
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Chem. , 21 December 2020
Sec. Supramolecular Chemistry
Volume 8 - 2020 | https://doi.org/10.3389/fchem.2020.616961
This article is part of the Research Topic Supramolecular Chirogenesis in Chemical and Related Sciences View all 15 articles
 Massimiliano Gaeta1
Massimiliano Gaeta1 Rosalba Randazzo1
Rosalba Randazzo1 Valentina Villari2
Valentina Villari2 Norberto Micali2
Norberto Micali2 Alessandro Pezzella3
Alessandro Pezzella3 Roberto Purrello1
Roberto Purrello1 Marco d'Ischia4*
Marco d'Ischia4* Alessandro D'Urso1*
Alessandro D'Urso1*Chiral porphyrin hetero-aggregates, produced from meso-tetrakis(4-N-methylpyridyl) porphyrin H2T4 and copper(II) meso-tetrakis(4-sulfonatophenyl)porphyrin CuTPPS by an imprinting effect in the presence of L-3,4-dihydroxyphenylalanine (L-DOPA), are shown herein to serve as templates for the generation of chiral structures during the oxidative conversion of the amino acid to melanin. This remarkable phenomenon is suggested to involve the initial role of L-DOPA and related chiral intermediates like dopachrome as templates for the production of chiral porphyrin aggregates. When the entire chiral pool from DOPA is lost, chiral porphyrin hetero-aggregate would elicit axially chiral oligomer formation from 5,6-dihydroxyindole intermediates in the later stages of melanin synthesis. These results, if corroborated by further studies, may open unprecedented perspectives for efficient strategies of asymmetric melanin synthesis with potential biological and technological applications.
L-3,4-Dihydroxyphenylalanine (L-DOPA; Figure 1A) is an aromatic amino acid produced in various organisms by the oxidative modification of L-tyrosine (Raper, 1927; Mason and Wright, 1949; Haneda and Watanabe, 1971; Prota, 1995; Ito, 2003; Slominski et al., 2012; Marchiosi et al., 2020). In the skin and other organs, like the ink sac of cephalopods, L-DOPA is an early intermediate in the synthesis of black eumelanin pigments from L-tyrosine by the action of the copper enzyme tyrosinase (Slominski et al., 2004; Ito and Wakamatsu, 2007; Simon et al., 2009; Della Vecchia et al., 2013; d'Ischia et al., 2014). In vivo, the process involves the oxidation of tyrosine to dopaquinone followed by intramolecular cyclization to an indoline intermediate, termed leucodopachrome or cyclodopa, which can enter a redox cycle by exchanging electrons with dopaquinone to produce L-DOPA and L-dopachrome. The latter, then, undergoes isomerization with or without decarboxylation and loss of the chiral center to give 5,6-dihydroxyindole (DHI) and/or 5,6-dihydroxyindole-2-carboxylic acid (DHICA), respectively (Pezzella et al., 1996; Edge et al., 2006; Ito and Wakamatsu, 2007, 2008; Ito et al., 2011; d'Ischia et al., 2013; Panzella et al., 2018). The oxidative polymerization of these latter intermediates leads to the deposition of black insoluble melanin polymers (Figure 1A). So far, knowledge of the intrinsic chiroptical features of melanin polymers is scant, and little attention has been paid to the possible generation of chiral structures under in vivo or in vitro conditions. The only evidence for the possible occurrence of chirality in melanins is due to the demonstration that oligomers from DHICA may display atropisomerism caused by hindered rotation about interunit bonds (Pezzella et al., 2003). However, current information on the chirality of DHICA oligomers during the polymerization process remains limited.
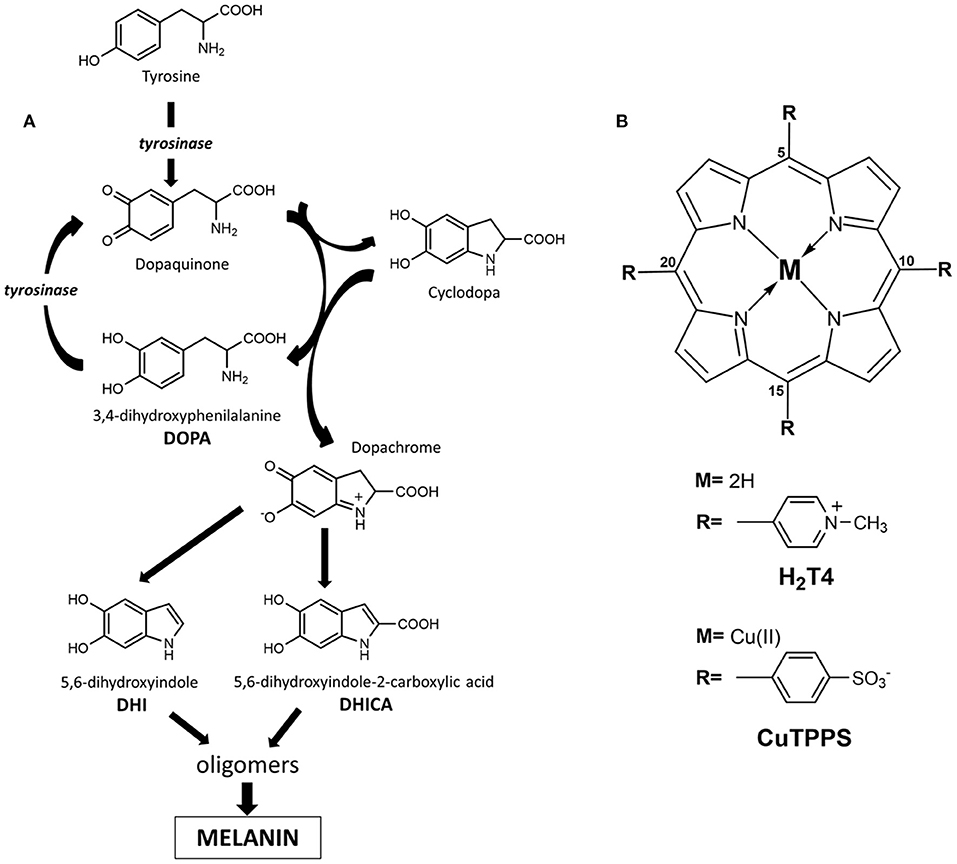
Figure 1. (A) Schematic illustration of dihydroxyphenylalanine (DOPA) oxidative polymerization to melanin (highlighted is the generation of L-3,4-dihydroxyphenylalanine (L-DOPA) by redox cycling between leucodopachrome and dopaquinone). (B) Molecular structures of porphyrinoids used, 5,10,15,20-tetrakis(4-N-methylpyridyl)porphyrin tetrachloride salt (H2T4) and copper(II) 5,10,15,20-tetrakis(4-sulfonatophenyl)porphyrin tetrasodium salt (CuTPPS).
Recently, a systematic investigation of the effect of porphyrin aggregation on melanin synthesis was undertaken, exploiting the well-known tendency of porphyrins to interact with amino acids (Angelini et al., 2005; Uemori et al., 2012; Charalambidis et al., 2016; Gaeta et al., 2016; Rananaware et al., 2016; Ryu et al., 2018) and polymeric chains (Borovkov et al., 2002a,b; De Luca et al., 2010; Occhiuto et al., 2015; Zhao et al., 2015; Gaeta et al., 2018), with a view to generating new bioinspired functional materials with tailored optical and chiral properties. Water-soluble porphyrins maintain their tendency to aggregate owing to the hydrophobic aromatic macrocycle, whereby binding suitable functional groups to the porphyrin ring may allow to realize self-assembled porphyrin systems in aqueous solution. Although supramolecular arrangements of achiral porphyrins in aqueous solution result in achiral supramolecular structures, chiral aggregates of porphyrins can be formed in the presence of chiral templates (Bellacchio et al., 1998; Onouchi et al., 2006; Toyofuku et al., 2007; Lauceri et al., 2008; Helmich et al., 2010).
Noteworthy, as a consequence of extensive network of interactions (electrostatic, solvophobic, etc.) that trap porphyrin aggregates in a quite deep local energy minimum ensuring kinetic inertia, the porphyrin supramolecular assembly is able to memorize the chiral information imprinted by the chiral template in aqueous solution (Mammana et al., 2007; Gaeta et al., 2016). In this context, porphyrin hetero-aggregates (built by opposite-charged porphyrins) proved to be an ideal system to store chiral information and may offer the possibility of designing switch of memory (Mammana et al., 2007). In this work, we show that porphyrin hetero-aggregates made up of 5,10,15,20-tetrakis(4-N-methylpyridyl)porphyrin H2T4 (Figure 1B) and copper(II) 5,10,15,20-tetrakis(4-sulfonatophenyl)porphyrin CuTPPS (Figure 1B) can drive the oxidative polymerization of DOPA to melanins with the unexpected generation of asymmetric structures.
5,10,15,20-Tetrakis(4-N-methylpyridyl)porphyrin tetrachloride salt (H2T4) and copper(II) 5,10,15,20-tetrakis(4-sulfonatophenyl)porphyrin tetrasodium salt (CuTPPS) were purchased from Mid-Century Company and used without further purification. Porphyrin mother solutions (about 4 × 10−4 M, stored in the dark at room temperature) were prepared dissolving the solid in ultrapure water obtained from Elga Purelab Flex system by Veolia. Then, the concentration was checked by spectrophotometric methods in water solution at neutral pH by means of their molar extinction coefficients at maximum of the Soret band: λmax(H2O) = 423 nm (ε = 224,000 M−1cm−1) for H2T4 and λmax(H2O) = 412 nm (ε = 416,000 M−1cm−1) for CuTPPS.
The phosphate buffered saline (PBS) tablets were purchased from Sigma-Aldrich Company, and the stock solution was prepared by dissolving one tablet in 200 ml of ultrapure water. PBS buffer (pH = 7.4) contains 10 mM of phosphate buffer sodium salt, 137 mM of sodium chloride, and 2.7 mM of potassium chloride.
L-DOPA and the respective D-enantiomer [D-3,4-dihydroxyphenylalanine (D-DOPA)] were purchased from Sigma-Aldrich Company and used without further purification. Solutions of both DOPA enantiomers were freshly prepared by solubilizing the proper amount of solid in PBS buffer in order to attain a final concentration of 0.5 mM.
The porphyrin hetero-aggregates in PBS buffer were obtained by filling with 2 ml of PBS solution a quartz cuvette (path length = 1 cm), then the proper volume of H2T4 mother solution was added to reach a 2 μM concentration of H2T4 in the sample solution. After 5 min, the amount of CuTPPS was added in order to reach again a 2 μM concentration of CuTPPS in the sample solution. After an additional 20 min, other aliquots of H2T4 and CuTPPS were added as illustrated before. The final work solution thus obtained was kept for 20 min before spectroscopic investigations.
The porphyrin hetero-aggregates in the presence of D- and L-DOPA were obtained by using the corresponding DOPA solution (0.5 mM in PBS) following the aforementioned procedure. In detail, the proper volume of H2T4 mother solution was added to D- or L-DOPA solution to reach a 2 μM concentration of H2T4, then after 5 min, the proper amount of CuTPPS was added to the sample solution in order to reach again a 2 μM concentration of CuTPPS. After an additional 20 min, other aliquots of H2T4 and CuTPPS were added as illustrated before. The final work solution thus obtained was kept for 20 min before spectroscopic investigations. For the long incubation time, each solution of DOPA and porphyrin hetero-aggregates was stored in sealed plastic cuvettes in order to limit the adhesion of both porphyrins and DOPA on the cuvette walls.
All solutions, both stock and sample solutions, are prepared in ultraclean conditions: (i) the operators wore a lab coat, hair cap, gloves, and mask during the preparation of samples and (ii) the tips of the pipettes and the plastic cells were washed three times with ultrapure water before being used.
Circular dichroism (CD) and Uv/Vis measurements were carried out at room temperature (quartz cuvette path length 1 cm) on a JASCO J-710 spectropolarimeter and JASCO V-530 spectrophotometer, respectively. Linear dichroism (LD) measurements were carried out on a modified JASCO J-500A spectropolarimeter (Micali et al., 2015) after proper calibration with an oriented polarizer. Linear birefringence of the instrument optics was also measured in order to evaluate the cross-talk contribution to CD.
In PBS buffer (pH = 7.4), the formation of porphyrin hetero-aggregates from equimolar amounts of tetra-cationic H2T4 and tetra-anionic CuTPPS was apparent by both the hypochromic effect and band broadening in the Soret region, as reported in UV/Vis spectrum (Supplementary Figure 1). In the absence of chiral inducers in solution and under ultraclean conditions, the building of achiral supramolecular structures, as expected, was denoted by zero optical activity in the porphyrin hetero-aggregate absorption region (Supplementary Figure 1 inset).
The construction of chiral multicomponent systems requires precise hierarchical rules (Elemans et al., 2003), whereby to prepare chiral porphyrin hetero-aggregates, cationic porphyrin H2T4 (4 μM) was to be added to a PBS solution of L-DOPA (0.5 mM) followed by the anionic counterpart CuTPPS (4 μM). UV/Vis spectra confirm the formation of porphyrin hetero-aggregate, showing a hypochromic effect and broadening of the Soret bands (Supplementary Figure 2). Noteworthy in the CD spectra, both a positive single Cotton effect of L-DOPA at about 280 nm and an induced bisignate CD signal in visible region, due to chiral exciton coupling of two porphyrin chromophores in hetero-aggregates, were observed (Figure 2). Mirror image was recorded when D-DOPA was used in place of levodopa, confirming that the CD signal in the porphyrin absorption region was induced by the interaction with DOPA via a chirality transfer process (Figure 2).
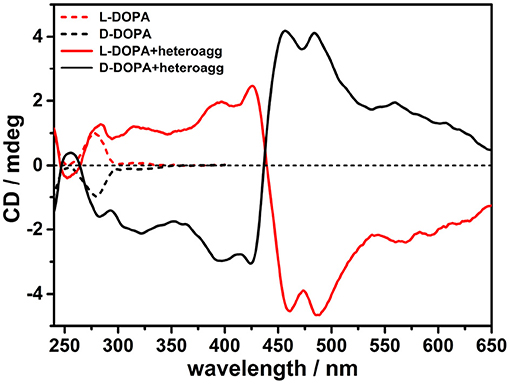
Figure 2. Circular dichroism (CD) spectra in phosphate buffered saline (PBS) buffer (pH = 7.4) of porphyrin hetero-aggregates [5,10,15,20-tetrakis(4-N-methylpyridyl)porphyrin tetrachloride salt (H2T4) = 4 μM, copper(II) 5,10,15,20-tetrakis(4-sulfonatophenyl)porphyrin tetrasodium salt (CuTPPS) = 4 μM] in the presence of L-3,4-dihydroxyphenylalanine (L-DOPA; red solid curve) and D-3,4-dihydroxyphenylalanine (D-DOPA; black solid curve) as prepared. The CD spectra for dihydroxyphenylalanine (DOPA) alone in PBS buffer are graphed in red dashed curve for L-enantiomer and in black dashed curve for D-enantiomer. In all samples, the concentration of DOPA was 0.5 mM.
The solutions containing chiral porphyrin hetero-aggregates and single DOPA enantiomers were incubated for 2 weeks in plastic cuvettes rather than in quartz cuvettes in order to minimize the sticking of DOPA-derived melanin products onto cuvette walls. Although the oxidative polymerization of DOPA evolved slowly in PBS buffer at pH 7.4 as is usually the case with catechol and catecholamine compounds (Bernsmann et al., 2011), after 2 weeks, significant variations in the DOPA absorption and CD spectra were observed. CD and UV spectra of the sample solutions were recorded after 24 h, 3, 7, and 15 days to follow the evolution of DOPA polymerization. In comparison with the initial situation, main changes observed concerned: i) the loss of the induced CD signal attributed to the porphyrin hetero-aggregates and, simultaneously, ii) a marked change in the dichroic signal of DOPA (Figure 3, Supplementary Figure 3).
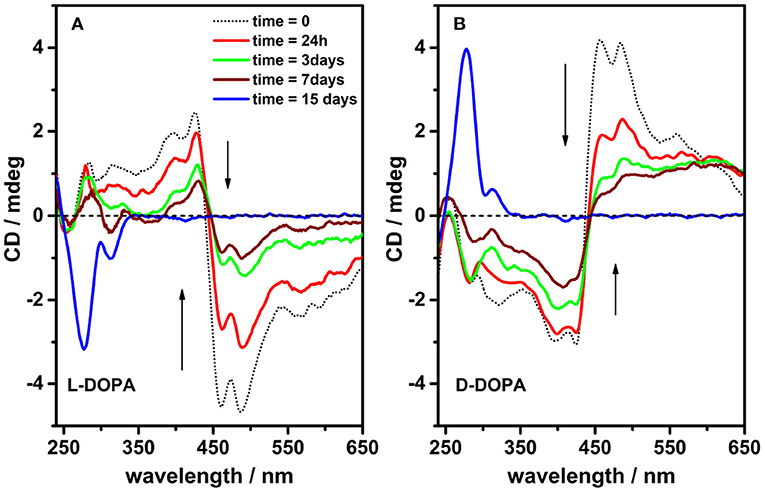
Figure 3. Circular dichroism spectra of incubated solutions [phosphate buffered saline (PBS) buffer, pH = 7.4] containing porphyrin hetero-aggregates [5,10,15,20-tetrakis(4-N-methylpyridyl)porphyrin tetrachloride salt (H2T4) = 4 μM, copper(II) 5,10,15,20-tetrakis(4-sulfonatophenyl)porphyrin tetrasodium salt (CuTPPS) = 4 μM] in the presence of L-3,4-dihydroxyphenylalanine (L-DOPA) 0.5 mM (A) and D-3,4-dihydroxyphenylalanine (D-DOPA) 0.5 mM (B) as prepared (dotted black curves) and after 24 h, 3, 7, and 15 days (red, green, wine, and blue curves in that order).
Since the chirality transfer mechanism implies a close-range contact between chiral inducer and achiral building blocks (Borovkov et al., 2001; Mammana et al., 2007; Zeng et al., 2009; Randazzo et al., 2019; Ustrnul et al., 2019), the disappearance of the induced chirality may be associated to a de-aggregation of the porphyrin hetero-aggregate owing to the polymerization of DOPA and the associated loss of chirality. In line with this conclusion, the UV/Vis spectra of incubated hetero-aggregates evolved with the growth of the CuTPPS Soret band (λmax = 412 nm) (Supplementary Figure 3), whereas, conversely, no detectable band associated with H2T4 (λmax = 423 nm) was observed (Supplementary Figure 3 inset) presumably due to embedment into the developing melanin matrix whose carboxylate residues are deprotonated (thus anionic charged) at pH value of PBS buffer. Indeed, adding acid solution to melanin precipitate (separated from the solution) in order to protonate the carboxylate residues, a band at 450 nm, ascribable to protonated form of H2T4, was detected (Supplementary Figure 4). These spectroscopic data suggested that the porphyrin hetero-aggregate in PBS at high ionic strength does not exhibit similar stability as previously demonstrated in water (Mammana et al., 2007; Gaeta et al., 2016). It is likely that ionic strength modulates electrostatic interactions between opposite-charged porphyrins, affecting the stability of the hetero-aggregate in PBS.
Remarkably, drastic changes in the CD signals at 450–500 nm are observed with time (1 week timescale; Figure 3), which are paired to a later increase of the signal at 280 nm (2 weeks timescale). Such profile evolution is a clear signature of the generation of asymmetric structures, likely driven by chirally imprinted porphyrin hetero-aggregates during melanin synthesis, while the original chiral information from DOPA was completely consumed because of its conversion into 5,6-dihydroxyindoles. Noteworthy, contributions from LD are negligible; however, the possibility that a component of differential scattering might affect the measurements owing to the presence of melanin particles cannot be ruled out.
To support the above conclusions, the spectroscopic behavior of L-DOPA in PBS buffer was monitored in the absence of porphyrins. The progress of the oxidative polymerization was denoted by the simultaneous decrease of the absorbance (λmax = 280 nm) and related CD signal of L-DOPA (Figure 4, Supplementary Figure 5). After several days, the final dark solution did not display almost any residual chirality suggesting the formation of achiral melanin.

Figure 4. Circular dichroism spectra of L-3,4-dihydroxyphenylalanine (L-DOPA; 0.5 mM) alone in phosphate buffered saline (PBS) buffer (pH = 7.4, 298K) as prepared (dotted black curve) and after 24 h, 3, 7, and 15 days (red, green, wine, and blue curves, respectively).
Further evidences of the role played by porphyrin hetero-aggregate as chiral templating agent of melanin oligomers have been gained performing a clear-cut experiment, reversing the order of addition of the components. In detail, we added L-DOPA to a solution of preformed achiral porphyrin hetero-aggregate (Supplementary Figure 6). After 1 week, the CD spectrum of L-DOPA looked similar to the CD spectrum of L-DOPA alone in PBS (Supplementary Figure 6 inset), confirming that chiral porphyrin hetero-aggregate plays a key role in inducing chiral melanin oligomer formation.
To conclude, these results disclose a rare example of temporary chiral mediation in which a chirally imprinted aggregate is decomposed while serving in turn as template for the chiral imprinting of developing oligomer aggregates from non-chiral decomposition products of a chiral precursor (Scheme 1).
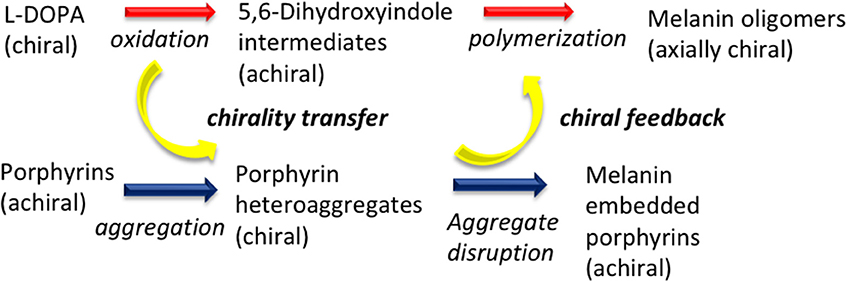
Scheme 1. Chirality transfer dynamics in the oxidative polymerization of L-3,4-dihydroxyphenylalanine (L-DOPA) in the presence of porphyrin hetero-aggregate.
These results open a new promising area of investigation on the organization of melanin pigments with applications ranging from biology and medicine to nanotechnology and material science.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
AD'U, Md'I, AP, and RP contributed to the conception and design of the study. Experimental work was carried out by MG, RR, VV, and NM (CD, UV/Vis, LD, differential scattering) under supervision of AD'U. MG wrote the manuscript and prepared images with contributions of AD'U, Md'I, and AP. All authors participated in the analysis and discussion of obtained results.
Ministero dell'Istruzione, dell'Università e della Ricerca (MIUR) PRIN project code: 2017YJMPZN-005; programma ricerca di ateneo UNICT 2016-18 linea 1 and 2; Dipartimento di Scienze Chimiche finanziamento giovani ricercatori 2020; programma ricerca di ateneo UNICT 2020-2022 linea 2; Fondi di Ateneo 2020-2022, Università di Catania, linea Open Access.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fchem.2020.616961/full#supplementary-material
Angelini, N., Micali, N., Mineo, P., Scamporrino, E., Villari, V., and Vitalini, D. (2005). Uncharged water-soluble Co(II) - Porphyrin: a receptor for aromatic α-amino acids. J. Phys. Chem. B 109, 18645–18651. doi: 10.1021/jp052408u
Bellacchio, E., Lauceri, R., Gurrieri, S., Scolaro, L. M., Romeo, A., and Purrello, R. (1998). Template-imprinted chiral porphyrin aggregates. J. Am. Chem. Soc. 120, 12353–12354. doi: 10.1021/ja9820893
Bernsmann, F., Ball, V., Addiego, F., Ponche, A., Michel, M., Gracio, J. J. D. A., et al. (2011). Dopamine-melanin film deposition depends on the used oxidant and buffer solution. Langmuir 27, 2819–2825. doi: 10.1021/la104981s
Borovkov, V. V., Lintuluoto, J. M., and Inoue, Y. (2001). Supramolecular chirogenesis in zinc porphyrins: mechanism, role of guest structure, and application for the absolute configuration determination. J. Am. Chem. Soc. 123, 2979–2989. doi: 10.1021/ja0032982
Borovkov, V. V., Lintuluoto, J. M., and Inoue, Y. (2002a). Stoichiometry-controlled supramolecular chirality induction and inversion in bisporphyrin systems. Org. Lett. 4, 169–171. doi: 10.1021/ol016870i
Borovkov, V. V., Lintuluoto, J. M., Sugiura, M., Inoue, Y., and Kuroda, R. (2002b). Remarkable stability and enhanced optical activity of a chiral supramolecular bis-porphyrin tweezer in both solution and solid state. J. Am. Chem. Soc. 124, 11282–11283. doi: 10.1021/ja026884z
Charalambidis, G., Georgilis, E., Panda, M. K., Anson, C. E., Powell, A. K., Doyle, S., et al. (2016). A switchable self-assembling and disassembling chiral system based on a porphyrin-substituted phenylalanine-phenylalanine motif. Nat. Commun. 7, 1–11. doi: 10.1038/ncomms12657
De Luca, G., Romeo, A., Scolaro, L. M., and Pasternack, R. F. (2010). Conformations of a model protein revealed by an aggregating CuII porphyrin: sensing the difference. Chem. Commun. 46, 389–391. doi: 10.1039/B918433C
Della Vecchia, N. F., Avolio, R., Alf,è, M., Errico, M. E., Napolitano, A., and d'Ischia, M. (2013). Building-block diversity in polydopamine underpins a multifunctional eumelanin-type platform tunable through a quinone control point. Adv. Funct. Mater. 23, 1331–1340. doi: 10.1002/adfm.201202127
d'Ischia, M., Napolitano, A., Ball, V., Chen, C. T., and Buehler, M. J. (2014). Polydopamine and eumelanin: from structure-property relationships to a unified tailoring strategy. Acc. Chem. Res. 47, 3541–3550. doi: 10.1021/ar500273y
d'Ischia, M., Wakamatsu, K., Napolitano, A., Briganti, S., Garcia-Borron, J. C., Kovacs, D., et al. (2013). Melanins and melanogenesis: methods, standards, protocols. Pigment Cell Melanoma Res. 26, 616–633. doi: 10.1111/pcmr.12121
Edge, R., D'Ischia, M., Land, E. J., Napolitano, A., Navaratnam, S., Panzella, L., et al. (2006). Dopaquinone redox exchange with dihydroxyindole and dihydroxyindole carboxylic acid. Pigment Cell Res. 19, 443–450. doi: 10.1111/j.1600-0749.2006.00327.x
Elemans, J. A. A. W., Rowan, A. E., and Nolte, R. J. M. (2003). Mastering molecular matter. Supramolecular architectures by hierarchical self-assembly. J. Mater. Chem. 13, 2661–2670. doi: 10.1039/B304972H
Gaeta, M., Oliveri, I., Pietro Fragal,à, M. E., Failla, S., D'Urso, A., Di Bella, S., et al. (2016). Chirality of self-assembled achiral porphyrins induced by chiral Zn(II) Schiff-base complexes and maintained after spontaneous dissociation of the templates: a new case of chiral memory. Chem. Commun. 52, 8518–8521. doi: 10.1039/C6CC04018G
Gaeta, M., Raciti, D., Randazzo, R., Gangemi, C. M. A., Raudino, A., D'Urso, A., et al. (2018). Chirality enhancement of porphyrin supramolecular assembly driven by a template preorganization effect. Angew. Chemie Int. Ed. 57, 10656–10660. doi: 10.1002/anie.201806192
Haneda, K., and Watanabe, S. (1971). Synthesis of L-3,4-dihydroxyphenylalanine from L-tyrosine by microorganisms. Appl. Microbiol. 22, 721–722. doi: 10.1128/AEM.22.4.721-722.1971
Helmich, F., Lee, C. C., Schenning, A. P. H. J., and Meijer, E. W. (2010). Chiral memory via chiral amplification and selective depolymerization of porphyrin aggregates. J. Am. Chem. Soc. 132, 16753–16755. doi: 10.1021/ja1077602
Ito, S. (2003). IFPCS presidential lecture: a chemist's view of melanogenesis. Pigment Cell Res. 16, 230–236. doi: 10.1034/j.1600-0749.2003.00037.x
Ito, S., and Wakamatsu, K. (2007). “Chemistry of Melanins,” in The Pigmentary System: Physiology and Pathophysiology: 2nd edn, eds J. J. Nordlund, R. E. Boissy, V. J. Hearing, R. A. King, W. S. Oetting, J.-P. Ortonne (Oxford: Blackwell Publishing Ltd), 282–310.
Ito, S., and Wakamatsu, K. (2008). Chemistry of mixed melanogenesis - pivotal roles of dopaquinone. Photochem. Photobiol. 84, 582–592. doi: 10.1111/j.1751-1097.2007.00238.x
Ito, S., Wakamatsu, K., d'Ischia, M., and Napolitano, A. P. (2011). “Structure of melanins,” in Melanins and Melanosomes: Biosynthesis, Biogenesis, Physiological, and Pathological Functions, eds J. Riley, P. A. Borovansky (Weinheim: Wiley-VCH Verlag GmbH), 167-181. Available at: https://onlinelibrary.wiley.com/doi/pdf/10.1002/9783527636150#page=185 (accessed October 7, 2020).
Lauceri, R., Fasciglione, G. F., D'Urso, A., Marini, S., Purrello, R., and Coletta, M. (2008). Kinetic investigation of porphyrin interaction with chiral templates reveals unexpected features of the induction and self-propagation mechanism of chiral memory. J. Am. Chem. Soc. 130, 10476–10477. doi: 10.1021/ja803426q
Mammana, A., D'Urso, A., Lauceri, R., and Purrello, R. (2007). Switching off and on the supramolecular chiral memory in porphyrin assemblies. J. Am. Chem. Soc. 129, 8062–8063. doi: 10.1021/ja071447b
Marchiosi, R., Soares, A. R., Abrahão, J., dos Santos, W. D., and Ferrarese-Filho, O. (2020). “L-DOPA and dopamine in plant metabolism,” in Neurotransmitters in Plant Signaling and Communication. Signaling and Communication in Plants, eds F. Baluška, S. Mukherjee, A. Ramakrishna (Cham: Springer), 141–167.
Mason, H. S., and Wright, C. I. (1949). The chemistry of melanin; oxidation of dihydroxyphenylalanine by tyrosinase. J. Biol. Chem. 180, 235–247.
Micali, N., Vybornyi, M., Mineo, P., Khorev, O., Häner, R., and Villari, V. (2015). Hydrodynamic and thermophoretic effects on the supramolecular chirality of pyrene-derived nanosheets. Chem. Eur. J. 21, 9505–9513. doi: 10.1002/chem.201500932
Occhiuto, I. G., Samperi, M., Trapani, M., De Luca, G., Romeo, A., Pasternack, R. F., et al. (2015). Aggregates of a cationic porphyrin as supramolecular probes for biopolymers. J. Inorg. Biochem. 153, 361–366. doi: 10.1016/j.jinorgbio.2015.09.013
Onouchi, H., Miyagawa, T., Morino, K., and Yashima, E. (2006). Assisted formation of chiral porphyrin homoaggregates by an induced helical poly(phenylacetylene) template and their chiral memory. Angew. Chem. 118, 2441–2444. doi: 10.1002/ange.200504162
Panzella, L., Ebato, A., Napolitano, A., and Koike, K. (2018). The late stages of melanogenesis: exploring the chemical facets and the application opportunities. Int. J. Mol. Sci. 19, 1753. doi: 10.3390/ijms19061753
Pezzella, A., Napolitano, A., D'Ischia, M., and Prota, G. (1996). Oxidative polymerisation of 5,6-dihydroxyindole-2-carboxylic acid to melanin: a new insight. Tetrahedron 52, 7913–7920. doi: 10.1016/0040-4020(96)00362-6
Pezzella, A., Vogna, D., and Prota, G. (2003). Synthesis of optically active tetrameric melanin intermediates by oxidation of the melanogenic precursor 5,6-dihydroxyindole-2-carboxylic acid under biomimetic conditions. Tetrahedron Asymmetry 14, 1133–1140. doi: 10.1016/S0957-4166(03)00156-3
Prota, G. (1995). “The chemistry of melanins and melanogenesis,” in Fortschritte der Chemie organischer Naturstoffe. Progress in the Chemistry of Organic Natural Products. Progrès Dans la Chimie des Substances Organiques Naturelles,eds W. Herz, G.W. Kirby, R.E. Moore, W. Steglich, C. Tamm (Vienna: Springer), 93–148.
Rananaware, A., La, D. D., Al Kobaisi, M., Bhosale, R. S., and Bhosale, S. V. (2016). Controlled chiral supramolecular assemblies of water soluble achiral porphyrins induced by chiral counterions. Chem. Commun. 52, 10253–10256. doi: 10.1039/C6CC04427A
Randazzo, R., Gaeta, M., Gangemi, C. M. A., Fragal,à, M. E., Purrello, R., and D'Urso, A. (2019). Chiral recognition of L- and D-amino acid by porphyrin supramolecular aggregates. Molecules 24, 84. doi: 10.3390/molecules24010084
Raper, H. S. (1927). The tyrosinase-tyrosine reaction: production from tyrosine of 5: 6-dihydroxyindole and 5:6-dihydroxyindole-2-carboxylic acid-the precursors of melanin. Biochem. J. 21, 89–96. doi: 10.1042/bj0210089
Ryu, J. H., Messersmith, P. B., and Lee, H. (2018). Polydopamine surface chemistry: a decade of discovery. ACS Appl. Mater. Interfaces 10, 7523–7540. doi: 10.1021/acsami.7b19865
Simon, J. D., Peles, D., Wakamatsu, K., and Ito, S. (2009). Current challenges in understanding melanogenesis: bridging chemistry, biological control, morphology, and function. Pigment Cell Melanoma Res. 22, 563–579. doi: 10.1111/j.1755-148X.2009.00610.x
Slominski, A., Tobin, D. J., Shibahara, S., and Wortsman, J. (2004). Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol. Rev. 84, 1155–1228. doi: 10.1152/physrev.00044.2003
Slominski, A., Zmijewski, M. A., and Pawelek, J. (2012). L-tyrosine and L-dihydroxyphenylalanine as hormone-like regulators of melanocyte functions. Pigment Cell Melanoma Res. 25, 14–27. doi: 10.1111/j.1755-148X.2011.00898.x
Toyofuku, K., Alam, M. A., Tsuda, A., Fujita, N., Sakamoto, S., Yamaguchi, K., et al. (2007). Amplified chiral transformation through helical assembly. Angew. Chemie - Int. Ed. 46, 6476–6480. doi: 10.1002/anie.200701668
Uemori, Y., Munakata, H., Kitazawa, S., Osada, A., and Imai, H. (2012). Optically active J-aggregate formed from water-soluble porphyrin with phenylalanine. J. Porphyr. Phthalocyanines 16, 1285–1292. doi: 10.1142/S1088424612501441
Ustrnul, L., Kaabel, S., Burankova, T., Martõnova, J., Adamson, J., Konrad, N., et al. (2019). Supramolecular chirogenesis in zinc porphyrins by enantiopure hemicucurbit[n] urils (n = 6, 8). Chem. Commun. 55, 14434–14437. doi: 10.1039/C9CC07150D
Zeng, L., He, Y., Dai, Z., Wang, J., Cao, Q., and Zhang, Y. (2009). Chiral induction, memory, and amplification in porphyrin homoaggregates based on electrostatic interactions. Chemphyschem 10, 954–962. doi: 10.1002/cphc.200800810
Zhao, L., Liu, M., Li, S., Li, A., An, H., Ye, H., et al. (2015). Aggregation and supramolecular chirality of 5,10,15,20-tetrakis-(4-sulfonatophenyl)-porphyrin on an achiral poly(2-(dimethylamino)ethyl methylacrylate)-grafted ethylene-vinyl alcohol membrane. J. Mater. Chem. C 3, 3650–3658. doi: 10.1039/C5TC00037H
Keywords: eumelanin, DOPA, porphyrin, supramolecular aggregates, circular dichroism
Citation: Gaeta M, Randazzo R, Villari V, Micali N, Pezzella A, Purrello R, d'Ischia M and D'Urso A (2020) En Route to a Chiral Melanin: The Dynamic “From-Imprinted-to-Template” Supramolecular Role of Porphyrin Hetero-Aggregates During the Oxidative Polymerization of L-DOPA. Front. Chem. 8:616961. doi: 10.3389/fchem.2020.616961
Received: 13 October 2020; Accepted: 23 November 2020;
Published: 21 December 2020.
Edited by:
Victor Borovkov, South-Central University for Nationalities, ChinaReviewed by:
Claudio Baggiani, University of Turin, ItalyCopyright © 2020 Gaeta, Randazzo, Villari, Micali, Pezzella, Purrello, d'Ischia and D'Urso. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marco d'Ischia, ZGlzY2hpYUB1bmluYS5pdA==; Alessandro D'Urso, YWR1cnNvQHVuaWN0Lml0
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.