
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Chem. , 15 September 2020
Sec. Supramolecular Chemistry
Volume 8 - 2020 | https://doi.org/10.3389/fchem.2020.588201
This article is part of the Research Topic Host-Guest Chemistry of Macrocycles View all 17 articles
In the present work, new host-guest binding motifs based on a water-soluble pillar[6]arene dodecyl-ammonium chloride (CP6) with two aromatic sulfonic acids in aqueous media were fabricated. In accordance with the integrated results of 1H NMR, 2D NOESY, and florescence titration experiments, it was demonstrated that the host-guest binding of CP6 with the two aromatic sulfonic acids in aqueous solution not only has high binding constants but also has pH-responsiveness.
In the supramolecular chemistry field, stimuli-responsive molecular host-guest recognition motifs are attracting much attention because of their wide range of applications in the fabrication of various fascinating and important supramolecular systems, such as molecular devices and machines (Palmer and Rebek, 2004; Han and Chen, 2007; Zhang et al., 2014), responsive supramolecular polymers (Xu et al., 2013; Cantekin et al., 2015), and other smart supramolecular materials (Avestro et al., 2012; Guo and Liu, 2012; Li et al., 2012; Vukotic and Loeb, 2012). Up to now, pH, temperature, light, redox reagents, enzymes, and other external stimuli have been widely utilized for the fabrication of various responsive host-guest complexation systems (Guo and Liu, 2014; Han et al., 2014; Ma and Tian, 2014). Among these stimuli, pH response is very interesting for special applications in electronic devices, gene delivery, and drug delivery (Credit et al., 1997; Badjic et al., 2004; Yu et al., 2012; Duan et al., 2013; Zhang et al., 2013). Therefore, it is of particular importance to construct pH-responsive molecular host-guest complexation systems.
Pillararenes (Ogoshi et al., 2008, 2016; Cao et al., 2009, 2014; Xue et al., 2012; Yao et al., 2012; Si et al., 2014; Wang et al., 2019), as a new kind of supramolecular macrocyclic hosts, have gained growing attention due to their intrinsic unique rigid and symmetrical pillar-shaped architecture, tunable cavity size, easy modification, and superior host-guest properties. Pillararenes endowed with these outstanding features have been used to construct numerous supramolecular systems, such as supramolecular polymers (Zhang et al., 2011; Guan et al., 2012; Li, 2014), daisy chains (Zhang et al., 2012), transmembrane channels (Si et al., 2014), drug-release systems (Cao et al., 2014; Chang et al., 2014; Hu et al., 2016), and other advanced functional materials (Ni et al., 2016; Wang et al., 2018; Xiao et al., 2018; Zhou et al., 2020). Practically, a series of water-soluble pillararenes have been synthesized and demonstrated to act as scaffolding hosts to various guests (Ogoshi et al., 2012; Hu et al., 2016; Yakimova et al., 2016). Among these water-soluble pillararenes, pH-responsive ones have been reported in the construction of plenty of supramolecular systems (Yu et al., 2012; Cao et al., 2014; Hu et al., 2016; Xiao et al., 2019). Recently, a pillar[6]arene dodecyl-ammonium chloride (CP6) with good water solubility was prepared by our group (Duan et al., 2019). The CP6 with 12 – groups on both rims has response to acid/base reagent pairs (such as HCl and NaOH). Searching new guests for this positively charged pillar[6]arene to fabricate pH-responsive host-guest binding motifs is thus of great interest. In the present manuscript, two aromatic sulfonic acids, i.e., p-toluenesulfonic acid (p-TSA) and 2-naphthalenesulfonic acid (2-NA) were selected as guests, owing to their wide uses in rubbers, dyestuffs, insecticides, varnishes, and pharmaceuticals (Wu et al., 2011). The design and fabrication of pH-responsive host-guest recognition motifs are elaborated in Scheme 1. We show that CP6 can form highly stable host-guest complexes (p-TSA⊂CP6 and 2-NA⊂CP6) with p-TSA and 2-NA, respectively. Based on these two molecular recognition motifs, pH-responsive host-guest complexes were demonstrated.
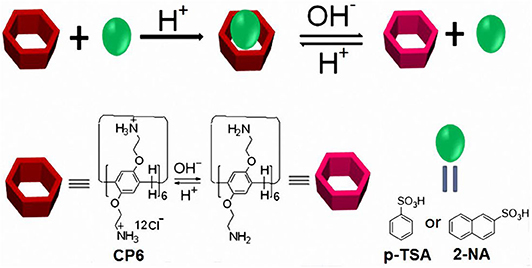
Scheme 1. Chemical structures and cartoon representation of pillararene CP6 and guests (p-TSA and 2-NA); illustration of the acid/base controlled dethreading/rethreading process.
All reagents were commercially available and used as supplied without further purification. CP6 (Duan et al., 2019) was prepared according to the published procedures. NMR spectra were conducted on Bruker Avance III HD 400 spectrometer with the use of the deuterated solvent as the lock and the residual solvent as the internal reference. Fluorescence spectra were performed on an Agilent Cary Eclipse fluorescence spectrophotometer. 0.1 M phosphate buffer solution (PBS, pH = 6.0) was prepared by mixing 12 mL 1 M Na2HPO4 and 88 mL 1 M NaH2PO4 solution. The D2O solutions were adjusted to pD 6.0 by DCl or NaOD. The pH and pD values were verified on a Mettler Toledo pH meter calibrated with two standard buffer solutions. pH readings were converted to pD by adding 0.4 units (Glasoe and Long, 1960).
The host-guest complexation of CP6 with p-TSA in D2O was first investigated by 1H NMR spectroscopy. As shown in Figure 1B, when adding about 0.1 equiv. of the host CP6, the signals of protons (a-c) on the guest p-TSA demonstrate significant upfield shifts against free guest p-TSA proton signals (Δδ = −0.15, −0.39, and −0.37 ppm for proton a, b and c, respectively). Strong upfield chemical shifts (Δδ) of the aromatic and methyl protons indicate that the p-TSA guest was fully threaded into the host cavity, forming a stable threaded host-guest complex. And the presence of only one set of peaks for the solution of CP6 and p-TSA (Figure 1B) suggests that the host-guest complex formation is a fast exchange process on the NMR time scale. The host-guest binding of CP6 and p-TSA in water was then examined by 2D NOESY analysis. From the 2D NOESY spectrum (Figure 2), NOE correlation signals were observed between Ha, Hb, and Hc on p-TSA and protons H1−4 on CP6, respectively, supporting the assignment of a threaded structure p-TSA⊂CP6.
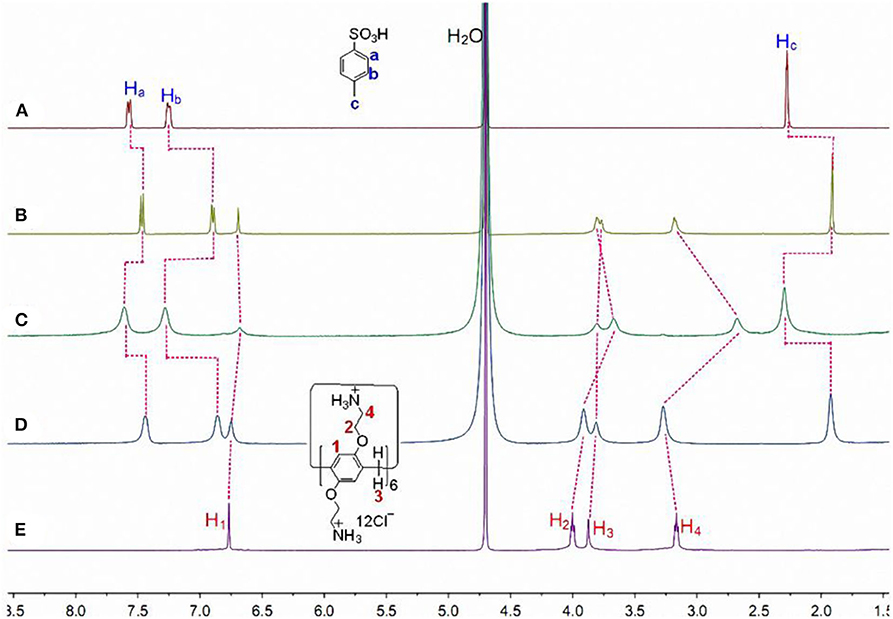
Figure 1. Partial 1H NMR spectra (400 MHz, D2O, 298 K) of (A) 20.00 mM p-TSA, (B) 2.00 mM CP6 and 20.00 mM p-TSA, (C) after addition of 1.0 μL of aqueous NaOD solution (30%) to b, (D) after addition of 2.0 μL of aqueous DCl solution (20%) to c, and (E) 2.00 mM CP6 at pD 6.0.
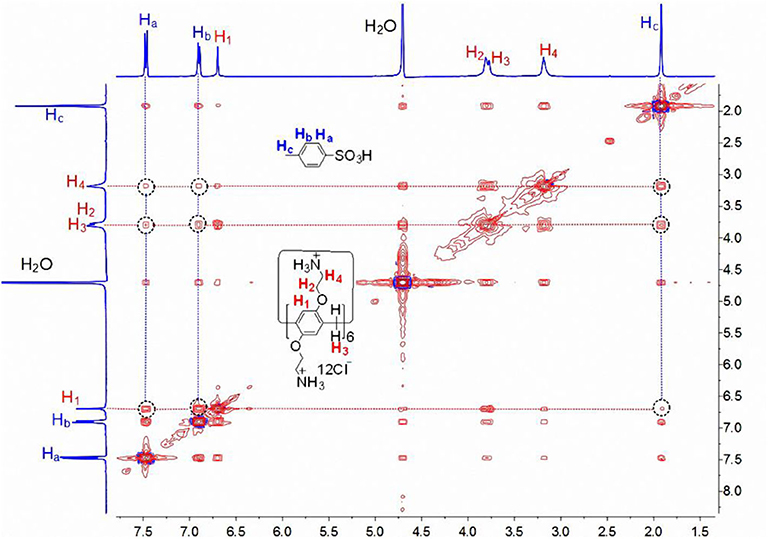
Figure 2. 2D NOESY NMR (400 MHz, D2O, 298 K, mixing time = 300 ms) spectrum of a solution of CP6 (2.00 mM) and p-TSA (20.00 mM).
Subsequently, the complexation of CP6 by the larger 2-NA guest was also investigated. Similarly, 1H NMR experiments were also performed to investigate the host-guest complexation of CP6 with 2-NA in D2O and MeOD. Figures 3A,B show the 1H NMR spectra of 2-NA in D2O recorded in the absence and the presence of about 0.1 equiv. of the host CP6, respectively. In the presence of CP6 (Figure 3B), the signals of protons (a-g) on the guest 2-NA exhibit substantial upfield shifts compared to those of the free 2-NA (Δδ = −0.24 to −0.16 ppm) (Figure 3A), suggesting the inclusion of the naphthalene moiety of 2-NA into the hydrophobic CP6 cavity. The assignment of these naphthyl proton signals of the inclusion complex can be verified by the analysis of the 1H-1H COZY data (correlation spectroscopy; see Supplementary Figure 1). Furthermore, these shifts appeared due to fast proton exchange observed for complexation in the 1H NMR timescale. The 2D NOESY data (Figure 4) show the NOE correlations between the aromatic protons (Ha−g) of the entrapped 2-NA and the aromatic proton H1 of CP6, which also revealed the interpenetrated geometry.
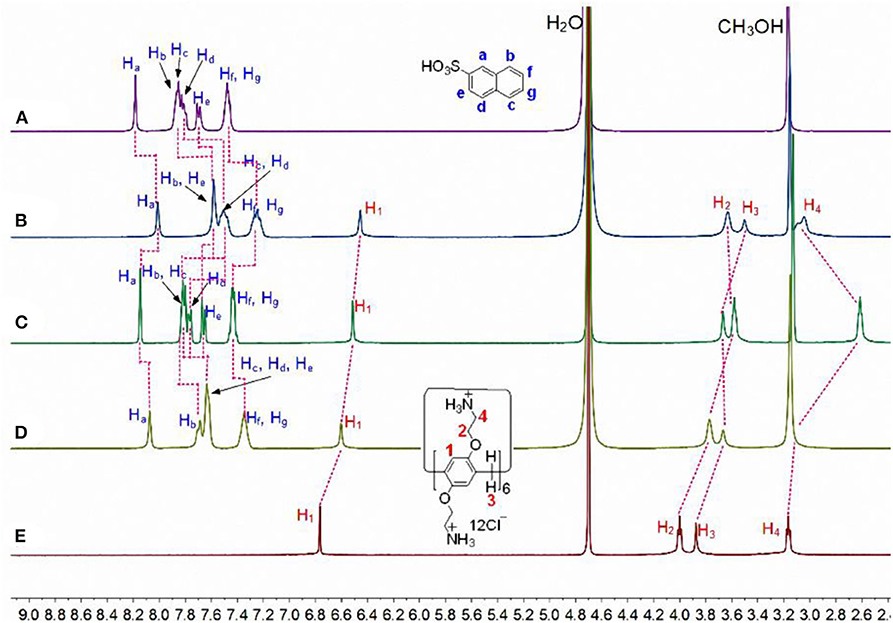
Figure 3. Partial 1H NMR spectra (400 MHz, D2O/CD3OD = 1/1, v/v, 298 K) of (A) 20.00 mM 2-NA, (B) 2.00 mM CP6 and 20.00 mM 2-NA, (C) after addition of 1.0 μL of aqueous NaOD solution (30%) to b, (D) after addition of 2.0 μL of aqueous DCl solution (20%) to c, and (E) 2.00 mM CP6 at pD 6.0.
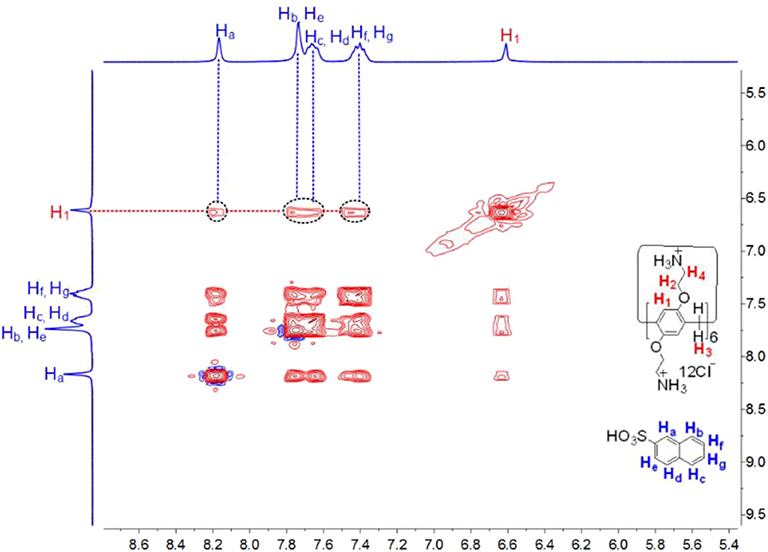
Figure 4. 2D NOESY NMR (400 MHz, D2O, 298 K, mixing time = 300 ms) spectrum of a solution of CP6 (2.00 mM) and 2-NA (20.00 mM).
To quantitatively estimate the binding behaviors of p-TSA and 2-NA with host CP6, fluorescence titrations were conducted at 298 K in a PBS of pH 6.0. Job plots (Supplementary Figures 2,3) based on the fluorescence titrations data indicated that CP6 and the two guests form a 1:1 host-guest complex in aqueous solution, respectively. By using a non-linear curve-fitting method (Supplementary Figures 4,5), the association constants (Ka) were calculated to be (2.23 ± 0.15) × 104 M−1 and (1.97 ± 0.28) × 104 M−1 for p-TSA and 2-NA, respectively. According to the pKa values of the two aromatic sulfonic acids (p-TSA: −2.1; 2-NA: −1.8), it can be concluded that the sulfonic groups of the two aromatic sulfonic acids should be in the deprotonated form at pH 6.0. Thus, we conclude that the interaction mechanism of CP6 with the two aromatic sulfonic acids is that the acidic aromatic sulfonic acids with one sulfonate anion could bind positively charged CP6 bearing 12 – groups in aqueous solutions at pH 6.0, where the electrostatic interactions between sulfonate anion of the two acidic aromatic sulfonic acids and the cationic portals of the host CP6 play a dominant role in formation of the present host-guest complexation. By comparing p-TSA and 2-NA, we can investigate the capability of these guests to form host-guest complexes because of the changed size of the hydrophobic part. The Ka value for p-TSA is almost same as that for 2-NA. Although 2-NA has one more benzene ring than p-TSA and thus the larger π-conjugated system could afford a stronger π-π stacking interaction with the host cavity (Gómez et al., 2014), the electrostatic attractive forces are dominant in these two host-guest complexes.
Additionally, both the obtained p-TSA⊂CP6 and 2-NA⊂CP6 have pH-responsiveness, i.e., the dynamic behavior for the inclusion process of the two aromatic sulfonic acids and CP6 can be reversed upon the addition of HCl and NaOH aqueous solutions. Proton NMR studies were conducted to affirm these two reverse processes (Figures 1, 3). As shown in Figures 1C, 3C, when adding NaOD to the mixed solution of p-TSA⊂CP6 and 2-NA⊂CP6, respectively, both signals of p-TSA and 2-NA returned to almost their uncomplexed positions, suggesting that both p-TSA and 2-NA dethreaded from the cavity of CP6. The reason is apparent: the addition of an aqueous NaOD solution yielded a basic solution and the groups on CP6 were deprotonated to produce the neutral amino groups, resulting in the disappearance of the electrostatic attractive forces between p-TSA or 2-NA and CP6. However, after adding DCl to these solutions, 1H NMR spectra similar to those of the original solutions of p-TSA⊂CP6 and 2-NA⊂CP6 were obtained (Figures 1D, 3D), resulting from the protonation of the amino groups and the regeneration of the complexes between p-TSA or 2-NA and CP6 in this solution. Thus, the host-guest complexes p-TSA⊂CP6 and 2-NA⊂CP6 can be reversed by the sequential addition of a base and an acid (NaOH and HCl, respectively) between its complexed and decomplexed states. In a word, the host-guest complexation between p-TSA or 2-NA and CP6 is pH-responsive and its reversible property can be used to serve as excellent motifs for a variety of controlled molecular release applications.
In summary, novel pH-responsive host-guest recognition motifs based on a water-soluble pillar[6]arene dodecyl-ammonium chloride CP6 with p-TSA or 2-NA were successfully constructed. It was established that CP6 could form a stable 1:1 inclusion complex with the two aromatic sulfonic acids, p-TSA and 2-NA, respectively. Furthermore, both these host-guest complexes can be controlled reversibly through the sequential addition of a base and an acid (NaOH and HCl, respectively) between its complexed and decomplexed states. Consequently, the findings of this work enrich the fields of controlled pillararene chemistry. Our further work will focus on expanding these new pH-responsive host-guest binding motifs to construct smart supramolecular materials in the treatment of sulfonated aromatic pollutants in aqueous media.
All datasets generated for this study are included in the article/Supplementary Material.
QD, FW, and KL designed the work. QD made contributions to the experiments and collective data. The paper was written by QD. All authors extensively discussed the results, reviewed the manuscript, and approved the final version of the manuscript to be submitted.
This work was supported by the National Natural Science Foundation of China (no. 21402040), Henan Province Science and Technology Research Program (no. 192102210197), and Program for Innovative Research Team (in Science and Technology) in University of Henan Province (no. 20IRTSTHN008). Financial support from the Program of 543 team and Research and Cultivation Foundation of Henan University of Engineering (PYXM202009) is gratefully acknowledged.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fchem.2020.588201/full#supplementary-material
Avestro, A.-J., Belowich, M. E., and Stoddart, J. F. (2012). Cooperative self-assembly: producing synthetic polymers with precise and concise primary structures. Chem. Soc. Rev. 41, 5881–5895. doi: 10.1039/C2CS35167F
Badjic, J. D., Balzani, V., Credi, A., Silvi, S., and Stoddart, J. F. (2004). A molecular elevator. Science 303, 1845–1849. doi: 10.1126/science.1094791
Cantekin, S., Markvoort, A. J., Elemans, J. A. A. W., Rowan, A. E., and Nolte, R. J. M. (2015). Allosterically controlled threading of polymers through macrocyclic dimers. J. Am. Chem. Soc. 137, 3915–3923. doi: 10.1021/jacs.5b00431
Cao, D., Kou, Y., Liang, J., Chen, Z., Wang, L., and Meier, H. (2009). A facile and efficient preparation of pillararenes and a pillarquinone. Angew. Chem. Int. Ed. 48, 9721–9723. doi: 10.1002/anie.200904765
Cao, Y., Hu, X.-Y., Li, Y., Zou, X., Xiong, X., Lin, C., et al. (2014). Multistimuli-responsive supramolecular vesicles based on water-soluble pillar[6]arene and SAINT complexation for controllable drug release. J. Am. Chem. Soc. 136, 10762–10769. doi: 10.1021/ja505344t
Chang, Y., Yang, K., Wei, P., Huang, S., Pei, Y., Zhao, W., et al. (2014). Cationic vesicles based on amphiphilic pillar[5]arene capped with ferrocenium: a redox-responsive system for drug/siRNA co-delivery. Angew. Chem. Int. Ed. 53, 13126–13130. doi: 10.1002/anie.201407272
Credit, A., Balzani, V., Langford, S. J., and Stoddart, J. F. (1997). Logic operations at the molecular level. An XOR gate based on a molecular machine. J. Am. Chem. Soc. 119, 2679–2681. doi: 10.1021/ja963572l
Duan, Q., Cao, Y., Li, Y., Hu, X., Xiao, T., Lin, C., et al. (2013). pH-responsive supramolecular vesicles based on water-soluble pillar[6]arene and ferrocene derivative for drug delivery. J. Am. Chem. Soc. 135, 10542–10549. doi: 10.1021/ja405014r
Duan, Q., Zhang, H., Mai, W., Wang, F., and Lu, K. (2019). Acid/base- and base/acid-switchable complexation between anionic-/cationic-pillar[6]arenes and a viologen ditosylate salt. Org. Biomol. Chem. 17, 4430–4434. doi: 10.1039/c9ob00398c
Glasoe, P. K., and Long, F. A. (1960). Use of glass electrodes to measure acidities in deuterium oxide. J. Phys. Chem. 64, 188–190. doi: 10.1021/j100830a521
Gómez, B., Francisco, V., Fernández-Nieto, F., Garcia-Rio, L., Paleo, M. R., and Sardina, F. J. (2014). Host-guest chemistry of a water-soluble Pillar[5]arene: evidence for an ionic-exchange recognition process and different complexation modes. Chem. Eur. J. 20, 12123–12132. doi: 10.1002/chem.201403194
Guan, Y., Ni, M., Hu, X., Xiao, T., Xiong, S., Lin, C., et al. (2012). Pillar[5]arene-based polymeric architectures constructed by orthogonal supramolecular interactions. Chem. Commun. 48, 8529–8531. doi: 10.1039/c2cc33943a
Guo, D.-S., and Liu, Y. (2012). Calixarene-based supramolecular polymerization in solution. Chem. Soc. Rev. 41, 5907–5921. doi: 10.1039/C2CS35075K
Guo, D.-S., and Liu, Y. (2014). Supramolecular chemistry of p-sulfonatocalix[n]arenes and its biological applications. Acc. Chem. Res. 47, 1925–1934. doi: 10.1021/ar500009g
Han, T., and Chen, C.-F. (2007). Formation of ternary complexes between a macrotricyclic host and hetero-guest pairs: an acid-base controlled selective complexation process. Org. Lett. 9, 4207–4210. doi: 10.1021/ol701770h
Han, Y., Meng, Z., Ma, Y.-X., and Chen, C.-F. (2014). Iptycene-derived crown ether hosts for molecular recognition and self-assembly. Acc. Chem. Res. 47, 2026–2040. doi: 10.1021/ar5000677
Hu, X.-Y., Liu, X., Zhang, W., Qin, S., Yao, C., Li, Y., et al. (2016). Controllable construction of biocompatible supramolecular micelles and vesicles by water-soluble phosphate pillar[5,6]arenes for selective anti-cancer drug delivery. Chem. Mater. 28, 3778–3788. doi: 10.1021/acs.chemmater.6b00691
Li, C. (2014). Pillararene-based supramolecular polymers: from molecular recognition to polymeric aggregates. Chem. Commun. 50, 12420–12433. doi: 10.1039/c4cc03170a
Li, S.-L., Xiao, T., Lin, C., and Wang, L. (2012). Advanced supramolecular polymers constructed by orthogonal self-assembly. Chem. Soc. Rev. 41, 5950–5968. doi: 10.1039/C2CS35099H
Ma, X., and Tian, H. (2014). Stimuli-responsive supramolecular polymers in aqueous solution. Acc. Chem. Res. 47, 1971–1981. doi: 10.1021/ar500033n
Ni, M., Zhang, N., Xia, W., Wu, X., Yao, C., Liu, X., et al. (2016). Dramatically promoted swelling of a hydrogel by pillar[6]arene-ferrocene complexation with multi-stimuli responsiveness. J. Am. Chem. Soc. 138, 6643–6649. doi: 10.1021/jacs.6b03296
Ogoshi, T., Kanai, S., Fujinami, S., Yamagishi, T.-A., and Nakamoto, Y. (2008). para-Bridged symmetrical pillar[5]arenes: their Lewis acid catalyzed synthesis and host-guest property. J. Am. Chem. Soc. 130, 5022–5023. doi: 10.1021/ja711260m
Ogoshi, T., Shiga, R., and Yamagishi, T.-A. (2012). Reversibly tunable lower critical solution temperature utilizing host-guest complexation of pillar[5]arene with triethylene oxide substituents. J. Am. Chem. Soc. 134, 4577–4580. doi: 10.1021/ja300989n
Ogoshi, T., Yamagishi, T.-A., and Nakamoto, Y. (2016). Pillar-shaped macrocyclic hosts pillar[n]arenes: new key players for supramolecular chemistry. Chem. Rev. 116, 7937–8002. doi: 10.1021/acs.chemrev.5b00765
Palmer, L. C., and Rebek, J. Jr. (2004). The ins and outs of molecular encapsulation. Org. Biomol. Chem. 2, 3051–3059. doi: 10.1039/B412510J
Si, W., Li, Z.-T., and Hou, J.-L. (2014). Voltage-driven reversible insertion into and leaving from a lipid bilayer: tuning transmembrane transport of artificial channels. Angew. Chem. Int. Ed. 53, 4578–4581. doi: 10.1002/anie.201311249
Vukotic, V. N., and Loeb, S. J. (2012). Coordination polymers containing rotaxane linkers. Chem. Soc. Rev. 41, 5896–5906. doi: 10.1039/C2CS35141B
Wang, S., Xu, Z., Wang, T., Xiao, T., Hu, X.-Y., Shen, Y.-Z., et al. (2018). Warm/cool-tone switchable thermochromic material for smart windows by orthogonally integrating properties of pillar[6]arene and ferrocene. Nat. Commun. 9:1737. doi: 10.1038/s41467-018-03827-3
Wang, X., Liu, Z.-J., Hill, E. H., Zheng, Y., Guo, G., Wang, Y., et al. (2019). Organic-inorganic hybrid pillarene-based nanomaterial for label-free sensing and catalysis. Matter 1, 848–861. doi: 10.1016/j.matt.2019.03.005
Wu, T., Cai, X., Tan, S., Li, H., Liu, J., and Yang, W. (2011). Adsorption characteristics of acrylonitrile, p-toluenesulfonic acid, 1-naphthalenesulfonic acid and methyl blue on graphene in aqueous solutions. Chem. Eng. J. 173, 144–149. doi: 10.1016/j.cej.2011.07.050
Xiao, T., Qi, L., Zhong, W., Lin, C., Wang, R., and Wang, L. (2019). Stimuli-responsive nanocarriers constructed from pillar[n]arene-based supra-amphiphiles. Mater. Chem. Front. 3, 1973–1993. doi: 10.1039/C9QM00428A
Xiao, T., Xu, L., Zhong, W., Zhou, L., Sun, X.-Q., Hu, X.-Y., et al. (2018). Advanced functional materials constructed from pillar[n]arenes. Isr. J. Chem. 58, 1219–1229. doi: 10.1002/ijch.201800026
Xu, J.-F., Chen, Y.-Z., Wu, D., Wu, L.-Z., Tung, C.-H., and Yang, Q.-Z. (2013). Photoresponsive hydrogen-bonded supramolecular polymers based on a stiff stilbene unit. Angew. Chem. Int. Ed. 52, 9738–9742. doi: 10.1002/anie.201303496
Xue, M., Yang, Y., Chi, X., Zhang, Z., and Huang, F. (2012). Pillararenes, a new class of macrocycles for supramolecular chemistry. Acc. Chem. Res. 45, 1294–1308. doi: 10.1021/ar2003418
Yakimova, L. S., Shurpik, D. N., Gilmanova, L. H., Makhmutova, A. R., Rakhimbekova, A., et al. (2016). Highly selective binding of methyl orange dye by cationic water-soluble pillar[5]arenes. Org. Biomol. Chem. 14, 4233–4238. doi: 10.1039/c6ob00539j
Yao, Y., Xue, M., Chen, J., Zhang, M., and Huang, F. (2012). An amphiphilic pillar[5]arene: synthesis, controllable self-assembly in water, and application in calcein release and TNT adsorption. J. Am. Chem. Soc. 134, 15712–15715. doi: 10.1021/ja3076617
Yu, G., Zhou, X., Zhang, Z., Han, C., Mao, Z., Gao, C., et al. (2012). Pillar[6]arene/paraquat molecular recognition in water: high binding strength, pH-responsiveness, and application in controllable self-assembly, controlled release, and treatment of paraquat poisoning. J. Am. Chem. Soc. 134, 19489–19497. doi: 10.1021/ja3099905
Zhang, H., Zhou, B., Li, H., Qu, D. H., and Tian, H. (2013). A ferrocene-functionalized [2]rotaxane with two fluorophores as stoppers. J. Org. Chem. 78, 2091–2098. doi: 10.1021/jo302107a
Zhang, M., Yan, X., Huang, F., Niu, Z., and Gibson, H. W. (2014). Stimuli-responsive host–guest systems based on the recognition of cryptands by organic guests. Acc. Chem. Res. 47, 1995–2005. doi: 10.1021/ar500046r
Zhang, Z., Han, C., Yu, G., and Huang, F. (2012). A solvent-driven molecular spring. Chem. Sci. 3, 3026–3031. doi: 10.1039/C2SC20728A
Zhang, Z., Luo, Y., Chen, J., Dong, S., Yu, Y., Ma, Z., et al. (2011). Formation of linear supramolecular polymers that is driven by C-H···π interactions in solution and in the solid state. Angew. Chem. Int. Ed. 50, 1397–1401. doi: 10.1002/anie.201006693
Keywords: pillararenes, supramolecular chemistry, binding motif, pH-responsiveness, aromatic sulfonic acids
Citation: Duan Q, Wang F, Zhang H and Lu K (2020) pH-Responsive Host-Guest Complexations Between a Water-Soluble Pillar[6]arene Dodecyl-Ammonium Chloride and Aromatic Sulfonic Acids. Front. Chem. 8:588201. doi: 10.3389/fchem.2020.588201
Received: 28 July 2020; Accepted: 13 August 2020;
Published: 15 September 2020.
Edited by:
Tangxin Xiao, Changzhou University, ChinaReviewed by:
Shao-Lu Li, Tianjin Polytechnic University, ChinaCopyright © 2020 Duan, Wang, Zhang and Lu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qunpeng Duan, cXBkdWFuQGhhdWUuZWR1LmNu; Kui Lu, bHVja3lsdWtlQGhhdWUuZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.