
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Chem. , 14 October 2020
Sec. Inorganic Chemistry
Volume 8 - 2020 | https://doi.org/10.3389/fchem.2020.536907
One Zn-Nd complex [Zn2Nd4L2(OAc)10(OH)2(CH3OH)2] (1) was synthesized from Schiff base ligand bis(3-methoxysalicylidene)ethylene-1,2-phenylenediamine (H2L). 1 shows nanoscale rectangular structure with sizes of about 0.8 × 1.1 × 2.8 nm. 1 exhibits typical near-infrared luminescence of Nd(III) under the excitation of UV-visible light. Further study shows that the complex displays luminescent response behavior to anions and nitro explosives, especially with high sensitivity to H2 and 2,4,6-trinitrophenol.
Construction of heterometallic d-f nanoclusters has received much interest during recent years because of their unique chemical properties (Peng et al., 2012; Wang et al., 2013; Yang et al., 2014; Andruh, 2015; Wen et al., 2019). Fluorescent response to ions and small molecules has received great attention because of the potential application in many areas such as medicine, biology, and environment (Jankolovits et al., 2011; Sun et al., 2015; Qi et al., 2017). As we know, luminescent lanthanide complexes can show emissions in both visible and near-infrared (NIR) ranges (900–1,600 nm) with sharp emission bands, large Stokes shifts, and long lifetimes (Hu et al., 2017; Ning et al., 2018). At present, many visible luminescent complexes with Tb(III) and Eu(III) ions have been used to detect analytes (Guo et al., 2011; Liu et al., 2013; Shi et al., 2015). However, compared with NIR fluorescent probes based on organic fluorophores (Yuan et al., 2013; Guo et al., 2014), very few NIR luminescent lanthanide complexes with Yb(III), Nd(III), and Er(III) ions have been reported to be used as sensors for the detection (Shi et al., 2019).
Phosphates play a key role in biological energy storage and signal transduction, and nitro explosives such as 2,4,6-trinitrophenol (TNP) are very common ingredients of industrial explosives. Thus, many efforts have been made to design fluorescent sensors for phosphates (Yang et al., 2015; Sedaghat et al., 2019) and nitro explosives (Nagarkar et al., 2014; Liu et al., 2017). Our current research interests are in the design of lanthanide-based complexes with luminescent response to various ions and explosives (Jiang et al., 2018; Shi et al., 2019; Liu et al., 2020). Thus, we report here the synthesis and NIR luminescence properties of a Zn-Nd complex [Zn2Nd4L2(OAc)10(OH)2(CH3OH)2] (1) with Schiff base ligand bis(3-methoxysalicylidene)ethylene-1,2-phenylenediamine (H2L, Scheme 1). 1 has nanoscale rectangular structure with diameters of 0.8 × 1.1 × 2.8 nm. The complex shows interesting NIR luminescent response behavior to anions and explosives, especially to H2 and 2,4,6-trinitrophenol (TNP) at ppm level.
Zn(OAc)2·2H2O (0.30 mmol, 0.0658 g), NdCl3·6H2O (0.60mmol, 0.2154 g), and H2L (0.30 mmol, 0.0324 g) were dissolved in 50 mL MeOH at room temperature, and a solution of triethylamine in EtOH (1.0 mol/L, 1 mL) was then added. The mixture was stirred and heated under reflux for 30 min and then filtered. The yellow crystalline product of 1 was obtained by the slow diffusion of diethyl ether into the filtrate at room temperature after 1 month. The crystalline product was collected by filtering and then dried at 120°C in the oven for 2 h. Yield: 0.0981 g (25 %). m. p. > 200°C (dec.). Elemental analysis: found: C, 32.91; H, 4.12; N, 2.50%. Calc. for C72H104Zn4Nd4N4O40Cl4 ([Zn2Nd4L2(OAc)10(OH)2(CH3OH)2]·[ZnCl2(H2O)CH3OH)]): C, 32.68; H, 3.96; N, 2.12%. IR (CH3CN, cm−1): 1,703 (s), 1,551 (s), 1,484 (s), 1,289 (s), 1,239 (s), 1,193 (s), 1,072 (m), 963 (s), and 845 (s).
A Smart APEX CCD diffractometer was used to collect the X-ray data of 1 at 190 K. The structure was solved by the direct method (SHELX 97 program) (Sheldrick, 1997). Non-hydrogen atoms were refined anisotropically. Hydrogen atoms were included in the structure factor calculation but not refined. See http://www.rsc.org/suppdata/cc/ for the crystallographic data of 1 in CIF format (CCDC no. 1971956). The selected bond lengths and angles for the structure of 1 are listed in Supplementary Table 1.
For 1: C72H104Zn4Nd4N4O40Cl4, triclinic, space group P-1, a = 9.6222(19), b = 14.771(3), c = 18.303(4) Å, α = 104.25(3)°, β = 98.18(3)°, γ = 103.42(3)°, V = 2,397.0(8) Å3, Z = 1, Dc = 1.833 g cm−3, μ(Mo-Kα) = 3.305 mm−1, F(000) = 1,312, T = 190 K. R1 = 0.0731, wR2 = 0.1934, GOF = 1.036.
The Schiff-base ligand H2L was prepared according to the literature report (Lam et al., 1996; Liu et al., 2020). For the synthesis of d-f complexes, the proportion of raw materials in the reaction may affect the composition of the product. Reaction of H2L with Zn(OAc)2·2H2O and NdCl3·6H2O in a molar ratio of 1:1:2 gave 1, in which the ratio of L2−:Zn2+:Nd3+ is 1:1:2. The slow diffusion of diethyl ether into the reaction solution led to the formation of yellow crystalline product of 1. This diffusion way helps to produce pure product of 1, but results in the low yield (25%), because there are still a large number of product in the mother solution. In the crystalline solid product, zinc chloride ([ZnCl2(H2O)CH3OH)]) coexists with the complex. The crystal structure of 1 is shown in Figure 1. It is centrosymmetric with two equivalent ZnNd2L moieties linked by two OAc− anions. The molecular dimensions of 1 are about 0.8 × 1.1 × 2.8 nm. In the ZnNd2L moiety, Zn2+ ion exhibits a square pyramidal geometry, coordinated with the O2N2 cavity of L2−. The coordination number of Nd3+ ions is nine, with a single cap square antiprism geometry. The L2− ligand is coordinated with metal ions using two nitrogen and two oxygen atoms. In 1, the average distance between neighboring Nd3+ ions is 4.088 Å. The bond lengths of Zn-N, Zn-O, and Nd-O are 2.036–2.047, 1.994–2.012, and 2.314–2.679 Å, respectively.
Because of the volatilization of solvent molecules in the product, the complex loses about 5% of the weight when heated before 100°C (Supplementary Figure 2, thermogravimetric analysis). It is stable until the heating temperature is about 200°C. Molar conductivity study indicates that 1 is neutral in CH3CN, in agreement with its crystal structure. This suggests that 1 remains its unique molecular structure in solution.
The free H2L shows UV-visible (UV-vis) absorption bands originated from the π − π* transition, which are red-shifted in 1 due to the perturbation of metal ions to the transition (Figure 2). Metal organometallic chromophores with Zn(II) ion in 1 may efficiently transfer energy to lanthanide ions and sensitize lanthanide luminescence (“antenna effect”) (Xu et al., 2010). Thus, excited by ligand-centered absorption bands, 1 shows typical NIR luminescence of Nd3+ ( transitions, j = 9, 11, and 13), and the most intense line is at 1,054 nm () (Figure 3). The complex exhibits broad ligand-centered excitation bands, indicating the ligand-to-metal energy transfer (LMET) in 1. The NIR emission lifetime (τ) and quantum yield (Φem) of 1 in CH3CN are found to be 6.2 μs and 0.8%, respectively.
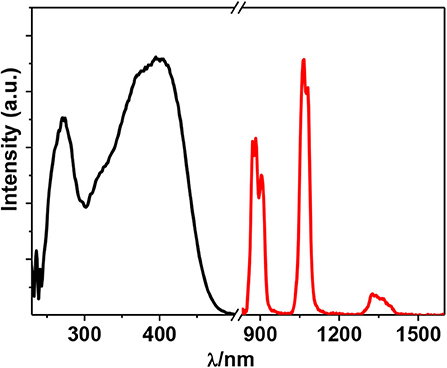
Figure 3. Excitation (λem = 1,054 nm) and emission (λex = 395 nm) spectra of 1 (50 μM) in CH3CN at 298 K.
The luminescent response of 1 toward anions H2, F−, CN−, OH−, , OAc−, Cl−, Br−, , and , and nitro explosives 2,4,6-trinitrophenol (TNP), 4-nitrochlorobenzene (4-NBC), 2-nitrophenol (2-NP), nitrobenzene (NB), 4-nitrobenzaldehyde (4-NBAP), 4-nitrotoluene (4-NT), 1,3-dinitrobenzene (1,3-DNB), and 4-nitrobenzyl chloride (4-NCB) (Supplementary Scheme 1) has been studied in CH3CN. It was found that the addition of all anions and explosives results in a quenching of the lanthanide luminescence (Supplementary Figures 4, 5). It is noticeable that the addition of H2 and TNP causes a more rapid decrease of the luminescence intensities than the addition of other anions and explosives (Figures 4, 5). For example, the emission intensities at 1,054 nm of 1 are decreased more than 50% when the concentrations of added H2 and TNP are 1.8 and 5.6 μM, respectively, which are much lower than those of other anions and explosives (Supplementary Figures 4, 5). These results indicate that 1 shows high selectivity to H2 and TNP through lanthanide luminescent response.
The addition of anions and explosives with low concentrations, such as <5 μM for H2 and TNP, results in a linear luminescence quenching response of 1. Thus, the luminescence quenching efficiencies (KSV) of the complex to these analytes can be calculated using Stern–Volmer (S-V) equation KSV = (I0/I – 1)/[A] (Xiao et al., 2010). As shown in Figures 6, 7, the KSV values of 1 to H2 and TNP are 4.67 × 105 M−1 and 1.46 × 105 M−1, respectively, which are much higher than other anions and explosives. The S–V plots of 1 can be explained by static and dynamic quenching models. The static luminescence quenching is generally associated with the formation of ground-state molecular associations upon addition of the analytes, whereas the dynamic luminescence quenching is under diffusion control, where collisions between analytes and excited fluorophores result in deactivation of the excited states. Basically, the formation of molecular associations in the static quenching model cannot affect the emission lifetimes of fluorophores, however, the collisions in dynamic luminescence quenching may reduce the emission lifetimes. The luminescence lifetimes of 1 are reduced to 4.1 and 5.2 μs with the addition of 1.8 μM of H2 and 5.6 μM of TNP, respectively, indicating the dominance of dynamic luminescence quenching in 1.
The selectivity of 1 to H2 and TNP in the presence of other anions and explosives was investigated. As shown in Figures 8, 9, the existence of another anion and explosive with the same concentration does not affect the high quenching percentage of 1 to H2 and TNP. These results indicate that 1 shows high selectivity to H2 and TNP even in the presence of other anions and explosives, respectively.
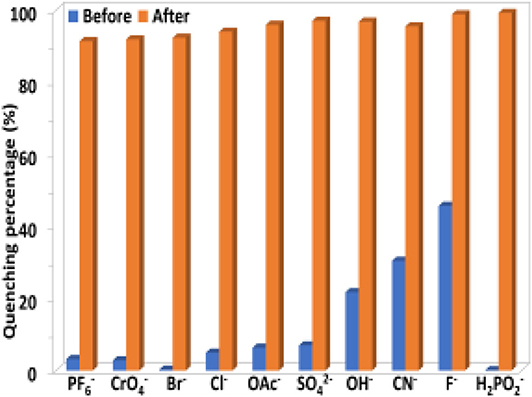
Figure 8. The luminescence quenching percentages of 1 (0.5 μM) before and after the addition of H2 (5 μM) in the presence of other anions (5 μM) in CH3CN.
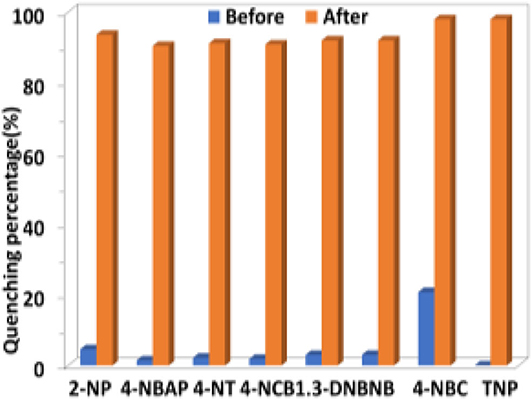
Figure 9. The luminescence quenching percentages of 1 (0.5 μM) before and after the addition of TNP (10 μM) in the presence of other explosives (10 μM) in CH3CN.
For luminescent lanthanide complexes, the LMET plays a key role in the intensities of luminescence. The electronic structure and excited state of the Schiff base ligand may be disturbed by the added anions, resulting in the change of the LMET process in 1 (Parker et al., 1998; Parker, 2000). In addition, the possible intermolecular electron transfer from anions to Schiff base ligands may also consume the excitation energy of lanthanide ions and in turn decreases the luminescence of 1 (Guha and Saha, 2010). The intermolecular interaction between the added anions and 1 is crucial to the lanthanide luminescent response. The interaction between H2 anion and 1 was studied by UV-vis spectral titration (Liu et al., 2017). The red-shift of the absorption bands of 1 indicates the formation of interaction between the added H2 anion and 1 (Figure 10). It is noticeable that the addition of H2 anion decreases the absorption of 1 at the excitation wavelength (λex = 395 nm), which is not advantageous for the Schiff base ligand to absorb light energy and further decreases the lanthanide luminescence (Feng et al., 2019).
Usually, the luminescence quenching of lanthanide complexes arisen by the addition of nitro explosives can be explained by photoinduced electron transfer mechanism (Li et al., 2013; Nagarkar et al., 2013; Qin et al., 2015). According to the literature (Xie et al., 2016), the approximate LUMO energy level of H2L in 1 is shown in Scheme 2, which is higher than those of explosives. Thus, the excited electrons of the Schiff base ligand can transfer to the LUMO orbitals of the explosives. TNP has the lowest LUMO energy level among the explosives, which helps in the electron transfer process. Meanwhile, the UV-vis spectra exhibit that TNP has the highest molar absorption at λex = 395 nm, compared with other explosives (Figure 11). This indicates that TNP may compete with 1 for the excitation energy, resulting in the further decrease of lanthanide luminescence.
In brief, one Zn-Nd complex 1 with dimensions of 0.8 × 1.1 × 2.8 nm was constructed from Schiff base ligand H2L. The structure of 1 is determined by X-ray crystallography. 1 shows the typical emission of Nd(III) under the excitation of UV-vis light. The addition of anions and nitro explosives leads to a quenching of the luminescence, with high sensitivity of 1 to H2 and TNP. UV-vis spectral titration confirms the formation of interaction between H2 anion and 1, and the addition of H2 anion decreases the absorption of 1 at the excitation wavelength of lanthanide luminescence. TNP has the lowest LUMO energy level among the added explosives. The addition of TNP results in the competition of excitation energy between the explosive and 1. Further investigations focused on the construction and luminescent response properties of lanthanide-based complexes are in progress.
The datasets generated for this study can be found in the Cambridge Crystallographic Data Centre (CCDC number: 1971956).
XY conceived and designed the experiments. XL, YM, JL, and DS performed the experiments. MN and DS analyzed the data. All authors contributed to the article and approved the submitted version.
This work was supported by the National Natural Science Foundation of China (No. 21771141).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fchem.2020.536907/full#supplementary-material
Andruh, M. (2015). The exceptionally rich coordination chemistry generated by Schiff-base ligands derived from o-vanillin. Dalton Trans. 44, 16633–16653. doi: 10.1039/C5DT02661J
Feng, D., Zhao, Y., Wang, X., Fang, D., Tang, J., Fan, L., et al. (2019). Two novel metal–organic frameworks based on pyridyl-imidazole-carboxyl multifunctional ligand: selective CO2 capture and multiresponsive luminescence sensor. Dalton Trans. 48, 10892–10900. doi: 10.1039/C9DT01430F
Guha, S., and Saha, S. (2010). Fluoride ion sensing by an anion–π interaction. J. Am. Chem. Soc. 132, 17674–17677. doi: 10.1021/ja107382x
Guo, Z., Park, S., Yoon, J., and Shin, I. (2014). Recent progress in the development of near-infrared fluorescent probes for bioimaging applications. Chem. Soc. Rev. 43, 16–29. doi: 10.1039/C3CS60271K
Guo, Z., Xu, H., Su, S., Cai, J., Dang, S., Xiang, S., et al. (2011). A robust near infrared luminescent ytterbium metal–organic framework for sensing of small molecules. Chem. Commun. 47, 5551–5553. doi: 10.1039/c1cc10897b
Hu, J. Y., Ning, Y. Y., Meng, Y. S., Zhang, J., Wu, Z. Y., Gao, S., et al. (2017). Highly near-IR emissive ytterbium(III) complexes with unprecedented quantum yields. Chem. Sci. 8, 2702–2709. doi: 10.1039/C6SC05021B
Jankolovits, J., Andolina, C. M., Kampf, J. W., Raymond, K. N., and Pecoraro, V. L. (2011). Assembly of near-infrared luminescent lanthanide host(host-guest) complexes with a metallacrown sandwich motif. Angew Chem. Int. Ed. 50, 9660–9664. doi: 10.1002/anie.201103851
Jiang, D., Yang, X., Zheng, X., Bo, L., Zhu, T., Chen, H., et al. (2018). Self-assembly of luminescent 12-metal Zn–Ln planar nanoclusters with sensing properties towards nitro explosives. J. Mater. Chem. C 6, 8513–8521. doi: 10.1039/C8TC02426J
Lam, F., Xu, J. X., and Chan, K. S. (1996). Binucleating ligands: synthesis of acyclic achiral and chiral Schiff base-pyridine and Schiff base-phosphine ligands. J. Org. Chem. 61, 8414–8418. doi: 10.1021/jo961020f
Li, X., Xu, H., Kong, F., and Wang, R. (2013). A cationic metal–organic framework consisting of nanoscale cages: capture, separation, and luminescent probing of Cr2O through a single-crystal to single-crystal process. Angew Chem. Int. Ed. 52, 13769–13773. doi: 10.1002/anie.201307650
Liu, C. L., Zhou, L. P., Tripathy, D., and Sun, Q. F. (2017). Self-assembly of stable luminescent lanthanide supramolecular M4L6 cages with sensing properties toward nitroaromatics. Chem. Commun. 53, 2459–2462. doi: 10.1039/C7CC00189D
Liu, G. L., Qin, Y. J., Jing, L., Wei, G. Y., and Li, H. (2013). Two novel MOF-74 analogs exhibiting unique luminescent selectivity. Chem. Commun. 49, 1699–1701. doi: 10.1039/C2CC37140E
Liu, X., Yang, X. P., Ma, Y. N., Liu, J. N., Shi, D. L., and Schipper, D. (2020). Construction of a nano-rectangular Zn-Nd complex with near-infrared luminescent response towards metal ions. Chin. Chem. Lett. doi: 10.1016/j.cclet.2020.03.016. [Epub ahead of print].
Nagarkar, S., Desai, A., and Ghosh. (2014). A fluorescent metal–organic framework for highly selective detection of nitro explosives in the aqueous phase. Chem. Commun. 50, 8915–8918. doi: 10.1039/C4CC03053B
Nagarkar, S. S., Joarder, B., Chaudhari, A. K., Mukherjee, S., and Ghosh, S. K. (2013). Highly selective detection of nitro explosives by a luminescent metal–organic framework. Angew Chem. Int. Ed. 52, 2881–2885. doi: 10.1002/anie.201208885
Ning, Y. Y., Tang, J., Liu, Y. W., Jing, J., Sun, Y. S., and Zhang, J. L. (2018). Highly luminescent, biocompatible ytterbium (III) complexes as near-infrared fluorophores for living cell imaging. Chem. Sci. 9, 3742–3753. doi: 10.1039/C8SC00259B
Parker, D. (2000). Luminescent lanthanide sensors for pH, pO2 and selected anions. Coord. Chem. Rev. 205, 109–130. doi: 10.1016/S0010-8545(00)00241-1
Parker, D., Senanayake, P. K., and Williams, J. G. (1998). Luminescent sensors for pH, pO2, halide and hydroxide ions using phenanthridine as a photosensitiser in macrocyclic europium and terbium complexes. J. Chem. Soc. Perkin Trans. 2, 2129–2140. doi: 10.1039/a801270i
Peng, J. B., Zhang, Q. C., Kong, X., Zheng, Y. Z., Ren, Y. P., Long, L. S., et al. (2012). High-nuclearity 3d-4f clusters as enhanced magnetic coolers and molecular magnets. J. Am. Chem. Soc. 134, 3314–3317. doi: 10.1021/ja209752z
Qi, X., Jin, Y., Li, N., Wang, Z., Wang, K., and Zhang, Q. (2017). A luminescent heterometallic metal–organic framework for the naked-eye discrimination of nitroaromatic explosives. Chem. Commun. 53, 10318–10321. doi: 10.1039/C7CC05345B
Qin, J. H., Ma, B., Liu, X. F., Lu, H. L., Dong, X. Y., Zang, S. Q., et al. (2015). Aqueous- and vapor-phase detection of nitroaromatic explosives by a water-stable fluorescent microporous MOF directed by an ionic liquid. J. Mater. Chem. A 3, 12690–12697. doi: 10.1039/C5TA00322A
Sedaghat, S., Jeong, S., Zareei, A., Peana, S., Glassmaker, N., and Rahimi, R. (2019). Development of a nickel oxide/oxyhydroxide-modified printed carbon electrode as an all solid-state sensor for potentiometric phosphate detection. New J. Chem. 43, 18619–18628. doi: 10.1039/C9NJ04502C
Sheldrick, G. H. (1997). SHELX 97, A Software Package for the Solution and Refinement of X-Ray Data. Göttingen: University of Göttingen.
Shi, D. L., Yang, X. P., Chen, H. F., Jiang, D. M., Liu, J. N., Ma, Y. N., et al. (2019). Large Ln42 coordination nanorings: NIR luminescence sensing of metal ions and nitro explosives. Chem. Commun. 55, 13116–13119. doi: 10.1039/C9CC07430A
Shi, P. F., Hu, H. C., Zhang, Z. Y., Xiong, G., and Zhao, B. (2015). Heterometal–organic frameworks as highly sensitive and highly selective luminescent probes to detect I− ions in aqueous solution. Chem. Commun. 51, 3985–3988. doi: 10.1039/C4CC09081K
Sun, X., Wang, Y., and Lei, Y. (2015). Fluorescence based explosive detection: from mechanisms to sensory materials. Chem. Soc. Rev. 44, 8019–8061. doi: 10.1039/C5CS00496A
Wang, B., Zang, Z., Wang, H., Dou, W., Tang, X., Liu, W., et al. (2013). Multiple lanthanide helicate clusters and the effects of anions on their configuration. Angew Chem. Int. Ed. 52, 3756–3759. doi: 10.1002/anie.201210172
Wen, Q., Tang, Y., Li, K., and Zheng, Y. (2019). Structural and optical features of lanthanide species-derived functional hydrogel. Soft Mater. 17, 350–358. doi: 10.1080/1539445X.2019.1606013
Xiao, Y., Cui, Y., Zheng, Q., Xiang, S., Qian, G., and Chen, B. (2010). A microporous luminescent metal–organic framework for highly selective and sensitive sensing of Cu2+ in aqueous solution. Chem. Commun. 46, 5503–5505. doi: 10.1039/c0cc00148a
Xie, R., Tu, M. B., and Elder, T. (2016). Substituent effect of phenolic aldehyde inhibition on alcoholic fermentation by saccharomyces cerevisiae. Energy Fuels 30, 3078–3084. doi: 10.1021/acs.energyfuels.5b03034
Xu, H. B., Wen, H. M., Chen, Z. H., Li, J., Shi, L. X., and Chen, Z. N. (2010). Square structures and photophysical properties of Zn2Ln2 complexes (Ln = Nd, Eu, Sm, Er, Yb), Dalton Trans. 39, 1948–1953. doi: 10.1039/B919170D
Yang, J., Dai, Y., Zhu, X., Wang, Z., Li, Y., Zhuang, Q., et al. (2015). Metal–organic frameworks with inherent recognition sites for selective phosphate sensing through their coordination-induced fluorescence enhancement effect. J. Mater. Chem. A 3, 7445–7452. doi: 10.1039/C5TA00077G
Yang, X. P., Jones, R. A., and Huang, S. M. (2014). Luminescent 4f and d-4f polynuclear complexes and coordination polymers with flexible salen-type ligands. Coord. Chem. Rev. 273, 63–75. doi: 10.1016/j.ccr.2013.11.012
Keywords: lanthanide complex, Schiff base ligand, nanoscale structure, NIR luminescence, luminescent sensing
Citation: Liu X, Yang X, Ma Y, Liu J, Shi D, Niu M and Schipper D (2020) One Nanoscale Zn(II)-Nd(III) Complex With Schiff Base Ligand: NIR Luminescent Sensing of Anions and Nitro Explosives. Front. Chem. 8:536907. doi: 10.3389/fchem.2020.536907
Received: 02 March 2020; Accepted: 31 August 2020;
Published: 14 October 2020.
Edited by:
Sidney J. L. Ribeiro, São Paulo State University, BrazilReviewed by:
Marie-Joëlle Menu, Université Toulouse III Paul Sabatier, FranceCopyright © 2020 Liu, Yang, Ma, Liu, Shi, Niu and Schipper. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoping Yang, eHB5YW5nQHd6dS5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.