
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Chem. , 08 January 2021
Sec. Nanoscience
Volume 8 - 2020 | https://doi.org/10.3389/fchem.2020.628273
This article is part of the Research Topic Advanced Nanomaterials for Light-Emitting Diodes and Solar Cells View all 11 articles
The effects of substitution of BaF2 for BaO on physical properties and 1. 8 μm emission have been systematically investigated to improve spectroscopic properties in Tm3+ doped gallium tellurite glasses for efficient 2.0 μm fiber laser. It is found that refractive index and density gradually decrease with increasing BaF2 content from 0 to 9 mol.%, due to the generation of more non-bridging oxygens. Furthermore, OH− absorption coefficient (αOH) reduces monotonically from 3.4 to 2.2 cm−1 and thus emission intensity near 1.8 μm in gallium tellurite glass with 9 mol.% BaF2 is 1.6 times as large as that without BaF2 while the lifetime becomes 1.7 times as long as the one without BaF2. Relative energy transfer mechanism is proposed. The maximum emission cross section and gain coefficient at around 1.8 μm of gallium tellurite glass containing 9 mol.% BaF2 are 8.8 × 10−21 cm2 and 3.3 cm−1, respectively. These results indicate that Tm3+ doped gallium tellurite glasses containing BaF2 appear to be an excellent host material for efficient 2.0 μm fiber laser development.
Over the past few decades, fiber lasers operating in eye-safe 2.0 μm spectral region have attracted a great deal of attention due to strong absorption band of several chemical compounds (H2O, CO2, N2O, etc.) in this region (Chen et al., 2010). Therefore, there are some potential applications in eye-safe laser radar, material processing, laser surgery, remote sensing and effective pump sources as mid-infrared lasers and optical parametric oscillators (Geng et al., 2011; Geng and Jiang, 2014; Slimen et al., 2019; Wang et al., 2019). Up to now, active ions for 2.0 μm laser have been mainly focused on Tm3+ and Ho3+ ions arising from and transition. Compared with Ho3+, Tm3+ owns very strong absorption band of transition and thus can be effectively pumped by commercial high-power 808 nm laser diode. Under the pump scheme, a quantum efficiency of 200% can be expected from “two-for-one” cross relaxation process ) (Richards et al., 2010). In addition, broad emission bandwidth of transition about 300 nm is advantageous to the generation of femtosecond pulse (Agger et al., 2004).
In pursuit of efficient 2.0 μm laser, different glass hosts have been extensively investigated and the laser operation has been demonstrated in silicate, fluoride, germanate and tellurite glasses (Richards et al., 2010; He et al., 2013; Wang et al., 2019). Among these glass hosts, tellurite glasses own a lot of advantage such as broad infrared transmission region, lower phonon energy, high rare-earth ion solubility, high refractive index (~2) and easy fabrication with low melting temperature(Richards et al., 2010). Recently, our groups have exploited several new tellurite glass systems such as TeO2-Ga2O3-BaO (TGB) and TeO2-Ga2O3-ZnO (TGZ) with excellent glass-forming ability, thermal stability and 2.0 μm spectroscopic properties (Li et al., 2019; Mao et al., 2020). To further improve 2.0 μm emission properties, it is very essential to reduce the hydroxyl content in glasses because OH− groups are the main energy loss channels for active ions and can result in strong 2.0 μm fluorescence quenching (Terra et al., 2006). We found that the strength of interaction between Tm3+ and OH− (12.9 × 10−19 cm4/s) was stronger than that between Er3+ and OH− (1.9 × 10−19 cm4/s) (Yuan et al., 2014).
Herein, based on the composition of TGB glass with good thermal stability, we systematically investigate the effects of substitution of BaF2 for BaO on physical properties and 1.8 μm emission properties. Density, refractive index, Raman spectra, absorption spectra and emission spectra were measured along with the lifetime of Tm3+:3F4 energy level. Moreover, energy transfer mechanism is proposed and emission cross section and gain coefficient of transition in TGB glass with 9 mol.% BaF2 are determined.
Tm3+ doped gallium tellurite glasses (TGB) with the molar compositions of 80TeO2-10Ga2O3-(9-x)BaO-xBaF2-1Tm2O3 (x = 0, 3, 6, and 9) were prepared by the conventional melt-quenching method. TeO2, Ga2O3, BaO, BaF2 and Tm2O3 with 99.99% purity (Aladdin) were used as raw chemicals. Appropriate amounts of these chemicals (~20 g) were well mixed and then melted in an alumina crucible with an alumina lid at ~950°C for 30 min. Afterwards, the melts were poured onto a preheated graphite mold and further annealed at 330°C for 2 h, after which they were cooled slowly inside the furnace to room temperature. The annealed samples for the optical property measurements need to be double-sided polishing into 10 × 10 × 1.5 mm3 cylinders. Densities of glasses were determined by the Archimedes' principle using the distilled water as the medium. The refractive index of all the samples was measured by the prism coupling method (Metricon Model 2010) at 633, 1,309, and 1,533 nm with an error of ±5 × 10−4. The infrared transmittance spectra were obtained using Vector 33 Fourier transform infrared (FTIR) spectrophotometer (Bruker, Switzerland). The Raman spectra were measured by Raman spectrometer (Renishawin Via, Gloucestershire, UK) and 532 nm laser as the excitation source. Optical absorption spectra measurements were performed on a Perkin-Elmer Lambda 900/UV/VIS/NIR spectrophotometer. The fluorescence spectra were recorded by a computer-controlled Triax 320 type spectrofluorimeter (Jobin-Yvon Corp.) equipped with an InAs detector upon the excitation of an 808 nm LD. After exciting the samples with an 808 nm LD, InAs detector was used to detect the lifetime of Tm3+:3F4 energy level (1.8 μm) along with a digital phosphor oscilloscope (TDS3012C, Tektronix, America) and signal generator. All of the measurements were carried out at room temperature.
Table 1 presents the refractive index (n) and density (ρ) of TGB glasses with different BaF2 contents. It is found that the refractive index and density monotonously decrease when BaF2 content increases from 0 to 9 mol.% in step of 3 mol.%. This indicates that the addition of BaF2 makes glass network looser (Yang et al., 2017), which is demonstrated by the Raman spectra as shown Figure 1. It is noted that three major bands appear in TGB glasses with different BaF2 amounts. The peak A at ~466 cm−1 is assigned to the symmetrical stretching or bending vibrations of Te-O-Te linkages at corner sharing sites (Murugan and Ohishi, 2004; Jose et al., 2007). The peak B at ~682 cm−1 is ascribed to the anti-symmetric stretching vibrations of Te-O-Te linkages constructed by two un-equivalent Te-O bonds containing bridging oxygens (BO) in TeO4 trigonal bipyramid and the peak C is due to the symmetrical stretching vibrations of Te-O− and Te=O bonds with non-bridging oxygens (NBO) in TeO3 trigonal pyramid and TeO3+1 polyhedra (Murugan and Ohishi, 2004; Jose et al., 2007). It is worth noting that the position of peak C slightly shifts from 769 to 787 cm−1 and normalized intensity of peak B declines with the increment of BaF2 from 0 to 9 mol.%, revealing that glass network structure is broken and more non-bridging oxygens arise. Such low phonon energy of TGB glasses is able to effectively decrease non-radiative relaxation in favor of the enhancement of 2.0 μm emission intensity.
Figure 2 shows the typical absorption spectra of TGB glasses in the wavelength range from 350 to 2,100 nm. The absorption spectrum consists of five absorption bands of Tm3+ centered at 473, 687, 794, 1,214, and 1,700 nm, corresponding to respective transitions from the 3H6 ground state to excited states 1G4, 3F2,3, 3H4, 3H5, and 3F4. Energy levels above 1G4 energy level are not clearly identified because of strong intrinsic bandgap absorption in the host glass. It is also found that the position and shape of five absorption peaks are almost constant with the addition of BaF2.
When BaF2 is added, F− ions crack O-H bond in glass network and produce HF gas so that OH− content is reduced. OH− content is reflected by OH− absorption coefficient (αOH) (Wang et al., 2013).
where l represents the thickness of glass samples, T0 and T are the incident and transmitted intensity, respectively. According to FTIR spectra, OH− absorption coefficient of TGB glasses is determined and presented in Figure 3. There are two absorption bands centered at 3.1 and 4.4 μm, corresponding to stretching mode of free Te-OH groups and/or stretching mode of molecular water and stretching mode of strong hydrogen-bonded Te-OH groups, respectively (Wang et al., 2019). αOH at 3.1 μm is obviously higher than the value at 4.4 μm. Moreover, αOH monotonically decreases from 3.4 to 2.2 cm−1 with increasing BaF2 content from 0 to 9 mol.% in step of 3 mol.%, which is beneficial to improve 1.8 μm emission properties of Tm3+ ions.
Figure 4 compares the fluorescence spectra and decay curves of transition in TGB glasses with different BaF2 amounts pumped by 808 nm LD. From Figure 4A, it is clear that the spectra are characterized by two emission peaks located at 1,488 and 1,808 nm, corresponding to and transitions, respectively. Emission intensity at 1,488 nm is obviously weaker than that at 1,808 nm, which is attributed to effective cross relaxation process ). Moreover, emission intensity at 1,488 nm remains almost unchanged and that near 1.8 μm gradually increases with the increment of BaF2 concentration. The peak value near 1.8 μm in TGB glasses with 9 mol.% BaF2 is 1.6 times as high as that without BaF2 because the reduction of OH− content weakens the interaction between Tm3+ and OH− and thus enhances radiative transition probability of transition. Figure 4B describes fluorescence decay curves of Tm3+:3F4 energy level monitored at 1,808 nm in TGB glasses with different proportions of BaF2. It is clearly noted that the lifetime of 3F4 energy level gradually prolongs from 337.4 to 577.8 μs when BaF2 content increases from 0 to 9 mol.% in step of 3 mol.%. The lifetime in TGB glass with 9 mol.% BaF2 is 1.7 times as long as the value without BaF2. These results mean that the addition of BaF2 can greatly improve 1.8 μm emission properties.
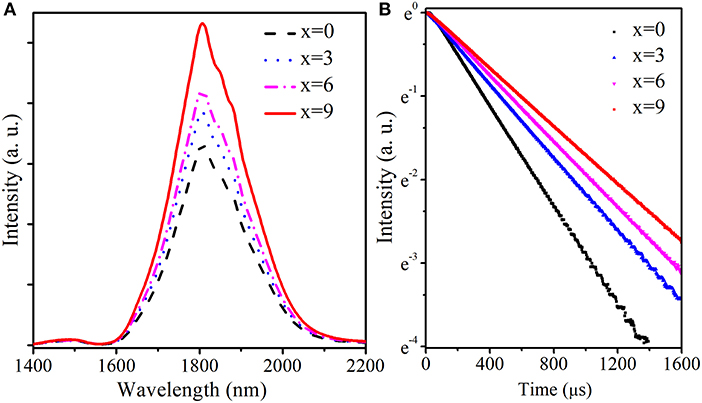
Figure 4. (A) Fluorescence spectra and (B) decay curves of Tm3+:3F4 energy level in TGB glasses with different proportions of BaF2 pumped by 808 nm LD.
In general, the total decay rate (W) of Tm3+:3F4 energy level is defined as the reciprocal of the measured decay lifetime (τm) and is described by the following equations (Zhou et al., 2010).
where Ar represents the radiative decay rate, WOH is the energy transfer rate between Tm3+ and OH−, Wmp is the multiphonon decay rate, WET is the energy transfer rate between Tm3+ ions, NTm is the total concentration of Tm3+ ions and αOH is OH− absorption coefficient. kOH−Tm is defined as the strength of interaction between Tm3+ and OH− and doesn't rely on the concentrations of Tm3+ and OH−. Figure 5 represents a good linear relationship between the total decay rate and αOH. From this fit, kOH−Tm is determined and equals to 2.82 × 10−18 cm4/s, which is larger than kOH−Er (1.9 × 10−19 cm4/s) (Zhou et al., 2010) and lower than kOH−Tm (7.89 × 10−18 cm4/s) in germanate glasses (Wang et al., 2014).
Based on above-mentioned results, Figure 6 shows energy transfer mechanism. Under excitation at 808 nm LD, Tm3+ ions are motivated to 3H4 state from the 3H6 ground state. Then, a few Tm3+ ions return radiatively to 3F4 state with 1,488 nm photon. However, the majority of ions relax nonradiatively to 3F4 state via muliphonon relaxation process and efficient cross relaxation process (CR) between two adjacent Tm3+ ions ). Finally, Tm3+ ions in the excited 3F4 state return to the 3H6 ground state, emitting fluorescence at 1.8 μm. Significantly, the residual OH− in TGB glasses can impair 1.8 μm emission via two OH− ions, indicating that it is essential to decrease the hydroxyl content for improving 1.8 μm emission.
Both absorption and emission cross sections of Tm3+ ions are very crucial parameters to evaluate the potential of TGB glasses as 2 μm laser material. Based on the Beer-Lambert equation and Fuchtbauer-Ladenburg equation (Chen et al., 2007), absorption and emission cross sections of transition in TGB glass with 9 mol.% BaF2 are calculated and presented in Figure 7A. The maximum absorption cross section of Tm3+ reaches 5.3 × 10−21 cm2 at 1,706 nm, which is higher than that of silicate glass (1.5 × 10−21 cm2) (Li et al., 2012), fluorophosphate glass (3.0 × 10−21 cm2) (Li et al., 2015), tellurium germanate glass (3.2 × 10−21 cm2) (Gao et al., 2015) and germanate glass (4.1 × 10−21 cm2) (Yu et al., 2009). Moreover, corresponding maximum emission cross section is 8.8 × 10−21 cm2 at 1,814 nm, which is higher than that of silicate glass (3.6 × 10−21 cm2) (Li et al., 2012), fluorophosphate glass (5.5 × 10−21 cm2) (Li et al., 2015), tellurium germanate glass (6.8 × 10−21 cm2) (Gao et al., 2015), germanate glass (5.5 × 10−21 cm2) (Yu et al., 2009) and zinc tellurite glass (7.3 × 10−21 cm2) (Yuan and Xiao, 2018). The high emission cross section of TGB glass with 9 mol.% BaF2 is helpful to provide high laser gain.
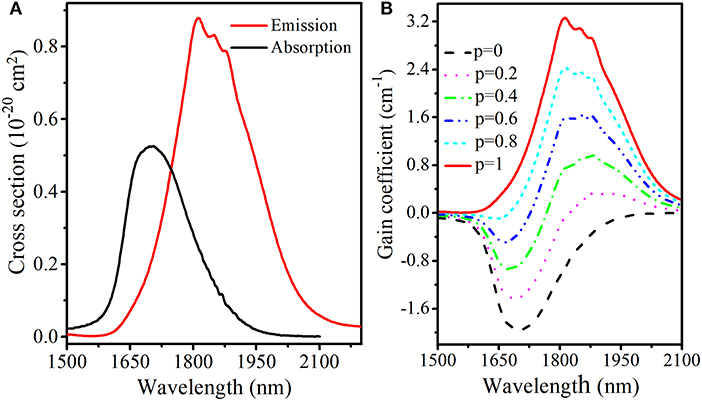
Figure 7. (A) Absorption, emission cross section and (B) calculated gain coefficient of TGB glass with 9 mol.% BaF2.
Once absorption and emission cross sections are determined and it is supposed that Tm3+ ions are only in either the 3H6 or 3F4 state, the gain coefficient G(λ) of Tm3+ near 1.8 μm can be obtained by the following equation (Zou and Toratani, 1996).
where N represents the total concentration of Tm3+ ions and p is the inversion factor given by the ratio between the population of lasing upper level (3F4) and the total concentration that ranges from 0 to 1. Figure 7B shows gain coefficient spectrum of TGB glass with 9 mol.% BaF2. It is found that the gain peak shifts to shorter wavelength with increasing p, which is a typical feature of the quasi-three-level system. Moreover, gain coefficient starts to be greater than zero in the wavelength range from 1,824 to 2,100 nm when p ≥ 0.2 and the maximum value is 3.3 cm−1 at 1,814 nm, which is larger than that of silicate glass (2.57 cm−1) (Tang et al., 2016), germanate glass (2.11 cm−1) (Slimen et al., 2019) and tellurite glass (0.91 cm−1) (Tian et al., 2019). This means that TGB glass with 9 mol.% BaF2 is a promising host material for efficient 2.0 μm fiber laser development.
In summary, the effects of BaF2 contents on density, refractive index, Raman spectra, OH− absorption coefficient and 1.8 μm spectroscopic properties of Tm3+ doped gallium tellurite glasses are studied in detail. When BaF2 content increases from 0 to 9 mol.% in step of 3 mol.%, refractive index and density gradually reduce. Meanwhile, αOH monotonically decreases from 3.4 to 2.2 cm−1, which makes emission peak value near 1.8 μm in TGB glass with 9 mol.% BaF2 being 1.6 times as large as that without BaF2 while the lifetime becomes 1.7 times as long as the value without BaF2. The maximum emission cross section at around 1.8 μm of TGB glass with 9 mol.% BaF2 reaches 8.8 × 10−21 cm2. In addition, positive gain coefficient in the wavelength range from 1,824 to 2,100 nm is achieved when p ≥ 0.2 and the maximum gain coefficient is 3.3 cm−1 at 1,814 nm. As a result, TGB glass with 9 mol.% BaF2 appears to be a highly promising host material for efficient 2.0 μm fiber laser development.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
JY, PX, and WW conceived the idea. JY and PX wrote the paper. TD, YY, DO, JC, and SY advised the paper. All authors contributed to the article and approved the submitted version.
The authors gratefully acknowledge the financial support from the National Natural Science Foundation of China (Grant Nos. 51902053 and 61804029), Natural Science Foundation of Guangdong province (Grant Nos. 2019A1515011988 and 2018A030310353), Guangdong Basic and Applied Basic Research Foundation (Grant No. 2019A1515110002), the Foundation for Distinguished Young Talents in Higher Education of Guangdong (Grant No. 2019KQNCX172), the Project of Foshan Education Bureau (Grant No. 2019XJZZ02), Guangdong-Hong Kong-Macao Intelligent Micro-Nano Optoelectronic Technology Joint Laboratory (Grant No. 2020B1212030010), and the Open Fund of the State Key Laboratory of Luminescent Materials and Devices (South China University of Technology, Grant No. 2020-skllmd-13).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Agger, S., Povlsen, J. H., and Varming, P. (2004). Single-frequency thulium-doped distributed-feedback fiber laser. Opt. Lett. 29, 1503–1505. doi: 10.1364/OL.29.001503
Chen, G. X., Zhang, Q. Y., Yang, G. F., and Jiang, Z. H. (2007). Mid-infrared emission characteristic and energy transfer of Ho3+-doped tellurite glass sensitized by Tm3+. J. Fluoresc. 17, 301–307. doi: 10.1007/s10895-007-0173-5
Chen, Y. J., Lin, Y. F., Gong, X. H., Zhu, H. M., Luo, Z. D., and Huang, Y. D. (2010). 805-nm diode-pumped continuous-wave 2-μm laser performance of Tm3+:BaGd2(MoO4)4 cleavage plate. Appl. Phys. B. 98, 55–60. doi: 10.1007/s00340-009-3704-8
Gao, S., Kuan, P. W., Liu, X. Q., Chen, D. P., Liao, M. S., and Hu, L. L. (2015). ~2-μm single-mode laser output in Tm3+-doped tellurium germanate double-cladding fiber. IEEE Photon. Technol. Lett. 27, 1702–1704. doi: 10.1109/LPT.2015.2438077
Geng, J. H., and Jiang, S. B. (2014). Fiber lasers:the 2 μm market heats up. Opt. Photonics News. 25, 35–41. doi: 10.1364/OPN.25.7.000034
Geng, J. H., Wang, Q., and Jiang, S. B. (2011). 2 μm fiber laser sources and their applications. Proc. SPIE. 8164:816409. doi: 10.1117/12.896259
He, X., Xu, S. H., Li, C., Yang, C. S., Yang, Q., Mo, S. P., et al. (2013). 1.95 μm kHz-linewidth single-frequency fiber laser using self-developed heavily Tm3+-doped germanate glass fiber. Opt. Exp. 21, 20800–20805. doi: 10.1364/OE.21.020800
Jose, R., Arai, Y., and Ohishi, Y. (2007). Raman scattering characteristics of the TBSN-based tellurite glass system as a new Raman gain medium. J. Opt. Soc. Am. B. 24,1517–1526. doi: 10.1364/JOSAB.24.001517
Li, L. X., Wang, W. C., Zhang, C. F., Liu, J. L., Zhang, Q. Y., and Jiang, Z. H. (2019). Exploration of the new tellurite glass system for efficient 2 μm luminescence. J. Non Cryst. Solids 508, 15–20. doi: 10.1016/j.jnoncrysol.2018.12.018
Li, M., Bai, G. X., Guo, Y. Y., Hu, L. L., and Zhang, J. J. (2012). Investigation on Tm3+-doped silicate glass for 1.8 μm emission. J. Lumin. 132, 1830–1835. doi: 10.1016/j.jlumin.2012.02.022
Li, R. B., Tian, C., Tian, Y., Tao, W., Li, B. P., Jing, X. F., et al. (2015). Mid-infrared emission properties and energy transfer evaluation in Tm3+ doped fluorophosphate glasses. J. Lumin. 162, 58–62. doi: 10.1016/j.jlumin.2015.02.016
Mao, L. Y., Liu, J. L., Li, L. X., and Wang, W. C. (2020). TeO2-Ga2O3-ZnO ternary tellurite glass doped with Tm3+ and Ho3+ for 2 μm fiber lasers. J. Non Cryst. Solids 531:119855. doi: 10.1016/j.jnoncrysol.2019.119855
Murugan, G. S., and Ohishi, Y. (2004). Raman spectroscopic studies of TeO2-BaO-SrO-Nb2O5 glasses: structure-property correlations. J. Appl. Phys. 96, 2437–2442. doi: 10.1063/1.1772890
Richards, B., Jha, A., Tsang, Y., Binks, D., Lousteau, J., Fusari, F., et al. (2010). Tellurite glass lasers operating close to 2 μm. Laser Phys. Lett. 7, 177–193. doi: 10.1002/lapl.200910131
Slimen, F. B., Chen, S. X., Lousteau, J., Jung, Y. M., White, N., Alam, S., et al. (2019). Highly efficient Tm3+ doped germanate large mode area single mode fiber laser. Opt. Mater. Exp. 9, 4115–4125. doi: 10.1364/OME.9.004115
Tang, G. W., Zhu, T. T., Liu, W. W., Qiao, T., Sun, M., Chen, D. D., et al. (2016). Tm3+ doped lead silicate glass single mode fibers for 2.0 μm laser applications. Opt. Mater. Exp. 6, 2147–2157. doi: 10.1364/OME.6.002147
Terra, I. A., A; Camargo, A. S. S., Nunes, L. A. O., Carvalho, R. A., and Li, M. S. (2006). Evaluation of the OH− influence on visible and near-infrared quantum efficiencies of Tm3+ and Yb3+ codoped sodium aluminophoshate glasses. J. Appl. Phys. 100:123103. doi: 10.1063/1.2400510
Tian, Y., Li, B. P., Wang, J. R., Liu, Q. H., Chen, Y. L., Zhang, J. J., et al. (2019). The mid-infrared emission properties and energy transfer of Tm3+/Er3+ codoped tellurite glass pumped by 808/980 nm laser diodes. J. Lumin. 214:116586. doi: 10.1016/j.jlumin.2019.116586
Wang, R. S., Meng, X. W., Yin, F. X., Feng, Y., Qin, G. S., and Qin, W. P. (2013). Heavily erbium-doped low-hydroxyl fluorotellurite glasses for 2.7 μm laser applications. Opt. Mater. Exp. 3, 1127–1136. doi: 10.1364/OME.3.001127
Wang, W. C., Yuan, J., Liu, X. Y., Chen, D. D., Zhang, Q. Y., and Jiang, Z. H. (2014). An efficient 1.8 μm emission in Tm3+ and Yb3+/Tm3+ doped fluoride modified germanate glasses for a diode-pump mid-infrared laser. J. Non Cryst. Solids 404, 19–25. doi: 10.1016/j.jnoncrysol.2014.07.026
Wang, W. C., Zhou, B., Xu, S. H., Yang, Z. M., and Zhang, Q. Y. (2019). Recent advances in soft optical glass fiber and fiber lasers. Progr. Mater. Sci. 101, 90–171. doi: 10.1016/j.pmatsci.2018.11.003
Yang, X. L., Wang, W. C., and Zhang, Q. Y. (2017). BaF2 modified Cr3+ /Ho3+ co-doped germanate glass for efficient 2.0 μm fiber lasers. J. Non Cryst. Solids 482, 147–153. doi: 10.1016/j.jnoncrysol.2017.12.031
Yu, S. L., Yang, Z. M., and Xu, S. H. (2009). Judd–Ofelt and laser parameterization of Tm3+-doped barium gallo-germanate glass fabricated with efficient dehydration methods. Opt. Mater. 31, 1723–1728. doi: 10.1016/j.optmat.2009.05.002
Yuan, J., Wang, W. C., Chen, D. D., Peng, M. Y., Zhang, Q. Y., and Jiang, Z. H. (2014). Enhanced 1.8 μm emission in Yb3+/Tm3+ codoped tungsten tellurite glasses for a diode-pump 2.0 μm laser. J. Non Cryst. Solids 402, 223–230. doi: 10.1016/j.jnoncrysol.2014.06.008
Yuan, J., and Xiao, P. (2018). Compositional effects of Na2O, GeO2, and Bi2O3 on 1.8 μm spectroscopic properties of Tm3+ doped zinc tellurite glasses. J. Lumin. 196, 281–284. doi: 10.1016/j.jlumin.2017.12.054
Zhou, Y. X., Gai, N., Chen, F., and Yang, G. B. (2010). Effect of hydroxyl groups in erbium-doped tellurite- and bismuth-based glasses. Opt. Fiber Technol. 16, 318–322. doi: 10.1016/j.yofte.2010.08.002
Keywords: gallium tellurite glass, Tm3+ doped, OH−, 1.8 μm emission, BaF2
Citation: Yuan J, Wang W, Ye Y, Deng T, Ou D, Cheng J, Yuan S and Xiao P (2021) Effect of BaF2 Variation on Spectroscopic Properties of Tm3+ Doped Gallium Tellurite Glasses for Efficient 2.0 μm Laser. Front. Chem. 8:628273. doi: 10.3389/fchem.2020.628273
Received: 11 November 2020; Accepted: 10 December 2020;
Published: 08 January 2021.
Edited by:
Baiquan Liu, Sun Yat-sen University, ChinaCopyright © 2021 Yuan, Wang, Ye, Deng, Ou, Cheng, Yuan and Xiao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peng Xiao, eGlhb3BlbmdAZm9zdS5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.