
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Chem. , 15 January 2021
Sec. Medicinal and Pharmaceutical Chemistry
Volume 8 - 2020 | https://doi.org/10.3389/fchem.2020.592152
 Yusra Habib Khan1†
Yusra Habib Khan1† Ambreen Malik Uttra2†
Ambreen Malik Uttra2† Sumera Qasim3†
Sumera Qasim3† Tauqeer Hussain Mallhi1*†
Tauqeer Hussain Mallhi1*† Nasser Hadal Alotaibi1†
Nasser Hadal Alotaibi1† Maria Rasheed4†
Maria Rasheed4† Abdulaziz Ibrahim Alzarea1†
Abdulaziz Ibrahim Alzarea1† Muhammad Shahid Iqbal5*†
Muhammad Shahid Iqbal5*† Nabil Khulaif Alruwaili6†
Nabil Khulaif Alruwaili6† Salah-Ud-Din Khan7†
Salah-Ud-Din Khan7† Abdullah Salah Alanazi1†
Abdullah Salah Alanazi1†World Health Organization (WHO) estimated breast cancer as one of the most prevailed malignancy around the globe. Its incident cases are gradually increasing every year, resulting in considerable healthcare burden. The heterogeneity of breast cancer accounts for its differential molecular subtyping, interaction between pathways, DNA damaging, and chronic inflammation. Matrix metalloproteinases (MMPs) are a group of zinc-containing, calcium dependent endopeptidases which play a substantial role in breast carcinogenesis through several mechanisms. These mechanisms include remodeling of extracellular matrix (ECM), cell proliferation, and angiogenesis which promote metastasis and result in tumor progression. In this context, compounds bearing MMP inhibitory potential can serve as potent therapeutic agents in combating MMPs provoked breast cancer. Current systematic review aimed to encompass the details of potent natural lead molecules that can deter MMPs-provoked breast cancer. Following the critical appraisal of literature, a total of n = 44 studies that explored inhibitory effect of phytochemicals on MMPs were included in this review. These phytoconstituents include alkaloids (n = 11), flavonoids (n = 23), terpenoids (n = 7), and lignans (n = 2). The most common inhibitory methods used to evaluate efficacy of these phytoconstituents included Gelatin Zymography, Western Blotting, and real time polymerase chain reaction (RT-PCR) analysis. Moreover, current limitations, challenges, and future directions of using such compounds have been critically discussed. This review underscores the potential implications of phytochemicals in the management of breast cancer which could lessen the growing encumbrance of disease.
Breast cancer has been characterized as the most prevalent type of cancer among women across the world. Majority of cancer related deaths observed among women of age ranging from 35 to 55 are due to breast cancer. It has been estimated that one among nine women will suffer from this life threatening breast cancer and about 130 thousand women each year die from this cancer. Invasive breast cancers have been categorized into three different categories based on histological features. These include well-differentiated cancer (Grade 1), moderately differentiated (Grade 2) and poorly differentiated cancer (Grade 3) (Abdulrahman and Rahman, 2012). It is pertinent to mention that primary reason of death from any type of cancer has been attributed to the distant metastasis. Extracellular matrix (ECM) degradation has been considered to be an important attributor for distant metastasis permitting cancer cells to enter local tissue, intra- and extravasate blood vessels and form different metastatic growths. ECM degradation occurs principally due to proteinases secreted by the tumor (Köhrmann et al., 2009). Currently four classes of proteinases are well-defined which includes matrix metalloproteinases, cysteine proteinases, serine proteinases, and aspartic proteinases. All these proteinases collectively contribute to the degradation of ECM. During physiological conditions including ovulation, tissue remodeling, angiogenesis, wound healing, a balance is maintained between proteolytic degradation and regulatory inhibition of proteolysis (Page-McCaw et al., 2007). This physiological balance might be disrupted during the tumor. Matrix metalloproteinases (MMPs) up-regulation has been observed in each type of cancer and found to be associated with the poor prognosis among cancerous patients. Previously conducted studies have linked this up-regulation to an advanced stage of breast cancer, enhanced tumor cells invasion and building of metastatic formations (Duffy et al., 2000).
Matrix metalloproteinases (MMPs) belong to zinc dependent endopeptidases, responsible for degradation and remodeling of extracellular matrix (ECM) during organogenesis, wound healing, angiogenesis, apoptosis, cell proliferation, and cancer progression. MMPs (except MP-11) are secreted as inactive zymogens and become activated outside the cell by the virtue of other MMPs or serine proteases (Nagase et al., 2006). MMPs induced ECM degradation facilitates the tumor invasion (Köhrmann et al., 2009). In particular, cancer development through MMPs is dependent on several factors including tumor site, tumor stage, substrate profile, and enzyme localization (Decock et al., 2008). Currently, 24 members of MMPs have been identified in vertebrates, including 23 in human beings. Based on substrate specificity, these MMPs are classified into six broad groups including collagenases (MMP-1,−8,−13,−18), gelatinases (MMP-2,−9), stromelysins (MMP-3,−10,−11), matrilysins (MMP-7,−26), membrane-type (MMP-14,−15,−16,−17,−24,−25), and non-classified MMPs (MMP-12,−19,−20,−21,−23,−27,−28). These different types of MMPs are located in cytosol, subcellular organelles, nucleus, and extracellular regions and serve different role at different stages including cell growth, differentiation, survival, and motility. MMPs induced ECM degradation not only supports tumor invasion but also alter behavior of tumor cell, thereby leads to cancer metastasis and ultimately disease progression. Therefore, inhibition of MMPs activity can serve as a useful therapeutic strategy in combating this life threatening cancer (Yu and Stamenkovic, 2000).
MMPs along with directly facilitating tumor invasion by degrading basement membrane also facilitate the release of factors promoting tumor growth or inhibiting apoptosis. Dysregulated MMPs activity facilitates cellular processes leading to DNA damage thus stimulate genomic instability. MMPs play critical roles in the tumor microenvironment. They provide nutrients and oxygen to the growing tumor as well as avenues for metastasis through MMP-mediated blood and lymph vessel formation. They generate tissue disruptive fibrotic stroma through MMP-induced activation of stromal fibroblasts. Moreover, action of MMPs on adipocytes stimulate tumor-promoting metabolism. In addition, phenotypic changes associated with the epithelial-mesenchymaltransition (EMT) are also induced by the direct action of MMPs. The EMT is a developmental process which activates during the tumor progression (Radisky and Radisky, 2015).
The expression of MMPs (MMP-1,−2,−8 to−13,−15,−19,−23,−24,−27, and−28) is comparatively much stronger in cancer tissues than normal tissues of the breast. Degradation of physical barriers during invasion of cancer cell at distant location is regulated by proteolytic activity of MMPs. Invasion is promoted by localization of MMPs to specialized cell surface structure called invadopodia (Weaver, 2006). MMP-1,−2,−3,−9, and−14 are primarily implicated as contributing factors in tumor invasion, metastasis and angiogenesis. Moreover, the progression of breast cancer is attributed to MMP-2,−7,−9,−10,−11,−13,−14, and−15 (Weaver, 2006). MMPs contribute to tumor cell proliferation through release of insulin-like growth factors (IGFs) and epidermal growth factor receptor (EGFR) that promote proliferation. Cancer cell proliferation is linked with the MMP-1,−2,−7, and−9, whereas breast tumor metastasis is linked with the MMP-1,−2,−3,−7, and−9 to−18. Furthermore, owing to its tendency to dissolve in bone matrix, MMP-13 stays in breast bone and contributes breast-bone metastasis. Moreover, the anti-apoptotic effect is regulated by activation of serine/threonine kinase Akt/protein kinase B through signaling cascades of EGFR and IGFR (Gialeli et al., 2009). Furthermore, available evidences support the pivotal role of MMPs−1,−2,−7, and−9 in the tumor angiogenesis (Benson et al., 2013). A brief description of induction of cancer via matrix metalloproteinase has been summarized in Figure 1.
The tissue inhibitors of metalloproteinases (TIMPs) regulate the MMPs activity. Currently, four distinct endogenous specific inhibitors of MMPs have been cloned and sequenced. They include TIMP-1,−2,−3, and−4. TIMPs have high affinity toward active MMPs and inhibit protease activity by forming complex. TIMP-1 has affinity for pro-MMP-9 resulting in complex formation. TIMP-2 possesses affinity for precursor of MMP-2. It is important to note that few TIMPs perform various functions at the same time, thus exhibiting multifunctional properties. In this context, TIMP-1 and TIMP-2 are able to stimulate the cell proliferation at least in vitro in addition to the inhibitory action for MMP activities. Moreover, studies have demonstrated the inhibitory effects of TIMP-1 and TIMP-2 against apoptosis (Duffy et al., 2000).
This review is a systematic search of literature with specific inclusion and exclusion criteria. All the published scholarly manuscripts describing the predefined objectives and published in English are included in the current review. However, opinions, perspectives, commentaries, viewpoints, case studies, and manuscript in language other than English are excluded from the review. PubMed, Scopus, Google Scholar, SciFinder, Natural Products Updates (NPU), Scientific Electronic Library Online (SciELO), ScienceDirect, Cochrane Library, and SciVerse were utilized for the literature search. Literature search was subjected without any time limit in order to include all relevant information from the date of inception. The following descriptors were used with various Boolean operators: Flavonoids; Alkaloids; Phytochemicals; Chemical structure; Breast cancer; MMPs; metalloproteinases; Matrix metalloproteinases; Tumor; Metastasis; Tumorigenic process; TIMPs; Lignans; Glycosides; Terpenoids. The process of data screening was initiated in January 2020. All the searched articles were based on the abstract analysis. Full texts of the selected article were retrieved and 44 manuscripts were selected following the critical appraisal. All the relevant data was extracted from the articles and tabulated. The information tabulated includes phytochemicals including alkaloids, flavonoids, lignans, glycosides, and terpenoids. For each phytochemical, name of compound, its source, chemical structure, inhibitory mechanism, assay method, and relevant reference were included in the Table 1.
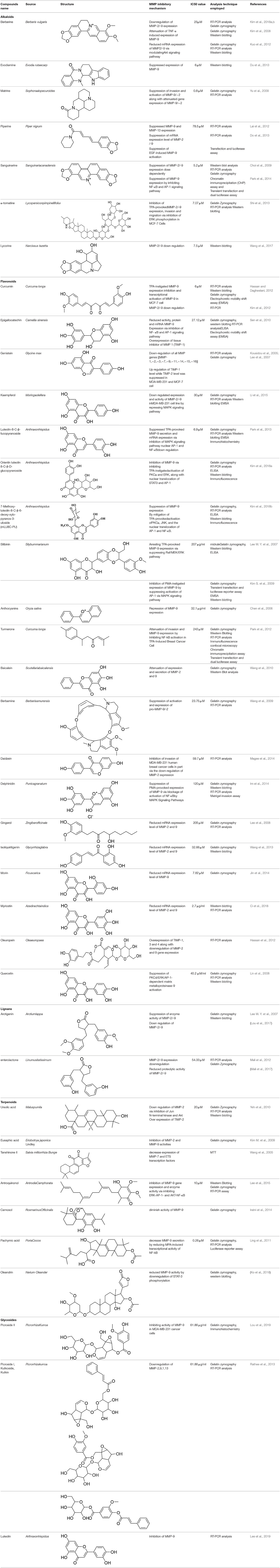
Table 1. Phytochemicals modulating MMP activity by inhibiting its activation and associated signaling pathways.
Alkaloids are documented to be the diverse class of natural compounds bearing a ring structure with nitrogen atom (Lu et al., 2012). Several alkaloids exhibit proven pharmacological attributes including ephedrine's pain relieving potential for asthmatics, morphine's analgesic attribute, and vinblastine's anticancer attributes (Benyhe, 1994; Li W. et al., 2007; Lee, 2011). Alkaloids are considered to be highly active constituents in natural flora, and some of them have previously been effectively established into anti-cancer drugs such as camptothecin (CPT), a well-known inhibitor of topoisomerase I (TopI) (Huang et al., 2007) and vinblastine, which produces its anti-cancerous impact via interacting with tubulin (Li W. et al., 2007).
Berberine, a quaternary ammonium isoquinoline alkaloids, can be found mostly in plant species belonging to the Berberis genus (Bashar et al., 2014). Berberine possesses an array of biological activities. Diversified studies have pointed out anti-tumorigenic potential of berberine by virtue of in vitro and in vivo experimental techniques. The evaluation of structure activity relationship (SAR) of substituents at R position of berberine revealed that their derivative possesses better cytotoxic potential as compared to parent compound. Of 20 newly synthesized compounds, 9-O-[4-ethyl-1-(naphthalene-2-ylmethyl)-1H-1,2,3,triazolel berberrubine chloride exhibits optimal anticancer activity against human breast cancer cell MCF-7 with (IC50 = 41.536 ± 3.4 um). The increased anticancer activity of berberine derivative is due to the activation of tumor necrosis factor alpha (TNF-α) which in turn produces substantial increase in MMP-9 activity. This elevated expression of TNF-α and MMP-9 as well as MMP-9 activity is promisingly attenuated by Berberine (Kim et al., 2008; Jin et al., 2015). TNFα-induced activator protein-1 (AP-1) DNA binding activity is also inhibited by berberine. Moreover, berberine can cause significant reduction in TNFα-provoked cell invasion. Taken together, TNF-α provoked MMP-9 activation leading to cell invasion is observed to be reduced by berberine via suppressing activity of AP-1 DNA (Kim et al., 2008). AP-1 is a transcriptional factor of MMP-9 and as such, suppression of AP-1 DNA binding activity can lead to suppression of MMP-9. It is important to note that MMP-9 secretion is elevated in several types of human cancer, and such elevation is correlated with poor prognosis (Roomi et al., 2009). However, another study concluded that berberine induced suppression of MMP-2 and MMP-9 involved the inhibition of the Akt/nuclear factor kappa B (NF-kappaB) and activator protein-1 (AP-1) signaling pathways (Kuo et al., 2012).
Evodiamine, a major bioactive quinolone alkaloid isolated from Evodiarutaecarpa (Lu et al., 2012), proves its anti-cancerous potential in both in vitro and in vivo models by arresting cell cycle, inducing apoptosis, preventing angiogenesis, invasion, and metastasis in a variety of cancer cell lines (Ogasawara et al., 2001, 2002; Fei et al., 2003; Zhang et al., 2003). The derivatives become more cytotoxic after an appropriate substitution on N13-position. Higher antitumor and broad spectrum activity has been observed by the one with a benzoyl group at N13-position against MDA-MB-435 cells (Dong et al., 2010). Evodiamine derivative promisingly inhibits cell migration and invasion via down regulation of MMP-9, urokinase-type plasminogen activator (uPA), and uPAR expression. Moreover, evodiamine down regulates p-ERK and p-p38 MAPK expression (Du et al., 2013).
Matrine exhibits various pharmacological properties including antiviral, antibacterial, anti-inflammatory, antiarrhythmic, anti-asthmatic, anti-obesity, diuretic, anticancer, choleretic, hepatoprotective, and cardioprotective effects (Lu et al., 2012). Matrine significantly attenuates tumor invasion, activation of MMP-9/MMP-2, Akt phosphorylation, DNA binding activity, nuclear factor-κB expression and mRNA levels of MMP-9, MMP2, EGF, and VEGFR1 in MDA-MB-231 cells. Matrine thus inhibits cancer cell proliferation and invasion via EGF/VEGF-VEGFR1-Akt-NF-κB signaling pathway (Yu et al., 2009).
Piperine, a piperidine alkaloid isolated from Piper nigrum and Piper longum, is a compound found in famous spices that have been used for centuries. Piperine possesses anti-inflammatory, antioxidant, antidiarrheal, antimutagenic, anticonvulsant, hypolipidemic, and tumor inhibitory activities (Lu et al., 2012). Piperine inhibits PMA-induced MMP-9 expression through down regulation of PKCα/extracellular signal-regulated kinase (ERK) 1/2 and reduction of NF-κB/AP-1 activation (Lai et al., 2012; Do et al., 2013).
Sanguinarine, isolated from family papaveracea, is a benzophenanthridine with proven biological activities including antifungal, antibacterial, antiplatelet, antischistosomal, anti-cancerous, and anti-inflammatory. It has been used extensively for controlling schistosomiasis (Lu et al., 2012). Sanguinarine displays its anti-invasive impact on MDA-MB−231breast cancer cell lines via tightening the tight junctions (TJs) and reducing mRNA expression of MMP-2/-9 (Choi et al., 2009).
Flavonoids are hydroxylated phenolic plant components, produced in response to microbial infection. Flavonoids have provoked colossal awareness in the earlier decade by virtue of their multifaceted health effects in humans and animals (Karak, 2019). They are termed as “functional components” and “health supporting biomolecules” due to their probable role in upholding health and averting the chronic deteriorating diseases (Nijveldt et al., 2001). Flavonoids are subdivided into different groups commonly flavones, flavonols, flavanones, flavanols, anthocyanins, and isoflavones.
Quercetin, a natural dietary flavonoid is obtained from barks of many plants, vegetables, fruits and employs antioxidant, anticancer, and antiinflammatory properties. Methyl capping of one or more free hydroxyl group improve compound cytotoxicity e.g., 3-O-methylquercetin and tamarexetin exhibited increased cytoxicity (Phatak, 2016). They control p38 MAPK signaling that persuade apoptosis in MCF-7 and MDA-MB-231 breast carcinoma cells (Ranganathan et al., 2015). Moreover, Quercetin derivatives significantly down-regulate Akt/MMP-9 pathway and suppress PKCd/ERK/AP-1-dependent matrix metalloproteinase-9 activation, thereby proved its anti-MMP activity confirmed through western blotting, gelatin zymography and RT-PCR analysis (Lin et al., 2008).
Kaempferol, a dietary flavonoid present in edible plants (beans, kale, tea, endive, broccoli, tomato, cabbage, and grapes) has been used as anticancer, antimicrobial, antioxidant, cardioprotective, neuroprotective, and anti-allergic compound (Kim and Choi, 2013). Kaempferol averts breast tumor infiltration and progression in MDA-MB-231 breast carcinoma cells by reducing MMP-2 and 9 expressions and subdual of AP-1 and MAPK signaling (Kim et al., 2016).
Delphinidin, found primarily in pomegranate extract, is consumed as dietary supplement. The presence of ortho-dehydroxyphenyl structure on β-ring and a free hydroxyl group at position-3 is responsible for highest inhibitory potency (Tang et al., 2015). A latest study explained that delphinidin treatment represses cell propagation and persuades apoptosis in triple-negative, ER-positive and HER2-overexpressing breast tumor cell lines, devoid of any toxic outcomes in normal breast cells (Ozbay and Nahta, 2011). In addition, MAPK signaling is repressed in both triple-negative and ER-negative breast carcinoma cells but not in MCF-10A normal cells. Delphinidin inhibits Ras-ERK MAPKs and PI3K/AKT/mTOR/p70S6K pathways (Hertog et al., 1993). Delphinidin also possess anti-angiogenic and anti-invasive properties by diminishing MMP-9 activity in ER+ MCF-7 cells by blocking NF-κB via MAPK signaling pathways (Im et al., 2014).
Daidzein, an isoflavonoid that can be isolated from various plants and herbs, has proven antiproliferative impact on breast cancer cells via inhibiting TNF-α expression, down-regulation of MMP-9 mRNA expression, as evident through RT-PCR analysis (Magee et al., 2014).
Genistein, isolated from soybean possesses chemopreventive potential against various cancer cells including prostate and breast cancer. Genistein has proven inhibitory potential against breast cancer cell proliferation, growth, invasion, and metastasis in various in vitro and in vivo models (Hsieh et al., 1998). NF-κB signaling pathway plays an imperative role not only in angiogenesis but also in cell growth, inflammation, apoptosis, and invasion. Genistein treatment considerably subdued MMP-9 activation by NF-κB signaling inactivation (Gupta et al., 2010). Genistein also displays down-regulation of all MMP genes (MMP-1,−2,−3,−7,−9,−11,−14,−15,−16) along with up-regulation of TIMP-1 level while TIMP-2 level is being suppressed in MDA-MB-231 and MCF-7 cancer cell lines (Kousidou et al., 2005; Lee W. Y. et al., 2007).
Lignans or phytoestrogens constitutes a group of natural compounds that manifest biological attributes via interfering with estrogen metabolism. This interference in estrogen metabolism is produced due to estrogenic as well as anti-estrogenic properties of lignans. Daily supplementation of lignans by females has been associated with hormonal changes that are favorable for women of all ages. Owing to reported benefits, lignans gained considerable attention as chemotherapeutic agent (Ezzat et al., 2018). Pre-clinical studies have been conducted to explore the anti-cancerous potential of lignans and reported their benefits in suppressing the early tumorigenesis (Landström et al., 1998; Bylund et al., 2000) along with actions on tumor growth inhibition, angiogenesis, and disease progression (Jungeström et al., 2007; Lin et al., 2008). Flaxseeds are thought to be the richest source of lignans and contain secoisolariciresinoldiglucoside (SDG), which is a major lignan possessing proven health benefits such as cardio protection, antioxidant, and anticancer effects (Alphonse and Aluko, 2015). SDG in mammalian body is metabolized to Enterodiol (ED) and Enterolactone (EL) by intestinal bacterias (Fuentealba et al., 2014). These mammalian lignans derived from SDG have shown promising benefits in breast cancer (Flower et al., 2014). The α-linolenic acid (ALA) is a precursor of PUFA omega 3 family. Eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) are formed by PUFA omega-3. ALA and omega 3 fatty acid are main constituents of flaxseed which possess anti-cancer property (Calado et al., 2018). Mammalian lignans have structure similarities to the estrogen with significant antioxidant potential but weak estrogenic action (Calado et al., 2018). Findings of RT-PCR and gelatin zymography assay show that SDG metabolite entereolactone significantly downregulates mRNA expression of MMP-9 and MMP-2 (Mali et al., 2012). Arctigenin, another natural lignan, derived from Arctium lappa also demonstrates inhibitory potential against MMP-9 and MMP-2 confirmed through western blotting and gelatin zymography (Lou et al., 2017). Taken together, lignans can be considered as useful natural remedies against breast cancer by suppressing MMP- activity, thereby helpful in preventing ECM degradation.
Glycosides comprise a large group of secondary metabolites that are widely distributed in plants. These have been employed as dietary agent or complementary medicine for management and treatment of cancer (Khan et al., 2019). The anti-metastatic effects of Picroside II, an iridoid glycoside derived from a high valued medicinal herb Picrorhiza Kurroa, was assessed on human breast cancer MDA-MB-231 cells with a focus on matrix metalloprotenaise (MMP) activity. Zymography experiments showed that the Picroside II glycosides led to suppression of MMP-9 activity in MDA-MB-231 cells in dose dependent manner (Lou et al., 2019).
Picroside Kurroa extract and its isolated iridoid glycosides Kutkin, Kutkoside and Picroside I exhibit cytotoxic potential in a dose dependent manner. This cytotoxic activity is primarily attributed to downregulation of gelatinases (MMP-2 and MMP-9) and collagenases (MMP-1 and MMP-13), decreased expression of MMPs at mRNA and protein level, inhibited migration, and invasion of human breast MCF-7 cells as detected by gelatin zymography and RT-PCR (Rathee et al., 2013).
A study showed that leuteolin glycosides, obtained from Arthraxonhispidus, have anti-metastatic activity through suppression of MMP-9 and inhibition of invasion and migration in MDA-MB-231 cells (Lee et al., 2019).
Oleandrin and odosroside are extracted from the Nerium oleander. These compounds comprise of lactone ring at carbon position 17 (C17) and a steroidal nucleus linked with sugar at position 3 (C3). A study has revealed reduction in mortality among breast cancer patients following the treatment with these compounds (Pongrakhananon, 2013). Oleandrin suppresses MMP-9 activity and octamer binding transcription factor 3/4 (OCT3/4) through inhibition of phosphor-signal transducer and activator of transcription (STAT)-3 at 50 and 100 nM doses, respectively (Ko et al., 2018).
Terpenoids, also known as isoprenoids, are the most numerous and structurally diverse natural products found in many plants. Several studies, in vitro, preclinical, and clinical have confirmed that this class of compounds displays a wide array of very important pharmacological properties. This diverse class has variety of functional roles in the field of food, medicine and cosmetics. Existing evidence suggests thee anticancer potential of terpenoids attributing to their inhibitory actions on cancer cells (Perveen and Al-Taweel, 2018).
Ursolic acid (UA) is natural triterpinoid obtained from apple (Malluspumila) peel and has potential to prevent and treat breast cancer. N-acylimidazole and N-alkylimidazole derivatives of UA with α,β-unsaturated ketone exhibit significant anti-cancer activity (Chen et al., 2015). Migration and invasion of highly metastatic MDA-MB-231 cells have been suppressed by dose and time dependent effect of urosolic acid derivatives. The down-modulation of MMP-2 and uPA expression is associated with inhibition of JNK-Akt and reduction of NF-Kb p65 in nucleus (Yeh et al., 2010).
Loquat extract (Eriobotrya japonica) has found its clinical application in reducing breast cancer metastasis and invasion. A study showed that its leaf extract has more potent chemopreventive effect than seed extract. The findings from gelatin zymographic assay reveal that its leaf extract inhibits the growth of MDA-MB-231 and activities of MMP-2 and MMP-9 more significantly than seed extract (Kim M. et al., 2009).
Tanshinone IIA (Tan IIA) is a member of the major lipophilic components extracted from the root of Salvia miltiorrhiza Bunge, which is currently used in China and other neighboring countries to treat patients suffering from myocardial infarction (MI), angina pectoris, stroke, diabetes, sepsis, and other conditions. It has anti-oxidant and potential cytotoxic activities. Tanshinone IIA suppresses MMP-7 and epithelium specific (ETS) transcription factors involved in tumorigenesis relevant to human breast cancer cells (Wang et al., 2005).
The ubiquinon derivative isolated from Antrodia Camphorata is Antroquinonol. It has been found to have anti-tumor effect by suppressing ERK-AP-1 and AKT-NF-kB dependent MMP-9 activity (Lee et al., 2015).
A natural constituent derived from Rosmarinus officinalis is carnosol which has anti-carcinogenic and antioxidant properties. The invasion and migration of B16/F10 cells is significantly inhibited by carnosol. Furthermore, carnosol suppresses MMP-9 expression through inhibition of extracellular signal regulated kinase (ERK) 1/2, AKT, and JNK signaling pathway (Huang et al., 2005).
Matrix metalloproteinases (MMPs) supports cancer invasion not only by degradation of basement membrane but also through release of some factors supporting cancer cell growth and preventing apoptosis. Several clinically approved MMP inhibitors have MMP-modulating potential but have limited use amid associated unwanted effects. The suppression of MMPs during cancer metastasis proves beneficial which can be done by blocking their activation or activity at transcription level via interfering with extracellular factors and signal transduction pathways (i.e., NF-kB or AP-1). Herbal medications have gained much attention during the past decades in healthcare systems. Phytochemicals derived from plant sources are a great source for novel drug development due to their safety and efficacy. These secondary metabolites bear the potential of modulating different pathological pathways responsible for number of disorders. Current review underscores the potential implications of phytochemicals in the management of breast cancer via direct inhibition of MMPs activation or by modulation of signaling pathways associated with MMPs activation. These phytoconstituents can serve as useful modalities for prevention of breast cancer invasion and metastasis. Natural MMPs inhibiting agents are now used for the development of alternative therapeutic strategies for treatment of breast cancer as they complement the action of conventional treatment strategies through their multi-targeting capacity. Modulation of MMPs is an emerging and promising area of research which could be translated to develop new therapeutic options for various subtypes of breast cancer.
AU, YK, SQ, and MR: conception or design of the work. NA, TM, AU, and AIA: analysis or interpretation of data for the work. YK, AU, SQ, and NA: drafting the work. TM, MI, MR, S-U-DK, and ASA: revising the manuscript critically for important intellectual content. All authors: provided approval for publication of the content and are accountable for all aspects of the work.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abdulrahman, G. O., and Rahman, G. A. (2012). Epidemiology of breast cancer in Europe and Africa. J. Cancer Epidemiol. 2012:915610. doi: 10.1155/2012/915610
Alphonse, P., and Aluko, R. (2015). A review on the anti-carcinogenic and anti-metastatic effects of flax seed lignan secolariciresinol diglucoside (SDG). Phytomedicine 1:6919427. doi: 10.15562/phytomedicine.2015.24
Bashar, A. A., Hossan, M. S., Jahan, R., Al-Nahain, A., Haque, A. M., and Rahmatullah, M. (2014). Berberine: a potential therapeutic candidate for breast cancer. J. Pharm. Pharm. Sci. 3, 1858–1869.
Benson, C. S., Babu, S. D., Radhakrishna, S., Selvamurugan, N., and Sankar, B. R. (2013). Expression of matrix metalloproteinases in human breast cancer tissues. Dis. Mark. 34, 395–405. doi: 10.3233/DMA-130986
Benyhe, S. (1994). Morphine: new aspects in the study of an ancient compound. Life Sci. 55, 969–979. doi: 10.1016/0024-3205(94)00631-8
Bylund, A., Zhang, J. X., Bergh, A., Damber, J. E., Widmark, A., Johansson, A., et al. (2000). Rye bran and soy protein delay growth and increase apoptosis of human LNCaP prostate adenocarcinoma in nude mice. Prostate 42, 304–314. doi: 10.1002/(SICI)1097-0045(20000301)42:4<304::AID-PROS8>3.0.CO;2-Z
Calado, A., Neves, P. M., Santos, T., and Ravasco, P. (2018). The effect of flaxseed in breast cancer: a literature review. Front. Nutr. 5:4. doi: 10.3389/fnut.2018.00004
Chen, H., Gao, Y., Wang, A., Zhou, X., Zheng, Y., and Zhou, J. (2015). Evolution in medicinal chemistry of ursolic acid derivatives as anticancer agents. Eur. J. Med. Chem. 92, 648–655. doi: 10.1016/j.ejmech.2015.01.031
Chen, P. N., Kuo, W. H., Chiang, C. L., Chiou, H. L., Hsieh, Y. S., and Chu, S. C. (2006). Black rice anthocyanins inhibit cancer cells invasion via repressions of MMPs and u-PA expression. Chem. Biol. Interact. 163, 218–229. doi: 10.1016/j.cbi.2006.08.003
Choi, Y. H., Choi, W. Y., Hong, S. H., Kim, S. O., Kim, G. Y., Lee, W. H., et al. (2009). Anti-invasive activity of sanguinarine through modulation of tight junctions and matrix metalloproteinase activities in MDA-MB-231 human breast carcinoma cells. Chem. Biol. Interact. 179, 185–191. doi: 10.1016/j.cbi.2008.11.009
Ci, Y., Zhang, Y., Liu, Y., Lu, S., Cao, J., Li, H., et al. (2018). Myricetin suppresses breast cancer metastasis through down-regulating the activity of matrix metalloproteinase (MMP)-2/9. Phytother. Res. 32, 1373–1381. doi: 10.1002/ptr.6071
Decock, J., Hendrickx, W., Vanleeuw, U., Van Belle, V., Van Huffel, S., Christiaens, M. R., et al. (2008). Plasma MMP1 and MMP8 expression in breast cancer: protective role of MMP8 against lymph node metastasis. BMC Cancer 8:77. doi: 10.1186/1471-2407-8-77
Do, M. T., Kim, H. G., Choi, J. H., Khanal, T., Park, B. H., Tran, T. P., et al. (2013). Antitumor efficacy of piperine in the treatment of human HER2-overexpressing breast cancer cells. Food Chem. 141, 2591–2599. doi: 10.1016/j.foodchem.2013.04.125
Dong, G., Sheng, C., Wang, S., Miao, Z., Yao, J., and Zhang, W. (2010). Selection of evodiamine as a novel topoisomerase I inhibitor by structure-based virtual screening and hit optimization of evodiamine derivatives as antitumor agents. J. Med. Chem. 53, 7521–7531. doi: 10.1021/jm100387d
Du, J., Wang, X. F., Zhou, Q. M., Zhang, T. L., Lu, Y. Y., Zhang, H., et al. (2013). Evodiamine induces apoptosis and inhibits metastasis in MDA-MB-231 human breast cancer cells in vitro and in vivo. Oncol. Rep. 30, 685–694. doi: 10.3892/or.2013.2498
Duffy, M. J., Maguire, T. M., Hill, A., McDermott, E., and O'Higgins, N. (2000). Metalloproteinases: role in breast carcinogenesis, invasion and metastasis. Breast Cancer Res. 2, 1–6. doi: 10.1186/bcr65
Ezzat, S. M., Shouman, S. A., Elkhoely, A., Attia, Y. M., Elsesy, M. S., El Senousy, A. S., et al. (2018). Anticancer potentiality of lignan rich fraction of six Flaxseed cultivars. Sci. Rep. 8:544. doi: 10.1038/s41598-017-18944-0
Fei, X. F., Wang, B. X., Li, Tashiro, J. S., Minami, M., Xing, D. J., et al. (2003). Evodiamine, a constituent of Evodiae Fructus, induces anti-proliferating effects in tumor cells. Cancer Sci. 94, 92–98. doi: 10.1111/j.1349-7006.2003.tb01358.x
Flower, G., Fritz, H., Balneaves, L. G., Verma, S., Skidmore, B., Fernandes, R., et al. (2014). Flax and breast cancer: a systematic review. Integr. Cancer Ther. 13, 181–192. doi: 10.1177/1534735413502076
Fuentealba, C., Figuerola, F., Estévez, A. M., Bastías, J. M., and Muñoz, O. (2014). Bioaccessibility of lignans from flaxseed (Linum usitatissimum L.) determined by single-batch in vitro simulation of the digestive process. J. Sci. Food Agr. 94, 1729–1738. doi: 10.1002/jsfa.6482
Gialeli, C., Kletsas, D., Mavroudis, D., Kalofonos, H., Tzanakakis, G., and Karamanos, N. (2009). Targeting epidermal growth factor receptor in solid tumors: critical evaluation of the biological importance of therapeutic monoclonal antibodies. Curr. Med. Chem. 16, 3797–3804. doi: 10.2174/092986709789177984
Gupta, S. C., Kim, J. H., Prasad, S., and Aggarwal, B. B. (2010). Regulation of survival, proliferation, invasion, angiogenesis, and metastasis of tumor cells through modulation of inflammatory pathways by nutraceuticals. Cancer Metastasis Rev. 29, 405–434. doi: 10.1007/s10555-010-9235-2
Hassan, Z. K., and Daghestani, M. H. (2012). Curcumin effect on MMPs and TIMPs genes in a breast cancer cell line. Asian Pac. J. Cancer Prev. 13, 3259–3264. doi: 10.7314/APJCP.2012.13.7.3259
Hassan, Z. K., Elamin, M. H., Daghestani, M. H., Omer, S. A., Al-Olayan, E. M., Elobeid, M. A., et al. (2012). Oleuropein induces anti-metastatic effects in breast cancer. Asian Pac. J. Cancer Prevent. 13, 4555–4559. doi: 10.7314/APJCP.2012.13.9.4555
Hertog, M. G., Hollman, P. C., Katan, M. B., and Kromhout, D. (1993). Intake of potentially anticarcinogenic flavonoids and their determinants in adults in The Netherlands. Nutr. Cancer 20, 21–29. doi: 10.1080/01635589309514267
Hsieh, C. Y., Santell, R. C., Haslam, S. Z., and Helferich, G. W. (1998). Estrogenic effects of genistein on the growth of estrogen receptor-positive human breast cancer (MCF-7) cells in vitro and in vivo. Cancer Res. 58, 3833–3838.
Huang, M., Gao, H., Chen, Y., Zhu, H., Cai, Y., Zhang, X., et al. (2007). Chimmitecan, a novel. 9-substituted camptothecin, with improved anticancer pharmacologic profiles in vitro and in vivo. Clin Cancer Res. 13, 1298–1307. doi: 10.1158/1078-0432.CCR-06-1277
Huang, S. C., Ho, C. T., Lin-Shiau, S. Y., and Lin, J. K. (2005). Carnosol inhibits the invasion of B16/F10 mouse melanoma cells by suppressing metalloproteinase-9 through down-regulating nuclear factor-kappaB and c-Jun. Biochem. Pharmacol. 69, 221–232. doi: 10.1016/j.bcp.2004.09.019
Im, N. K., Jang, W. J., Jeong, C. H., and Jeong, G. S. (2014). Delphinidin suppresses PMA-induced MMP-9 expression by blocking the NF-κB activation through MAPK signaling pathways in MCF-7 human breast carcinoma cells. J. Med. Food. 17, 855–861. doi: 10.1089/jmf.2013.3077
Iratni, R., Al Dhaheri, Y., Attoub, S., Karuventevida, N., and Arafat, K. (2014). P0174 Anti-metastatic and anti-tumour growth effects of carnosol on breast cancer through autophagy and apoptosis. Eur. J. Cancer 50:e59. doi: 10.1016/j.ejca.2014.03.218
Jin, H., Lee, W. S., Eun, S. Y., Jung, J. H., Park, H. S., Kim, G., et al. (2014). Morin, a flavonoid from Moraceae, suppresses growth and invasion of the highly metastatic breast cancer cell line MDA-MB-231 partly through suppression of the Akt pathway. Int. J. Oncol. 45, 1629–1637. doi: 10.3892/ijo.2014.2535
Jin, X., Yan, L., Li, H. J, Wang, R. L., Hu, Z. L., Jiang, Y. Y., Cao, Y. B., et al. (2015). Novel triazolyl berberine derivatives prepared via CuAAC click chemistry: synthesis, anticancer activity and structure-activity relationships. Anti-Cancer Agents Med. Chem. 15, 89–98. doi: 10.2174/1871520614666141203142012
Jungeström, M. B., Thompson, L. U., and Dabrosin, C. (2007). Flaxseed and its lignans inhibit estradiol-induced growth, angiogenesis, and secretion of vascular endothelial growth factor in uman breast cancer xenografts in vivo. Clin. Cancer Res. 13, 1061–1067. doi: 10.1158/1078-0432.CCR-06-1651
Karak, P. (2019). Biological activities of flavonoids: an overview. IJPSR 3, 1567–1574. doi: 10.13040/IJPSR.0975-8232.10(4).1567-74
Khan, H., Saeedi, M., Nabavi, S. M., Mubarak, M. S., and Bishayee, A. (2019). Glycosides from medicinal plants as potential anticancer agents: emerging trends towards future drugs. Curr. Med. Chem. 26, 2389–2406. doi: 10.2174/0929867325666180403145137
Kim, J. M., Noh, E. M., Kwon, K. B., Kim, J. S., You, Y. O., Hwang, J. K., et al. (2012). Curcumin suppresses the TPA-induced invasion through inhibition of PKCα-dependent MMP-expression in MCF-7 human breast cancer cells. Phytomedicine 19, 1085–1092. doi: 10.1016/j.phymed.2012.07.002
Kim, M. S., You, M. K., Rhuy, D. Y., Kim, Y. J., Baek, H. Y., and Kim, H. A. (2009). Loquat (Eriobotrya japonica) extracts suppress the adhesion, migration and invasion of human breast cancer cell line. Nutr. Res. Pract. 3, 259–264. doi: 10.4162/nrp.2009.3.4.259
Kim, S., Choi, J. H., Kim, J. B., Nam, S. J., Yang, J. H., Kim, J. H., et al. (2008). Berberine suppresses TNF-α-induced MMP-9 and cell invasion through inhibition of AP-1 activity in MDA-MB-231 human breast cancer cells. Molecules 13, 2975–2985. doi: 10.3390/molecules13122975
Kim, S., Choi, J. H., Lim, H. I., Lee, S. K., Kim, W. W., Kim, J. S., et al. (2009). Silibinin prevents TPA-induced MMP-9 expression and VEGF secretion by inactivation of the Raf/MEK/ERK pathway in MCF-7 human breast cancer cells. Phytomedicine 16, 573–580. doi: 10.1016/j.phymed.2008.11.006
Kim, S. H., and Choi, K. C. (2013). Anti-cancer effect and underlying mechanism (s) of kaempferol, a phytoestrogen, on the regulation of apoptosis in diverse cancer cell models. Toxicol. Res. 29, 229–234. doi: 10.5487/TR.2013.29.4.229
Kim, S. H., Hwang, K. A., and Choi, K. C. (2016). Treatment with kaempferol suppresses breast cancer cell growth caused by estrogen and triclosan in cellular and xenograft breast cancer models. J. Nutr. Biochem. 28, 70–82. doi: 10.1016/j.jnutbio.2015.09.027
Kim, S. J., Pham, T. H., Bak, Y., Ryu, H. W., Oh, S. R., and Yoon, D. Y. (2018a). Orientin inhibits invasion by suppressing MMP-9 and IL-8 expression via the PKCα/ERK/AP-1/STAT3-mediated signaling pathways in TPA-treated MCF-7 breast cancer cells. Phytomedicine 50, 35–42. doi: 10.1016/j.phymed.2018.09.172
Kim, S. J., Pham, T. H., Bak, Y., Ryu, H. W., Oh, S. R., and Yoon, Y. D. (2018b). 7-Methoxy-luteolin-8-C-β-6-deoxy-xylo-pyranos-3-uloside exactly (mLU8C-PU) isolated from Arthraxon hispidus inhibits migratory and invasive responses mediated via downregulation of MMP-9 and IL-8 expression in MCF-7 breast cancer cells. Environ. Toxicol. 33, 1143–1152. doi: 10.1002/tox.22620
Ko, Y. S., Rugira, T., Jin, H., Park, S. W., and Kim, J. H. (2018). Oleandrin and its derivative odoroside a, both cardiac glycosides, exhibit anticancer effects by inhibiting invasion via suppressing the stat-3 signaling pathway. Int. J. Mol. Sci. 19:3350. doi: 10.3390/ijms19113350
Köhrmann, A., Kammerer, U., Kapp, M., Dietl, J., and Anacker, J. (2009). Expression of matrix metalloproteinases (MMPs) in primary human breast cancer and breast cancer cell lines: new findings and review of the literature. BMC Cancer 9:188. doi: 10.1186/1471-2407-9-188
Kousidou, O. C., Mitropoulou, T., Roussidis, A., Kletsas, D., Theocharis, A., and Karamanos, N. (2005). Genistein suppresses the invasive potential of human breast cancer cells through transcriptional regulation of metalloproteinases and their tissue inhibitors. Int. J. Oncol. 26, 1101–1109. doi: 10.3892/ijo.26.4.1101
Kuo, H. P., Chuang, T. C., Tsai, S. C., Tseng, H. H., Hsu, S. C., Chen, Y. C., et al. (2012). Berberine, an isoquinoline alkaloid, inhibits the metastatic potential of breast cancer cells via Akt pathway modulation. J. Agric. Food Chem. 60, 9649–9658. doi: 10.1021/jf302832n
Lai, L. H., Fu, Q. H, Liu, Y., Jiang, K., Guo, Q. M., Chen, Q. Y., et al. (2012). Piperine suppresses tumor growth and metastasis in vitro and in vivo in a 4T1 murine breast cancer model. Acta Pharmacol. Sin. 33, 523–530. doi: 10.1038/aps.2011.209
Landström, M., Zhang, J. X., Hallmans, G., Äman, P., Bergh, A., Damber, J. E., et al. (1998). Inhibitory effects of soy and rye diets on the development of Dunning R3327 prostate adenocarcinoma in rats. Prostate 36, 151–161. doi: 10.1002/(SICI)1097-0045(19980801)36:3<151::AID-PROS2>3.0.CO;2-K
Lee, H. S., Seo, E. Y., Kang, N. E., and Kim, K. W. (2008). [6]-Gingerol inhibits metastasis of MDA-MB-231 human breast cancer cells. J. Nutr. Biochem. 19, 313–319. doi: 10.1016/j.jnutbio.2007.05.008
Lee, J., Park, S. H., Lee, J., Chun, H., Choi, M. K., Yoon, J. H., et al. (2019). Differential effects of luteolin and its glycosides on invasion and apoptosis in MDA-MB-231 triple-negative breast cancer cells. EXCLI J. 18, 750−763. doi: 10.17179/excli2019-1459
Lee, M. (2011). The history of Ephedra (ma-huang). JR Coll Physicians Edinb. 41, 78–84. doi: 10.4997/JRCPE.2011.116
Lee, S. O., Jeong, Y. J., Im, H. G., Kim, C. H., Chang, Y. C., and Lee, I. S. (2007). Silibinin suppresses PMA-induced MMP-9 expression by blocking the AP-1 activation via MAPK signaling pathways in MCF-7 human breast carcinoma cells. Biochem. Biophys. Res. Commun. 354, 165–171. doi: 10.1016/j.bbrc.2006.12.181
Lee, W. T., Lee, T. H., Cheng, C. H., Chen, K. C., Chen, Y. C., and Lin, C. W. (2015). Antroquinonol from Antrodia Camphorata suppresses breast tumor migration/invasion through inhibiting ERK-AP-1-and AKT-NF-κB-dependent MMP-9 and epithelial-mesenchymal transition expressions. Food Chem. Toxicol. 78, 33–41. doi: 10.1016/j.fct.2015.01.012
Lee, W. Y., Huang, S. C., Tzeng, C. C., Chang, T. L., and Hsu, K. F. (2007). Alterations of metastasis-related genes identified using an oligonucleotide microarray of genistein-treated HCC1395 breast cancer cells. HNUC 58, 239–246. doi: 10.1080/01635580701328636
Li, C., Zhao, Y., Yang, D., Yu, Y., Guo, H., Zhao, Z., et al. (2015). Inhibitory effects of kaempferol on the invasion of human breast carcinoma cells by downregulating the expression and activity of matrix metalloproteinase-9. Biochem. Cell Biol. 93, 16–27. doi: 10.1139/bcb-2014-0067
Li, W., Shao, Y., Hu, L., Zhang, X., Chen, Y., Tong, L., et al. (2007). BM6, a new semi-synthetic vinca alkaloid, exhibits its potent in vivo anti-tumor activities via its high binding affinity for tubulin and improved pharmacokinetic profiles. Cancer Biol. Ther. 6, 787–794. doi: 10.4161/cbt.6.5.4006
Lin, C. W., Hou, W. C., Shen, S. C., Juan, S. H., Ko, C. H., Wang, L. M., et al. (2008). Quercetin inhibition of tumor invasion via suppressing PKCδ/ERK/AP-1-dependent matrix metalloproteinase-9 activation in breast carcinoma cells. Carcinogenesis 29, 1807–1815. doi: 10.1093/carcin/bgn162
Ling, H., Zhang, Y., Ng, K. Y., and Chew, E. H. (2011). Pachymic acid impairs breast cancer cell invasion by suppressing nuclear factor-κB-dependent matrix metalloproteinase-9 expression. Breast Cancer Res. Treat. 126, 609–620. doi: 10.1007/s10549-010-0929-5
Lou, C., Zhu, Z., Xu, X., Zhu, R., Sheng, Y., and Zhao, H. (2019). Picroside II. an iridoid glycoside from Picrorhiza kurroa, suppresses tumor migration, invasion, and angiogenesis in vitro and in vivo. Biomed. Pharmacother. 120:109494. doi: 10.1016/j.biopha.2019.109494
Lou, C., Zhu, Z., Zhao, Y., Zhu, R., and Zhao, H. (2017). Arctigenin, a lignan from Arctium lappa L., inhibits metastasis of human breast cancer cells through the downregulation of MMP-2/-9 and heparanase in MDA-MB-231 cells. Oncol. Rep. 37, 179–184. doi: 10.3892/or.2016.5269
Lu, J. J., Bao, J. L., Chen, X. P., Huang, M., and Wang, Y. T. (2012). Alkaloids isolated from natural herbs as the anticancer agents. Evid. Based Complement Alternat. Med. (2012) 2012:485042. doi: 10.1155/2012/485042
Magee, P. J., Allsopp, P., Samaletdin, A., and Rowland, R. I. (2014). Daidzein, R-(+) equol and S-(–) equol inhibit the invasion of MDA-MB-231 breast cancer cells potentially via the down-regulation of matrix metalloproteinase-2. Eur. J. Nutr. 53, 345–350. doi: 10.1007/s00394-013-0520-z
Mali, A., Wagh, U., Hegde, M., Chandorkar, S., Surve, S., and Patole, M. (2012). In vitro anti-metastatic activity of enterolactone, a mammalian lignan derived from flax lignan, and down-regulation of matrix metalloproteinases in MCF-7 and MDA MB 231 cell lines. Indian J. Cancer. 49, 181–187. doi: 10.4103/0019-509X.98948
Mali, A. V., Joshi, A. A., Hegde, M. V., and Kadam, S. S. (2017). Enterolactone suppresses proliferation, migration and metastasis of MDA-MB-231 breast cancer cells through inhibition of uPA induced plasmin activation and MMPs-mediated ECM remodeling. Asian Pac.c J. Cancer Prev. 18, 905–915. doi: 10.22034/APJCP.2017.18.4.905
Nagase, H., Visse, R., and Murphy, G. (2006). Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc. Res. 69, 562–573. doi: 10.1016/j.cardiores.2005.12.002
Nijveldt, R. J., Van Nood, E., Van Hoorn, D. E., Boelens, P. G., Van Norren, K., and Van Leeuwen, A. P. (2001). Flavonoids: a review of probable mechanisms of action and potential applications. Am. J. Clin. Nutr. 74, 418–425. doi: 10.1093/ajcn/74.4.418
Ogasawara, M., Matsubara, T., and Suzuki, H. (2001). Inhibitory effects of evodiamine on in vitro invasion and experimental lung metastasis of murine colon cancer cells. Biol. Pharm. Bull. 24, 917–920. doi: 10.1248/bpb.24.917
Ogasawara, M., Matsunaga, T., Takahashi, S., Saiki, I., and Suzuki, H. (2002). Anti-invasive and metastatic activities of evodiamine. Biol. Pharm. Bull. 25, 1491–1493. doi: 10.1248/bpb.25.1491
Ozbay, T., and Nahta, R. (2011). Delphinidin inhibits HER2 and Erk1/2 signaling and suppresses growth of HER2-overexpressing and triple negative breast cancer cell lines. Breast Cancer. 5, 143–154. doi: 10.4137/BCBCR.S7156
Page-McCaw, A., Ewald, A. J., and Werb, Z. (2007). Matrix metalloproteinases and the regulation of tissue remodelling. Nat. Rev. Mol. Cell Biol. 8, 221–233. doi: 10.1038/nrm2125
Park, S. H., Kim, J. H., Lee, D. H., Kang, J. W., Song, H. H., Oh, S. R., et al. (2013). Luteolin. 8-C-β-fucopyranoside inhibits invasion and suppresses TPA-induced MMP-9 and IL-8 via ERK/AP-1 and ERK/NF-κB signaling in MCF-7 breast cancer cells. Biochimie. 95, 2082–2090. doi: 10.1016/j.biochi.2013.07.021
Park, S. Y., Jin, M. L., Kim, Y. H., Lee, S. J., and Park, G. (2014). Sanguinarine inhibits invasiveness and the MMP-9 and COX-2 expression in TPA-induced breast cancer cells by inducing HO-1 expression. Oncol. Rep. 31, 497–504. doi: 10.3892/or.2013.2843
Park, S. Y., Kim, Y. H., Kim, Y., and Lee, J. S. (2012). Aromatic-turmerone attenuates invasion and expression of MMP-9 and COX-2 through inhibition of NF-κB activation in TPA-induced breast cancer cells. J. Cell. Biochem. 113, 3653–3662. doi: 10.1002/jcb.24238
Phatak, J. (2016). Structure-Function Relationship Between Quercetin, Its Methylated Derivatives and Cytotoxicity in Triple Negative Breast Cancer Cells California, CA.
Pongrakhananon, V. (2013). “Anticancer properties of cardiac glycosides,” in Cancer Treatment-Conventional and Innovative Approaches, ed L. Rangel (Rijeka: Intech), 65-83.
Radisky, E. S., and Radisky, D. C. (2015). Matrix metalloproteinases as breast cancer drivers and therapeutic targets. Front. Biosci. 20:1144. doi: 10.2741/4364
Ranganathan, S., Halagowder, D., and Sivasithambaram, D. N. (2015). Quercetin suppresses twist to induce apoptosis in MCF-7 breast cancer cells. PLoS ONE. 10:e0141370. doi: 10.1371/journal.pone.0141370
Rathee, D., Thanki, M., Bhuva, S., Anandjiwala, S., and Agrawal, R. (2013). Iridoid glycosides-Kutkin, Picroside I. and Kutkoside from Picrorrhiza kurroa Benth inhibits the invasion and migration of MCF-7 breast cancer cells through the down regulation of matrix metalloproteinases: 1st Cancer Update. Arab. J. Chem. 6, 49–58. doi: 10.1016/j.arabjc.2011.01.011
Roomi, M., Monterrey, J., Kalinovsky, T., Rath, M., and Niedzwiecki, A. (2009). Patterns of MMP-2 and MMP-9 expression in human cancer cell lines. Oncol. Rep. 21, 1323–1333. doi: 10.3892/or_00000358
Sen, T., Dutta, A., and Chatterjee, A. (2010). Epigallocatechin-3-gallate (EGCG) downregulates gelatinase-B (MMP-9) by involvement of FAK/ERK/NFκB and AP-1 in the human breast cancer cell line MDA-MB-231. Anticancer. Drugs. 21, 632–644. doi: 10.1097/CAD.0b013e32833a4385
Shi, M. D., Shih, Y. W., Lee, Y. S., Cheng, Y. F., and Tsai, L. Y. (2013). Suppression of. 12-O-tetradecanoylphorbol-13-acetate-induced MCF-7 breast adenocarcinoma cells invasion/migration by α-tomatine through activating PKCα/ERK/NF-κB-dependent MMP-2/MMP-9 expressions. Cell Biochem. Biophys. 66, 161–174. doi: 10.1007/s12013-012-9465-8
Tang, J., Oroudjev, E., Wilson, L., and Ayoub, G. (2015). Delphinidin and cyanidin exhibit antiproliferative and apoptotic effects in MCF7 human breast cancer cells. Integr. Cancer Sci. Ther. 2, 82–86. doi: 10.15761/ICST.1000119
Wang, J., Xu, J., and Xing, G. (2017). Lycorine inhibits the growth and metastasis of breast cancer through the blockage of STAT3 signaling pathway. Acta Biochim. Biophys. Sin. 49, 771–779. doi: 10.1093/abbs/gmx076
Wang, K. L., Hsia, S. M., Chan, C. J., Chang, F. Y., Huang, C. Y., Bau, D. T., et al. (2013). Inhibitory effects of isoliquiritigenin on the migration and invasion of human breast cancer cells. Expert Opin. Ther. Targets 17, 337–349. doi: 10.1517/14728222.2013.756869
Wang, L., Ling, Y., Chen, Y., Li, C. L., Feng, F., You, Q. D., et al. (2010). Flavonoid baicalein suppresses adhesion, migration and invasion of MDA-MB-231 human breast cancer cells. Cancer Lett. 297, 42–48. doi: 10.1016/j.canlet.2010.04.022
Wang, S., Liu, Q., Zhang, Y., Liu, K., Yu, P., Liu, K., et al. (2009). Suppression of growth, migration and invasion of highly-metastatic human breast cancer cells by berbamine and its molecular mechanisms of action. Mol. Cancer 8:81. doi: 10.1186/1476-4598-8-81
Wang, X., Wei, Y., Yuan, S., Liu, G., Lu, Y., Zhang, J., et al. (2005). Potential anticancer activity of tanshinone IIA against human breast cancer. Int. J. Cancer 116, 799–807. doi: 10.1002/ijc.20880
Weaver, A. M. (2006). Invadopodia: specialized cell structures for cancer invasion. Clin. Exp. Metastasis 23, 97–105. doi: 10.1007/s10585-006-9014-1
Yeh, C. T., Wu, C. H., and Yen, C. G. (2010). Ursolic acid, a naturally occurring triterpenoid, suppresses migration and invasion of human breast cancer cells by modulating c-Jun N-terminal kinase, Akt and mammalian target of rapamycin signaling. Mol. Nutr. Food Res. 54, 1285–1295. doi: 10.1002/mnfr.200900414
Yu, P., Liu, Q., Liu, K., Yagasaki, K., Wu, E., and Zhang, G. (2009). Matrine suppresses breast cancer cell proliferation and invasion via VEGF-Akt-NF-κB signaling. Cytotechnology 59, 219–229. doi: 10.1007/s10616-009-9225-9
Yu, Q., and Stamenkovic, I. (2000). Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-β and promotes tumor invasion and angiogenesis. Genes Dev. 14, 163–176.
Keywords: cancer, breast cancer, phytochemical, matrix metalloproteinase, tumor, Metalloproteinase (MMP), Metalloproteinase (aMMP-8)
Citation: Khan YH, Uttra AM, Qasim S, Mallhi TH, Alotaibi NH, Rasheed M, Alzarea AI, Iqbal MS, Alruwaili NK, Khan S-U-D and Alanazi AS (2021) Potential Role of Phytochemicals Against Matrix Metalloproteinase Induced Breast Cancer; An Explanatory Review. Front. Chem. 8:592152. doi: 10.3389/fchem.2020.592152
Received: 06 August 2020; Accepted: 20 November 2020;
Published: 15 January 2021.
Edited by:
Muhammad Ajaz Hussain, University of Sargodha, PakistanReviewed by:
Muhammad Arshad Raza, Government College University, PakistanCopyright © 2021 Khan, Uttra, Qasim, Mallhi, Alotaibi, Rasheed, Alzarea, Iqbal, Alruwaili, Khan and Alanazi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tauqeer Hussain Mallhi, dGF1cWVlci5odXNzYWluLm1hbGxoaUBob3RtYWlsLmNvbQ==; Muhammad Shahid Iqbal, ZHJtbXNpcWJhbEBnbWFpbC5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.