
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Chem. , 14 January 2021
Sec. Electrochemistry
Volume 8 - 2020 | https://doi.org/10.3389/fchem.2020.626490
This article is part of the Research Topic Design, Synthesis, and Modification of Metal Oxides for Energy Storage View all 12 articles
In this paper, thiourea was successfully grafted onto the surface of acid preprocessed graphite felts [sulfuric acid-treated graphite felt (SA-GFs)] by thiol-carboxylic acid esterification. The thiourea-grafted graphite felts (TG-GFs) were investigated as the positive electrode for vanadium redox flow battery (VRFB). X-ray photoelectron spectroscopy results suggested that thiourea was grafted into the surface of graphite felts. The cyclic voltammetry showed that the peak potential separation decreased by 0.2 V, and peak currents were greatly enhanced on TG-GF electrode compared with SA-GF electrode, implying improved electro-catalytic activity and reversibility of TG-GF electrode toward VO2+/ redox reaction. The initial capacity of TG-GF-based cell reached 55.6 mA h at 100 mA cm−2, 22.6 mA h larger than that of SA-GF-based cell. The voltage and energy efficiency for TG-GF-based cell increased by 4.9% and 4.4% compared with those of SA-GF-based cell at 100 mA cm−2, respectively.
Vanadium redox flow battery (VRFB) as energy storage system has caused more and more attention because VRFB displays some advanced characteristics, such as long cycle life, high energy efficiency (EE), and excellent electrochemical reversibility (Bhushan et al., 2019; Li et al., 2019; Xiang and Daoud, 2019; He et al., 2020; Lv et al., 2020). The electrodes play a central role where redox reactions occur (Ding et al., 2018; Ye et al., 2018). Although the commercial graphite felts can be used as electrode materials for VRFB, the electrochemical activity is not enough for practical application (He et al., 2015).
The introduction of functional groups is one of the effective surface functional treatments to improve the electrochemical properties of the graphite felts. Among the functional groups, oxygen-containing groups, such as -COOH, -OH, and C=O, have been widely studied by various methods including heat treatment (Zhang et al., 2020), acid treatment (Sun and Skyllas-Kazacos, 2010), electrochemical oxidation (Xiao-Gang et al., 2007), and microwave treatment (Wu et al., 2014). In addition, the nitrogen-containing groups also have been reported to be active toward vanadium redox reactions. Tao et al. (2012) reported a hydrothermal ammoniated treatment for graphite felt used as the positive electrode for VRFB. The introduction of the polar nitrogenous groups can facilitate the charge transfer rate between electrode and vanadium ions. He et al. (2013) added two organic additives in positive electrolyte, which provided -NH2 group on the surface of the graphite felt and could be employed as active sites for vanadium ion reactions. Recently, Lee et al. (2015) reported that the supercapacitor performance based on thiourea (NH2CSNH2)-grafted graphene could be greatly improved due to introducing the amine and sulfur functional groups into graphene. In addition, the electrocatalytic properties of multi-walled carbon nanotubes toward the VO2+/ redox couple were also improved by surface functional treatments using thiourea as nitrogen and sulfur sources (Li et al., 2017).
In this work, we report a novel, simple, and mild method for in situ functionalizing graphite felt electrode by grafting thiourea for VRFB. The -NH2 and C-S functional groups were successfully introduced onto the surface of graphite felts. The VRFB using the thiourea-grafted graphite felt as positive electrode showed larger discharge capacity (DC) and EE.
Polyacrylonitrile (PAN)-based graphite felts (GFs) (thickness: 6 mm; Beijing Jinglong Carbon Technology Co., Ltd.) were pretreated with 98% sulfuric acid at room temperature for 24 h. In order to obtain thiourea-grafted graphite felts, the sulfuric acid-treated graphite felts (SA-GFs) were placed in a beaker containing 30 ml of 150 mg ml−1 thiourea solution, and then the beaker was kept in a water bath at 80°C for 10 h (Lee et al., 2015).
Morphology of samples was characterized by scanning electron microscopy (SEM, S-4800, Hitachi, Japan). X-ray photoelectron spectroscopy (XPS) was carried out (K-Alpha 1063, Thermo Fisher Scientific, UK) for characterization of the surface chemistry of samples. Raman spectra were recorded on a laser Raman spectrometer (Thermo Electron DXR, USA).
The electrochemical measurements [cyclic voltammogram (CV) and electrochemical impedance spectroscopy (EIS)] of the prepared electrode (area: 1 cm−2) were carried out on IM6e Zennium electrochemical workstation (Zahner Scientific Instruments, Germany) using Pt electrode and saturated calomel electrode (SCE) as the counter and reference electrodes, respectively. The electrolyte consisted of 0.1 M VOSO4 and 3 M H2SO4. The scan rate of CV test was 1 mV s−1. The frequency range of EIS was 10−2-106 Hz.
The charge–discharge performance for TG-GF electrode was assessed in a static cell using CT2001A (LAND, Wuhan) battery test system. The cells were assembled using TG-GF and SA-GF (3 × 3 cm2) as positive electrode, SA-GF as corresponding negative electrode, and perfluorinated ion-exchange membrane as separator in 1.2 M V(III)/V(IV) + 3 M H2SO4 electrolyte.
As shown in Figure 1A, thione (C=S) in thiourea exists a resonance structure thiol (C-S-H). The amine groups connected with the C-S-H can react with carboxylic acid (COOH) on the graphite felts via thiol-carboxylic acid esterification (Lee et al., 2015). SEM images for SA-GF and TG-GF (Figure 1A) show no obvious change of morphology. Figures 1B,C show the C1s high-resolution XPS spectra of SA-GF and TG-GF. Two samples contain C=C (284.4 eV), C-C (285.4 eV), C-S/C-O (286.5 eV), and COOH (288.6 eV) functional groups (Liu et al., 2015; Kabtamu et al., 2017). The peak at 286.5 eV represents carbon in SA-GF bound to one oxygen or sulfur (e.g., C-O, C-S) (Gattrell et al., 2006). XPS spectra for S2p shown in Figures 1D,E indicate that TG-GF sample exhibits S2p3/2 and S2p1/2 signals at 163.9 and 165.1 eV, respectively, as well as a trace peak at 168.2 eV (Baker et al., 2004; Huang et al., 2014). As shown in Table 1, compared with SA-GF, TG-GF has more C-S groups but lower COOH functional groups, which is attributed to the reaction between the carboxyl groups on SA-GF surface and the grafting of the amine and sulfur functional groups on thiourea, accompanied by the introduction of C-S and -NH2 groups on the surface of SA-GF. The O atomic percentage of TG-GF decreases to 12.8% from 24.1% after grafting thiourea group onto SA-GF. Meanwhile, S atomic percentage of TG-GF increases to 1.8% from 1.1%, and N atomic percentage increases to 8.1% from 5.9%. The trace peak at 168.2 eV is ascribed to sulfone species (Huang et al., 2014; Lee et al., 2015). XPS full spectra of SA-GF and TG-GF (Figure 1F) show a very clear S2p signal appearance for TG-GF, while for SA-GF, without S2p signal. Figure 1G shows Raman spectra of both samples. SA-GF and TG-GF samples give similar Raman scattering patterns with peaks at 1,380 (D band) and 1,600 cm−1 (G band). The intensity ratio of D to G band (ID/IG) that represents the extent of defects in carbon materials is different. The increase of ID/IG value from 1.00 for SA-GF to 1.06 for TG-GF means the decrease in the ordered graphite crystal structure after SA-GF grafting thiourea (Lee et al., 2015).
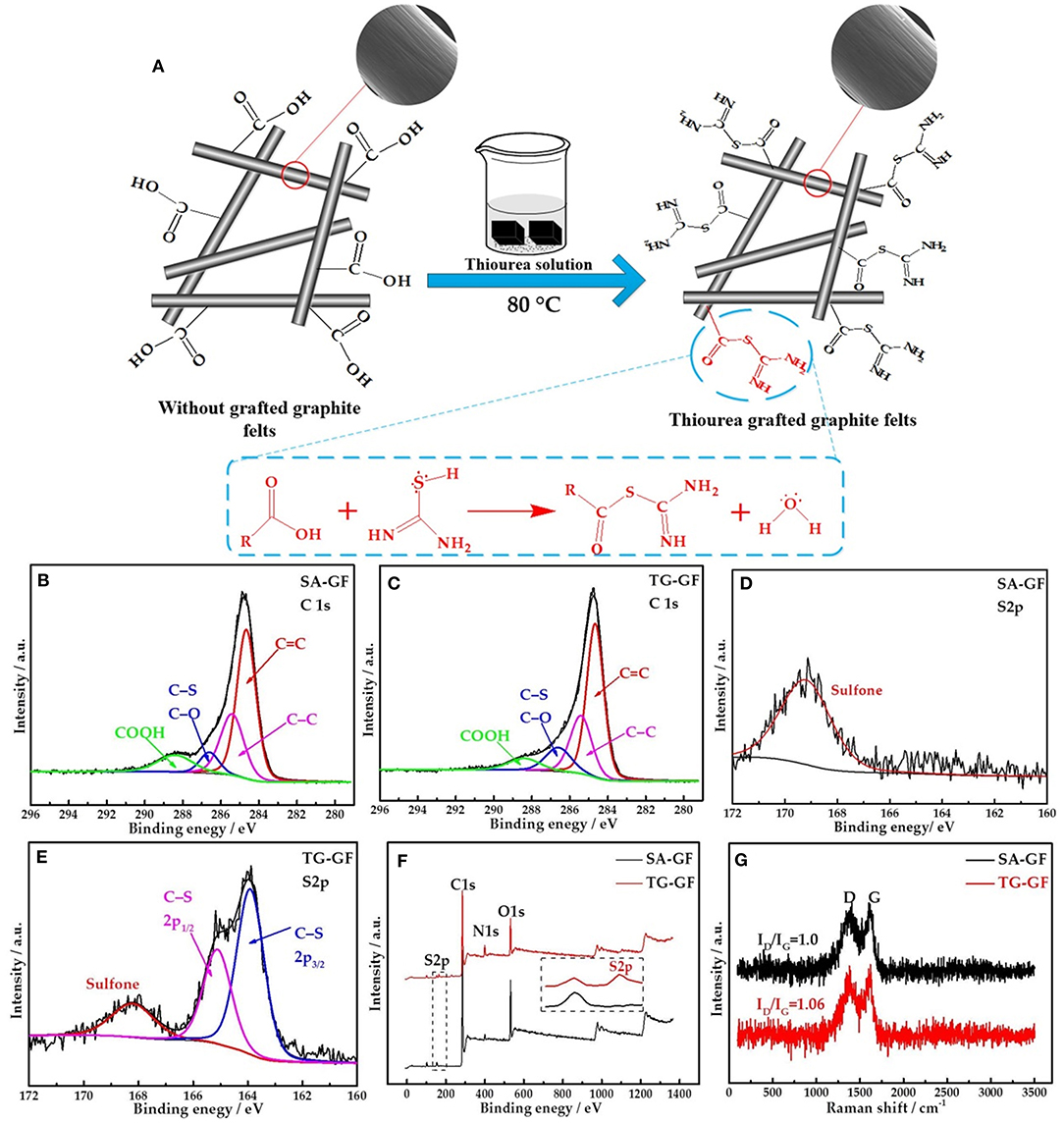
Figure 1. Mechanism of thiourea-grafted graphite felt (TG-GF) (including SEM photos) (A). X-ray photoelectron spectroscopy (XPS) C1s spectra of sulfuric acid-treated graphite felt (SA-GF) (B) and TG-GF (C). S2p spectra of graphite felt (GF) (D) and TG-GF (E). Survey spectrum of SA-GF and TG-GF (F). Raman spectra of SA-GF and TG-GF (G).
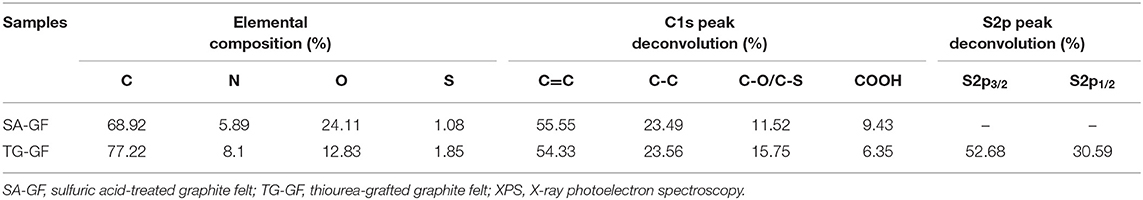
Table 1. Elemental composition and chemical composition of functional groups based on C1s and S2p XPS spectra.
CV curves of all electrodes (Figure 2A) appear two peaks, which correspond to oxidation and reduction reactions of VO2+/ couple. Compared with SA-GF, the redox peak potential separation of TG-GF dramatically decreases from 0.686 to 0.483 V. The peak currents are in the order of TG-GF > SA-GF > GF, showing that sulfuric acid pretreatment can slightly improve the performance of GF. However, thiourea grafting can greatly enhance the electrochemical activity and reversibility toward the VO2+/ redox reaction. The little peak appearing at 1.3–1.5 V for TG-GF is ascribed to the slight oxygen evolution reaction.
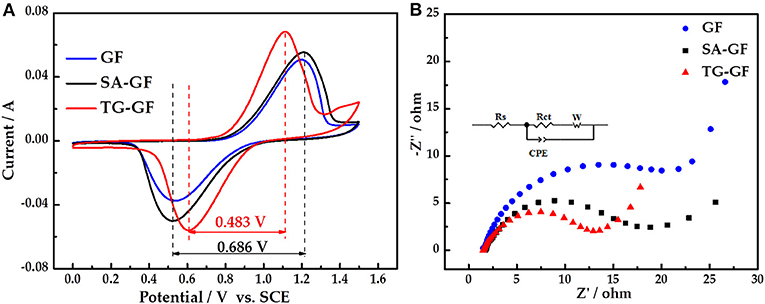
Figure 2. Cyclic voltammogram (CV) (A) and Nyquist plot (B) curves of graphite felt (GF), sulfuric acid-treated graphite felt (SA-GF), and thiourea-grafted graphite felt (TG-GF) in 0.1 M VOSO4 + 3 M H2SO4 electrolyte.
Figure 2B shows Nyquist plots of three electrodes. A semicircle and a straight line are observed at high and low frequencies, respectively. Rs is attributed to the resistance of electrolyte and electrode. Rct represents Faradaic interfacial charge-transfer resistance. The constant-phase element (CPE) is attributed to the double-layer capacitance, and W is Warburg impedance (Li et al., 2017). According to fitting results, the Rs values for GF, SA-GF, and TG-GF were almost equivalent. Rct of GF (25.8 Ω) is higher than that of other electrodes, suggesting poorer electrochemical activity of GF. The decrease of Rct value from 15.50 Ω for SA-GF to 10.25 Ω for TG-GF indicates that grafting thiourea onto SA-GF can reduce the electrochemical polarization.
Figure 3A shows the charge–discharge curves of the cells at 30 mA cm−2. Compared with SA-GF-based cell, TG-GF-based cell delivers longer charge–discharge time, lower charge voltage, and higher discharge voltage, which leads to the improvement of the DC and EE. Figure 3B presents the DC dependence on cycle number at 30 mA cm−2. TG-GF-based cell shows higher DC than that of SA-GF-based cell. For example, in the first cycle, DC of TG-GF-based cell is 81.2 mA h, 18.7 mA h larger than that of SA-GF-based cell. Meanwhile, the 87.4% DC retention and 87.3% average EE for TG-GF-based cell are 6.0 and 2.5% larger than those of SA-GF-based cell, respectively (Figure 3C).
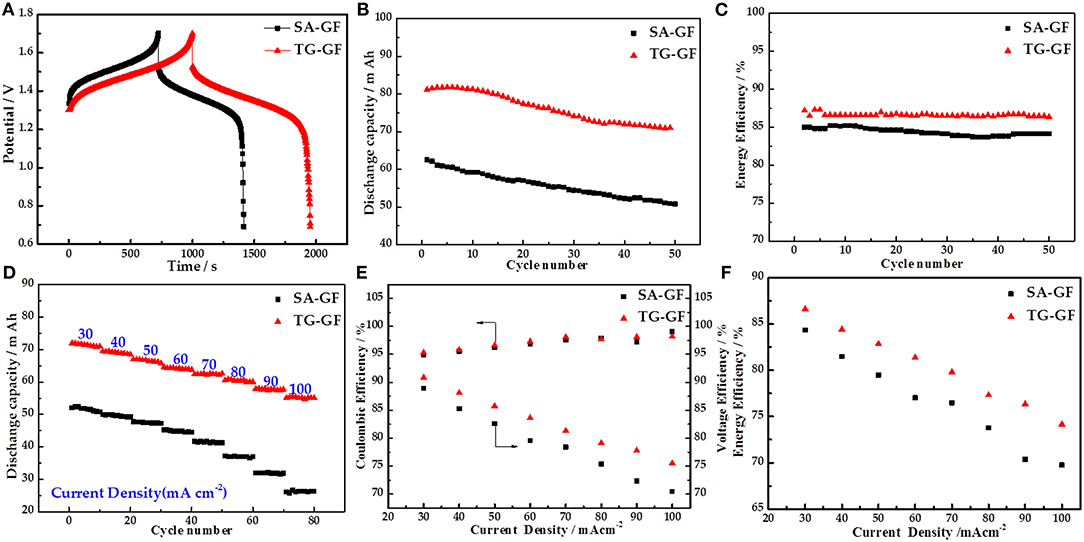
Figure 3. Electrochemical performances of vanadium redox flow battery (VRFB) cells with sulfuric acid-treated graphite felt (SA-GF) and thiourea-grafted graphite felt (TG-GF): (A) charge–discharge curves, (B) discharge capacity, and (C) energy efficiency (EE) of VRFB at the current density of 30 mA cm−2; (D) discharge capacity, (E) coulombic efficiency (CE), voltage efficiency (VE), and (F) EE of VRFB at the different current densities.
Figure 3D presents the DC of both cells at different current densities. The DCs of TG-GF-based cell are improved significantly at different current densities. For example, DC of TG-GF-based cell is 55.6 mA h at 100 mA cm−2, which is much larger than that of SA-GF-based cell (22.6 mA h). Figures 3E,F show the coulombic efficiency (CE), voltage efficiency (VE), and EE of the cells at different current densities. The CE values for two cells are almost the same, while the VE and EE of TG-GF-based cell are much higher than those of SA-GF-based cell at all current densities, especially at high current density. For example, the VE and EE of TG-GF-based cell are 75.5 and 74.1% at 100 mA cm−2, which are 4.9 and 4.4% higher than those of SA-GF-based cell, respectively.
In order to improve the performance of the electrode, thiourea was grafted onto the surface of SA-GF by thiol-carboxylic acid esterification. Both electrochemical activity and reversibility of the modified electrode toward VO2+/ redox reaction are improved. Compared with SA-GF-based cell, the cell using TG-GF electrode displays higher DC and VE due to a lower charge transfer resistance, particularly at a high current density.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
SW is mainly responsible for experimental operations and drafting paper. XL is mainly responsible for the collecting and processing experimental data. ZG is mainly responsible for collecting information and drafting paper. LW is mainly responsible for designing the experiment and the paper guidance. LD is mainly responsible for reviewing the final manuscript for publication. ZH is mainly responsible for making important modifications to the manuscript.
This work was financially supported by the Natural Science Foundation of China (51872090, 51772097).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Baker, W. S., Long, J. W., Stroud, R. M., and Rolison, D. R. (2004). Sulfur-functionalized carbon aerogels: a new approach for loading high-surface-area electrode nanoarchitectures with precious metal catalysts. J. Non Cryst. Solids 350, 80–87. doi: 10.1016/j.jnoncrysol.2004.07.088
Bhushan, M., Kumar, S., Singh, A. K., and Shahi, V. K. (2019). High-performance membrane for vanadium redox flow batteries: cross-linked poly(ether ether ketone) grafted with sulfonic acid groups via the spacer. J. Membr. Sci. 583, 1–8. doi: 10.1016/j.memsci.2019.04.028
Ding, M., Ling, X., Yuan, D., Cheng, Y. H., Wu, C., Chao, Z. S., et al. (2018). SPEEK membrane of ultrahigh stability enhanced by functionalized carbon nanotubes for vanadium redox flow battery. Front. Chem. 6:286. doi: 10.3389/fchem.2018.00286
Gattrell, M., Qian, J., Stewart, C., Graham, P., and MacDougall, B. (2006). The electrochemical reduction of VO2+ in acidic solution at high overpotentials. Electrochim. Acta 51, 395–407. doi: 10.1016/j.electacta.2005.05.001
He, Z., Cheng, G., Jiang, Y., Li, Y., Zhu, J., Meng, W., et al. (2020). Novel 2D porous carbon nanosheet derived from biomass: ultrahigh porosity and excellent performances toward V2+/V3+ redox reaction for vanadium redox flow battery. Int. J. Hydrog. Energy 45, 3959–3970. doi: 10.1016/j.ijhydene.2019.12.045
He, Z., Dai, L., Liu, S., Wang, L., and Li, C. (2015). Mn3O4 anchored on carbon nanotubes as an electrode reaction catalyst of V(IV)/V(V) couple for vanadium redox flow batteries. Electrochim. Acta 176, 1434–1440. doi: 10.1016/j.electacta.2015.07.067
He, Z. X., Liu, J. L., Han, H. G., Chen, Y., Zhou, Z., Zheng, S. J., et al. (2013). Effects of organic additives containing -NH2 and -SO3H on electrochemical properties of vanadium redox flow battery. Electrochim. Acta 106, 556–562. doi: 10.1016/j.electacta.2013.05.086
Huang, Y., Candelaria, S. L., Li, Y., Li, Z., Tian, J., Zhang, L., et al. (2014). Sulfurized activated carbon for high energy density supercapacitors. J. Power Sources 252, 90–97. doi: 10.1016/j.jpowsour.2013.12.004
Kabtamu, D. M., Chen, J. Y., Chang, Y. C., and Wang, C. H. (2017). Water-activated graphite felt as a high-performance electrode for vanadium redox flow batteries. J. Power Sources 341, 270–279. doi: 10.1016/j.jpowsour.2016.12.004
Lee, W. S. V., Leng, M., Li, M., Huang, X. L., and Xue, J. M. (2015). Sulphur-functionalized graphene towards high performance supercapacitor. Nano Energy 12, 250–257. doi: 10.1016/j.nanoen.2014.12.030
Li, C. C., Xie, B. S., Chen, J., He, J. J., and He, Z. X. (2017). Enhancement of nitrogen and sulfur co-doping on the electrocatalytic properties of carbon nanotubes for VO2+/ redox reaction. RSC Adv. 7, 13184–13190. doi: 10.1039/C6RA27734A
Li, Q., Liu, J., Bai, A., Li, P., Li, J., Zhang, X., et al. (2019). Preparation of a nitrogen-doped reduced graphene oxide-modified graphite felt electrode for VO2+/ reaction by freeze-drying and pyrolysis method. J. Chem. 2019, 1–9. doi: 10.1155/2019/8958946
Liu, T., Li, X., Nie, H., Xu, C., and Zhang, H. (2015). Investigation on the effect of catalyst on the electrochemical performance of carbon felt and graphite felt for vanadium flow batteries. J. Power Sources 286, 73–81. doi: 10.1016/j.jpowsour.2015.03.148
Lv, Y., Li, Y., Han, C., Chen, J., He, Z., Zhu, J., et al. (2020). Application of porous biomass carbon materials in vanadium redox flow battery. J. Colloid Interface Sci. 566, 434–443. doi: 10.1016/j.jcis.2020.01.118
Sun, B. T., and Skyllas-Kazacos, M. (2010). Chemical modification of graphite electrode materials for vanadium redox flow battery application—part II. Acid treatments. Electrochim. Acta 37, 1253–1260. doi: 10.1016/0013-4686(92)85064-R
Tao, W., Huang, K., Liu, S., Zhuang, S., Dong, F., Sha, L., et al. (2012). Hydrothermal ammoniated treatment of PAN-graphite felt for vanadium redox flow battery. J. Solid State Electrochem. 16, 579–585. doi: 10.1007/s10008-011-1383-y
Wu, X., Xu, H., Xu, P., Yang, S., Lu, L., Shi, J., et al. (2014). Microwave-treated graphite felt as the positive electrode for all-vanadium redox flow battery. J. Power Sources 263, 104–109. doi: 10.1016/j.jpowsour.2014.04.035
Xiang, Y., and Daoud, W. A. (2019). Binary NiCoO2-modified graphite felt as an advanced positive electrode for vanadium redox flow batteries. J. Mater. Chem. A 7, 5589–5600. doi: 10.1039/C8TA09650C
Xiao-Gang, L. I., Huang, K. L., Liu, S. Q., Tan, N., and Chen, L. Q. (2007). Characteristics of graphite felt electrode electrochemically oxidized for vanadium redox battery application. Transact. Nonferrous Metals Soc. China 17, 195–199. doi: 10.1016/S1003-6326(07)60071-5
Ye, J. Y., Lou, X. C., Wu, C., Wu, S. J., Ding, M., Sun, L. D., et al. (2018). Ion selectivity and stability enhancement of SPEEK/Lignin membrane for vanadium redox flow battery: the degree of sulfonation effect. Front. Chem. 6:549. doi: 10.3389/fchem.2018.00549
Keywords: vanadium redox flow battery, graphite felts, thiourea, grafted, energy storage
Citation: Wu S, Lv X, Ge Z, Wang L, Dai L and He Z (2021) Thiourea-Grafted Graphite Felts as Positive Electrode for Vanadium Redox Flow Battery. Front. Chem. 8:626490. doi: 10.3389/fchem.2020.626490
Received: 06 November 2020; Accepted: 07 December 2020;
Published: 14 January 2021.
Edited by:
Wei Xiao, Yangtze University, ChinaReviewed by:
Zhipeng Ma, Yanshan University, ChinaCopyright © 2021 Wu, Lv, Ge, Wang, Dai and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ling Wang, dHN3bGluZ0AxMjYuY29t; Zhangxing He, enhoZUBuY3N0LmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.