
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Chem., 12 April 2021
Sec. Supramolecular Chemistry
Volume 9 - 2021 | https://doi.org/10.3389/fchem.2021.636431
This article is part of the Research TopicSupramolecular Chemistry Editor’s Pick 2022View all 8 articles
Luminescent Pb2+-based metal–organic frameworks (MOFs) belong to a new class of multifunctional molecular materials with interesting luminescence properties and potential applications within a single crystalline phase. In this mini review, we present the recent advances that have been achieved in their applications as single-phase white-light emitting materials and chemosensors in the last decade. We focus on the trends in the modification of their structures and luminescence by various bridging ligands, and subsequently their multifunctional applications, which may affect the future development of the field.
Metal–organic frameworks (MOFs), a class of coordination crystalline materials involving metal nodes and multi-topic ligands, have attracted broad interest, as a result of their novel structures and various applications (Wang and Astruc, 2019). Until recent years, most of the reported MOFs were constructed on the basis of d-block and f-block metals (Cui et al., 2012; Zhou et al., 2015). In contrast, much less is understood on main-group MOFs, especially Pb2+-containing MOFs, mainly as a result of their flexible geometry and nonclassical coordination chemistry. As with other heavier metals, the toxicity of Pb2+, a heavy p-block element, has drawn certain environmental concerns. However, the interesting emission properties of Pb2+-based materials, which are highly dependent on the coordination environment, and thus their potential for different applications, have also attracted much interest. The optical and electronic properties of Pb2+ compounds have recently been explored in various applications, such as Pb2+-based perovskite (Nazarenko et al., 2018), white-light emitting material (Peng et al., 2018), X-ray scintillator (Lu et al., 2019), luminescent sensing (Wang et al., 2018), batteries (Hu et al., 2017), nonlinear optical materials (Chen et al., 2020), ferroelectric materials (Gao et al., 2017), and semiconductors (Terpstra et al., 1997). These fascinating properties are closely associated with its heavy atom effect, inert lone pair effect, large ionic radius, and its borderline position on the hard–soft acid–base scale (Chu et al., 2013). Pb2+-based MOFs exhibit frequently unique luminescent properties and applications which are seldom realized in other metal-based MOFs, and thus represent an interesting class of functional materials for the study of structure–property correlations. Since the luminescence properties of Pb2+-based MOFs are highly important to their functionality, especially in the application as fluorescent sensors, a brief introduction to the nature of their emission properties will be provided initially, which is followed by the discussion on their applications as white-light emitting materials and luminescent sensors.
Luminescent metal complexes of Ln3+, Zn2+, Cd2+, and Cu+, and noble metals (Ru2+, Os2+/6+, Ir3+, Pt2+, and Re+/5+) have been well documented, and their emission properties are usually predictable (Cui et al., 2012; Zhou et al., 2015). In contrast, the luminescence properties of Pb2+ compounds are more complicated, as they may exhibit simultaneously a variety of electronic transitions, including (1) the metal-centered (MC) s→p transition that usually occurs in hemi-directed Pb2+ compounds (Pan et al., 2018), (2) ligand-to-metal charge transfer (LMCT) (Pan et al., 2018), (3) Pb2+-perturbed ligand-centered π→π* transition (Pan et al., 2018), and (4) metal-to-ligand charge transfer (MLCT) (Chu et al., 2013). The emissions of Pb2+ compounds are usually phosphorescence, irrespective of their emission natures, as spin–orbital coupling is enhanced by the heavy atom effect of Pb2+. In addition, the luminescence of Pb2+-based MOFs is sensitive to the substituents on the ligands and the subtle changes in their macrostructures. Thus, interesting luminescence properties were often reported for Pb2+-based MOFs. For example, a novel 3D Pb2+ MOF Pb4(L1)3(μ4-O)(H2O) (1) (H2L1 = 1,3-benzenedicarboxylate) exhibiting an eight-connected bcu-type topological motif has been synthesized by Yang et al. (2008). Upon excitation at 374 nm, the Pb2+ MOF shows an emission at 424 nm, which is assigned to LMCT from delocalized π bonds of carboxylate groups to p orbitals of Pb2+ ion. In contrast, Pb2+-based MOFs with MLCT character are rare, as the Pb(III) state is not readily accessible. However, Sun and coworkers have recently reported two lead(II) carboxyphosphonate compounds [Pb2Cl3(H2L2)]·H2O (2) and [Pb2(HL2)(HL3)] (3) (H3L2 = 1-(phosphonomethyl)piperidine-4-carboxylic acid and H3L3 = 1,3,5-benzenetricarboxylic acid), which show a significant red shift and enhancement of the emission compared with the free H3L3 ligand, probably attributed to the MLCT transition (Chu et al., 2013). Moreover, owing to the presence of stereochemically active lone pair effect, the emissions from the metal-centered (MC) s→p transition are most commonly found in semidirectionally coordinated Pb2+-MOFs.
In this part, the recent development in Pb2+-based organic–inorganic hybrid materials with white-light emission (WLE) and their photophysical properties will be discussed in relation with the structures of the ligands and the MOFs. Materials with WLE have attracted immense interests as a consequence of their potential usage in displays and lightings. Currently, most of the white-light sources are fabricated by a combination of emissions from separate dopants or a blending of multiple components. However, these materials may bring along complications and higher cost in the fabrication process, together with intrinsic problems such as reabsorption, phase separation, and color variation. The construction of single-phase WLE materials is therefore considered an ideal approach to overcome these issues. To achieve high-quality white light, the single-phase materials must exhibit emission with the Commission Internationale de l’Eclairage (CIE) coordinates (0.33, 0.33). Pb2+-based MOFs are found to be potential single-phase materials for WLE, since the multiple emitting centers necessary for WLE could be achieved by suitable combination of organic moieties and Pb2+, which exhibits emissions of different origins.
Hybrid organic–inorganic lead halide perovskites were reported to be a special class of intrinsic broadband white-light emitters. The incorporation of structurally deformable PbmXn units into MOFs was a convenient method for the crystal engineering of optoelectric materials. In some cases, the emission solely originates from the lead halide units, and the ligands function only as bridging groups to stabilize the MOFs, whereas the introduction of π-conjugated aromatic moiety was suggested to significantly influence the emission properties by their readily accessible and modifiable charge-transfer bands. Several examples of Pb2+ halide perovskites bearing aliphatic dicarboxylate linkage groups were reported. For example, two 2D Pb2+ halide polymeric complexes [Pb2X2][L4] (X = Cl, 4 and Br, 5) with broadband WLE were obtained from the reactions of PbX2 and trans-1,4-cyclohexanedicarboxylic acid (H2L4) under hydrothermal conditions. These materials were chemically robust over a wide pH range (3–9) and exhibited undiminished luminescence upon UV excitation for 30 days. The WLE was suggested, by DFT calculations, to originate from the Pb–Pb dimerization and Cl–Cl pairing in the [Pb2X2]2+ (X = Cl/Br) layers (Supplementary Table S1) (Yin J. et al., 2019). Two cationic porous 3D organic–metal halide frameworks [Pb2Br2][L5] (6) and [Pb3Br4][L6] (7) were prepared from bromoplumbate and aliphatic dicarboxylate bridging ligands. These compounds exhibit high chemical resistance and intrinsic white-light emission (λex = 360 nm) at high quantum efficiency. The WLE spanned the whole visible-light spectrum and was suggested to arise from the electron–phonon coupling in the strongly deformable and anharmonic lattice (Peng et al., 2018). A series of cationic layered lead halide materials, formulated as [Pb2X2]2+[L5] (X = F, Cl and Br) (8–10), were later reported by the same group to exhibit intense broadband WLE in the bulk form at an external quantum efficiency up to 11.8% (Zhuang et al., 2017).
Aromatic dicarboxylate bridging ligands in lead halide perovskites were found to influence the photophysical properties significantly. Several Pb2+ MOFs have been prepared by using derivatized aromatic dicarboxylate bridging moieties, and the study of the dependence of their photophysical properties on the aromatic ring may provide more insights for the development of single-phase WLE materials. These compounds usually exhibit dual or multi-emission bands, in contrast to conventional luminescent materials. Owing to the lone pair effect, the emissions from the MC s→p transition are readily found in Pb2+ MOFs with a semi-directional geometry. On the other hand, suitable bridging ligands are crucial in making the ligand-centered and charge-transfer (LMCT/MLCT) transitions accessible in these Pb2+ MOFs. Three stable WLE-MOFs, [Pb2X3+][L7]2 [(CH3)2NH2+]3 (X = Cl/Br/I) (11–13), were afforded by bridging the deformable [Pb2X3]+ (X = Cl, Br, and I) 1D chains with 1,4-benzenedicarboxylate (H2L7) (Supplementary Figure S1). Upon near-UV excitation, these materials exhibit intrinsic broadband emissions with a high color-rendering index (CRI) of up to 89 (Peng et al., 2019). Whereas their emissions of 11–13 are mainly originated from the [Pb2X3+] moieties, the introduction of dicarboxylate linkers with rigid aromatic moiety was found to significantly enhance the contribution from the ligand-centered emission and was an effective means for tuning the luminescent properties of the MOFs. For example, single-component broadband photoemitters, [(Pb4X2)(L8)4·A2]n (X = Cl 14, Br 15, and I 16, A = (CH3)3NH+ and (CH3)2NH2+), were formed by bridging 1D haloplumbate chains with the rigid luminescent 2,6-naphthalene dicarboxylate (H2L8). The bromo and iodo analogs exhibited WLE, which were attributed to the ligand-centered blue emissions from the extended conjugation in L8 and the red emissions from the hemi-directed haloplumbate centers (Lin et al., 2020). Xu and coworkers synthesized two emissive 3D networks, PbL9 (17) and PbL10 (18), with 1,4-benzenedicarboxylic acid modified, respectively, with CH3SCH2CH2S- (L9) and (S)-H3(OH)CHCH2S- (L10) at the 2 and 5 positions (Figure 1). The two compounds featured, respectively, a yellowish-green photoluminescence (17) and a bright WLE (18) resulting similarly from broadband dual emissions of different origins. The white emission of 18 was attributed to a suitable ratio of LMCT and s→p transitions, while in 17, the contribution from LMCT was more significant. A thin film of 18 was then applied onto a commercially available UV-LED lamp by a dip-coating procedure (Figure 1) and demonstrated to work in conventional lighting application (He et al., 2012). Wibowo and coworkers have recently synthesized two Pb2+-based MOFs, Pb(HL3)(1,4-dioxane)0.5 (19) and Pb2(HL3)2(H2O)5 (20), by using a dissolution–crystallization method (H3L3 = benzene-1,3,5-tricarboxylic acid). These complexes contained similar linear subunits that were interconnected by HL3 into three-dimensional porous MOFs. The two compounds exhibit broad emissions, probably originating from a mixture of ILCT, LMCT, and/or MLCT, upon excitation at λex = 350 nm. Particularly, the CIE coordinates (0.33, 0.36) of 20 are close to the ideal CIE coordinates (0.33, 0.33) for WLE (Al-Nubi et al., 2019).
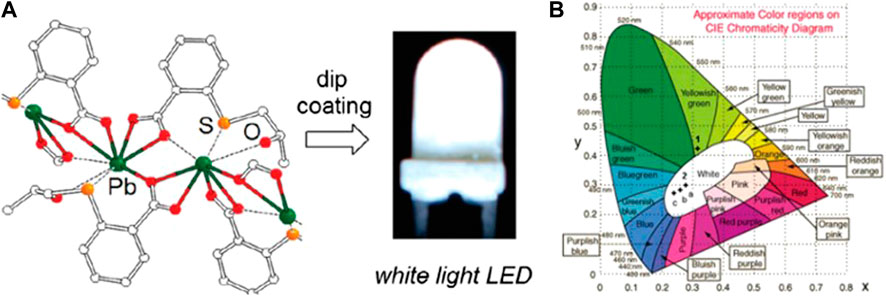
FIGURE 1. (A) Local coordination environment around the Pb2+ ion and white LED based on complex [Pb(L10)]. (B) CIE coordinates for emissions of [Pb(L9)] (λex = 365 nm) and [Pb(L10)].
In addition to carboxylate moieties, N-heterocycles have also emerged as important linkage groups in Pb2+ MOFs. Zeng and coworkers have recently reported three Pb2+ MOFs, [Pb2(L11)2(DMA)]·DMA (21), [Pb2(L11)2(DMF)]·1.5DMF (22), and [Pb2(L11)2(DMF)]·NEt3 (23) (H2L11 = 5-(pyridin-4-yl)isophthalic acid, DMA = N,N-dimethylacetamide) (Supplementary Figure S3). These bulk MOF materials had iso-reticular structures with 1D square or rhombic channels with subtle difference in Pb2+ coordination geometry. Optical experiments showed that 21 was an excellent white emitter with multiple advantages, including pure white color with the chromaticity coordinates (0.331, 0.347) (λex = 350 nm), high fluorescence intensity, and good compatibility to human visibility (Yin Z. et al., 2019). Hydrothermal reaction of pyridine-2,6-dicarboxylic acid (H2L12) and Pb(NO3)2 afforded a rhombic-like 2D polymer [Pb(L12)] (24) (Qi et al., 2018). Upon excitation at λex = 340 nm, 24 exhibits a high-energy emission at 441 nm along with two broad low-energy bands at ca. 553 and 662 nm with a high quantum efficiency of 52%. The emission is possibly assigned to a mixture of LMCT and s→p transition of the Pb2+ center. The high quantum yield and thermal stability, as well as CIE coordinate (0.28, 0.25), suggested the compound as a potential candidate for solid-state white luminescent materials. Reaction of the structurally related ligand, pyridine-2,5-dicarboxylate ligand (H2L13), with Pb(NO3)2 afforded a 3D [Pb(L13)(H2O)] (25) (Wibowo et al., 2010), which is formed by connecting the 1D chains of corner-shared distorted capped trigonal prisms with L13. 25 is also a single-phase WLE phosphor covering a wide spectral range; however, its luminescence origin remains unclear. Examples of WLE Pb2+ MOFs constructed from bridging ligands containing only N-heterocyclic donor moieties are relatively rare but are important for illustrating the influence of aromatic ligands with extended π-conjugation on their emission, because of the more efficient ligand-centered and LMCT transitions. For example, two Pb2+-based coordination polymers, [Pb(NO3)(L14)]n (26) and [Pb(L14)2]n (27), were synthesized from the reactions of 1-tetrazole-4-imidazole-benzene (HL14) and Pb2+ salt in different solvents (Chen et al., 2015). Both compounds exhibit dual emission resulting from different emission origins of LMCT and IL π-π* charge transfer. However, their photoluminescence is dependent on the excitation wavelengths. Recently, Peedikakkal and coworkers reported two Pb2+ MOFs, [Pb2(L15)(O2CCH3)2(O2CCH3)2]·H2O (28) and [Pb(L15)(O2CCF3)2]·1/2CHCl3 (29), as well as the mononuclear complex [Pb(L15-H)2(O2CCF3)4] (30) prepared from the neutral 4,4’-bipyridine (L15) (Peedikakkal et al., 2018). The solid-state photoluminescence of 28–30 was investigated at room temperature. Upon photoexcitation (λex = 329, 376, and 330 nm, respectively), the compounds showed near-white light emissions with CIE coordinates (0.24, 0.32) for 28, (0.33, 0.39) for 29, and (0.26, 0.31) for 30, which were attributed to mixed LMCT and MC transitions.
MOFs have been regarded as one of the promising candidates of fluorescent probes, as a result of the high sensitivity, short response time, portability, and ease of visualization (Kreno et al., 2012). Recent works on Pb2+-based MOFs showed that they exhibited the potential to serve as efficient sensor materials, since their luminescence intensity is found to change linearly with the concentration of the analytes, which are absorbed into the MOF structures. Among the Pb2+-based MOFs reported so far, multi-responsive luminescent MOFs which could probe more than one analyte were of particular interest.
As in the abovementioned WLE materials, carboxylates were often adopted as bridging ligands in luminescent Pb2+-based MOF sensors of ionic and organic analytes. Typical examples are the Pb2+-based MOFs containing pyridine-carboxylates. The addition of various functional groups, such as halides and non-coordinated heteroatoms (N or O) on the pyridyl moiety, were found to significantly alter the functions of Pb2+-based MOFs, by varying the interaction with the analytes. Recently, Guo and coworkers synthesized two Pb2+ complexes, [Pb(L16)2]n (31) and [Pb(L17)2]n (32) (HL16 = 5-chloronicotinic acid, and HL17 = 5-bromonicotinic acid), to investigate effect of the halo-substituents on the sensing properties. It was revealed that the chloro-containing 31 acted as a multi-response luminescent sensor toward Cr2O72‒, Fe3+, and TNP in DMF solution (Supplementary Table S2) (Guo et al., 2019; Miao, 2019). Upon substituting the halide groups by -NH2 and -OH groups, two 3D MOFs, {[Pb3(L18)2Cl5]·(H2O)}n (33) and [Pb2(L19)Cl2]n (34) (HL18 = 5-aminonicotinic acid; H2L19 = 5-hydroxynicotinic acid), have been synthesized, and their functions as luminescent sensors have been compared. Although the non-coordinated donor groups were expected to strengthen the interactions between the MOFs and analytes, very different activities were observed in the two MOFs. 33 was found to be a heterogeneous catalyst for Knoevenagel condensation reaction and exhibited no sensing properties, while 34 was found to be a luminescence sensor for Fe3+ with good recyclability (Zhang et al., 2019). Recently, a 2D framework [PbL18(NO3)]n (35), obtained from hydrothermal reaction of HL18 of Pb(NO3)2, acted not only as a luminescent sensor for picric acid but also as a temperature sensor (Wang, 2019). More recently, Gai reported a novel Pb2+-containing polymer, [Pb(L20)]·0.5H2O·0.5CH3OH (36), containing a zwitterionic ligand 4-carboxy-1-(3,4-dicarboxy-benzyl)-pyridinium chloride (H3L20Cl) (Supplementary Figure S2). Optical experiments indicated that 36 was a versatile turn-off luminescent sensor, which was multi-responsive toward Cr2O72−, CrO42−, Fe3+, and nitrobenzene with a fast response and a high selectivity (Zhao et al., 2020).
As extended π-conjugation in the bridging ligands was suggested to significantly influence the luminescence in Pb2+-based MOF, related complexes containing phthalate and its derivatives were also studied for their sensing properties. A pair of enantiomorphic luminescent MOFs, [Pb10(L21)7(NO3)6(H2O)2] (37) (1P and 1M) (H2L21 = 5-methylisophthalic acid), which possess a novel {Pb18} wheel and a chiral 3D inorganic connectivity, were reported to act as a rapid and highly selective sensor toward Co2+ (Han et al., 2014). Modification of the MOF polymeric structures and their sensing properties was also explored by the co-reaction with bridging ligands of other acidic moieties. Dong and coworkers have prepared two luminescent Pb2+-phosphonate MOFs, the 2D [Pb3(L22)2(HL23)(H2O)2] (38) and 3D [Pb2(L24)0.5 (L25)(H2O)2]·H2O (39) frameworks, which bore both aromatic carboxylates (H3L23 = 5-sulfosalicylic acid, and NaH2L25 = 5-sulfoisophthalic acid sodium) and amino methylenephosphonates (H2L22 = (morpholinomethyl)phosphonic acid and H4L24 = (piperazine-1,4-diylbis (methylene))bis (phosphonic acid)) as the bridging ligands, under hydrothermal conditions. The compounds were demonstrated to be highly selective fluorescent probes for sensing thymine and VO3−, respectively, via fluorescent quenching (Supplementary Figure S4) (Cai et al., 2018). Multi-responsive and multifunctional MOFs were also obtained on introduction of N-heterocycles onto the aromatic linkage moieties. The hydrothermal reactions of the 1,4-bis-(imidazol-1-yl)terephthalic acid (H2L26) with Pb2+ in different solvents afforded a 3D framework with two different isomeric forms, [Pb(L26)]n (40) ((4,5,6)-c net) and [Pb(L26)]n (41) (6-c pcu net), which could be used in fluorescent sensing of different ions, that is, Fe3+ and Cr2O72− (Wang et al., 2018). A new Pb2+-based 2D MOF, {[PbNa(L27)](H2O)(DMF)2}n (42) containing the π-conjugated ligand 4’-(1H-tetrazol-5-yl)-[1,10-biphenyl]-3,5-dicarboxylate (H3L27), was found to not only act as a luminescent sensor for the detection of nitroaromatic compounds and ferric ions but also show excellent activity for the photodegradation of methylene orange (Wu et al., 2018).
Recently, N-heterocycles, for example, imidazolyl and tetrazolyl, with extended π-conjugation, have been adopted as the building blocks in Pb2+-based MOF fluorescent probes. As the multiple donor atoms and the π-conjugation on these ligands are sensitive to environmental changes, the resultant MOF fluorescent probes would contain, in their polymeric structures, uncoordinated N-donor atoms which were suggested to interact with different analytes and instruments in multi-responsive luminescent probes for the simultaneous detection of different analytes. The Pb2+-based coordination polymer [Pb(L28)(NO3)2]n (43) (L28 = 1,4-bis(imidazol-1-yl)benzene), which featured a homochiral double stranded helical structure, was reported to be a luminescent sensor for detecting Fe3+ ions in aqueous solution with high sensitivity (Sun et al., 2019). A stable 3D MOF [Pb3O2L29] (44) was obtained from the hydrothermal reaction of 4-(1H-tetrazol-5-yl)phenol ligand (H2L29) and Pb2+ salt. The compound was a sensitive probe for multi-responsive detection of trace amounts of nitroaromatic compounds and Fe3+ in aqueous media, with visible color changes (Luo et al., 2017). A cage-containing chain [Pb5(L30)6(N3)2(OH)2]n (45) and an 1D double helical chain [Pb(L31)(N3)]n (46) with 1D channels were prepared by solvothermal reactions of the tetrazolyl ligands HL17 or HL18 with Pb2+ salts (Figure 2) (HL30 = 2-(1H-tetrazol-5-yl)quinoline; HL31 = 2-(1H-tetrazol-5-yl)-1,10-phenanthroline) (Xiang et al., 2016). Both MOF structures were reported to uptake different metal ions (Pb2+, Hg2+, Zn2+, and Cd2+) and exhibit varied luminescence responses which are transduced as the change in emission wavelengths and are distinguishable with naked eye, rendering them ideal candidates for sensing different heavy metal ions.
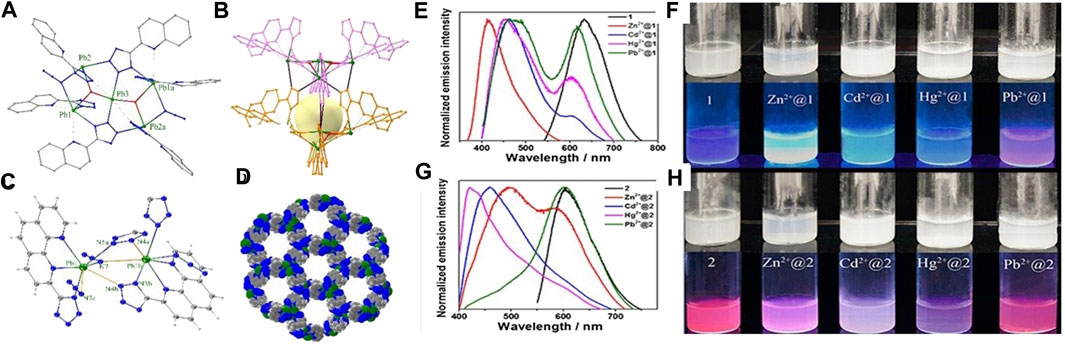
FIGURE 2. (A) Perspective view and (B) one of the cavities of 1D chain cage structure of [Pb5(L30)6(N3)2(OH)2] cluster. (C) Coordination environment of Pb2+ ions in two adjacent molecules and (D) 3D porous structure of [Pb(L31)(N3)]n. The emission spectra of (E) [Pb5(L30)6(N3)2(OH)2]n and (G) [Pb(L31)(N3)]n suspended in the aqueous solution and in 0.005 M MCl2 aqueous solutions (M = Zn2+, Cd2+, Hg2+, and Pb2+) with λex = 360 nm. The photographs of aqueous suspensions of (F) [Pb5(L30)6(N3)2(OH)2]n and (H) [Pb(L31)(N3)]n under normal lighting condition and UV excitation.
In this review, we have summarized the development of Pb2+-based photoluminescent MOFs. Compared with MOFs of block-d and block-f elements, multifunctional Pb2+-based MOFs are still a new area of research. These Pb2+ MOFs have shown the potentials for the unique applications, especially in single-phase WLE and ion/molecular sensing, owing to the special coordination features associated with their Pb2+ centers. At present, most of Pb2+-based MOFs are mainly constructed by bridging carboxylic acid ligands. The design and synthesis of novel functional Pb2+-based MOFs containing various N-heterocyclic ligands with multiple donor atoms, such as imidazole and tetrazole, are suggested to be an effective alternative to engineer and tailor their properties for a given purpose. Since these moieties have pKa values similar to those of carboxylic acid, their more versatile coordination modes and extended π-conjugated systems will also have a significant influence on the structures and emission properties of the resultant MOFs. In addition, their multiple donor atoms will also provide additional sites of interaction with substrates/analytes of different properties, which may thus result in alternative signal transduction processes and further expand the applications of hybrid organic–inorganic lead halide perovskites and related materials. Considering the toxicity of Pb2+ and associated environmental problems, future research will also focus on the synthesis of Pb2+-based multifunctional materials with good thermal stability and water stability, to prevent the leakage of Pb2+ ion and to realize their practical applications.
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors gratefully acknowledge the financial support of the Natural Science Foundation of China (21771026), the Hubei Provincial Natural Science Foundation of China (2018CFA047), and the Natural Science Foundation of Jingzhou (2019EC61-03). Financial support from the Education University of Hong Kong (04567) is also acknowledged.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fchem.2021.636431/full#supplementary-material.
Al-Nubi, M. A. A., Hamisu, A. M., Wardana, F. Y., Ariffin, A., Jo, H., Ok, K. M., et al. (2019). Lead-organic frameworks containing trimesic acid: facile dissolution-crystallization and near-white light emission. Cryst. Growth Des. 19 (11), 6274–6282. doi:10.1021/acs.cgd.9b00759
Cai, X.-O., Sun, M., Shao, Y.-J., Liu, F., Liu, Q.-L., Zhu, Y.-Y., et al. (2018). Two highly stable luminescent lead phosphonates based on mixed ligands: highly selective and sensitive sensing for thymine molecule and VO3-anion. ACS Omega 3 (12), 16443–16452. doi:10.1021/acsomega.8b02030
Chen, J., Zhang, Q., Liu, Z.-F., Wang, S.-H., Xiao, Y., Li, R., et al. (2015). Color tunable and near white-light emission of two solvent-induced 2D lead(ii) coordination networks based on a rigid ligand 1-tetrazole-4-imidazole-benzene. Dalton Trans. 44 (21), 10089–10096. doi:10.1039/C5DT00929D
Chen, X., Jo, H., and Ok, K. M. (2020). Lead mixed oxyhalides satisfying all fundamental requirements for high‐performance mid‐infrared nonlinear optical materials. Angew. Chem. Int. Ed. Engl. 132 (19), 7584–7590. doi:10.1002/anie.20200388210.1002/ange.202002291
Chu, W., Sun, Z.-G., Jiao, C.-Q., Zhu, Y.-Y., Sun, S.-H., Tian, H., et al. (2013). Two novel lead(ii) carboxyphosphonates with a layered and a 3D framework structure: syntheses, crystal structures, reversible dehydration/hydration, and luminescence properties. Dalton Trans. 42 (22), 8009–8017. doi:10.1039/C3DT00125C
Cui, Y., Yue, Y., Qian, G., and Chen, B. (2012). Luminescent functional metal-organic frameworks. Chem. Rev. 112 (2), 1126–1162. doi:10.1021/cr200101d
Gao, R., Reyes-Lillo, S. E., Xu, R., Dasgupta, A., Dong, Y., Dedon, L. R., et al. (2017). Ferroelectricity in Pb1+δZrO3 thin films. Chem. Mater. 29 (15), 6544–6551. doi:10.1021/acs.chemmater.7b02506
Guo, F., Su, C., Fan, Y., and Fu, W. (2019). Two Pb(II) coordination complexes based on 5-halonicotinate (Cl or Br): structural diversities and sensing performance. J. Solid State. Chem. 277, 83–92. doi:10.1016/j.jssc.2019.05.044
Han, Y.-H., Tian, C.-B., and Du, S.-W. (2014). An unusual chiral 3D inorganic connectivity featuring a (Pb18) wheel: rapid and highly selective and sensitive sensing of Co(ii). Dalton Trans. 43 (30), 11461–11464. doi:10.1039/C4DT00905C
He, J., Zeller, M., Hunter, A. D., and Xu, Z. (2012). White light emission and second harmonic generation from secondary group participation (SGP) in a coordination network. J. Am. Chem. Soc. 134 (3), 1553–1559. doi:10.1021/ja2073559
Hu, L., Lin, X.-M., Mo, J.-T., Lin, J., Gan, H.-L., Yang, X.-L., et al. (2017). Lead-based metal-organic framework with stable lithium anodic performance. Inorg. Chem. 56 (8), 4289–4295. doi:10.1021/acs.inorgchem.6b02663
Kreno, L. E., Leong, K., Farha, O. K., Allendorf, M., Van Duyne, R. P., and Hupp, J. T. (2012). Metal-organic framework materials as chemical sensors. Chem. Rev. 112 (2), 1105–1125. doi:10.1021/cr200324t
Lin, X.-L., Chen, B., Huang, Y.-R., Song, K.-Y., Zhou, P.-K., Zong, L.-L., et al. (2020). Achievement of intrinsic white light emission by hybridization-deformable haloplumbates with rigid luminescent naphthalene motifs. Inorg. Chem. Front. 7, 4477–4487. doi:10.1039/D0QI00995D
Lu, J., Xin, X.-H., Lin, Y.-J., Wang, S.-H., Xu, J.-G., Zheng, F.-K., et al. (2019). Efficient X-ray scintillating lead(ii)-based MOFs derived from rigid luminescent naphthalene motifs. Dalton Trans. 48 (5), 1722–1731. doi:10.1039/C8DT04587A
Luo, X., Zhang, X., Duan, Y., Wang, X., and Zhao, J. (2017). A novel luminescent Pb(ii) - organic framework exhibiting a rapid and selective detection of trace amounts of NACs and Fe3+ with excellent recyclability. Dalton Trans. 46 (19), 6303–6311. doi:10.1039/C7DT00715A
Miao, C. (2019). Design and construction of a 2D PbII coordination polymer as a multi-response luminescent sensor for Fe3+, Cr2O72−, and TNP. J. Mol. Struct. 1193, 286–293. doi:10.1016/j.molstruc.2019.05.031
Nazarenko, O., Kotyrba, M. R., Yakunin, S., Aebli, M., Rainò, G., Benin, B. M., et al. (2018). Guanidinium-formamidinium lead iodide: a layered perovskite-related compound with red luminescence at room temperature. J. Am. Chem. Soc. 140 (11), 3850–3853. doi:10.1021/jacs.8b00194
Pan, M., Liao, W.-M., Yin, S.-Y., Sun, S.-S., and Su, C.-Y. (2018). Single-phase white-light-emitting and photoluminescent color-tuning coordination assemblies. Chem. Rev. 118 (18), 8889–8935. doi:10.1021/acs.chemrev.8b00222
Peedikakkal, A. M. P., Quah, H. S., Chia, S., Jalilov, A. S., Shaikh, A. R., Al-Mohsin, H. A., et al. (2018). Near-white light emission from lead(II) metal-organic frame works. Inorg. Chem. 57 (18), 11341–11348. doi:10.1021/acs.inorgchem.8b00637
Peng, C., Song, X., Yin, J., Zhang, G., and Fei, H. (2019). Intrinsic white‐light‐emitting metal-organic frameworks with structurally deformable secondary building units. Angew. Chem. Int. Ed. 58 (23), 7818–7822. doi:10.1002/anie.201903665
Peng, C., Zhuang, Z., Yang, H., Zhang, G., and Fei, H. (2018). Ultrastable, cationic three-dimensional lead bromide frameworks that intrinsically emit broadband white-light. Chem. Sci. 9 (6), 1627–1633. doi:10.1039/C7SC04118G
Qi, H.-X., Jo, H., and Ok, K. M. (2018). Pb[NC5H3(CO2)2]: a white light emitting single component coordination polymer revealing high quantum efficiency and thermal stability. Inorg. Chem. Front. 5 (6), 1273–1276. doi:10.1039/C8QI00217G
Sun, J., Liu, L., Cheng, F., Hu, M., Qin, W., Huang, R., et al. (2019). A homochiral lead(II) double-stranded helical coordination network as luminescent sensor for iron(III). J. Solid State. Chem. 277, 769–772. doi:10.1016/j.jssc.2019.05.049
Terpstra, H. J., De Groot, R. A., and Haas, C. (1997). The electronic structure of the mixed valence compound Pb3O4. J. Phys. Chem. Sol. 58 (4), 561–566. doi:10.1016/S0022-3697(96)00165-5
Wang, J., Gao, L., Zhang, J., Zhao, L., Wang, X., Niu, X., et al. (2018). Syntheses, gas adsorption, and sensing properties of solvent-controlled Zn(II) pseudo-supramolecular isomers and Pb(II) supramolecular isomers. Cryst. Growth Des. 19 (2), 630–637. doi:10.1021/acs.cgd.8b01077
Wang, L. (2019). A dual-functional lead(II) metal-organic framework based on 5-aminonicotinic acid as a luminescent sensor for selective sensing of nitroaromatic compounds and detecting the temperature. J. Inorg. Organomet. Polym. Mater. 30 (2), 291–298. doi:10.1007/s10904-019-01186-0
Wang, Q., and Astruc, D. (2019). State of the art and prospects in metal-organic framework (MOF)-Based and MOF-derived nanocatalysis. Chem. Rev. 120 (2), 1438–1511. doi:10.1021/acs.chemrev.9b00223
Wibowo, A. C., Vaughn, S. A., Smith, M. D., and zur Loye, H.-C. (2010). Novel bismuth and lead coordination polymers synthesized with pyridine-2,5-dicarboxylates: two single component “white” light emitting phosphors. Inorg. Chem. 49 (23), 11001–11008. doi:10.1021/ic1014708
Wu, X., Shen, X., Fan, S., Trivedi, M., Li, B., Kumar, A., et al. (2018). The utilization of a stable 2D bilayer MOF for simultaneous study of luminescent and photocatalytic properties: experimental studies and theoretical analysis. RSC Adv. 8 (42), 23529–23538. doi:10.1039/C8RA04145H
Xiang, J., Shen, C., Cheng, S.-C., Yu, F., Chu, W.-K., Feng, H., et al. (2016). Luminescence behaviour of Pb2+-based cage-containing and channel-containing porous coordination polymers. Dalton Trans. 45 (41), 16134–16138. doi:10.1039/C6DT02986H
Yang, E.-C., Li, J., Ding, B., Liang, Q.-Q., Wang, X.-G., and Zhao, X.-J. (2008). An eight-connected 3D lead(ii) metal-organic framework with octanuclear lead(ii) as a secondary building unit: synthesis, characterization and luminescent property. CrystEngComm 10 (2), 158–161. doi:10.1039/B709810C
Yin, J., Yang, H., and Fei, H. (2019). Robust, cationic lead halide layered materials with efficient broadband white-light emission. Chem. Mater. 31 (11), 3909–3916. doi:10.1021/acs.chemmater.8b05345
Yin, Z., Ma, W.-M., Wang, C., Luo, X.-P., Hu, X.-T., Cao, L.-H., et al. (2019). Color-tuning and near-sunlight white emission in highly stable rod-spacer MOFs with defective dicubane based lead(II)-carboxyl chains. Inorg. Chem. 58 (23), 16171–16179. doi:10.1021/acs.inorgchem.9b02697
Zhang, X., Zhang, R., Jin, Y., and Li, T. (2019). Two PbII-based coordination polymers based on 5-aminonicotinic acid and 5-hydroxynicotinic acid for Knoevenagel condensation reaction and luminescent sensor. J. Solid State Chem. 278, 120927. doi:10.1016/j.jssc.2019.120927
Zhao, X.-Y., Liang, B., Xiong, K.-C., Shi, Y.-W., Yang, S.-L., Wei, T.-Y., et al. (2020). Two novel lead-based coordination polymers for luminescence sensing of anions, cations and small organic molecules. Dalton Trans. 49 (17), 5695–5702. doi:10.1039/D0DT00533A
Zhou, J., Liu, Q., Feng, W., Sun, Y., and Li, F. (2015). Upconversion luminescent materials: advances and applications. Chem. Rev. 115 (1), 395–465. doi:10.1021/cr400478f
Keywords: metal-organic framework, lead, luminescence, sensor, white-light emitting materials
Citation: Wang L-X, Xiang J, Li C-H, Leung C-F and Xiang J (2021) Recent Advances on the Applications of Luminescent Pb2+-Containing Metal–Organic Frameworks in White-Light Emission and Sensing. Front. Chem. 9:636431. doi: 10.3389/fchem.2021.636431
Received: 01 December 2020; Accepted: 01 February 2021;
Published: 12 April 2021.
Edited by:
Xiaoming He, Shaanxi Normal University, ChinaReviewed by:
Jiang-Shan Shen, Huaqiao University, ChinaCopyright © 2021 Wang, Xiang, Li, Leung and Xiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chi-Fai Leung, Y2ZsZXVuZ0BlZHVoay5oaw==; Jing Xiang, eGlhbmdqaW5nQHlhbmd0emV1LmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.