavarga plants in traditional systems of medicine in Garhwal, Indian Himalaya. Int. J. Biodivers. Sci. Ecosyst. Serv. Manage. 6, 13–19. doi: 10.1080/21513732.2010.521490
Diaz, P., Jeong, S. C., Lee, S., Khoo, C., and Koyyalamudi, S. R. (2012). Antioxidant and anti-inflammatory activities of selected medicinal plants and fungi containing phenolic and flavonoid compounds. Chin. Med. 7, 26–28. doi: 10.1186/1749-8546-7-26
Gopal, B. B., Shankar, D. S., and Avishek, R. (2014). Study of antioxidant property of the rhizome extract of Roscoea purpurea Sm. (Kakoli) and its use in green synthesis of gold nanoparticles. Int. J. Res. Chem. Environ. 4, 174–180.
Gupta, P., Yadav, D. K., Siripurapu, K. B., Palit, G., and Maurya, R. (2007). Constituents of Ocimum sanctum with antistress activity. J. Nat. Prod. 70, 1410–1416. doi: 10.1021/np0700164
Hammami, S., Jannet, H. B., Bergaoui, A., Ciavatta, L., Cimino, G., and Mighri, Z. (2004). Isolation and structure elucidation of a flavone, a flavonone glycoside and vomifoliol from Echiochilon fruticosum growing in Tunisia. Molecules 9, 602–608. doi: 10.3390/90700602
Itokawa, H., Morita, H., Katou, I., Takeya, K., Cavalheiro, A. J., de Oliveira, R. C., et al. (1988). Cytotoxic diterpenes from the rhizomes of Hedychium coronarium. Planta Med. 54, 311–315. doi: 10.1055/s-2006-962442
Jaivel, N., Uvarani, C., Rajesh, R., Velmurugan, D., and Marimuthu, P. (2014). Natural occurrence of organofluorine and other constituents from Streptomyces sp. TC1. J. Nat. Prod. 77, 2–8. doi: 10.1021/np400360h
Kumar, S., Guru, S. K., Pathania, A. S., Kumar, A., Bhushan, S., and Malik, F. (2013). Autophagy triggered by magnolol derivative negatively regulates angiogenesis. Cell. Death Dis. 4:e889. doi: 10.1038/cddis.2013.399
Misra, A., Srivastava, S., Verma, S., and Rawat, A. K. S. (2015). Nutritional evaluation, antioxidant studies and quantification of poly phenolics, in Roscoea purpurea tubers. BMC. Res. Notes 8:324. doi: 10.1186/s13104-015-1290-x
Rawat, S., Andola, H., Giri, L., Dhyani, P., Jugran, A., Bhatt, I. D., et al. (2014). Assessment of nutritional and antioxidant potential of selected vitality strengthening Himalayan medicinal plants. Int. J. Food Prop. 17, 703–712. doi: 10.1080/10942912.2012.654563
Rawat, S., Bhatt, I. D., Rawal, R. S., and Nandi, S. K. (2016). Geographical and environmental variation in chemical constituents and antioxidant properties in Roscoea procera wall. J. Food Biochem. 40, 1–6. doi: 10.1111/jfbc.12302
Sahu, M. S., Mali, P. Y., Waikar, S. B., and Rangari, V. D. (2010). Evaluation of immunomodulatory potential of ethanolic extract of Roscoea procera rhizomes in mice. J. Pharm. Bioall. Sci. 4, 346–349. doi: 10.4103/0975-7406.72138
Sharma, B. D., and Balkrishna, A. V. (2005). Vitality Strengthening Astavarga Plants (Jeevaniya and Vayasthapan paudhe). Haridwar: Divya Publishers, Divya Yog Mandir.
Singh, G., and Rawat, G. S. (2011). Etnomedicinal survey of kedarnath wildlife sanctuary in western Himalaya, India. Indian J. Fundamen. App. Life Sci. 1, 35–46.
Srivastava, S., Ankita, M., Kumar, D., Srivastava, A., Sood, A., and Rawat, A. K. S. (2015). Reversed-phase high-performance liquid chromatography-ultraviolet phtodiode array detector validated simultaneous quantification of six bioactive phenolic acids in Roscoea purpurea tubers and their in vitro cytotoxic potential against various cell lines. Phcog. Mag. 11, 488–495. doi: 10.4103/0973-1296.168944
Yoshioka, T., Inokuchi, T., Fujioka, S., and Kimura, Y. (2004). Phenolic compounds and flavonoids as plant growth regulators from fruit and leaf of Vitex rotundifolia. Z. Naturforsch. C. 59, 509–514. doi: 10.1515/znc-2004-7-810
Keywords: Roscoea purpurea, Astavarga, Zingiberaceae, labdane diterpenes, cytotoxic, coronarin k, coronarin L
Citation: Singamaneni V, Lone B, Singh J, Kumar P, Gairola S, Singh S and Gupta P (2021) Coronarin K and L: Two Novel Labdane Diterpenes From Roscoea purpurea: An Ayurvedic Crude Drug. Front. Chem. 9:642073. doi: 10.3389/fchem.2021.642073
Received: 15 December 2020; Accepted: 25 March 2021;
Published: 21 April 2021.
Edited by:
Ramon Rios, University of Southampton, United KingdomReviewed by:
Guoxu Ma, Institute of Medicinal Plant Development, ChinaCopyright © 2021 Singamaneni, Lone, Singh, Kumar, Gairola, Singh and Gupta. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Prasoon Gupta, Z3VwdGFwQGlpaW0uYWMuaW4=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.