- 1College of Pharmacy, Gachon University, Incheon, South Korea
- 2College of Pharmacy, Yonsei University, Incheon, South Korea
- 3Department of Pharmacy, College of Pharmacy and Institute of Pharmaceutical Sciences, CHA University, Pocheon-si, South Korea
Arylnaphthalene lignan lactones belong to a class of natural lignans, and more than 60 analogs have been isolated. Their pharmacological activities as well as unique structural features have attracted considerable attention from medicinal and synthetic chemists. Since the first synthesis in 1895, many synthetic methodologies with ionic or pericyclic reaction mechanisms have been reported. Transition metal catalysts sometimes provide exceptional synthetic versatility for the syntheses of natural compounds. Recently, transition metal-mediated methodologies were investigated for the construction of basic scaffolds of arylnaphthalene lignan lactones. Five kinds of transition metal catalysts containing gold, manganese, nickel, palladium, and silver have been explored. Most of the metal catalysts successfully created arylnaphthalene lactones by intermolecular or intramolecular annulative cyclization. In this review, all reports of transition metal-mediated annulative construction of arylnaphthalene lignan lactones were compiled, and synthetic approaches, mechanistic aspects, and successful applications were discussed.
Introduction
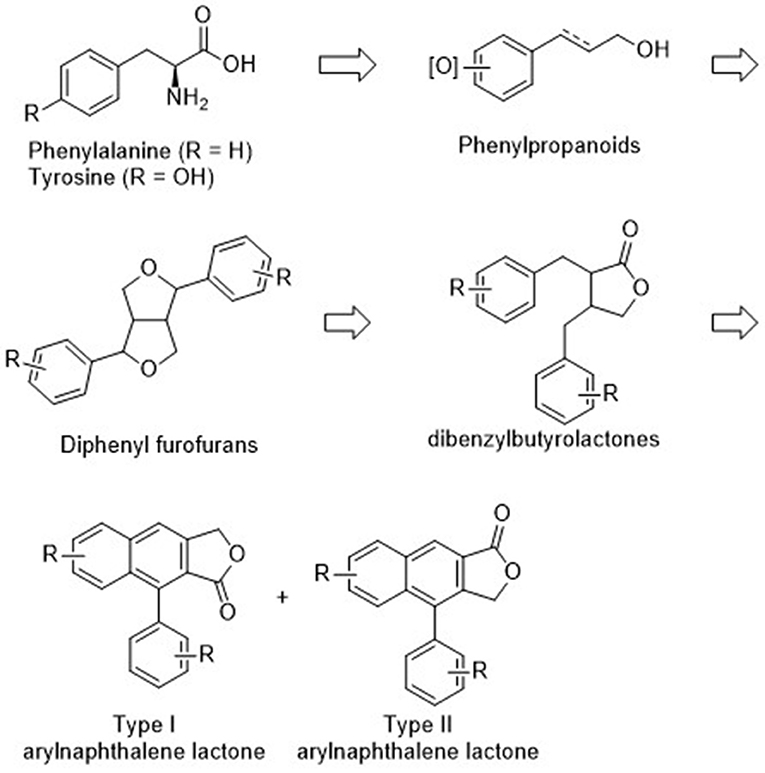
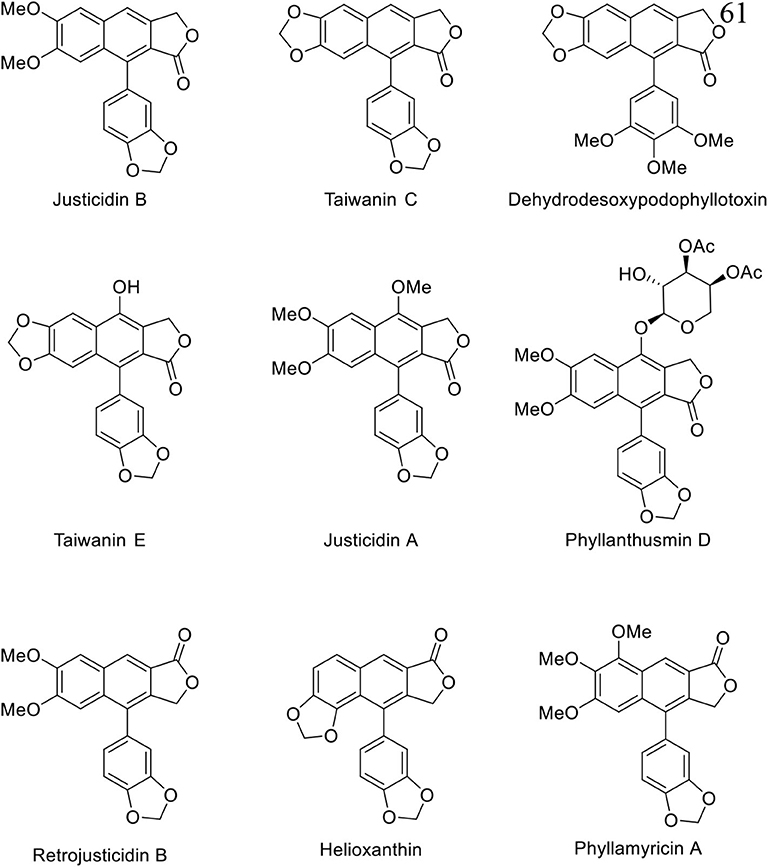
Natural arylnaphthalene lignan lactones are classified as lignans and isolated from a variety of dietary or medicinal plants, such as Phyllanthus, Justicia, Hapllophyllum, and Cleistanthus (Anjaneyulu et al., 1981; Batsuren et al., 1981; Khalid and Waterman, 1981; Ulubelen, 1985; Lin et al., 1995). They show broad pharmacological activities, including cytotoxic (Day et al., 2002; Yu et al., 2010; He et al., 2012; Deng et al., 2014; Luo et al., 2014; Ren et al., 2014, 2019; Won et al., 2015; Woo et al., 2017; Woodard et al., 2018; Yi et al., 2018; Young et al., 2018), antiplatelet (Chen et al., 1996; Weng et al., 2004), neuroprotective (Gu et al., 2016), antiviral (Asano et al., 1996; Cow et al., 2000), antifungal (Windayani et al., 2014), and anti-HIV (Chang et al., 1995; Zhang et al., 2017) activities. From the biosynthetic viewpoint, oxygenated phenylpropanoids, which are synthesized from phenylalanine or tyrosine, are condensed to bicyclic diphenyl furofurans. Reductive ring opening and oxidative cyclization delivers dibenzylbutyrolactones and final intramolecular Friedel–Crafts type cyclization produced arylnaphthalene lignan lactones (Teponno et al., 2016). Structurally, these compounds can be divided into Type I and Type II arylnaphthalene lignan lactones based on the position of lactones and phenyl groups (Figure 1). Thus far, more than 60 arylnaphthalene congeners and glycosylated products are reported. Diverse and significant pharmacological activities make them more attractive, and representative natural examples are listed in Figure 2.
Since the report of early efforts on the synthesis of arylnaphthalene lignan lactone in 1895 by Michael and Bucher (1895) and in 1910 by Bucher (1910) using the thermal cyclative condensation reaction of arylpropiolic acids, many synthetic methodologies for arylnaphthalene lignan lactones and their applications for natural arylnaphthalene lignan lactones have been investigated. Representative synthetic methodologies include intramolecular Diels–Alder type ring formation of arylpropiolic anhydride by Stevenson's group (Brown and Stevenson, 1964, 1965; Maclean and Stevenson, 1966; Holmes and Stevenson, 1970, 1971; Block and Stevenson, 1971, 1973; Stevenson and Block, 1971; Stevenson and Holmes, 1971; Stevenson and Weber, 1989, 1991; Anastas and Stevenson, 1991; Park et al., 2014), intermolecular Diels–Alder reaction of isobenzofurans and dimethyl acetylenedicarboxylate (De Silva et al., 1980; Plaumann et al., 1980), sequential Blaise reaction-intramolecular [4 + 2] reaction of 2-alkynylbenzonitriles (He et al., 2014), and Garratt–Braverman cyclization of substituted bis-propargyl ethers (Mondal et al., 2011, 2012). Photo-assisted cyclization methods provide arylnaphthalene lignan lactones efficiently (Block and Stevenson, 1971, 1973; Arnold et al., 1973; Yamamoto et al., 2015). Tandem conjugate addition-aldol reaction protocol (Ogiku et al., 1990; Kamal et al., 1994), benzoin condensation-thermal cyclization (Hayat et al., 2015), and electrophilic aromatic substitution protocols (González et al., 1978; Ogiku et al., 1995) have also been reported. Recently, synthetic approaches and biological activities of arylnaphthalene lignan lactones were reviewed by Zhao et al. (2018).
Transition metals have proven to be very useful in modern organic synthesis. The transition metal catalyzed reaction is particularly important for the formation of carbon–carbon bonds, and enables formation of bonds that are not possible with conventional methods. It also allows the formation of complex structures in a single reaction. In this review, all the research articles in which transition metals were utilized for the synthesis of arylnaphthalene lignan lactones are comprehensively discussed and the main advantages in each method are elucidated. There are five transition metals involved in the synthesis of arylnaphthalene lignan lactones, and each one is discussed in terms of the reaction mechanism and its applications for synthesis of arylnaphthalene lignan lactones.
Transition Metal-Mediated Synthesis of Arylnaphthalene Lignan Lactones
Gold-Catalyzed Cyclization
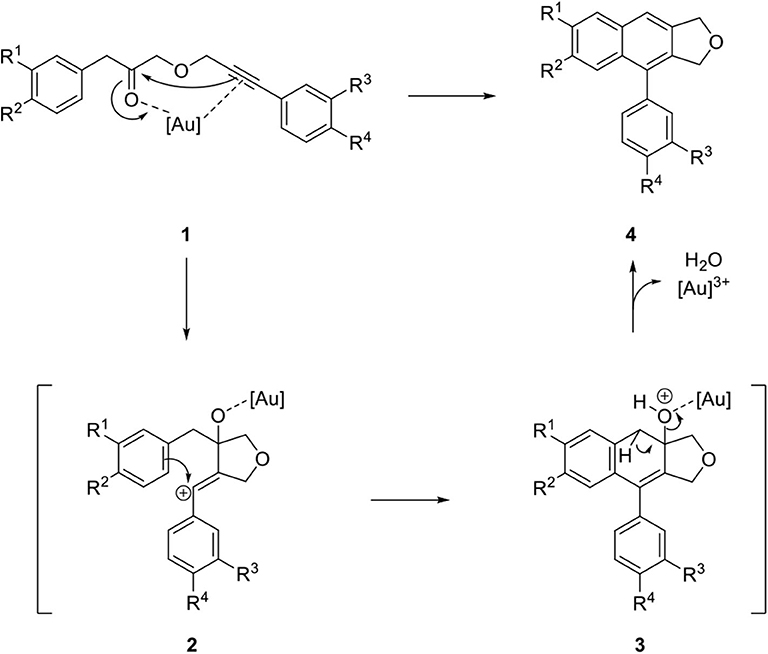
Recently, extensive studies have been devoted to gold-catalyzed reactions in organic synthesis (Hashmi, 2007; Li et al., 2008). Balamurugan's group focused on the intramolecular cyclization of alkynyl ester 1 by an Au catalyst in combination with AgSbF6 for the synthesis of an arylnaphthalene lignan lactone lignan scaffold (Gudla and Balamurugan, 2011). Catalysis of the cyclization begins with coordination of the distant carbonyl and alkyne group of 1 to a proximal position by the Au catalyst. Vinyl cation 2 was produced by subsequent attachment of the alkyne on the carbonyl group. Arylnaphthalene lignan lactone precursor 4 was synthesized by the removal of water and Au catalyst from 3 that was obtained from the electrophilic benzannulation reaction of 2 (Scheme 1).
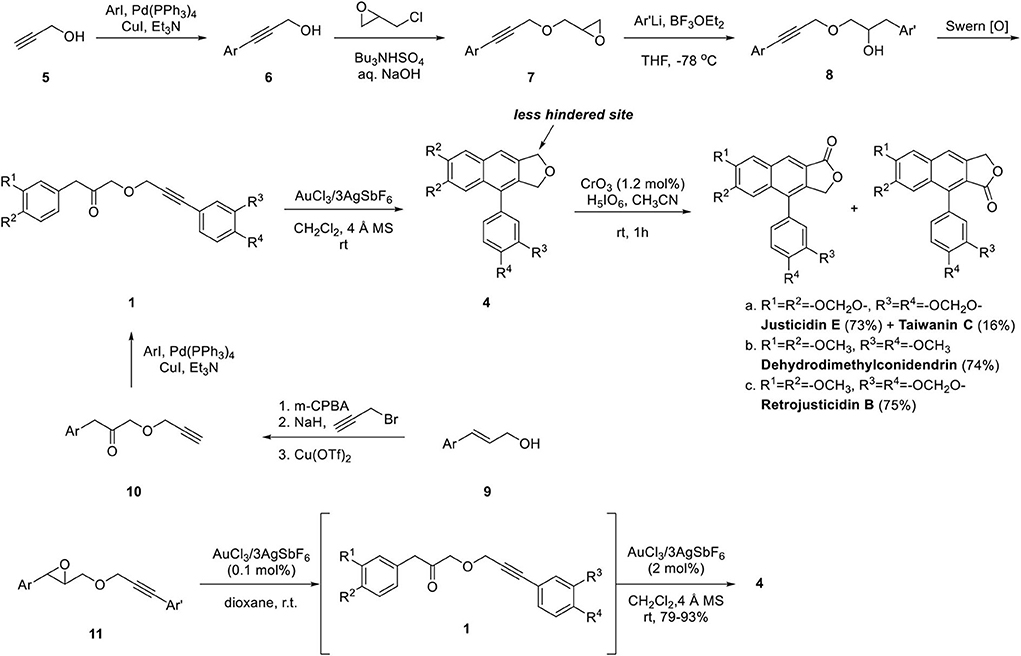
Application of this methodology to the synthesis of natural arylnaphthalene lignan lactone is summarized in Scheme 2. The key intermediate 1 was obtained from propargyl alcohol in four steps. Sonogashira coupling of propyn-1-ol 5 with substituted aryl iodide provided 6 and 7 was obtained by the substitution reaction. Boron trifluoride-mediated epoxide opening provided 8 and 1 was obtained by Swern oxidation. The ketone 1 can also be obtained by epoxidation of cinnamyl alcohol 9, propargylation, Cu(OTf)2-mediated Meinwald rearrangement and Sonogashira coupling with substituted aryl iodide albeit in low yield. The Au-catalyzed cyclization of 1a-1c was achieved in yields of 84, 68, and 73%, respectively. Regiocontrolled oxidation of 4 by CrO3/H5IO6 in acetonitrile (Yamazaki, 1999) afforded 4.5:1 mixture of type II and isomeric type I arylnaphthalene lignan lactone. The advantage of this synthetic approach is the highly mild reaction condition performed at room temperature and easy derivatization with late-stage introduction of the aryl group from intermediate 10. Two years later, Balamurugan's group developed the one-pot sequential Meinwald rearrangement of 11 and cyclization of ketone 1 to provide 4 in yields of 79–93% (Gudla and Balamurugan, 2013).
Manganese-Catalyzed Cyclization
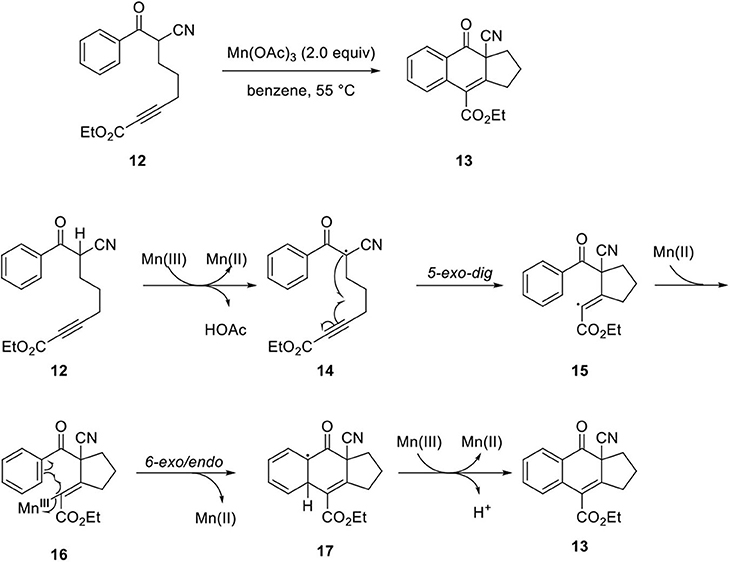
Shia's group studied Mn(OAc)3-mediated cyclization to synthesize arylnaphthalene lignan lactone (Wong et al., 2014; Kao et al., 2015). Initially, they investigated the feasibility of the oxidative cyclization of model system 12. The reaction proceeded under mild condition compared to the dehydro-Diels–Alder reaction, which requires high reaction temperatures of 160–300°C. The mild reaction conditions allow diverse functional groups, including Trimethylsilyl (TMS), ester, ketone, amide, phosphonate, and halogen. The reaction mechanism was proposed as follows. Abstraction of α-H in 12 produced Mn(III) enolate, and electron transfer generated Mn(II) and radical species 14. Then, the radical was added to the alkyne by 5-exo-dig type cyclization to provide 15. Next, the vinyl radical reacted with Mn(II) to form Mn(III)-vinyl complex 16, and naphthalene core 13 can be constructed by vinyl radical addition to benzene by 6-exo/endo type cyclization, and another electron transfer from 17 to Mn(III) ultimately generates an aromatic ring with loss of H+ (Scheme 3).
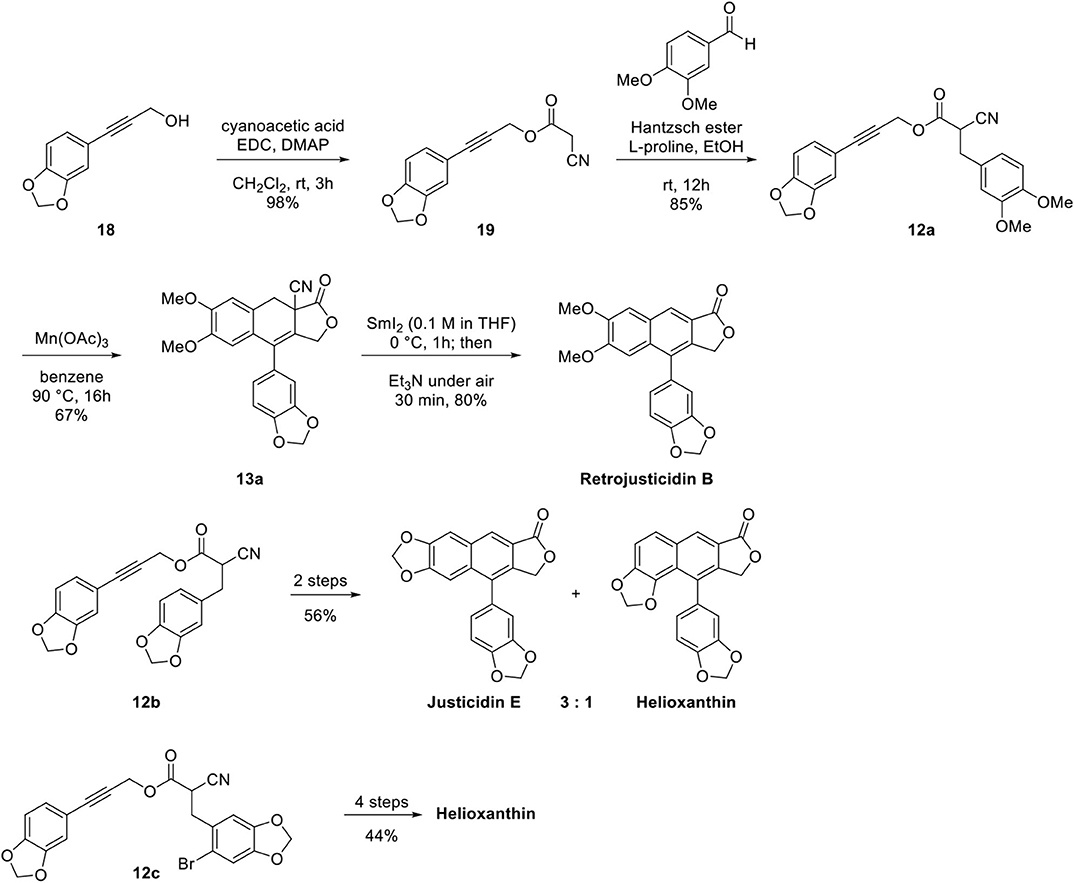
With these successful results in hand, they turned their attention to the natural products synthesis as shown in Scheme 4. Cyclization precursor 12a was synthesized by esterification of substituted phenyl propargyl alcohol 18 with cyanoacetic acid, one-pot Knoevenagel condensation with benzaldehyde, and Hantzsch ester mediated reduction. Mn(OAc)3-catalyzed cyclization provided inseparable 3:1 mixtures of 13a and its regioisomer (67:22%). Samarium iodide mediated reductive decyanation and air oxidation afforded retrojusticidin B at 80% yield. Synthesis of justicidin E and helioxanthin was also achieved from 13b following the same procedure affording a 59:20% mixture. Regioselective synthesis of helioxanthin was accomplished from 13c by introducing Br in the 2-position of piperonal to minimize regioisomer formation. The main advantage of this reaction may be functional group tolerance; however, the regioselectivity is not satisfactory unless Br is introduced in the 2-position to inhibit adverse cyclization, where Mn(OAc)3 is used in stoichiometric amount (2.0 equivalents).
Nickel-Catalyzed Cyclization
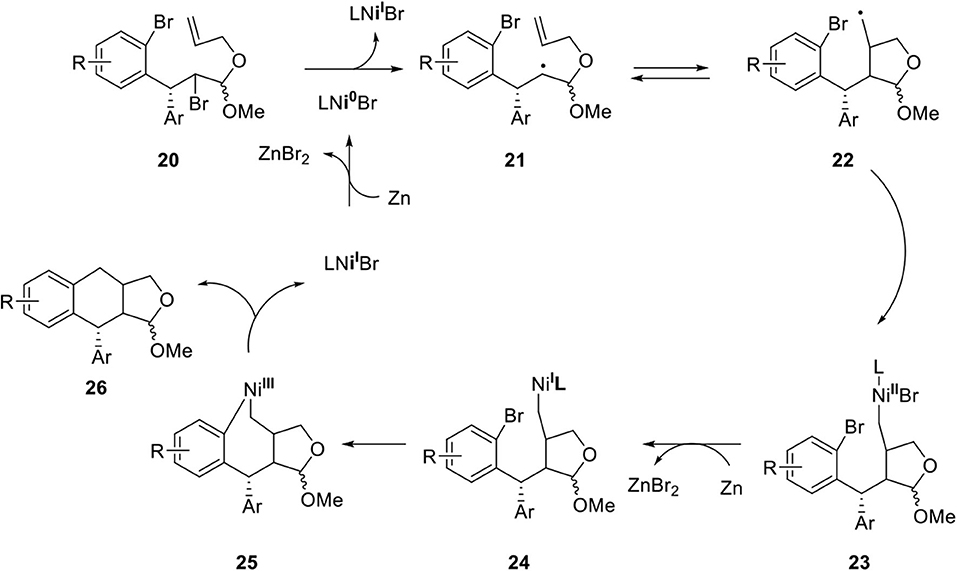
Peng's group developed a nickel-catalyzed cyclization to afford dihydronaphthalene and tetrahydronaphthalene in a diastereodivergent manner (Xiao et al., 2018a). Following on, they investigated the construction of an arylnaphthalene core for the synthesis of dehydrodesoxypodophyllotoxin (Xiao et al., 2018c). Key reaction is summarized in Scheme 5 although detailed stereochemical outcomes are not indicated here (Xiao et al., 2018b). Ni(I) is reduced to Ni(0) by Zinc(0) metal and the single electron transfer process produced alkyl radical 21 from 20. The secondary radical of 21 was added to the alkene in 5-exo-trig type producing 22 and then, the primary radical formed the Ni(III) species 2 following the known NiI-NiIII redox process via 23 and 24. Finally, reductive elimination provided 26 and regenerated the Ni(I) species.
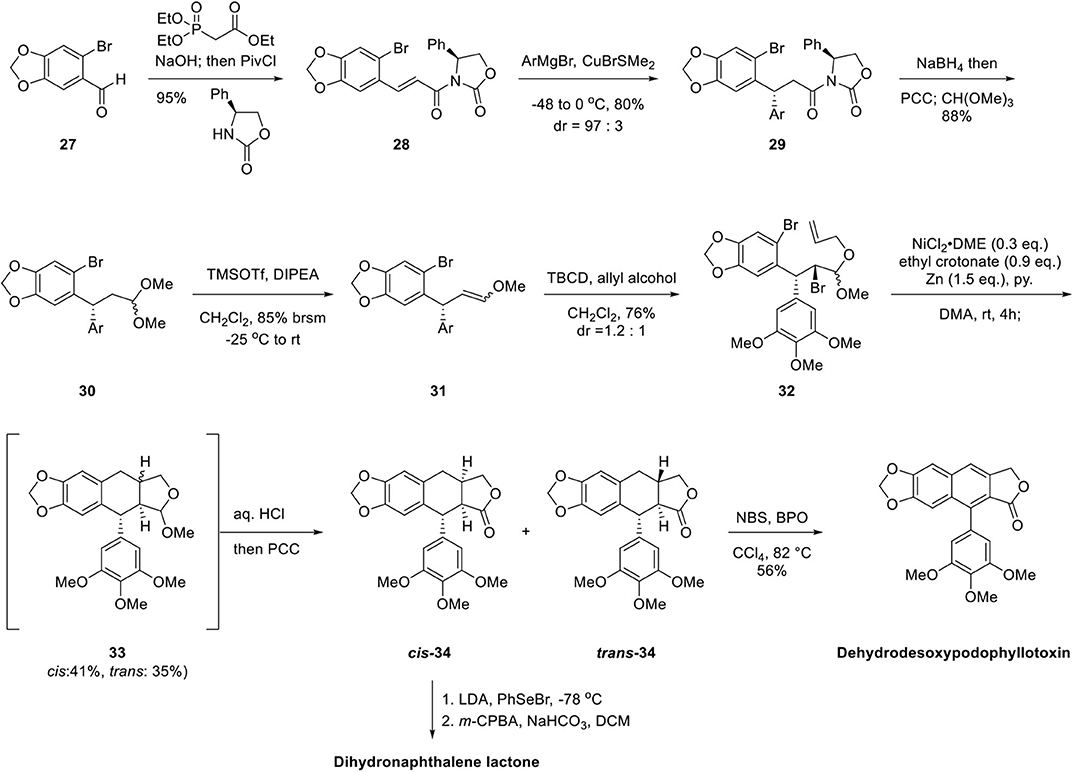
The synthesis of arylnaphthalene lignan lactones begins with oxazolidinone imide formation, as shown in Scheme 6. Oxazolidinone imide 28 was obtained by the Horner–Emmons reaction of 6-bromopiperonal 27 with triethyl phosphonoacetate, saponification, and imide formation by reaction of in situ generated pivaloyl anhydride and oxazolidinone. Gilman reagent was added to the β-position of enone diasteroselectively at a ratio of 97:3 affording 29 and subsequent reduction of the imide by NaBH4, oxidation with Pyridinium ChloroChromate (PCC) and acetal formation afforded 30. Enol ether 31 was generated in the presence of TMSOTf as Lewis acid. 32 was obtained as a 1.2:1 mixture by bromination with 2,4,4,6-tetrabromo-2,5-cyclohexadienone (TBCD) (Kato et al., 1976, 1984) and concomitant mixed acetal formation with allyl alcohol. Ni-catalyzed reductive cyclization afforded tetrahydronaphthalene lactol ether 33 with cis and trans mixture of 41 and 35% yield, respectively. Then, acetal 33 was hydrolyzed and oxidized to lactone by PCC to produce cis-34 and trans-34 in 68 and 62% yield, respectively. Dehydrodesoxypodophyllotoxin was obtained by radical halogenation, elimination reaction, and further oxidation from trans-34. Dihydronaphthalene lactone can be obtained from cis-34 by phenylselenylation at −78°C, m-CPBA oxidation, and selenoxide elimination. This Ni-mediated reductive cyclization is advantageous in terms of diastereodivergent synthesis for tetrahydronaphthalene containing diversity-oriented library synthesis.
Palladium-Mediated Cyclization
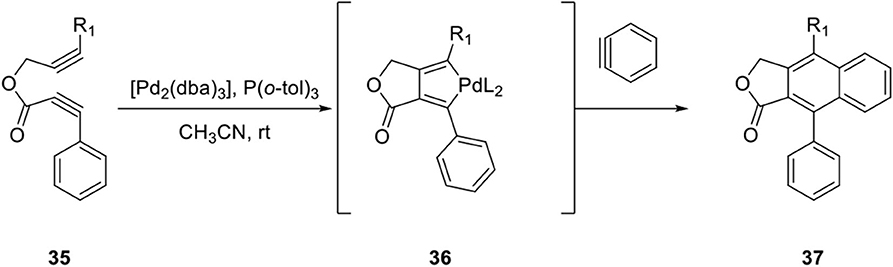
Pd-catalyzed copolymerization of diynes and arynes is well-known for the construction of an aromatic ring depending on the metal species (Peña et al., 1998). In continuation of arylnaphthalene lignan synthesis efforts (Mori et al., 1999; Sato et al., 1999), Mori et al. developed a new method for construction of the naphthalene ring by a Pd-catalyzed cocyclization of diyne esters and arynes (Sato et al., 2004). A plausible reaction mechanism can be explained by the formation of palladacycle intermediate 36 from diyne ester 35 and further reaction with benzyne to provide arylnaphthalene lignan lactone 37 (Peña et al., 1998; Scheme 7).
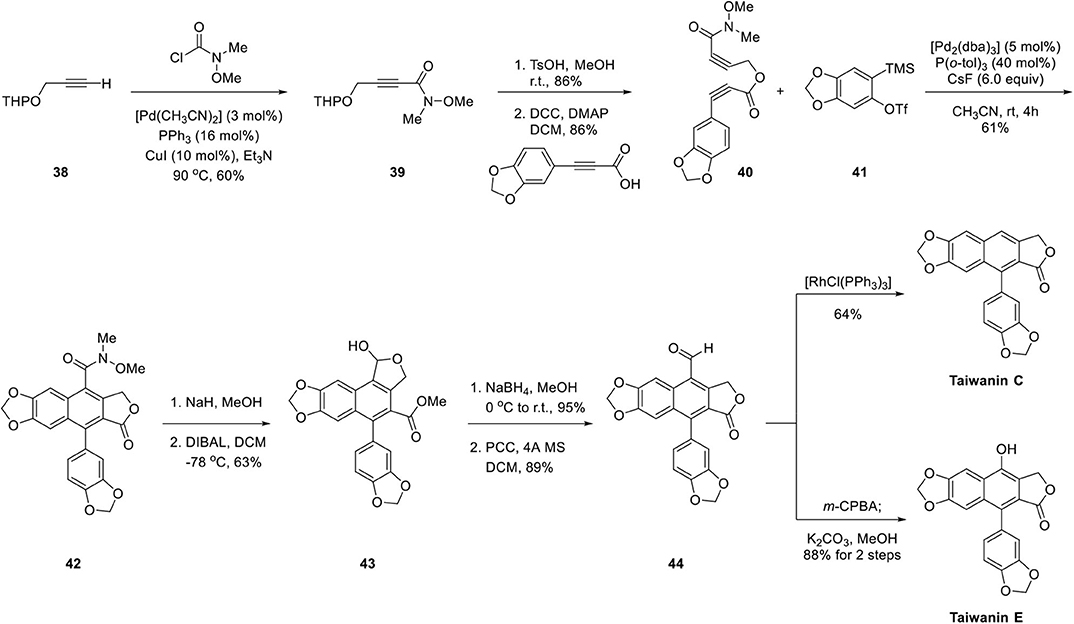
As depicted in Scheme 8, synthesis of diyne ester 40 was commenced with Pd-catalyzed coupling of N-methoxy-N-methylcarbamoyl chloride with protected propargyl alcohol 38 (Murakami et al., 1998). After deprotection of 39 in the acidic condition, the resulting alcohol was coupled with 3-[benzo(d)(1,3)dioxol-5-yl]propiolic acid, which was prepared by sequential Corey–Fuchs olefination, carboxylation under n-BuLi, and methyl chloroformate, and hydrolysis of methyl ester to afford diyne ester 40. Aryne precursor 41 was synthesized by silylation of 2-bromophenol, lithiation at low temperature with silyl group migration, and triflation of in situ generated phenol. Arylnaphthalene lignan lactone 42 was obtained with diyne ester 40 and aryne precursor 41 in the presence of CsF and Pd catalyst in 61% yield with only 5 mol% loading of Pd catalyst. However, the functional group interconversion of Weinreb amide of 42 was difficult compared to the ease of core ring formation. Direct reduction of amide with LiAl(OtBu)3H (Paris et al., 1998) and L-Selectride with MeOTf (Tsay et al., 1990) were attempted but were unsuccessful. Finally, it was converted to aldehyde with an additional four steps. Lactol 43 was obtained by methanolysis of lactone 42 and concomitant transesterification and partial reduction with DIBAL at low temperature. Reduction of lactol 43 with NaBH4 afforded diol, which underwent transesterification to provide primary alcohol and aldehyde 44 was obtained upon PCC oxidation. Then, the aldehyde was converted to taiwanin C by the Tsuji–Wilkinson decarbonylation reaction with rhodium catalyst (Tsuji and Ohno, 1965). Taiwanin E was obtained by Baeyer–Villiger oxidation and hydrolysis of the ester.
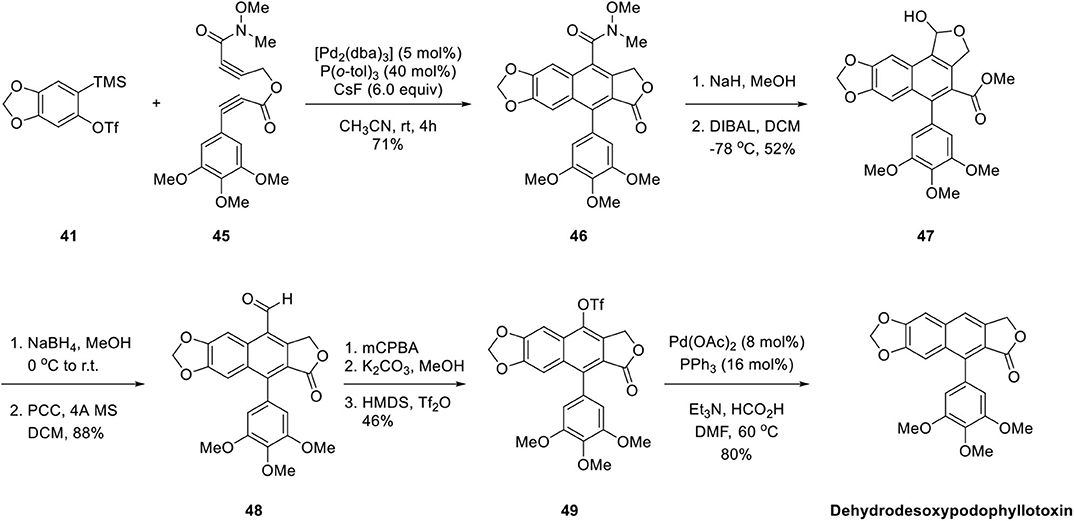
In 2007, Sato et al. (2007) applied the aforementioned methodology for the synthesis of arylnaphthalene lignan lactone. As shown in Scheme 9, Pd-catalyzed copolymerization of diyne ester 45 and aryne precursor 41 was also successful despite a multistep of reactions for conversion of Weinreb amide 46 to lactol 47 and aldehyde 48. The aldehyde group of 48 was converted to O-triflate 49 by a three-step sequence of Baeyer–Villiger oxidation, hydrolysis, and triflation of the resulting phenolic hydroxyl group. Finally, the triflate was eliminated by Pd-catalyzed hydrogenation to give arylnaphthalene lignan lactone. The desired dehydrodesoxypodophyllotoxin was synthesized from 46 in low yields of 17% through eight steps. In these two syntheses, constructions of the core ring were highly efficient and regioselective. Further manipulation of the functional groups successfully delivered natural arylnaphthalene lactone products.
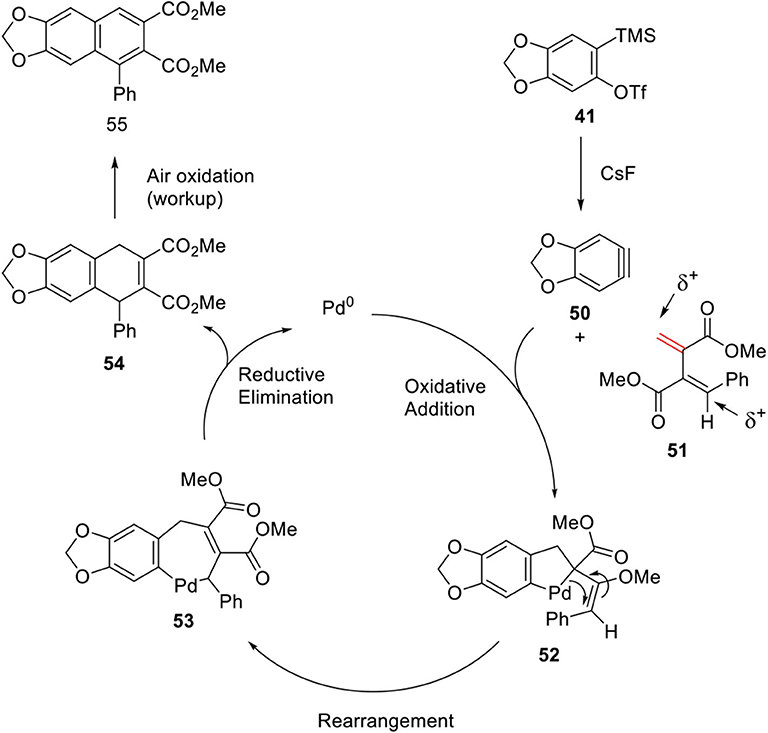
In 2013, Patel and Argade (2013) further developed Pd-catalyzed [2 + 2 + 2] cocyclization of aryne 50 and unsymmetrical conjugated diene 51 using palladium catalyst with N-heterocyclic carbene ligand. Initial trial of aryne 50 and diene 51 in the absence of catalyst resulted in only [2 + 2] cycloaddition adduct in which the less substituted olefin of 51 reacted with aryne 50. It was assumed that the desired [2 + 2 + 2] cycloadduct was not generated because of two electro-positive carbons in diene. Therefore, Argade's group systematically studied metal-mediated [2 + 2 + 2] cocyclization to construct a 6-membered ring. The reaction was ultimately optimized to 62% yield with Pd2(dba)3 and IMes.HCl as the ligands. The mechanistic pathway involves oxidative addition of Pd to aryne 50 and the less hindered alkene of 51 to form a five-membered palladacycle intermediate 52, and insertion of more substituted bonds to form a seven-membered palladacycle 53 via rearrangement. Finally, 55 was obtained by reductive elimination of 53 and spontaneous air oxidation of 54 during workup (Scheme 10).
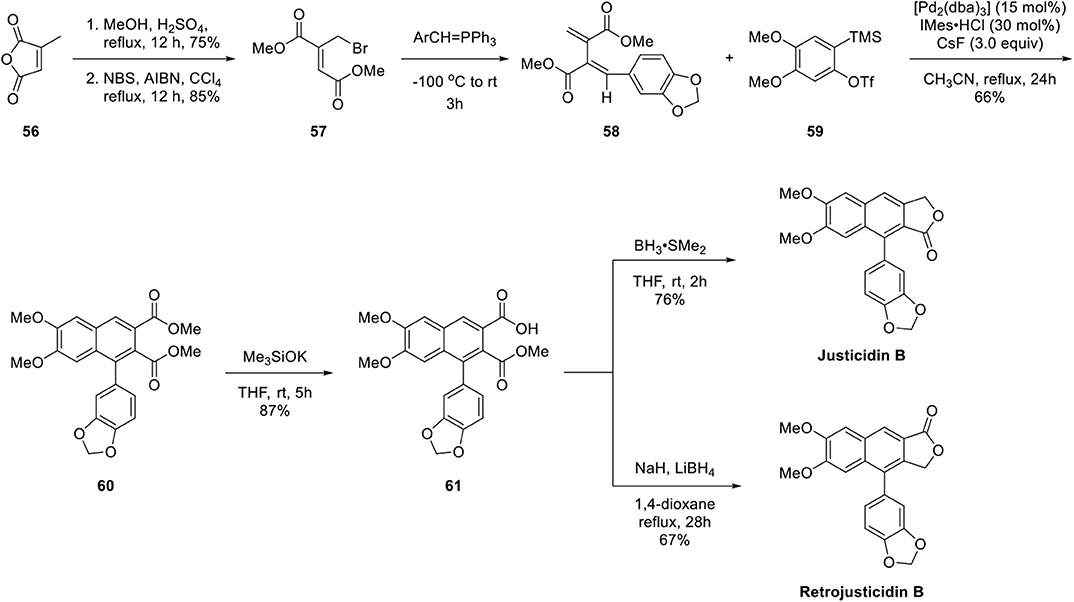
By applying this methodology, the authors successfully achieved total synthesis of Type I and Type II arylnaphthalene lignan lactones, justicidin B and retrojusticidin B, respectively, as presented in Scheme 11. The synthesis begins with methanolysis of citraconic anhydride 56 in acidic condition and then allylic bromination to afford 57 (Kar and Argade, 2002). Unsymmetrical diene 58 was synthesized by further reaction with Wittig reagent (Patel and Argade, 2007). Arylnaphthalene lignan lactone 60 was obtained in 66% yield by Pd-promoted copolymerization of 58 and 59. Regioselective hydrolysis of less-hindered ester afforded 61. Justicidin B was obtained by borane reduction of carboxylic acid and lactonization. Retrojusticidin B was obtained in 67% yield by selective ester group reduction in the presence of carboxylate salt with 28% yield of justicidin B as a regioisomer.
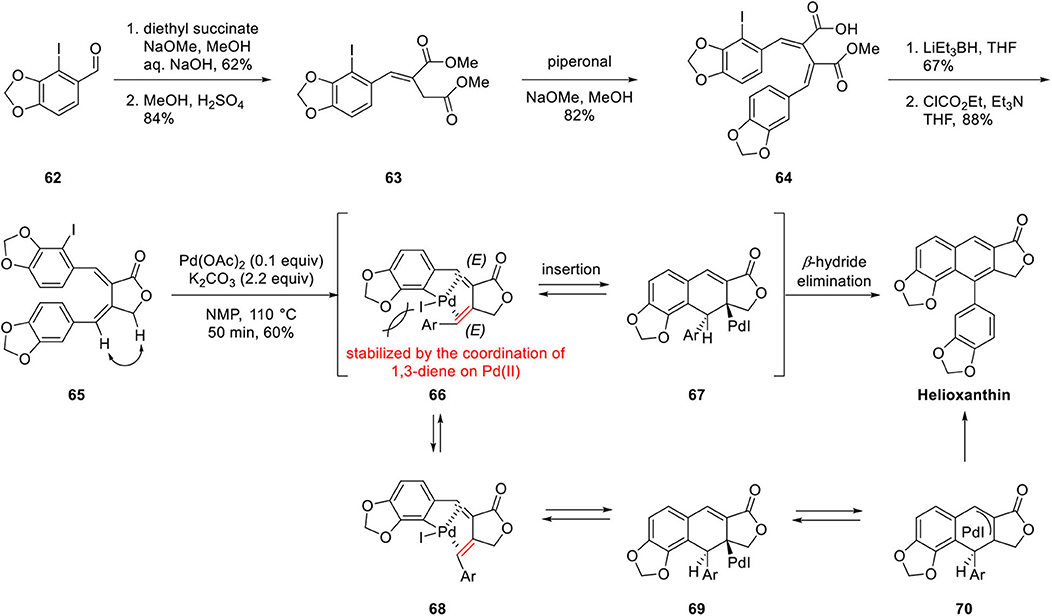
Mitsudera's group studied the construction of arylnaphthalene lignan lactone by a regiospecific Pd-catalyzed benzannulation of α, β-bisbenzylidene-γ-lactone for the synthesis of helioxanthin (Mizufune et al., 2001; Scheme 12). Structurally, helioxanthin is a 7,8-substituted naphthalene lignan lactone compound, whereas most natural arylnaphthalene lignan lactones bear 6,7-oxygen substituents. Therefore, regioselectivity is the most significant issue in this synthesis. Benzannulation precursor 65 was synthesized by Stobbe condensation of diethyl succinate with 2-iodopiperonal 62 and Fischer esterification to afford 63. Stobbe reaction of 63 with piperonal afforded 64, and lactone 65 was obtained by Super-Hydride-mediated ester reduction and concomitant lactonization. The geometry of the alkene was confirmed by analysis of the NOESY spectrum. With this substrate in hand, helioxanthin was obtained by benzannulation in 60% yield. The reaction mechanism can be explained as described below. Oxidative addition of Pd to aryl iodide 65 provided intermediate 66, which is stabilized by a coordination of 1,3-diene to Pd even in the presence of steric hindrance generated by the aryl group of the diene. Then syn insertion of the palladium complex to the alkene provided σ-dihydronaphthalene palladium complex 67 and syn β-hydride elimination afforded helioxanthin. In the other process, the geometry of alkene can be isomerized to (Z) in 68 specified in red color in Scheme 6. In that case, the β-hydride, which is positioned anti to Pd in 69 is eliminated by anti β-hydride elimination process by way of π-allylpalladium complex 70. In this synthesis, construction of the naphthalene ring is proceeded in relatively mild condition with only the catalytic amount of Pd catalyst in a concise, efficient manner with opposite regioselectivity usually obtained as a minor product using the Diels–Alder approach.
Silver-Catalyzed Cyclization
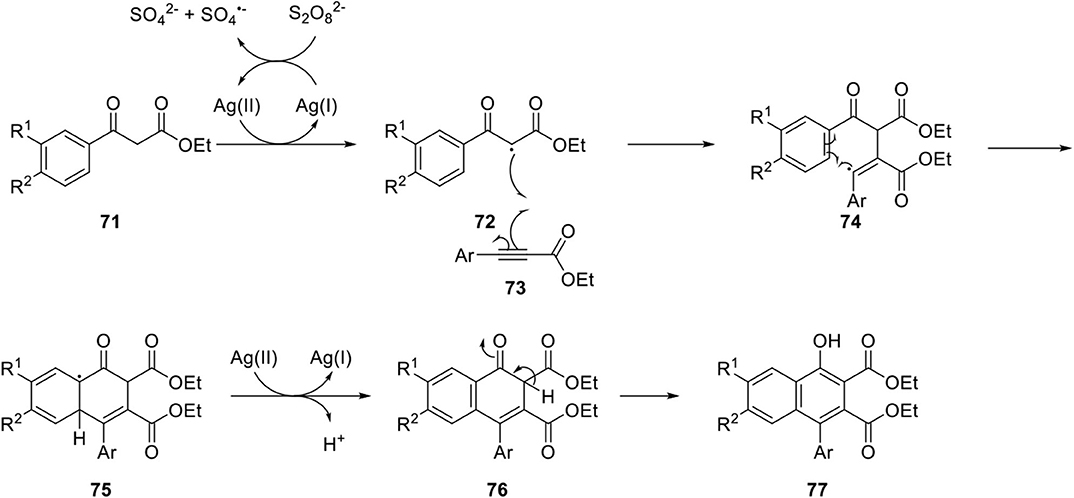
Narender et al. developed a novel route for regioselective synthesis of 4-aryl substituted α-naphthols by silver(I) catalyzed C-H functionalization of β-keto esters 71 and aryl propiolates 73 in an environmentally friendly manner (Naresh et al., 2015). The reaction mechanism was proposed to begin with disproportionation of persulfate dianion to persulfate radical anion producing Ag(II) ion. Then, β-ketoester 71 is oxidized by the Ag(II) ion to a highly stable radical species 72, and the radical is added to propiolate 73 to produce vinyl radical 74, which is stabilized by the neighboring aryl group (Yan et al., 2015). Finally, the radical 74 underwent addition to the benzene ring and synthesis of 4-arylnaphthol 77 was achieved by additional oxidation of 75 by Ag(II) ion, and tautomerization of 76 (Scheme 13).
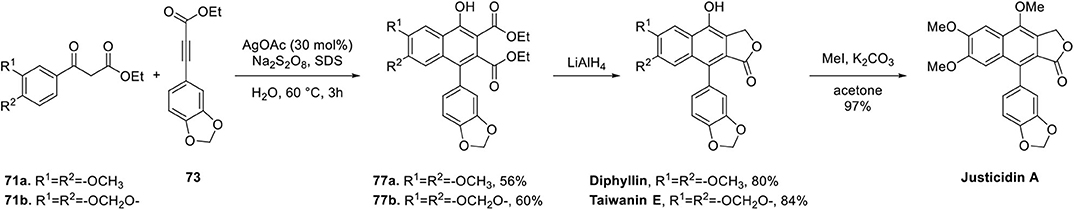
Successful and efficient applications for the synthesis of arylnaphthalene lignan lactone is summarized in Scheme 14. The reaction of substituted ethyl benzoyl acetate 71 and substituted ethyl phenylpropiolate 73 in the presence of silver acetate and Na2S2O8 furnished 4-aryl α-naphthol 77a and 77b in 56 and 60% yield, respectively. Surfactant (sodium dodecyl sulfate) was used to enhance water solubility and yield and reduced the use of organic solvent. Lithium Aluminum Hydride (LAH)-mediated reduction of ester located next to phenol and the spontaneous lactonization afforded diphyllin and taiwanin E in 80 and 84% yield, respectively. Justicidin A was also obtained by methylation of diphyllin in quantitative yield. This Ag-catalyzed cyclization proceeded in an atom economic manner, broad substrate scope, excellent regioselectivity, and environmental friendliness albeit with high loading of Ag catalyst.
Conclusion
Arylnaphthalene lignan lactones continuously intrigue synthetic organic chemists and medicinal chemists owing to their structural uniqueness and pharmacological activity as well as the possibility as privileged structures. This review focused on transition metal catalyzed approaches for the synthesis of arylnaphthalene lignan lactones, especially on the construction of arylnaphthalene cores by metal catalyst incorporating gold, manganese, nickel, palladium, and silver. Compared with the non-metal catalyzed synthesis of arylnaphthalene lignan lactones, synthetic methodologies using transition metal catalysts provide distinct advantages in terms of mild reaction conditions, chemical yields, and functional group tolerance. In addition, single step construction of a complex core structure through multiple bond formation is scientifically meaningful and provides utility in chemical library synthesis for drug discovery.
Author Contributions
DS conceptualized the manuscript. SP researched publications related to the review article. SP and J-HK prepared the schemes and figures. S-HK and DS wrote the draft of the manuscript and prepared the final version of the manuscript with considerable corrections. All authors approved the manuscript in its final form for publication.
Funding
This work was supported by grants from the Science Research Program through the National Research Foundation of Korea (NRF), Grant No. NRF-2018M3A9C8024360.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Anastas, P. T., and Stevenson, R. (1991). Synthesis of natural lignan arylnaphthalene lactones, daurinol and retrochinensin. J. Nat. Prod. 54, 1687–1691. doi: 10.1021/np50078a035
Anjaneyulu, A. S. R., Ramaiah, P. A., Row, L. R., Venkateswarlu, R., Pelter, A., and Ward, R. S. (1981). New lignans from the heartwood of cleistanthus collinus. Tetrahedron 37, 3641–3652. doi: 10.1016/S0040-4020(01)98893-3
Arnold, B. J., Mellows, S. M., and Sammes, P. G. (1973). Photochemical reactions. Part I. A new route to tetradehydropodophyllotoxin, taiwanin E, and related compounds. J. Chem. Soc. Perkin Trans. 1, 1266–1270. doi: 10.1039/p19730001266
Asano, J., Chiba, K., Tada, M., and Yoshii, T. (1996). Antiviral activity of lignans and their glycosides from Justicia procumbens. Phytochemistry 42, 713–717. doi: 10.1016/0031-9422(96)00024-6
Batsuren, D., Batirov, K., Malikov, V. M., Zemlyanskii, V. N., and Yagudaev, M. R. (1981). Arylnaphthalene lignans of Haplophyllum dauricum. The structure of daurinol. Chem. Nat. Compd. 17, 223–225. doi: 10.1007/BF00568506
Block, E., and Stevenson, R. (1971). Synthesis of model aryltetralin ligands: adducts of o-methylbenzophenone with unsymmetrical dienophiles. J. Chem. Soc. D Chem. Commun. 13, 711–712. doi: 10.1039/c29710000711
Block, E., and Stevenson, R. (1973). The irradiation of 2-methylbenzophenone in the presence of dienophiles. J. Chem. Soc. Perkin Trans. 1, 308–313. doi: 10.1039/p19730000308
Brown, D., and Stevenson, R. (1964). Action of N,N-dicyclohexylcarbodiimide on phenylpropiolic acids: synthesis of dehydro-otobain. Tetrahedron Lett. 5, 3213–3216. doi: 10.1016/0040-4039(64)83136-1
Brown, D., and Stevenson, R. (1965). Synthesis of dehydrootobain. J. Org. Chem. 30, 1759–1763. doi: 10.1021/jo01017a012
Bucher, J. E. (1910). The acids of the phenylpropiolic series and their condensation to naphthalene derivatives. J. Amer. Chem. Soc. 32, 212–221. doi: 10.1021/ja01920a008
Chang, C.-W., Lin, M.-T., Lee, S.-S., Liu, K. C. S. C., Hsu, F.-L., and Lin, J.-Y. (1995). Differential inhibition of reverse transcriptase and cellular DNA polymerase-α activities by lignans isolated from Chinese herbs, Phyllanthus myrtifolius Moon, and tannins from Lonicera japonica Thunb and Castanopsis hystrix. Antiviral Res. 27, 367–374. doi: 10.1016/0166-3542(95)00020-M
Chen, C.-C., Hsin, W.-C., Ko, F.-N., Huang, Y.-L., Ou, J.-C., and Teng, C.-M. (1996). Antiplatelet arylnaphthalide lignans from Justicia procumbens. J. Nat. Prod. 59, 1149–1150. doi: 10.1021/np960443+
Cow, C., Leung, C., and Charlton, J. L. (2000). Antiviral activity of arylnaphthalene and aryldihydronaphthalene lignans. Can. J. Chem. 78, 553–561. doi: 10.1139/v00-059
Day, S.-H., Lin, Y.-C., Tsai, M.-L., Tsao, L.-T., Ko, H.-H., Chung, M.-I., et al. (2002). Potent cytotoxic lignans from Justicia procumbens and yheir effects on nitric oxide and tumor necrosis factor-α production in mouse macrophages. J. Nat. Prod. 65, 379–381. doi: 10.1021/np0101651
De Silva, S. O., Denis, C. S., and Rodrigo, R. (1980). A regiocontrolled synthesis of some arylnaphthalide ligans. J. Chem. Soc.Chem. Commun. 21, 995–997. doi: 10.1039/c39800000995
Deng, Y., Chu, J., Ren, Y., Fan, Z., Ji, X., Mundy-Bosse, B., et al. (2014). The natural product phyllanthusmin C enhances IFN-γ production by human NK cells through upregulation of TLR-mediated NF-κB signaling. J. Immunol. 193, 2994–3002 doi: 10.4049/jimmunol.1302600
González, A. G., Pérez, J. P., and Trujillo, J. M. (1978). Synthesis of two arylnaphthalene lignans. Part 18 in the series Constituents of the Umbelliferae. Tetrahedron 34, 1011–1013. doi: 10.1016/0040-4020(78)88156-3
Gu, M.-Y., Kim, J., and Yang, H. O. (2016). The neuroprotective effects of justicidin A on amyloid beta25–35-induced neuronal cell death through inhibition of Tau hyperphosphorylation and induction of autophagy in SH-SY5Y cells. Neurochem. Res. 41, 1458–1467. doi: 10.1007/s11064-016-1857-5
Gudla, V., and Balamurugan, R. (2011). Synthesis of arylnaphthalene lignan scaffold by gold-catalyzed intramolecular sequential electrophilic addition and benzannulation. J. Org. Chem. 76, 9919–9933. doi: 10.1021/jo201918d
Gudla, V., and Balamurugan, R. (2013). Synthesis of 1-arylnaphthalenes by gold-catalyzed one-pot sequential epoxide to carbonyl rearrangement and cyclization with arylalkynes. Chem. Asian J. 8, 414–428. doi: 10.1002/asia.201200817
Hashmi, A. S. K. (2007). Gold-catalyzed organic reactions. Chem. Rev. 107, 3180–3211. doi: 10.1021/cr000436x
Hayat, F., Kang, L., Lee, C. Y., and Shin, D. (2015). Synthesis of arylnaphthalene lignan lactone using benzoin condensation, intramolecular thermal cyclization and Suzuki coupling. Tetrahedron 71, 2945–2950. doi: 10.1016/j.tet.2015.03.023
He, X.-L., Zhang, P., Dong, X.-Z., Yang, M.-H., Chen, S.-L., and Bi, M.-G. (2012). JR6, a new compound isolated from Justicia procumbens, induces apoptosis in human bladder cancer EJ cells through caspase-dependent pathway. J. Ethnopharmacol.144, 284–292. doi: 10.1016/j.jep.2012.09.010
He, Y., Zhang, X., and Fan, X. (2014). Synthesis of naphthalene amino esters and arylnaphthalene lactone lignans through tandem reactions of 2-alkynylbenzonitriles. Chem. Commun. 50, 5641–5643. doi: 10.1039/C4CC01738B
Holmes, T. L., and Stevenson, R. (1970). Synthesis of helioxanthin. Tetrahedron Lett. 11, 199–202. doi: 10.1016/0040-4039(70)80025-9
Holmes, T. L., and Stevenson, R. (1971). Arylnaphthalene lignans: synthesis of helioxanthin. J. Chem. Soc. C Org. 11, 2091–2094. doi: 10.1039/j39710002091
Kamal, A., Daneshtalab, M., and Micetich, R. G. (1994). A rapid entry into podophyllotoxin congeners: Synthesis of justicidin B. Tetrahedron Lett. 35, 3879–3882. doi: 10.1016/S0040-4039(00)76691-3
Kao, T. T., Lin, C. C., and Shia, K. S. (2015). The total synthesis of retrojusticidin B, justicidin E, and helioxanthin. J. Org. Chem. 80, 6708–6714. doi: 10.1021/acs.joc.5b00866
Kar, A., and Argade, N. P. (2002). A facile synthesis of natural products chaetomellic acid A and 1,7(Z)- nonadecadiene-2,3-dicarboxylic acid. J. Org. Chem. 67, 7131–7134. doi: 10.1021/jo020195o
Kato, T., Ichinose, I., Kamoshida, A., and Kitahara, Y. (1976). Cyclization of polyenes. Biogenetic-type synthesis of snyderols. J. Chem. Soc. Chem. Commun. 13, 518–519. doi: 10.1039/c39760000518
Kato, T., Mochizuki, M., Hirano, T., Fujiwara, S., and Uyehara, T. (1984). Selective brominative cyclization of polyenes assisted by acetonitrile. Application to the synthesis of acoratriene. J. Chem. Soc. Chem. Commun. 16, 1077–1078. doi: 10.1039/c39840001077
Khalid, S. A., and Waterman, P. G. (1981). Alkaloid, lignan and flavonoid constituents of Haplophyllum tuberculatum from Sudan. Planta Med. 43, 148–152. doi: 10.1055/s-2007-971491
Li, Z., Brouwer, C., and He, C. (2008). Gold-catalyzed organic transformations. Chem. Rev. 108, 3239–3265. doi: 10.1021/cr068434l
Lin, M.-T., Lee, S.-S., and Liu, K. C. S. C. (1995). Phyllamyricins A-C, three novel lignans from Phyllanthus myrtifolius. J. Nat. Prod. 58, 244–249. doi: 10.1021/np50116a013
Luo, J., Hu, Y., Kong, W., and Yang, M. (2014). Evaluation and structure-activity relationship analysis of a new series of arylnaphthalene lignans as potential anti-tumor agents. PLoS ONE 9:e93516. doi: 10.1371/journal.pone.0093516
Maclean, I., and Stevenson, R. (1966). Synthesis of (±)-otobain. J. Chem. Soc. C Org. 1717–1719. doi: 10.1039/J39660001717
Michael, A., and Bucher, J. E. (1895). Ueber die Einwirkung von Essigsäureanhydrid auf Säuren der Acetylenreihe. Ber. Deutsch. Chem. Ges. 28, 2511–2512. doi: 10.1002/cber.18950280337
Mizufune, H., Nakamura, M., and Mitsudera, H. (2001). The first regiospecific synthesis of helioxanthin by novel palladium-catalyzed benzannulation reaction of alpha,beta-bisbenzylidene-gamma-lactone. Tetrahedron Lett. 42, 437–439. doi: 10.1016/S0040-4039(00)01982-1
Mondal, S., Maji, M., and Basak, A. (2011). A Garratt–Braverman route to aryl naphthalene lignans. Tetrahedron Lett. 52, 1183–1186. doi: 10.1016/j.tetlet.2011.01.011
Mondal, S., Mitra, T., Mukherjee, R., Addy, P. S., and Basak, A. (2012). Garratt–Braverman cyclization, a powerful tool for C–C bond formation. Synlett 23, 2582–2602. doi: 10.1055/s-0032-1317321
Mori, S., Takechi, S., Shimizu, S., Kida, S., Iwakura, H., and Hajima, M. (1999). Convergent synthesis of S-8921, a new potent hypocholesterolemic arylnaphthalene lignan analog. Tetrahedron Lett. 40, 1165–1168. doi: 10.1016/S0040-4039(98)02554-4
Murakami, M., Hoshino, Y., Ito, H., and Ito, Y. (1998). Palladium-catalyzed coupling reactions of N-methoxy-N-methylcarbamoyl chloride for the synthesis of N-methoxy-N-methylamides. Chem. Lett. 27, 163–164. doi: 10.1246/cl.1998.163
Naresh, G., Kant, R., and Narender, T. (2015). Silver(I)-catalyzed regioselective construction of highly substituted alpha-naphthols and its application toward expeditious synthesis of lignan natural products. Org. Lett. 17, 3446–3449. doi: 10.1021/acs.orglett.5b01477
Ogiku, T., Seki, M., Takahashi, M., Ohmizu, H., and Iwasaki, T. (1990). A new 2-step synthesis of 1-arylnaphthalene lignans from cyanohydrins. Tetrahedron Lett. 31, 5487–5490. doi: 10.1016/S0040-4039(00)97879-1
Ogiku, T., Yoshida, S., Ohmizu, H., and Iwasaki, T. (1995). Efficient synthesis of 1-arylnaphthalene lactones and related-compounds from cyanohydrins. J. Org. Chem. 60, 4585–4590. doi: 10.1021/jo00119a041
Paris, M., Pothion, C., Heitz, A., Martinez, J., and Fehrentz, J.-A. (1998). Synthesis of N- and side chain protected aspartyl and glutamyl aldehyde derivatives. Reinvestigation of the reduction of Weinreb amides. Tetrahedron Lett. 39, 1341–1344. doi: 10.1016/S0040-4039(97)10813-9
Park, J. E., Lee, J., Seo, S. Y., and Shin, D. (2014). Regioselective route for arylnaphthalene lactones: convenient synthesis of taiwanin C, justicidin E, and daurinol. Tetrahedron Lett. 55, 818–820. doi: 10.1016/j.tetlet.2013.12.014
Patel, R. M., and Argade, N. P. (2007). Facile SN2‘coupling reactions of Wittig reagents with dimethyl bromomethylfumarate: synthesis of enes, dienes, and related natural products. J. Org. Chem. 72, 4900–4904. doi: 10.1021/jo070728z
Patel, R. M., and Argade, N. P. (2013). Palladium-promoted 2+2+2 cocyclization of arynes and unsymmetrical conjugated dienes: synthesis of justicidin B and retrojusticidin B. Org. Lett. 15, 14–17. doi: 10.1021/ol3028658
Peña, D., Escudero, S., Pérez, D., Guitián, E., and Castedo, L. (1998). Efficient palladium-catalyzed cyclotrimerization of arynes: synthesis of triphenylenes. Angew. Chem. Int. Ed. 37, 2659–2661. doi: 10.1002/(SICI)1521-3773(19981016)37:19<2659::AID-ANIE2659>3.0.CO;2-4
Plaumann, H. P., Smith, J. G., and Rodrigo, R. (1980). Potential isobenzofurans: their use in the synthesis of naturally occurring 1-arylnaphthalide lignans. J. Chem. Soc. Chem. Commun. 8, 354–355. doi: 10.1039/c39800000354
Ren, Y., Carcache de Blanco, E. J., Fuchs, J. R., Soejarto, D. D., Burdette, J. E., Swanson, S. M., et al. (2019). Potential anticancer agents characterized from selected tropical plants. J. Nat. Prod. 82, 657–679. doi: 10.1021/acs.jnatprod.9b00018
Ren, Y., Lantvit, D. D., Deng, Y., Kanagasabai, R., Gallucci, J. C., Ninh, T. N., et al. (2014). Potent cytotoxic arylnaphthalene lignan lactones from Phyllanthus poilanei. J. Nat. Prod. 77, 1494–1504. doi: 10.1021/np5002785
Sato, Y., Ohashi, K., and Mori, M. (1999). Synthesis of biaryls using nickel-catalyzed [2+2+2] cocyclization. Tetrahedron Lett. 40, 5231–5234. doi: 10.1016/S0040-4039(99)00945-4
Sato, Y., Tamura, T., Kinbara, A., and Mori, M. (2007). Synthesis of biaryls via palladium-catalyzed 2+2+2 cocyclization of arynes and diynes: application to the synthesis of arylnaphthalene lignans. Adv. Synth. Catal. 349, 647–661. doi: 10.1002/adsc.200600587
Sato, Y., Tamura, T., and Mori, M. (2004). Arylnaphthalene lignans through Pd-catalyzed 2+2+2 cocyclization of arynes and diynes: total synthesis of taiwanins C and E. Angew. Chem. Int. Ed. 43, 2436–2440. doi: 10.1002/anie.200453809
Stevenson, R., and Block, E. (1971). Lignan lactones. Synthesis of (±)-collinusin and justicidin B. J. Org. Chem. 36, 3453–3455. doi: 10.1021/jo00821a038
Stevenson, R., and Holmes, T. L. (1971). Arylnaphthalene lignans. Synthesis of justicidin E, taiwanin C, dehydrodimethylconidendrin, and dehydrodimethylretrodendrin. J. Org. Chem. 36, 3450–3453. doi: 10.1021/jo00821a037
Stevenson, R., and Weber, J. V. (1989). Improved methods of synthesis of lignan arylnaphthalene lactones via arylpropargyl arylpropiolate esters. J. Nat. Prod. 52, 367–375. doi: 10.1021/np50062a024
Stevenson, R., and Weber, J. V. (1991). Synthesis of lignan aryldihydronaphthalene lactones by cyclization of cinnamyl arylpropiolate esters-revised structure of beta apopolycamatin. J. Nat. Prod. 54, 310–314. doi: 10.1021/np50073a042
Teponno, R. B., Kusari, S., and Spiteller, M. (2016). Recent advances in research on lignans and neolignans. Nat. Prod. Rep. 33, 1044–1092. doi: 10.1039/C6NP00021E
Tsay, S.-C., Robl, J. A., and Hwu, J. R. (1990). New method for the selective reduction of amides. J. Chem. Soc. Perkin Trans. 1, 757–759. doi: 10.1039/p19900000757
Tsuji, J., and Ohno, K. (1965). Organic syntheses by means of noble metal compounds XXI. Decarbonylation of aldehydes using rhodium complex. Tetrahedron Lett. 6, 3969–3971. doi: 10.1016/S0040-4039(01)89127-9
Ulubelen, A. (1985). Alkaloids from Haplophyllum buxbaumii. Phytochemistry 24, 372–374. doi: 10.1016/S0031-9422(00)83563-3
Weng, J.-R., Ko, H.-H., Yeh, T.-L., Lin, H.-C., and Lin, C.-N. (2004). Two new arylnaphthalide lignans and antiplatelet constituents from Justicia procumbens. Arch. Pharm. 337, 207–212. doi: 10.1002/ardp.200300841
Windayani, N. R., Rukayadi, Y., Hakim, E. H., Ruslan, K., and Syah, Y. M. (2014). Antifungal activity of lignans isolated from Phyllanthus myrtifolius Moon against. Fusarium oxysporum. Phytochemistry 12, 33–39.
Won, S.-J., Yen, C.-H., Liu, H.-S., Wu, S.-Y., Lan, S.-H., Jiang-Shieh, Y.-F., et al. (2015). Justicidin A-induced autophagy flux enhances apoptosis of human colorectal cancer cells via class III PI3K and Atg5 pathway. J. Cell Physiol. 230, 930–946. doi: 10.1002/jcp.24825
Wong, Y.-C., Kao, T.-T., Huang, J.-K., Jhang, Y.-W., Chou, M.-C., and Shia, K.-S. (2014). Manganese(III)-catalyzed oxidative cyclization of aryl 1-cyanoalk-5-ynyl ketone systems: a convenient and general approach to cyclopenta[b]naphthalene derivatives. Adv. Synth. Catal. 356, 3025–3038. doi: 10.1002/adsc.201400257
Woo, J. K., Jung, H. J., Park, J. Y., Kang, J. H., Lee, B. I., Shin, D., et al. (2017). Daurinol blocks breast and lung cancer metastasis and development by inhibition of focal adhesion kinase (FAK). Oncotarget 8, 57058–57071. doi: 10.18632/oncotarget.18983
Woodard, J. L., Huntsman, A. C., Patel, P. A., Chai, H.-B., Kanagasabai, R., and Karmahapatra, S. (2018) Synthesis antiproliferative activity of derivatives of the phyllanthusmin class of arylnaphthalene lignan lactones. Bioorg. Med. Chem. 26, 2354–2364. doi: 10.1016/j.bmc.2018.03.033.
Xiao, J., Cong, X.-W., Yang, G.-Z., Wang, Y.-W., and Peng, Y. (2018a). Divergent asymmetric syntheses of podophyllotoxin and related family members via stereoselective reductive Ni-catalysis. Org. Lett. 20, 1651–1654. doi: 10.1021/acs.orglett.8b00408
Xiao, J., Cong, X.-W., Yang, G.-Z., Wang, Y.-W., and Peng, Y. (2018b). Stereoselective synthesis of a podophyllum lignan core by intramolecular reductive nickel-catalysis. Chem. Commun. 54, 2040–2043. doi: 10.1039/C8CC00001H
Xiao, J., Nan, G. M., Wang, Y. W., and Peng, Y. (2018c). Concise synthesis of (+)-β- and γ-apopicropodophyllins, and dehydrodesoxypodophyllotoxin. Molecules 23, 3037. doi: 10.3390/molecules23113037
Yamamoto, Y., Mori, S., and Shibuya, M. (2015). A combined transition-metal-catalyzed and photopromoted process: synthesis of 2,3-fused 4-phenylnaphthalen-1-yl carboxylates from 1,7-diaryl-1,6-diynes. Chem. Eur. J. 21, 9093–9100. doi: 10.1002/chem.201500978
Yamazaki, S. (1999). Chromium(VI) oxide-catalyzed benzylic oxidation with periodic acid. Org. Lett. 1, 2129–2132. doi: 10.1021/ol991175k
Yan, K., Yang, D., Wei, W., Wang, F., Shuai, Y., Li, Q., et al. (2015). Silver-mediated radical cyclization of alkynoates and α-keto acids leading to coumarins via cascade double C–C bond formation. J. Org. Chem. 80, 1550–1556. doi: 10.1021/jo502474z
Yi, L., Chen, L., Guo, X., Lu, T., Wang, H., Ji, X., et al. (2018). A synthetic disaccharide derivative of diphyllin, TAARD, activates human natural killer cells to secrete interferon-gamma via toll-like receptor-mediated NF-κB and STAT3 signaling pathways. Front. Immunol. 9:1509. doi: 10.3389/fimmu.2018.01509
Young, A. N., Herrera, D., Huntsman, A. C., Korkmaz, M. A., Lantvit, D. D., Mazumder, S., et al. (2018). Phyllanthusmin derivatives induce apoptosis and reduce tumor burden in high-grade serous ovarian cancer by late-stage autophagy inhibition. Mol. Cancer Ther. 17, 2123–2135. doi: 10.1158/1535-7163.MCT-17-1195
Yu, Z., Dan, W., Jie, H., and Li, Z. (2010). Synthesis and bioevaluation of novel analogues of justicidin A. Med. Chem. Res. 19, 71–76. doi: 10.1007/s00044-009-9172-1
Zhang, H.-J., Rumschlag-Booms, E., Guan, Y.-F., Liu, K.-L., Wang, D.-Y., Li, W.-F., et al. (2017). Anti-HIV diphyllin glycosides from Justicia gendarussa. Phytochem 136, 94–100. doi: 10.1016/j.phytochem.2017.01.005
Keywords: transition metal, arylnaphthalene, catalysis, lignan, synthesis, lactone
Citation: Park S, Kim J-H, Kim S-H and Shin D (2020) Transition Metal-Mediated Annulation Approaches for Synthesis of Arylnaphthalene Lignan Lactones. Front. Chem. 8:628. doi: 10.3389/fchem.2020.00628
Received: 04 April 2020; Accepted: 17 June 2020;
Published: 06 August 2020.
Edited by:
Nam-Jung Kim, Kyung Hee University, South KoreaReviewed by:
Yulin Ren, The Ohio State University, United StatesBen W. Greatrex, University of New England, Australia
Copyright © 2020 Park, Kim, Kim and Shin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Seok-Ho Kim, a3NoMzQxMEBjaGEuYWMua3I=; Dongyun Shin, ZHlzaGluQGdhY2hvbi5hYy5rcg==
†These authors have contributed equally to this work
 Sooyoung Park
Sooyoung Park Jin-Hee Kim2†
Jin-Hee Kim2† Seok-Ho Kim
Seok-Ho Kim Dongyun Shin
Dongyun Shin