- 1Faculty of Materials Metallurgy and Chemistry, Jiangxi University of Science and Technology, Ganzhou, China
- 2Institute of Resources Comprehensive Utilization, Guangdong Academy of Science, Guangzhou, China
The alkyl salicylaldoxime has attracted more and more attention recently due to the complex branched alkyl groups. In this study, a novel alkyl salicylaldoxime, tert-octylsalicylaldoxime, was successfully synthesized by the one-pot method. The yield and purity by the elemental analysis were 96.17 and 94.13%, respectively. The structure was confirmed by elemental analysis, FT-IR, 1H NMR (Nuclear Magnetic Resonance), 13C NMR spectroscopy, and MS. Results showed that tert-octylsalicylaldoxime with a new structure exhibited excellent extraction ability and selectivity for Cu(II) and can be successfully used to recover Cu from copper-nickel alloy electroplating wastewater. Thus, this product has the potential to be used as a powerful copper extractant in the future.
Introduction
Because copper is one of the most important base metals, the big economic development year by year will cause a serious copper supply and demand contradiction (Yu et al., 2020). Although there are many ways to extract and recover copper ions, solvent extraction is still the most effective and key technique (Farrell et al., 2010; Watling et al., 2014; Edebali and Pehlivan, 2016; Wang et al., 2018; Elizalde et al., 2019; Wang et al., 2020). Therefore, it is urgent to improve the solvent extraction technology and develop novel extractants to overcome this contradiction (Li et al., 2011). In nearly one hundred years of hydrometallurgy history, the importance of chemical factors in achieving the targeted performance of extraction has been widely recognized. Alkyl salicylaldoxime series (AS), the N-O type chelating organic agents, is widely used as extractants to extract copper, nickel, zirconium, and molybdenum (Moradi Ali, 2012; Jain et al., 2016). Jiang et al. have investigated the performance of DZ988N (a mixture of equal volumes of 5-nonylsalicylaldoxime and 2-hydroxy-5-nonyl-acetophenone oxime) in separating copper from sulfate solution containing Cu2+, Fe3+, and Zn2+ (Jiang et al., 2018). Lasheen et al. have investigated the recovery of Mo(VI) from sulfate leach liquor containing Mo(VI) and U(VI) using 5-nonylsalicylaldoxime in a kerosene system (Lasheen et al., 2014). Sastre and Alguacil have used Lix 622 as an extractant to co-extract and selectively strip copper(II) (Sastre and Alguacil, 2001). Gameiro et al. have used Lix 84-I as an extractant to extract copper from the ammoniacal medium (Gameiro et al., 2010). 5-Nonylsalicylaldoxime (NSO) (Zhang et al., 2010), a kind of AS extractant, has been most widely used to extract Cu(II) as the main effective component in the common commercial extractants (see Table 1). In addition, minor changes to the chemical structure of the extracting molecule can have a very significant effect on the extracting agent’s performance in the extraction process.
The focus of this study was to create novel salicylaldoximes with new structures for Cu(II) extraction. Tert-octylsalicylaldoxime (TOSO) was successfully synthesized by the one-pot method in the laboratory (Li et al., 2017). Moreover, its properties and extraction ability for Cu(II) were investigated using the extraction test. This study may provide a promising extractant for the recovery and separation of Cu(II).
The NSO was chosen as a reference extractant. Because the structures of NSO and TOSO are very similar and belong to salicylic oxime, and NSO is the most successful copper commercial extractant.
Experimental
Materials and Reagents
All chemicals used in this work were of analytical grade, and all solutions at specified concentrations were prepared or diluted by deionized water. The stock solutions (0.1 mol/L) of Cu(II), Co(II), Fe(III), and Ni(II) were prepared from the sulfate salts in 1% H2SO4. The working solutions of metals were obtained by diluting these stock solutions before use. Salicylaldoxime (SA) solution was prepared by dissolving SA in kerosene. The pH values were adjusted by 20% (v/v) sodium hydroxide solution or sulfuric acid solution. The reagent was prepared daily.
The copper-nickel alloy electroplating wastewater was kindly supplied by Chengdu Quanrui Technology Co., Ltd. (Sichuan, China), which is produced by electroplating a copper-nickel alloy in a sulfate system. The wastewater mainly contains copper ions, nickel ions, and a small amount of electroplating additives. The copper content was 3.21 g/L, the nickel content was 1.52 g/L, and the pH was 1–2.
Synthesis and Characterization of Tert-Octylsalicylaldoxime
Synthesis
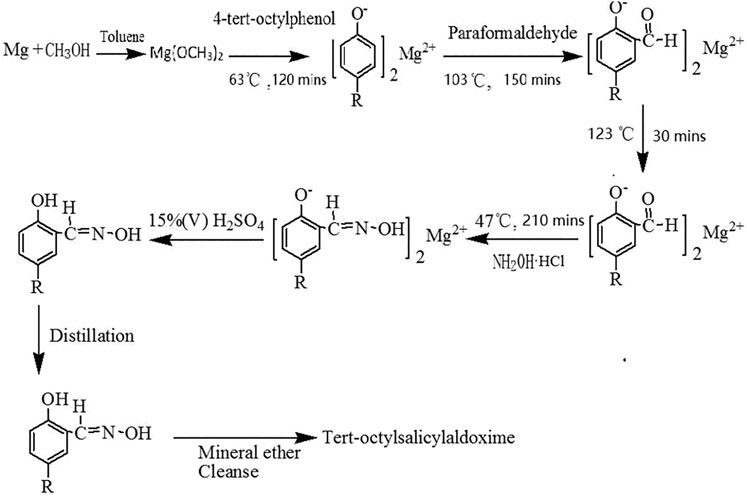
The synthetic routes to TOSO are outlined in Scheme 1. Tert-octylsalicylaldoxime is mainly synthesized by magnesium, 4-tert-octylphenol, paraformaldehyde, and hydroxylamine hydrochloride. The optimized mole ratio of the four reactants is Mg:4-tert-octylphenol:paraformaldehyde:hydroxylamine hydrochloride = 0.55:1:2.40:1.50.
Formylation
Magnesium ribbons (0.022 mol) were added to the solution consisting of anhydrous methanol (50 ml) and toluene (30 ml), and then the mixture was heated to 63°C for reaction with stirring until all the magnesium was dissolved and H2 generation stopped. Then, 4-tert-octylphenol (8.25 g, 0.04 mol) was added to the mixture and refluxed for 120 min. Finally, the paraformaldehyde (0.096 mol) was added and then heated to 103°C for reaction with stirring for 150 min and subsequently heated to 123°C for reaction with stirring for 30 min.
Oximation
Firstly, the mixture was cooled to 47°C. Secondly, the hydroxylamine hydrochloride (0.06 mol) dissolved in deionized water was added to the above mixture and reacted for 210 min. Finally, tert-octylsalicylaldoxime (9.58 g, purity of 94.13%), appearing as white needle solid, was obtained after additional processes, including acidification, reduced pressure distillation, and petroleum ether cleaning.
Analytical Methods
The concentrations of C, H, and N of the final product were measured by Elementar-Vario elemental analyzer (Elementar Co., Germany). The purity and yield can be calculated by Eq 1 and Eq 2, respectively. Fourier Transform Infrared Spectrum (FT-IR) of the synthesized tert-octylsalicylaldoxime was surveyed from 400 to 4000 cm−1 by AVATR-360 FT-IR infrared spectrophotometer (Nicolet Co., United States) using KBr pellet technique. The 1H NMR (Nuclear Magnetic Resonance) and 13C NMR spectroscopy of the final product were obtained by ADVANCE III 500 NMR spectrometer (Bruker Co., Germany) using deuterium chloroform as solvent. The mass spectrometry was recorded by a GCMS-QP2010 analyzer (Japan) (Li et al., 2019; Wang et al., 2019).
Solvent Extraction Experiments
All the extraction and stripping experiments were conducted in a programmable air bath shaker with the organic phase (volume ratio W/O = 1). The stirring speed was maintained at 300 rpm with a stirring time ranging from 1 to 5 min to obtain extraction equilibrium. Initial pH was adjusted in the range of 1.0–3.5 with sulfuric acid or sodium hydroxide. The organic phase (extract) was stripped with sulfuric acid at a volume ratio W/O = 1. Unless otherwise stated, all other experiments were carried out at room temperature. A small amount of the raffinate (1 ml) was drawn out and diluted to the appropriate concentration for analysis.
Effects of extraction time, equilibrium pH, volume content, and A:O were investigated. The extraction efficiencies (E) of Cu(II) can be calculated from the differences between the concentrations of Cu(II) in the aqueous phase before and after extraction, as expressed by Eq. 3. The separation factor of the extractants for copper(II) to one metal (βCu/metal) is evaluated by Eq. 4.
where E is the metal extraction efficiency, C1 and C2 are the metal concentrations in the aqueous phase before and after extraction, V1 and V2 are the volumes of the aqueous phase before and after the extraction; CCu(O) and CCu(A) indicate the concentration of copper(II) in the organic phase and aqueous phase (g/L), respectively, and Cmetal(O) and Cmetal(A) indicate the concentration of organic phase and aqueous phase (g/L), respectively. The concentration of metals in an aqueous solution is analyzed by Inductively Coupled Plasma-Atomic Emission Spectrometry (ICP-AES).
Results and Discussion
Characterization of Tert-Octylsalicylaldoxime
Chemical spectral data of TOSO were demonstrated as follows.
Element analysis results (%): (1) calculated, C 61.14, H 9.55, N8.92; (2) found, C 61.02, H 9.48, N 8.72. IR (KBr, cm−1): 3400 (OH), 2960 (CH3, CH2), 2800 (CH3, CH2), 1630(C=N), 1600 (aromatic CH), 1500 (aromatic CH), 1580 (aromatic CH), 1350 (NO).1H NMR (CDCl3, ppm): = 0.719 (t, 3H, J = 6.0 Hz, CH3), 1.343 (t,3H, J = 6.0 Hz, CH3), 1.701 (s, 1H, CH2), 7.13 (s, 1H, CH), 6.90 (d, 1H, CH),7.28 (d, 1H, J = 6.4 Hz, CH), 7.50 (t, 1H, J = 6.0 Hz, CH), 9.646–9.668 (s, 1H, OH), 8.233 (s, 1H, J = 6.4 Hz, OH).13C NMR (CDCl3, ppm): = 31.56, 31.79, 32.33, 56.89, 37.88,115.46, 129.47, 128.15, 141.47, 116.05, 154.88 and 153.62. MS for C15H23NO2 (M+): 249.35. Found: 249.
Extraction and Stripping of Cu(II) Ion
Effects of extraction time, equilibrium pH, volume content, and A:O on extraction efficiency are shown in Figure 1. Those experiments were carried out in H2SO4 media with an initial Cu(II) concentration of 1.92 g L−1, 15% (V/V) of extractants, and the phase ratio (aqueous phase to organic phase) of 1 and were single-stage.
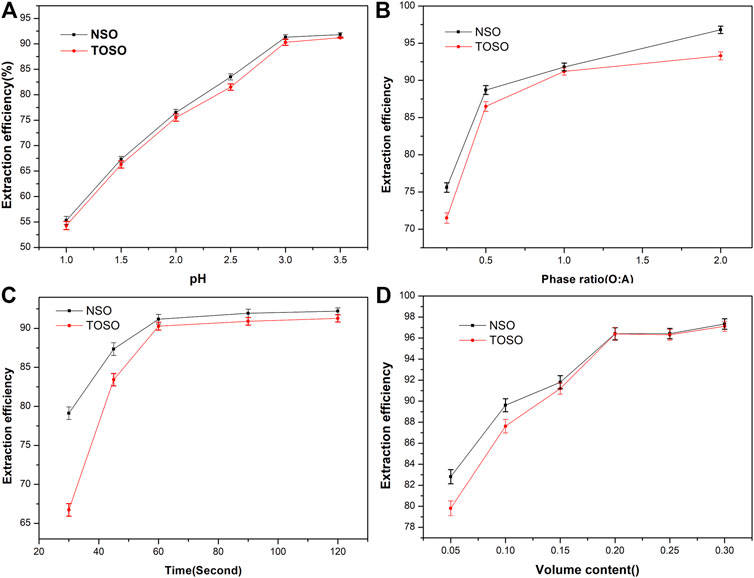
FIGURE 1. Effects of equilibrium pH (A), phase ratio A:O (B), extraction time (C), and volume content (D) on the recovery of Cu(II) with TOSO and NSO.
As can be seen in Figure 1A, extraction efficiency increased with pH under the experimental pH 1.0–3.5. At pH 3.0, TOSO and NSO extracted out 90.3 and 91.3% of Cu(II) from the aqueous phase, respectively. Figure 1B indicates that with the increase of phase ratio, TOSO and NSO exhibited more satisfactory extraction efficiency for Cu(II). When the phase ratios (O:A) were 0.5, 1, and 2, the copper extraction efficiencies of TOSO were 86.53, 91.20, and 94.32%, respectively, and those NSO were 88.71, 91.80, and 96.82%, respectively. It indicated that the extraction efficiency increased when the phase ratio (O:A) increased from 1 to 2. However, the operation with a higher O:A ratio has a potential risk of phase separation. Therefore, it is reasonable to use a phase ratio of 1 in the experiment. Figure 1C shows that TOSO and NSO have a similar tendency of extraction efficiency within a fixed time; more than 90% Cu(II) was recovered in 60 s. In addition, TOSO and NSO revealed similar extraction efficiency in high extractant concentrations, as shown in Figure 1D.
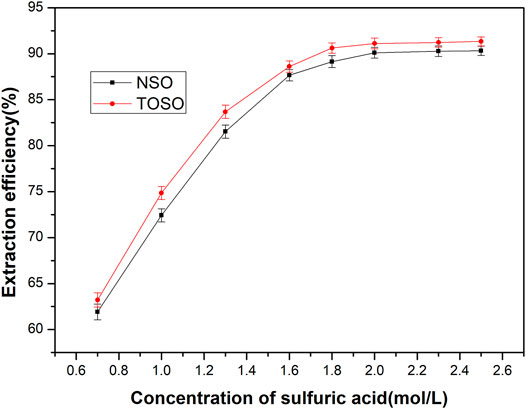
The copper-loaded organic was stripped at a 1:1 phase ratio with H2SO4 in a single stage. Results of these tests are listed in Figure 2. It is clear that the stripping efficiency of TOSO and NSO increased rapidly with the concentration of sulfuric acid and reached around 90% at the concentration of 1.8 mol/L. Further increase of sulfuric acid concentration did not have a significant effect on the stripping efficiency. Meanwhile, TOSO delivered better results than NSO in terms of stripping efficiency.
Copper(II) Selectivity
In industrial applications, there are many different types of metal ions present in a single hydrometallurgical process, which can significantly interfere with the extraction of Cu(II). Therefore, it is necessary to investigate the selectivity of extractants for metals.
The extraction experiments were conducted in H2SO4 media with a phase ratio (O:A) of 1. A single-stage extraction was applied with an extraction time of 3 min at room temperature. The pH of the solution was adjusted by 20% (v/v) sodium hydroxide solution or sulfuric acid solution. The concentration of Cu2+ was 0.03 mol/L, while the concentrations of Fe3+, Zn2+, Ni2+, and Co2+ were set as 0.02 mol/L.
The experimental results are shown in Figure 3. As shown in Figure 3A, the synthesized tert-octylsalicylaldoxime presents a good extraction selectivity of Cu from other metals in the H2SO4 medium. The selectivity increased with the increase of pH from 1 to 3. The separation factor can be ranked in the following order: βCu/Co > βCu/Ni > βCu/Zn ≈ βCu/Fe within the pH studied, indicating that TOSO can selectively extract Cu from the complex solution. The strong selectivity for Cu may be caused by the particular umbrella-like structure of TOSO, which can provide a stronger steric effect than that of other AS series extractants with straight-chain R groups.

FIGURE 3. Selectivity of TOSO and NSO for Cu2+/metal ion in H2SO4 medium. (A) Selectivity of TOSO; (B) selectivity of TOSO and NSO at pH 3.
As can been seen in Figure 3B, when pH was 3.0, the βCu/Fe, βCu/Zn, βCu/Ni, and βCu/Co values of TOSO in sulfuric acid are 1128, 1368, 15,375, and 16,787, respectively; the βCu/Fe, βCu/Zn, βCu/Ni, and βCu/Co values of NSO are 1009, 1251, 14,875, and 15,867, respectively. Compared with NSO, TOSO showed better selectivity for Cu/metal separation.
Tert-Octylsalicylaldoxime Application in Recovery of Cu(II) From Plating Wastewater
Considering the excellent separation performance of TOSO in Cu/Ni system, TOSO was selected for recovering Cu(II) from copper-nickel alloy electroplating wastewater. The experimental results, including extraction and stripping efficiency, extraction and stripping time, and βCu/Ni for all the copper extraction experiments, are summarized in Table 2.
The maximum extraction, maximum stripping efficiency, extraction separation time, stripping separation time, and separation factor of TOSO were 88.42%, 85.61%, 42 s, 45 s, and 131, respectively. Moreover, those of NSO were 89.21%, 84.83%, 35 s, 39 s, and 101, respectively. It indicated that NSO exhibited better performance in extraction efficiency and extraction and stripping phase separation time. However, TOSO showed advantages in separation factor and stripping efficiency.
Based on the above results, NSO has higher extraction ability and shorter phase separation time for Cu(II) than TOSO, while TOSO is superior to NSO in terms of the separation ability for metals (Ni, Co, Cu, Zn) and stripping efficiency. The alkyl side chains link of TOSO (C8) is shorter than that of NSO (C9), and the molecular sizes and Log P (Represents the hydrophobicity value) of TOSO are also smaller than those of NSO. This means that TOSO has a lower probability of capturing Cu(II) ions and weaker hydrophobicity, which results in a longer phase separation time. Compared with NSO, the better extraction selectivity of TOSO might be due to the particular umbrella-like structure of the tert-octyl group and shorter carbon chain (Mowafy and Mohamed, 2014).
Conclusion
In this article, the extraction behavior of tert-octylsalicylaldoxime (TOSO) for Cu has been investigated by extraction and stripping tests. Based on the experimental results, the following conclusions could be drawn:
1) TOSO, a novel AS series extractant for Cu(II), was successfully synthesized by a one-pot reaction with a yield of 96.17% and purity of 94.13%. The structure of the synthesized TOSO was verified by elemental analysis, FT-IR, 1H NMR, and 13C NMR spectroscopy.
2) The results showed that TOSO exhibited powerful extraction and stripping performance. Compared with 5-nonylsalicylaldoxime (NSO), tert-octylsalicylaldoxime (TOSO) presents a stronger affinity to Cu(II) than to Co, Ni, Zn, and Fe in H2SO4 media, indicating it is suitable to extract Cu from these complex solutions selectively. Moreover, TOSO has been proved effective as a special extractant in the copper-nickel alloy electroplating wastewater. Therefore, TOSO can be considered a powerful extractant candidate for copper extraction in the hydrometallurgical process, which is of great significance to the development and utilization of copper resources.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material; further inquiries can be directed to the corresponding authors.
Author Contributions
LL performed the experiments, analyzed all the data, drafted all the figures, and prepared the manuscript. FL and LY performed the experiments. CL and LL conceived and designed the experiments. FL revised the manuscript.
Funding
This research was supported by the National Natural Science Foundation of China (Nos. 21406097 and U1607108), the National Science Foundation for Postdoctoral Scientists of China (No. 2016M592118), the Jiangxi Province Postdoctoral Sustentation Fund of China (Nos. 2015KY11 and 2015RC17), the Jiangxi Province Funds for Distinguished Young Scientists (Nos. 20192BCB23016), and GDAS’ Project of Science and Technology Development (2020GDASYL-20200302004).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors thank Hong Zhong from Central South University in China for this research.
References
Alguacil, F. J., and Cobo, A. (1998). Extraction of Nickel from Ammoniacal/ammonium Carbonate Solutions Using Acorga M5640 in Iberfluid. Hydrometallurgy 50 (2), 143–151. doi:10.1016/s0304-386x(98)00047-4
Aminian, H., and Bazin, C. (2000). Solvent Extraction Equilibria in Copper (II)-Iron (III)-LIX984 System. Minerals Eng. 13 (6), 667–672. doi:10.1016/s0892-6875(00)00049-2
Edebali, S., and Pehlivan, E. (2016). Evaluation of Chelate and Cation Exchange Resins to Remove Copper Ions. Powder Technol. 301, 520–525. doi:10.1016/j.powtec.2016.06.011
Elizalde, M. P., Rúa, M. S., Menoyo, B., and Ocio, A. (2019). Solvent Extraction of Copper from Acidic Chloride Solutions with LIX 84. Hydrometallurgy 183, 213–220. doi:10.1016/j.hydromet.2018.12.013
Farrell, M., Perkins, W. T., Hobbs, P. J., Griffith, G. W., and Jones, D. L. (2010). Migration of Heavy Metals in Soil as Influenced by Compost Amendments. Environ. Pollut. 158, 55–64. doi:10.1016/j.envpol.2009.08.027
Gameiro, M. L. F., Machado, R. M., Ismael, M. R. C., Reis, M. T. A., and Carvalho, J. M. R. (2010). Copper Extraction from Ammoniacal Medium in a Pulsed Sieve-Plate Column with LIX 84-I. J. Hazard. Mater. 183, 165–175. doi:10.1016/j.jhazmat.2010.07.006
Jain, V., Pradip, , and Rai, B. (2016). Density Functional Theory Computations for Design of Salicylaldoxime Derivatives as Selective Reagents in Solvent Extraction of Copper. Trans. Indian Inst. Met. 69 (1), 135–141. doi:10.1007/s12666-015-0722-6
Jiang, F., Yin, S., Zhang, L., Peng, J., Ju, S., Miller, J. D., et al. (2018). Solvent Extraction of Cu(II) from Sulfate Solutions Containing Zn(II) and Fe(III) Using an Interdigital Micromixer. Hydrometallurgy 177, 116–122. doi:10.1016/j.hydromet.2018.03.004
Lasheen, T. A., Ibrahim, M. E., Hassib, H. B., and Helal, A. S. (2014). Recovery of Molybdenum from Uranium Bearing Solution by Solvent Extraction with 5-Nonylsalicylaldoxime. Hydrometallurgy 146, 175–182. doi:10.1016/j.hydromet.2014.03.011
Li, L., Wang, Y., An, W., and Bao, S. (2017). Effect of the Structure of Alkyl Salicylaldoxime on Extraction of Copper(II). Minerals 7 (4), 61–66. doi:10.3390/min7040061
Li, L., Zhao, J., Sun, Y., Yu, F., and Ma, J. (2019). Ionically Cross-Linked Sodium Alginate/ĸ-Carrageenan Double-Network Gel Beads with Low-Swelling, Enhanced Mechanical Properties, and Excellent Adsorption Performance. Chem. Eng. J. 372, 1091–1103. doi:10.1016/j.cej.2019.05.007
Li, L., Zhong, H., Cao, Z., and Yuan, L. (2011). Recovery of Copper(II) and Nickel(II) from Plating Wastewater by Solvent Extraction. Chin. J. Chem. Eng. 19 (6), 926–930. doi:10.1016/s1004-9541(11)60073-6
Moradi Ali, P. (2012). Characterization of 5-nonylsalicylaldoxime Production and the Effects of Modifiers on its Extracting/stripping Properties. Res. Chem. Intermed. 38, 2401–2409. doi:10.1007/s11164-012-0556-3
Mowafy, E. A., and Mohamed, D. (2014). Extraction Behavior of Trivalent Lanthanides from Nitric Acid Medium by Selected Structurally Related Diglycolamides as Novel Extractants. Separat. Purif. Technol. 128, 18–24. doi:10.1016/j.seppur.2014.03.005
Parija, C., and Bhaskara Sarma, P. (2000). Separation of Nickel and Copper from Ammoniacal Solutions through Co-extraction and Selective Stripping Using LIX84 as the Extractant. Hydrometallurgy 54 (2-3), 195–204. doi:10.1016/s0304-386x(99)00069-9
Sastre, A. M., and Alguacil, F. J. (2001). Co-extraction and Selective Stripping of Copper (II) and Molybdenum (VI) Using LIX 622. Chem. Eng. J. 81, 109–112. doi:10.1016/s1385-8947(00)00237-0
Wang, Y.-C., Wang, R.-X., Qiu, G., Zhou, H., Xie, W., and Liu, J.-B. (2019). ortho-Amide-directed 2,4-dibromohydration of Conjugated Enynes. Org. Chem. Front. 6, 2471–2479. doi:10.1039/c9qo00540d
Wang, Y., Chen, X., and Zhou, H. (2018). Disentangling Effects of Temperature on Microbial Community and Copper Extraction in Column Bioleaching of Low Grade Copper Sulfide. Bioresour. Technol. 268, 480–487. doi:10.1016/j.biortech.2018.08.031
Wang, Y., Li, J., Gao, Y., Yang, Y., Gao, Y., and Xu, Z. (2020). Removal of Aluminum from Rare-Earth Leaching Solutions via a Complexation-Precipitation Process. Hydrometallurgy 191, 105220. doi:10.1016/j.hydromet.2019.105220
Watling, H. R., Collinson, D. M., Li, J., Mutch, L. A., Perrot, F. A., Rea, S. M., et al. (2014). Bioleaching of a Low-Grade Copper Ore, Linking Leach Chemistry and Microbiology. Minerals Eng. 56, 35–44. doi:10.1016/j.mineng.2013.10.023
Yang, R., Wang, S., Duan, H., Yuan, X., Huang, Z., Guo, H., et al. (2016). Efficient Separation of Copper and Nickel from Ammonium Chloride Solutions through the Antagonistic Effect of TRPO on Acorga M5640. Hydrometallurgy 163, 18–23. doi:10.1016/j.hydromet.2016.03.006
Yu, B., Kou, J., Xing, Y., and Sun, C. (2020). Enhanced Extraction of Copper from Cupriferous Biotite by Organic Intercalation. Hydrometallurgy 192, 105286. doi:10.1016/j.hydromet.2020.105286
Keywords: tert-octylsalicylaldoxime, synthesis, characteristic, extraction performances, plating wastewater
Citation: Li L, Feng L, Liao C, Li F and Yang L (2021) Synthesis of Tert-Octylsalicylaldoxime and Its Application in Extraction of Cu(II). Front. Chem. 9:592760. doi: 10.3389/fchem.2021.592760
Received: 08 August 2020; Accepted: 13 July 2021;
Published: 13 August 2021.
Edited by:
Shenxu Bao, Wuhan University of Technology, ChinaReviewed by:
Wuping Liao, Changchun Institute of Applied Chemistry (CAS), ChinaZhenyue Zhang, Wuhan Institute of Technology, China
Copyright © 2021 Li, Feng, Liao, Li and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liqing Li, bGlsaXFpbmc3OUAxMjYuY29t; Fangxu Li, bGlmYW5neHUyOEAxNjMuY29t
 Liqing Li
Liqing Li Luo Feng
Luo Feng Chunfa Liao1
Chunfa Liao1 Fangxu Li
Fangxu Li