
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Chem. , 02 October 2020
Sec. Analytical Chemistry
Volume 8 - 2020 | https://doi.org/10.3389/fchem.2020.00800
This article is part of the Research Topic Functionalized Organic Fluorescent Materials for Biosensing and Bioimaging View all 6 articles
 Jie Zhang1
Jie Zhang1 Meng-Yu Chen1
Meng-Yu Chen1 Cui-Bing Bai1,2,3*
Cui-Bing Bai1,2,3* Rui Qiao1,2,3*
Rui Qiao1,2,3* Biao Wei1,3
Biao Wei1,3 Lin Zhang1,3
Lin Zhang1,3 Rui-Qian Li1,3
Rui-Qian Li1,3 Chang-Qing Qu4*
Chang-Qing Qu4*The fluorescent probe L, based on naphthalimide-modified coumarin, was designed, synthesized, and characterized, which could recognize Cu2+ from other cations selectively and sensitively in HEPES buffer (10 mM, Ph = 7. 4)/CH3CN (1:4, V/V). When the probe L interacted with Cu2+, the color and the fluorescent intensity changed obviously and it provided the naked-eye detection for Cu2+. The recognition mode between them was achieved by Job's plot, IR, MS, SEM, and 1HNMR. In addition, test strips made from L could still interact with Cu2+ in tap water effectively. The limit of detection (LOD) of L was 3.5 × 10−6 M. Additionally, the density functional theory (DFT) calculation method was used to analyze the action mechanism of L toward Cu2+. Importantly, the fluorescent probe L could demonstrate favorable selectivity toward Cu2+ in Caenorhabditis elegans. Thus, L was considered to have some potential for application in bioimaging.
As is known to all, copper ion (Cu2+) occupies an important place in a variety of fundamental physiological processes in organisms ranging from bacteria to mammals (Huang et al., 2014; Arjmand et al., 2018; Wang P. et al., 2018; Wang Y. et al., 2018; Zeng et al., 2019; Aydin et al., 2020; Wang Z. G. et al., 2020). However, Cu2+ can also lead to environmental pollution because it has been used widely in industrial and agricultural processes (Huang et al., 2014; Zhang et al., 2016; Gu et al., 2018; Wang et al., 2019; Zhu et al., 2020). So, it is urgent to develop some methods for recognition toward Cu2+. Owing to their advantage over other analytical methods which include atomic absorption spectrometer (ABS) and inductively coupled plasma mass spectrometry (ICP-MS), fluorescent probes have received more and more attention in the past few decades (Ge et al., 2013; Chen et al., 2017; Han et al., 2017; Wang et al., 2017; Zhou et al., 2017; Liu et al., 2018; Tian et al., 2019; Pipattanawarothai and Trakulsujaritchok, 2020). Therefore, various kinds of probes have been reported, such as rhodamines (Zhang et al., 2020), phenanthroline (Nawaz et al., 2018), anthracene (Shree et al., 2019), coumarin (Qi et al., 2018; Qu et al., 2019; Zhu et al., 2020), and BODIPY (Cetinkaya et al., 2019; Fang et al., 2019; Xia et al., 2019). In contrast with other derivatives, coumarin derivatives represent good fluorescence properties, excellent photostability, and easy preparation (Zhang et al., 2014; Hossain et al., 2017; Kumari et al., 2017). Consequently, many coumarin derivatives have been obtained to detect Cu2+ (Li et al., 2018; Roy et al., 2018; Wang Y. et al., 2018; Zhao et al., 2019; Joniak et al., 2020).
Formylcoumarins have been linked with aromatic amine through C=N to acquire the derivatives (Qin et al., 2016; He et al., 2019; Srivastava et al., 2019). Although few derivatives, which are connected by amide linkage, have been reported. It is obvious that the amide-modified derivatives have more potential sites to interact with Cu2+ by amide than by C=N, which might enhance their selectivity and sensitivity (Bai et al., 2019).
In this paper, a naphthalimide-modified probe L (Scheme 1) based on coumarin was designed and synthesized with amide. It is interesting that probe L could distinguish Cu2+ from other cations selectively and sensitively in HEPES buffer (10 mM, pH = 7.4)/CH3CN (1:4, V/V) observable by the naked-eye. In addition, test strips made from L could also detect Cu2+ successfully. Importantly, probe L could identify Cu2+ in Caenorhabditis elegans. From these data, probe L has potential applications in bioimaging.
1H NMR and 13C NMR spectra were both performed on a Bruker at 400 MHz using TMS as an internal standard (DMSO-d6 as the solvents). Infrared spectra were obtained on a Nicolete 5700 FT-IR spectrophotometer. Mass spectra were carried on with a Shimadzu LCMS-IT/TOF mass spectrometer. UV-Vis absorption spectra were studied on a Shimadzu UV-1601 spectrophotometer. Fluorescence spectrum was operated on a HORIBA FLUOROMAX-4-NIR spectrometer. Biological imaging was performed on a LEICA DM 2500. All reagents used were of analytical grade.
Compounds 1-3 were gained according to the previous work (Ma et al., 2004; Yu et al., 2011; Tanaka et al., 2019; Wang P. et al., 2020; Wang Z. G. et al., 2020). Then compound 3 (1 mmol, 304 mg) and 1.8-naphthalic anhydride (1 mmol, 198 mg) were dissolved in 25 mL DMF and heated to reflux for 6 h. After the reaction was complete, the reaction mixture was cooled to room temperature and poured into ice water to separate the solid. The solid was purified by silica gel chromatography (methylene chloride: methanol=30:1) to obtain probe L (Yield 75%). 1H NMR (400 MHz, DMSO-d6) δ 8.77 (t, J = 6.0 Hz, 1H), 8.52 (s, 1H), 8.44 (t, J = 8.3 Hz, 4H), 7.84 (t, J = 7.7 Hz, 2H), 7.60 (d, J = 9.0 Hz, 1H), 6.79 – 6.72 (m, 1H), 6.62 – 6.53 (m, 1H), 4.28 (t, J = 5.7 Hz, 2H), 3.66 (q, J = 5.9 Hz, 2H), 3.46 (q, J = 7.1 Hz, 4H), 1.32 – 1.20 (m, 1H), 1.12 (t, J = 7.0 Hz, 6H), 1.06 (s,1 H) (Supplementary Figure 1). 13C NMR (100 MHz, DMSO-d6) δ 164.12, 163.13, 161.75, 157.61, 152.81, 148.02, 134.64, 131.97, 131.76, 131.09, 127.96, 127.65, 122.71, 110.50, 109.91,107.99, 96.27, 44.78, 37.92, 12.77 (Supplementary Figure 2). HRMS (ESI) m/z: [L+H].+ Calcd for C28H26N3: 484.19; Found 484.15 (Supplementary Figure 3a).
Solutions of metal ions were prepared from their nitrates salts of K+, Fe2+, Ca2+, Na+, Ag+, Cu2+, Co2+, Mg2+, Cd2+, Ni2+, Ba2+, Pb2+, Al3+, Sr2+, Mn2+, Zn2+, Hg2+, Ce3+, Y3+, and Fe3+. The ligand concentration (L) was kept constantly at (1.0 × 10−5 M). The solution of the probe was prepared in HEPES buffer (10 mM, pH = 7.4)/CH3CN (1:4, V/V).
Physiological pH (e.g., in the human body) is between 7.35 and 7.45 (Lee et al., 2010), thus, pH 7.4 was used in the subsequent study, in which Cu2+ in adult C. elegans was detected. The effect of pH on the fluorescent signal was investigated (Supplementary Figure 10). When the solution of Cu2+ was added into the L solution, the maximum absorption peak shifted from 412 nm to 385 nm (Figure 1A). The solution color changed from faint yellow to colorless (Figure 1B). While other cations didn't cause any change. It was clear from the competitive experiment that other cations have little impact on the selectivity of L toward Cu2+ (Figure 2). According to UV-Vis spectroscopy, the L solution color change caused by Cu2+ could be observed directly by the naked-eye. So, the fluorescent probe L can demonstrate favorable selectivity toward Cu2+among other metals.
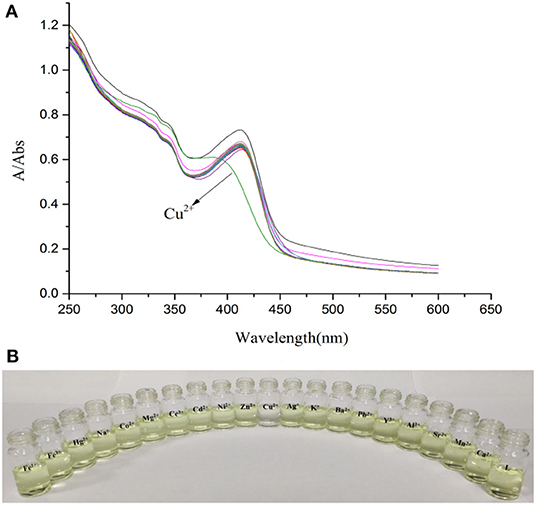
Figure 1. (A) Absorption spectra of L (1.0 × 10−5 M) in the presence of various metal ions K+, Na+, Ag+, Cu2+, Co2+, Ca2+, Cd2+, Mg2+, Ba2+, Pb2+, Sr2+, Fe2+, Ni2+, Zn2+, Mn2+, Hg2+, Al3+, Y3+, Ce3+, and Fe3+ (3.0 × 10−5 M) in HEPES buffer (10 mM, pH = 7.4)/CH3CN (1:4, V/V). (B) Photograph of L (1.0 × 10−5 M) in the presence of various metal ions (3.0 × 10−5 M) in HEPES buffer (10 mM, pH = 7.4)/CH3CN (1:4, V/V).
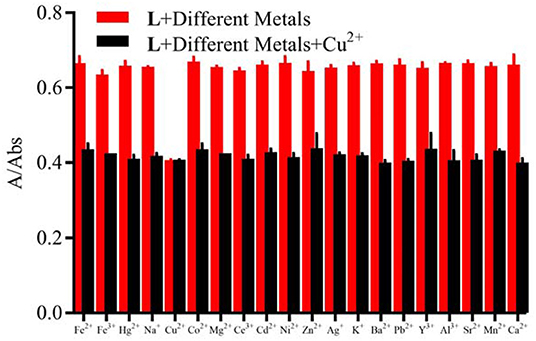
Figure 2. Competitive selectivity of L (1.0 × 10−5 M) toward Cu2+ (3.0 × 10−5 M) in the presence of other metal ions (3.0 × 10−5 M) in HEPES buffer (10 mM, pH = 7.4)/CH3CN (1:4, V/V), the absorbance at 412 nm.
When the probe L was excited by 412 nm, the fluorescent emission peak appeared at 465 nm. Interestingly, only Cu2+ caused the fluorescent intensity at 465 nm to reduce when the cation solution was added (Figure 3A). The fluorescent change induced by Cu2+ could also be observed easily by the naked-eye under a 365 nm UV lamp (Figure 3B). According to the competition experiment, other cations seldom interfered with the detection of L toward Cu2+ (Figure 4). Moreover, the limit of detection for L toward Cu2+ was calculated to be 3.5 × 10−6 M (Supplementary Figure 8). Based on the data above, it was concluded that L might recognize Cu2+ selectively and sensitively.
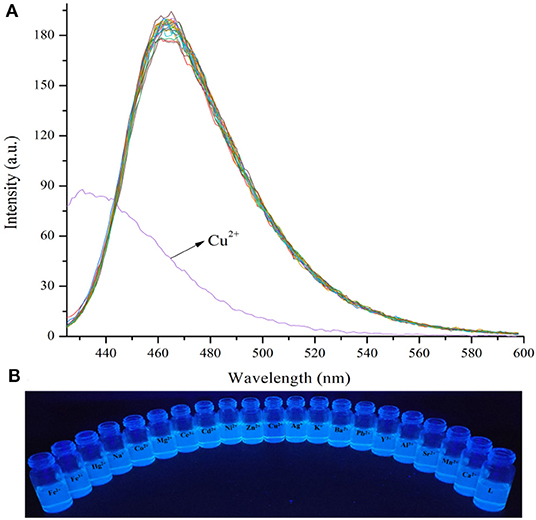
Figure 3. (A) Fluorescence spectra changes of L (1.0 × 10−5 M) in the presence of various metal ions (3.0 × 10−5 M) in HEPES buffer (10 mM, pH = 7.4)/CH3CN (1:4, V/V), λex =412 nm, detection from 425 to 600 nm. (B) Photograph of probe L (1.0 × 10−5 M) in the presence of various metal ions (3.0 × 10−5 M) in HEPES buffer (10 mM, pH = 7.4)/CH3CN (1:4, V/V) under a 365 nm UV lamp.
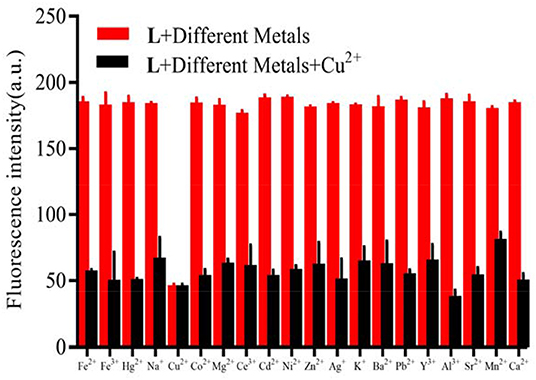
Figure 4. Competitive selectivity of L (1.0 × 10−5 M) toward Cu2+ in the presence of other metals (3.0 × 10−5 M) in HEPES buffer (10 mM, pH = 7.4)/CH3CN (1:4, V/V), λex = 412 nm, the fluorescent intensity at 465 nm.
In order to determine the stoichiometric ratio of L toward Cu2+, the molar method (Supplementary Figure 4) and the continuous variation method (Supplementary Figure 5) were both carried out. The results showed that the stoichiometric ratio was 1:1 between them. To our great joy, the result was supported by mass spectral analyses because the ion peak was detected at m/z 670.08 which was in accordance with [L+Cu2++] + (Supplementary Figure 3b). On the basis of the data, it was concluded that the stoichiometric ratio between them was 1:1 when L interacted with Cu2+. To study how Cu2+ changed the L aggregation morphology, a SEM experiment was performed. When L (1 equiv) combined with the Cu2+(2 equiv), it was discovered that the L morphology changed from the layer to the petal shape whose diameter was 1 um (Figure 5) which may be the result of the interaction between the fluorescent probe L and Cu2+.
To clarify the interaction mode between L and Cu2+, IR analyses and 1H NMR titration were conducted. From IR (Supplementary Figure 6), the absorption band at 1,704 cm−1 assigned to the C=O stretching vibration vanished when L (1 equiv) interacted with Cu2+ (2 equiv). The absorption band at 3,337 cm−1 assigned to the N-H stretching vibration also disappeared, were the absorption band at 1,537 cm−1 corresponding to the stretching vibration of C=N appeared. The amide group tautomerized to C=N once L associated with Cu2+. It is important that the conclusion from IR was in accordance with the 1H NMR titration. As the Cu2+ concentration increased, the chemical shift of N-H in the amide group at 8.50 disappeared by a degree (Supplementary Figure 7). From the above data, the interaction mode between L and Cu2+ was shown as (Figure 6).
To clarify the interaction mode between L and Cu2+, the orbital energy and spatial distribution levels of L and L-Cu2+ were gained with the DFT calculation (Figure 7). The electron density for L was mainly distributed over the coumarin groups in the highest occupied molecular orbital (HOMO), where the electron density for L-Cu2+ was focused on Cu2+ in the highest occupied molecular orbital (HOMO). The electron density was mainly located in the naphthalimide group in the lowest unoccupied molecular orbital (LUMO) of L and L-Cu2+. The energy gaps of the L and L-Cu2+ were calculated to be 2.9764 and 3.8984 eV, which were in accordance with the hypsochromic shift in the UV-Vis spectra after the Cu2+ solution was added into the L solution. The theoretical calculation results also confirmed the interaction mode between them.
To evaluate the practical application of L, test strips were made from L to detect Cu2+, in which the filer paper was soaked in the L solution (1 × 10−5 M) and dried in the air. After the test strips were immersed in the Cu2+ solution (1.0 × 10−5 M), the test strips color change was examined directly by the naked-eye under a 365 UV lamp (Figure 8). It meant that probe L could also recognize Cu2+ in the solid state as well. In addition, test strips were made from L to detect Cu2+ in tap water (Supplementary Figure 9). It is interesting that only the aqueous solution containing the Cu2+ faded and the fluorescence decreased which shows that probe L could potentially identify Cu2+ water pollution.
To explore the application of L in the biological system, the ability of probe L to sense Cu2+ in adult C. elegans was studied (Figure 9). Bright field and fluorescent images of the C. elegans nematodes are shown in Figures 9A,D. The nematodes cultured with L exhibited blue fluorescence (Figures 9B,E). The fluorescence reduced obviously after the nematodes were cultured with Cu2+ (Figures 9C,E). This result showed the applicability of probe L to in vivo studies.
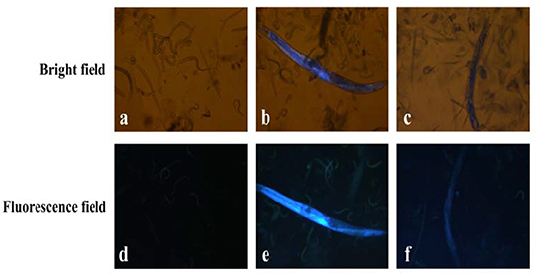
Figure 9. Bright field and fluorescent images of C. elegans: (a,d) C. elegans were incubated with agaragar for 30 min; (b,e) C. elegans were incubated with agaragar after pre-incubation with L (10 μM) for 30 min. (c,f) C. elegans were incubated with L (10 μM) for 30 min after pre-incubation with Cu2+ (10 μM) for 30 min.
In summary, probe L, based on naphthalimide-modified coumarin derivatives, was designed, synthesized, and characterized. Probe L showed good selectivity and high sensitivity toward Cu2+ while other metal ions did not cause interference. At the same time, the solution color change was observed directly by the naked-eye. The proposed interaction mode between them was confirmed by UV-Vis spectroscopy, fluorescence, Job's plot, 1H NMR titration, ESI-MS, and SEM. In addition, probe L has a good application prospect for detecting Cu2+ qualitatively. The LOD of L was 3.5 × 10−6 M. Additionally, a DFT calculation method was utilized to analyze the action mechanism of L toward Cu2+. Furthermore, the successful detection of Cu2+ in the living system using L also suggests its potential utilization in practical applications.
All datasets generated for this study are included in the article/Supplementary Material.
JZ and RQ designed the work and wrote the manuscript. JZ, M-YC, BW, LZ, and C-QQ carried out the experiments. R-QL performed the spectroscopic experiments. C-BB revised and edited the manuscript. All authors reviewed the manuscript and have agreed to its publication.
This work was supported by the National Natural Science Foundation of China (No. 21302019), the Anhui Provincial Natural Science Foundation (No. 1908085QB78), the Key Projects of Natural Science Research of Anhui Province Colleges and Universities (No. KJ2019ZD38), the Key Projects of Support Program for Outstanding Young Talents in Anhui Province Colleges and Universities (No. gxyqZD2016068), the Key Program for Young Talents of Fuyang Normal University (No. rcxm201902), the Anhui Province Undergraduate Training Programs for Innovation and Entrepreneurship (No. S201910371042), and the Horizontal Cooperation Project of Fuyang Municipal Government and Fuyang Normal University (Nos. XDHX201730 and XDHX201731). This work was also supported by the Open Project Grant of Key Laboratory of Photochemical Conversion and Optoelectronic Materials (PCOM202002), TIPC, Chinese Academy of Sciences.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fchem.2020.00800/full#supplementary-material
Arjmand, F., Afsan, Z., and Roisnel, T. (2018). Design synthesis and characterization of novel chromone based-copper (II) antitumor agents with N, N-donor ligands: comparative DNA/RNA binding profifile and cytotoxicity. RSC. Adv. 8, 37375–37390. doi: 10.1039/C8RA06722H
Aydin, Z., Yan, B., and Wei, Y. (2020). A novel near-infrared ratiometric fluorescent probe capable of copper(II) ion determination in living cells. Chem. Commun. 56, 6043–6046. doi: 10.1039/D0CC01481H
Bai, B. C., Fan, Y. H., Qiao, R., Wang, N. S., Wei, B., Meng, Q., et al. (2019). Synthesis of methionine methyl ester-modified coumarin as the fluorescent-colorimetric chemosensor for selective detection Cu2+ with application in molecular logic gate. Spectrochim. Acta. A. 216, 45–51. doi: 10.1016/j.saa.2019.03.016
Cetinkaya, Y., Yurt, M. N. Z., AvniOktem, H., and Yilmaz, M. D. (2019). A Monostyryl Boradiazaindacene (BODIPY)-based Lanthanide-free Colorimetric and Fluorogenic Probe for Sequential Sensing of Copper (II) Ions and Dipicolinic Acid as a Biomarker of Bacterial Endospores. J. Hazard. Mater. doi: 10.1016/j.jhazmat.2019.05.108
Chen, S., Kuang, Y., Zhang, P., Huang, Y., Wen, A., Zeng, X., et al. (2017). A dual-functional spectroscopic probe for simultaneous monitoring Cu2+ and Hg2+ ions by two different sensing nature based on novel fluorescent gold nanoclusters. Sens. Actuators B Chem. 253, 283–291, doi: 10.1016/j.snb.2017.06.140
Fang, T., Jiang, X. D., Sun, C., and Li, Q. (2019). BODIPY-based naked-eye fluorescent on-off probe with high selectivity for H2S based on thiolysis of dinitrophenyl ether. Sens. Actuators B Chem. 290, 551–557. doi: 10.1016/j.snb.2019.03.141
Ge, F., Ye, H., Luo, J. Z., et al. (2013). A new fluorescent and colorimetric chemosensor for Cu(II) based on rhodamine hydrazone and ferrocene unit. Sens. Actuators B Chem. 181, 215–220. doi: 10.1016/j.snb.2013.01.048
Gu, B., Huang, L., Xu, Z., Tan, Z., Meng, H., Yang, Z., et al. (2018). A reaction-based, colorimetric and near-infrared flfluorescent probe for Cu2+ and its applications, Sens. Actuators B Chem. 273, 118–125. doi: 10.1016/j.snb.2018.06.032
Han, X., Song, X., Yu, F., and Chen, L. (2017). A Ratiometric Near-Infrared Fluorescent Probe for Quantification and Evaluation of Selenocysteine-Protective Effects in Acute Inflammation. Adv. Funct. Mater. 27, 1700769. doi: 10.1002/adfm.201700769
He, G., Hua, X., Yang, N., Li, L., Xu, J., Yang, L., et al. (2019). Synthesis and application of a “turn on” fluorescent probe for glutathione based on a copper complex of coumarin hydrazide Schiff base derivative. Bioorg. Chem. 91, 103176. doi: 10.1016/j.bioorg.2019.103176
Hossain, M. S., Singh, K., Lakma, A., Pradhan, N. R., and Singh, K. A. (2017). A schiff base ligand of coumarin derivative as an ICT-Based fluorescence chemosensor for Al3+, Sens. Actuators B Chem. 239, 1109–1117. doi: 10.1016/j.snb.2016.08.093
Huang, J., Liu, M., Ma, X., et al. (2014). A highly selective turn-off fluorescent probe for Cu(II) based on a dansyl derivative and its application in living cell imaging. RSC. Adv. 4, 22964. doi: 10.1039/C4RA02050B
Joniak, J., Stankovičov,á, H., Filo, J., Gaplovská-Kyselá, K., Garaj, V., and Cigán, M. (2020). Small-molecule coumarin fluorescent pH probes for extremely acidic conditions. Sens. Actuators B Chem. 307, 127646. doi: 10.1016/j.snb.2019.127646
Kumari, C., Sain, D., Kumar, A., Debnath, S., Sahac, P., and Dey, S. (2017). Intracellular detection of hazardous Cd2+ through a fluorescence imaging technique by using a nontoxic coumarin based sensor. Dalton. Trans. 46, 2524–2531. doi: 10.1039/C6DT04833A
Lee, M. H., Giap, T. V., Kim, S. H., et al. (2010). A novel strategy to selectively detect Fe(III) in aqueous media driven by hydrolysis of a rhodamine 6G Schiff base. Chem. Commun. 46, 1407–1409. doi: 10.1039/B921526C
Li, H., Sun, X., Zheng, T., Xu, Z., Song, Y., and Gu, X. (2018). Coumarin-based multifunctional chemosensor for arginine/lysine and Cu2+/Al3+ ions and its Cu2+ complex as colorimetric and fluorescent sensor for biothiols. Sens. Actuators B Chem. 279, 400–409. doi: 10.1016/j.snb.2018.10.017
Liu, S., Liu, Y., Pan, H., Chen, H., and Li, H. (2018). Novel fluorescent probe bearing triarylimidazole and pyridine moieties for the rapid and naked-eye recognition of Cu2+. Tetrahedron Lett. 59, 108–112. doi: 10.1016/j.tetlet.2017.11.059
Ma, Y., Luo, W., Quinn, P. J., Liu, Z., and Hider, R. C. (2004). Design, Synthesis, Physicochemical Properties, and Evaluation of Novel Iron Chelators with Fluorescent Sensors. J. Med. Chem. 47, 6349–6362. doi: 10.1021/jm049751s
Nawaz, H., Tian, W., Zhang, J., Jia, R., Chen, Z., and Zhang, J. (2018). Cellulose-Based Sensor Containing Phenanthroline for the Highly Selective and Rapid Detection of Fe2+ Ions with Naked Eye and Fluorescent Dual Modes. ACS Appl. Mater. Inter. 10, 2114–2121. doi: 10.1021/acsami.7b17342
Pipattanawarothai, A., and Trakulsujaritchok, T. (2020). Hybrid polymeric chemosensor bearing rhodamine derivative prepared by sol-gel technique for selective detection of Fe3+ ion. Dyes Pigments. 173, 107946. doi: 10.1016/j.dyepig.2019.107946
Qi, S., Li, Q., Liu, W., Ren, H., Zhang, H., Wu, J., et al. (2018). Coumarin/fluorescein-fused fluorescent dyes for rapidly monitoring mitochondrial pH changes in living cells. Spectrochim. Acta. A. 204, 590–597. doi: 10.1016/j.saa.2018.06.095
Qin, J., Fan, L., and Yang, Z. (2016). A small-molecule and resumable two-photon fluorescent probe for Zn2+ based on a coumarin Schiff-base. Sens. Actuators B Chem. 228, 156–161. doi: 10.1016/j.snb.2016.01.031
Qu, P., Ma, X., Chen, W., Zhu, D., Bai, H., Wei, X., et al. (2019). A coumarin-based fluorescent probe for ratiometric detection of hydrazine and its application in living cells. Spectrochim. Acta. A. 210, 381–386. doi: 10.1016/j.saa.2018.11.007
Roy, D., Chakraborty, A., and Ghosh, R. (2018). Coumarin based colorimetric and fluorescence on-off chemosensor for F−, CN− and Cu2+ ions. Spectrochim. Acta. A. 191, 69–78. doi: 10.1016/j.saa.2017.09.071
Shree, G. J., Sivaraman, G., Siva, A., and Chellappa, D. (2019). Anthracene- and pyrene-bearing imidazoles as turn-on fluorescent chemosensor for aluminum ion in living cells. Dyes Pigments, 163, 204–212. doi: 10.1016/j.dyepig.2018.11.061
Srivastava, P., Verma, M., Sivakumar, S., and Patra, A. K. (2019). A smart FRET probe exhibiting a molecular keypad lock device based on rapid detection of nitric oxide mediated by Cu2+ ion. Sens. Actuators B Chem. 291, 478–484. doi: 10.1016/j.snb.2019.04.093
Tanaka, T., Shiraishi, M., Matsuda, A., and Mizuno, M. (2019). Efficient, solventless N-Boc protection of amines carried out at room temperature using sulfamic acid as recyclable catalyst. Tetrahedron Lett. 60, 151106.
Tian, Z. N., Wu, D. Q., Sun, X. J., Liu, T. T., and Xing, Z. Y. (2019). A Benzothiazole-Based Fluorescent Probe for Ratiometric Detection of Al3+and Its Application in Water Samples and Cell Imaging. Int. J. Mol. Sci. 20, 5993. doi: 10.3390/ijms20235993
Wang, P., Fu, J., Yao, K., Chang, Y., Xu, K., and Xu, Y. (2018). A novel quinoline-derived fluorescent “turn-on” probe for Cu2+ with highly selectivity and sensitivity and its application in cell imaging. Sens. Actuators B Chem. 273, 1070–1076. doi: 10.1016/j.snb.2018.07.028
Wang, P., Xue, S., and Yang, X. (2020). Highly selective and sensitive detection of hydrogen sulfide in aqueous medium and live cells using peptide-based bioprobe to mimic the binding sites of the ceruloplasmin for Cu(II) ions. Biosens. Bioelectron. 163, 112283. doi: 10.1016/j.bios.2020.112283
Wang, Y., Mao, P. D., Wu, W. N., Mao, X. J., Zhao, X. L., Xu, Z. Q., et al. (2017). A novel colorimetric and ratiometric fluorescent Cu2+ sensor based on hydrazone bearing1,8-naphthalimide and pyrrole moieties. Sens. Actuators B Chem. 251, 813–820. doi: 10.1016/j.snb.2017.05.134
Wang, Y., Wu, H., Wu, W. N., Li, S. J., Xu, Z. H., Xu, Z. Q., et al. (2018). An AIE active Schiff base bearing coumarin and pyrrole unit: Cu2+detection in either solution or aggregation states. Sens. Actuators B Chem. 260, 106–115. doi: 10.1016/j.snb.2017.12.201
Wang, Y., Zhang, L., and Chen, L. (2019). Glutathione peroxidase-activatable two-photon ratiometric fluorescent probe for redox mechanism research in aging and mercury exposure mice models. Anal. Chem. 92, 1997–2004. doi: 10.1021/acs.analchem.9b04381
Wang, Z. G., Wang, Y., Ding, X. J., Sun, Y. X., Liu, H. B., Xie, C. Z., et al. (2020). A highly selective colorimetric and fluorescent probe for quantitative detection of Cu2+/Co2+: The unique ON-OFF-ON fluorimetric detection strategy and applications in living cells/zebrafish. Spectrochim. Acta. A. 117, 117763. doi: 10.1016/j.saa.2019.117763
Xia, S., Fang, M., Wang, J., Bi, J., Mazi, W., Zhang, Y., et al. (2019). Near-infrared Fluorescent Probes with BODIPY Donors and Rhodamine and Merocyanine Acceptors for Ratiometric Determination of Lysosomal pH Variance. Sens. Actuators B Chem. 294, 1–13. doi: 10.1016/j.snb.2019.05.005
Yu, C., Chen, L., Zhang, J., Li, J., Liu, P., Wang, W., et al. (2011). “Off-On” based fluorescent chemosensor for Cu2+ in aqueous media and living cells. Talanta, 85, 1627–1633. doi: 10.1016/j.talanta.2011.06.057
Zeng, H. H., Zhi, Y. Z., Fang, L., Jie, D., Shu, Y. H., Guo, P. L., et al. (2019). Design and Synthesis of Vanadate-Based Ratiometric Fluorescence Probe for Sequential Recognition of Cu2+ and Biothiol. Analyst. 144, 7368–7377. doi: 10.1039/C9AN01518C
Zhang, B., Diao, Q., Ma, P., Liu, X., Song, D., and Wang, X. (2016). A sensitive fluorescent probe for Cu2+ based on rhodamine B derivatives and its application to drinking water examination and living cells imaging. Sens. Actuators B Chem. 225, 579–585. doi: 10.1016/j.snb.2015.11.069
Zhang, M., Shen, C., Jia, T., Qiu, J., Zhu, H., and Gao, Y. (2020). One-step synthesis of rhodamine-based Fe3+ fluorescent probes via Mannich reaction and its application in living cell imaging. Spectrochim. Acta. A. 231, 118105. doi: 10.1016/j.saa.2020.118105
Zhang, Y. G., Shi, Z. H., Yang, L. Z., Tang, X. L., An, Y. Q., Ju, Z. H., et al. (2014). A facile fluorescent probe based on coumarin-derived Schiff base for Al3+ in aqueous media. Inorg. Chem. Commun. 39, 86–89. doi: 10.1016/j.inoche.2013.10.035
Zhao, C., Chen, J., Cao, D., Wang, J., and Ma, W. (2019). Novel coumarin-based containing denrons selective fluorescent chemosesor for sequential recognition of Cu2+ and PPi. Tetrahedron Lett. 75, 1997–2003. doi: 10.1016/j.tet.2019.02.024
Zhou, F., Leng, T. H., and Liu, Y. J. (2017). Water-soluble rhodamine-based chemosensor for Fe3+, with high sensitivity, selectivity and anti-interference capacity and its imaging application in living cells. Dyes Pigments. 142, 429–436. doi: 10.1016/j.dyepig.2017.03.057
Keywords: coumarin, fluorescent probe, Cu2+, test strips, bioimaging
Citation: Zhang J, Chen M-Y, Bai C-B, Qiao R, Wei B, Zhang L, Li R-Q and Qu C-Q (2020) A Coumarin-Based Fluorescent Probe for Ratiometric Detection of Cu2+ and Its Application in Bioimaging. Front. Chem. 8:800. doi: 10.3389/fchem.2020.00800
Received: 22 June 2020; Accepted: 30 July 2020;
Published: 02 October 2020.
Edited by:
Ruilong Sheng, University of Madeira, PortugalReviewed by:
Mingming Yu, Zhengzhou University, ChinaCopyright © 2020 Zhang, Chen, Bai, Qiao, Wei, Zhang, Li and Qu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cui-Bing Bai, YmFpY3VpYmluZ0BmeW51LmVkdS5jbg==; Rui Qiao, cWlhb3J1aUBmeW51LmVkdS5jbg==; Chang-Qing Qu, cXVjcTUxOEAxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.