- 1IRCCS SDN, Naples, Italy
- 2Andrological and Urogynecological Clinic, Santa Maria Terni Hospital, University of Perugia, Terni, Italy
About 70% of bladder cancers (BCs) are diagnosed as non-muscle-invasive BCs (NMIBCs), while the remaining are muscle-invasive BCs (MIBCs). The European Association of Urology (EAU) guidelines stratify NMIBCs into low, intermediate, and high risk for treatment options. Low-risk NMIBCs undergo only the transurethral resection of the bladder (TURB), whereas for intermediate-risk and high-risk NMIBCs, the transurethral resection of the bladder (TURB) with or without Bacillus Calmette-Guérin (BCG) immune or chemotherapy is the standard treatment. A minority of NMIBCs show unfavorable prognosis. High-risk NMIBCs have a high rate of disease recurrence and/or progression to muscle-invasive tumor and BCG treatment failure. The heterogeneous nature of NMIBCs poses challenges for clinical decision-making. In 2020, the EAU made some changes to NMIBCs BCG failure definitions and treatment options, highlighting the need for reliable molecular markers for improving the predictive accuracy of currently available risk tables. Nowadays, next-generation sequencing (NGS) has revolutionized the study of cancer biology, providing diagnostic, prognostic, and therapy response biomarkers in support of precision medicine. Integration of NGS with other cutting-edge technologies might help to decipher also bladder tumor surrounding aspects such as immune system, stromal component, microbiome, and urobiome; altogether, this might impact the clinical outcomes of NMBICs especially in the BCG responsiveness. This review focuses on NMIBCs with unfavorable prognoses, providing molecular prognostic factors from tumor immune and stromal cells, and the perspective of urobiome and microbiome profiling on therapy response. We provide information on the cornerstone of immunotherapy and new promising bladder-preserving treatments and ongoing clinical trials for BCG–unresponsive NMIBCs.
Introduction
In the United States, urothelial carcinoma of the bladder represents the most frequent urothelial neoplasm, and the fourth most common cancer in men (McConkey and Lerner, 2019; Siegel et al., 2020). Approximatively 70% of bladder cancer (BC) are non-muscle-invasive (NMIBCs), also known as “superficial” cancer, whereas advanced stages are muscle-invasive BCs (MIBCs) or metastatic BCs (Kamat et al., 2016). According to the European Association of Urology (EAU) guidelines, NMIBCs are distinct into low, intermediate, and high risk (Babjuk et al., 2019, 2020). The standard of care recommends for low-risk NMIBCs only the transurethral resection of the bladder (TURB); for intermediate- and high-risk NMIBCs, standard of care recommends the transurethral resection of the bladder (TURB) with or without Bacillus Calmette-Guérin (BCG) immune or chemotherapy (Babjuk et al., 2019). On the contrary, MIBCs undergo radical cystectomy, radiotherapy, and/or chemotherapy even alone (Babjuk et al., 2019; McConkey and Lerner, 2019). Genitourinary cancers are the most likely responsive to immunotherapy (Lalani and Sonpavde, 2019); however, about 20–30% of BCs have unfavorable to very unfavorable prognoses (Babjuk et al., 2019). High-risk NMIBCs show a greater propensity for disease recurrence and/or progression to muscle-invasive tumor, even after optimal BCG immunotherapy (Tse et al., 2019). NMIBCs require a better risk stratification due to clinical and molecular heterogeneity also in BCG responsiveness, which poses a major challenge for clinical decision-making. In 2020, the EAU made changes for NMIBC BCG failure definitions and treatment options (Table 1), since novel promising bladder-preserving treatments are currently under evaluation. Moreover, it has highlighted the need for reliable molecular markers for improving the predictive accuracy of currently available risk tables (Babjuk et al., 2019; Soukup et al., 2020).
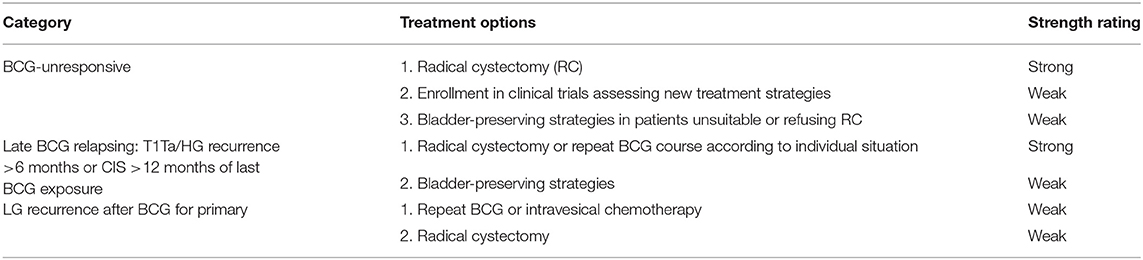
Table 1. European Association of Urology (EAU) 2020 guidelines for the treatment of Bacillus Calmette-Guérin (BCG) failure (Table 7.7 EAU guidelines).
In the last decades, the increasing number of biobanks infrastructures (Coppola et al., 2019) has allowed the prompt availability of quality-controlled biological samples to be processed using next-generation sequencing (NGS) technologies (Chakraborty et al., 2018). The advent of NGS technologies and radiomics has revolutionized the approach to study disease biology, augmenting precision oncology (Incoronato et al., 2017; Zanfardino et al., 2019a,b; Castaldo et al., 2020). In BC research, the integration of NGS technologies with cutting-edge approaches might help to decipher also other tumor-relevant aspects such as immune and stromal component, microbiome, and urobiome modifications. This might impact the clinical outcomes of NMIBCs, especially for the BCG responsiveness.
This review focuses on NMIBCs with unfavorable prognosis providing interesting molecular prognostic factors from tumor closely related immune and stroma cells and the perspective of urobiome and microbiome profiling on therapy response. We illustrate the cornerstone of immunotherapy and new promising bladder-preserving treatments for BCG-unresponsive including the ongoing Food and Drug Administration (FDA)-approved clinical trials.
Need For Reliable Molecular Bladder Cancer Biomarkers and Cutting-Edge Approaches
Currently, NMIBC risk stratification is based especially on clinical–pathological parameters such as grade (Babjuk et al., 2019). As opposed to other cancers such as prostate, BCs lack prognostic molecular markers used in clinical practice. NMIBC new prognostic factors are coming from tumor–host biology studies (Cooley et al., 2020). Indeed, innate and adaptive immune cells as well as stromal components surrounding tumor may have a prognostic value especially for evaluating BCG responsiveness in NMIBCs. Recently, Mezheyeuski et al. (2020) analyzed five cancer-associated fibroblast (CAF) markers, stroma-based, alpha smooth muscle actin (ASMA), CD90/Thy-1, fibroblast activation protein (FAP), platelet-derived growth factor receptor-alpha and -beta (PDGFRa-b) with survival and histopathological characteristics in 344 BC patients (231 NMIBCs, 113 MIBCs). Cluster analysis of stromal marker-based patient stratification identified a FAP-dominant patient cluster as an independent marker for shorter 5-year survival [Hazard Ratio, HR (95% confidence interval) 2.25 (1.08–4.67), p = 0.030]. Other studies on immunomodulatory properties rely on CD8a and revealed a potential minority of cases with CD90-defined stroma and high CD8a T cell infiltration showing a good prognosis of more than 80% 5-year survival (Mezheyeuski et al., 2020). Chu et al. (2020) described an innovative approach using indoleamine 2,3-dioxgenase 1 (IDO1) inhibitors in combination with immunotherapies in MIBCs and NMIBCs; the use of specific molecules such as indoximod, epacadostat, and linrodostat permit the immune microenvironment manipulation and increase of sensitivity to existing therapies with the goal of preventing the immune escape of cancer (Chu et al., 2020). In a retrospective study of high-grade pT1NMIBCs after TURB, the abundance of stromal tumor-infiltrating lymphocytes (TILs) associated with tumor invasion depth (Rouanne et al., 2019). Guillamón et al. (2019) isolated natural killer cells (NK) from BC peripheral blood for classifying patient risk which may be combined with BC histopathology. The mutation rate of genetic alterations may have an important prognostic value. Telomerase reverse transcriptase gene promoter (TERTp) mutations represent a frequent genetic event in BC. Batista et al. (2020) screened 125 NMIBC high-risk patients treated with BCG therapy (referred as BCG-NMIBC) for TERTp mutations, TERT rs2853669 single-nucleotide polymorphism, and fibroblast growth factor receptor 3 (FGFR3) hot spot mutations. TERTp mutations were found in 56% of BCG-NMIBC and were not associated with tumor stage or grade. FGFR3 mutations were found in 44.9% of the cases and were not associated with tumor stage or grade nor with TERTp mutations. The TERT rs2853669 single-nucleotide polymorphism was associated with tumors of a higher grade. The specific c.1-146 G > A TERTp mutation was an independent predictor of non-recurrence after BCG therapy (Hazard Ratio, HR 0.382; 95% confidence interval—0.150–0.971, p = 0.048) (Batista et al., 2020). In clinical practice, to date, we are still far from reliable molecular prognostic biomarkers due especially to heterogeneous results.
Perspectives of Bladder Microbiome and Urobiome: a New Opportunity for a Predictive Response?
NGS technologies enable the study of the microbiome (Marchesi et al., 2016; Yoshida et al., 2018; Forkosh and Ilan, 2019). Microbial dysbiosis impacts several human diseases including bladder carcinogenesis (Bajic et al., 2019b). However, the mechanistic links between microbiome signatures and bladder pathogenesis are still unknown. Urobiome studies may open the way to understand how bladder microbiota affects immunotherapy response leading to BCG-unresponsive patients (Wolfe and Brubaker, 2019).
Urinary microbiome through the 16S ribosomal RNA sequencing and expanded quantitative urine culture (EQUC) enable unculturable and/or rare microbes detection (Ferreira et al., 2010; Karstens et al., 2018; Govender et al., 2019). The core step in microbiome analysis is the taxonomic classification of the representative sequences and clustering of operational taxonomic units (OTUs). To assess the association between microbiome signatures and clinical phenotype, useful bioinformatic tools are PERMANOVA-S (Tang et al., 2016) and MiRKAT (Zhao et al., 2015). Bladder microbiome analysis showed that the most abundant genera are Lactobacillus (15%), followed by Corynebacterium (14.2%), Streptococcus (11.9%), Actinomyces (6.9%), and Staphylococcus (6.9%) (Bersanelli et al., 2019). Innovative drug strategies based on precise antimicrobial peptides might boost the host immune system to ensure bladder microbiome homeostasis (de la Fuente-Nunez et al., 2017; Gaglione et al., 2019). Novel urinary biomarkers proved to be endowed with prognostic and diagnostic value in urological malignancies derived from exosomes, such as H2B1K and alpha-1 antitrypsin (Wu et al., 2019).
Recent studies evaluated microbiome composition using 16S ribosomal RNA sequencing of urine specimens from healthy individuals vs. urothelial carcinoma patients with heterogeneous results especially because both studies used a small number of patients (Xu et al., 2014; Bučević Popović et al., 2018). Other studies have clearly shown the increase in bacterial species in BC patients compared to control patients, correlating them with the high risk of disease progression and indicating them as possible new biomarkers to further stratify patients. Although exciting, urinary microbiome signature needs precautions due to several issues associated with sample collections and management of the biological sample, sex factors such as age, menopausal status, sex steroid hormones, and body mass index (Karstens et al., 2018; Bajic et al., 2019b).
Modern Anticancer Therapy
A panel of potential drug targets underlying BC signaling (Krämer et al., 2014) is schematically represented in Figure 1. In recent years, there has been a growing demand for BCG-unresponsive salvage treatments as alternative to radical cystectomy. Moreover, immunotherapies and new therapeutic options, listed in Table 2, may be administered as single arm (monotherapy) but also as a combination and even multitarget drugs could be available in the near future (see below).
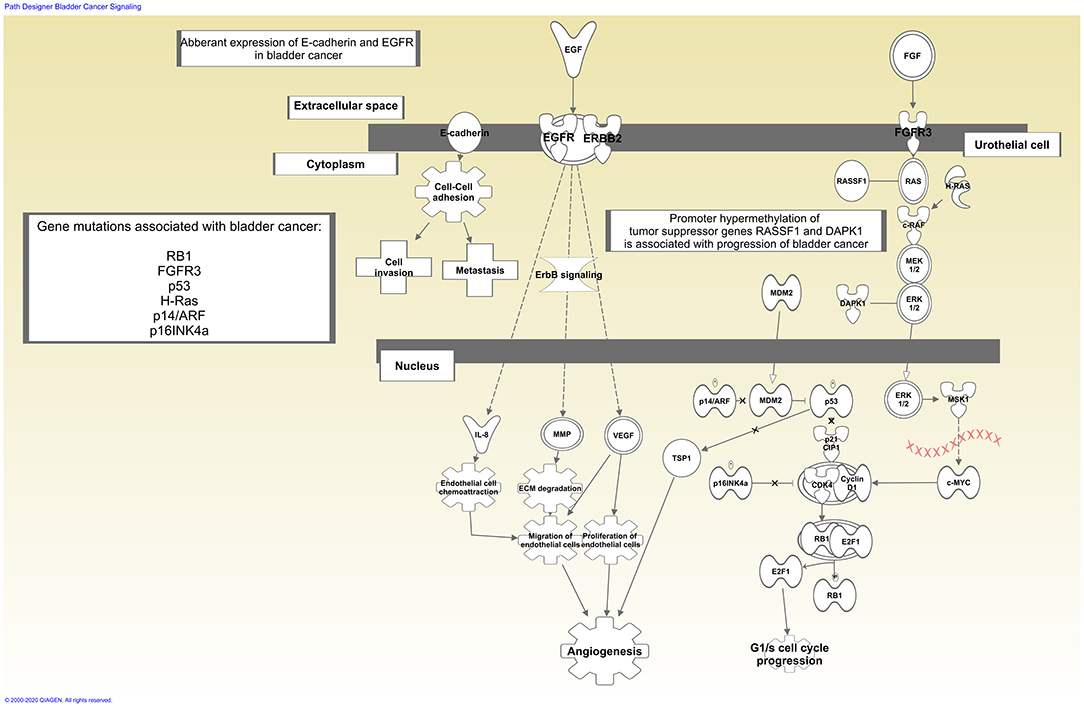
Figure 1. Schematic representation of bladder cancer signaling. Drug therapy based on immunotherapy affects tumor biology enhancing host immune response. In contrast, single arm or multi-target drugs may affect one single target or more targets within the same or multiple biological pathways underlying bladder cancer. Signaling generated through the use of IPA (QIAGEN Inc., https://www.qiagenbioinformatics.com/products/ingenuity-pathway-analysis).
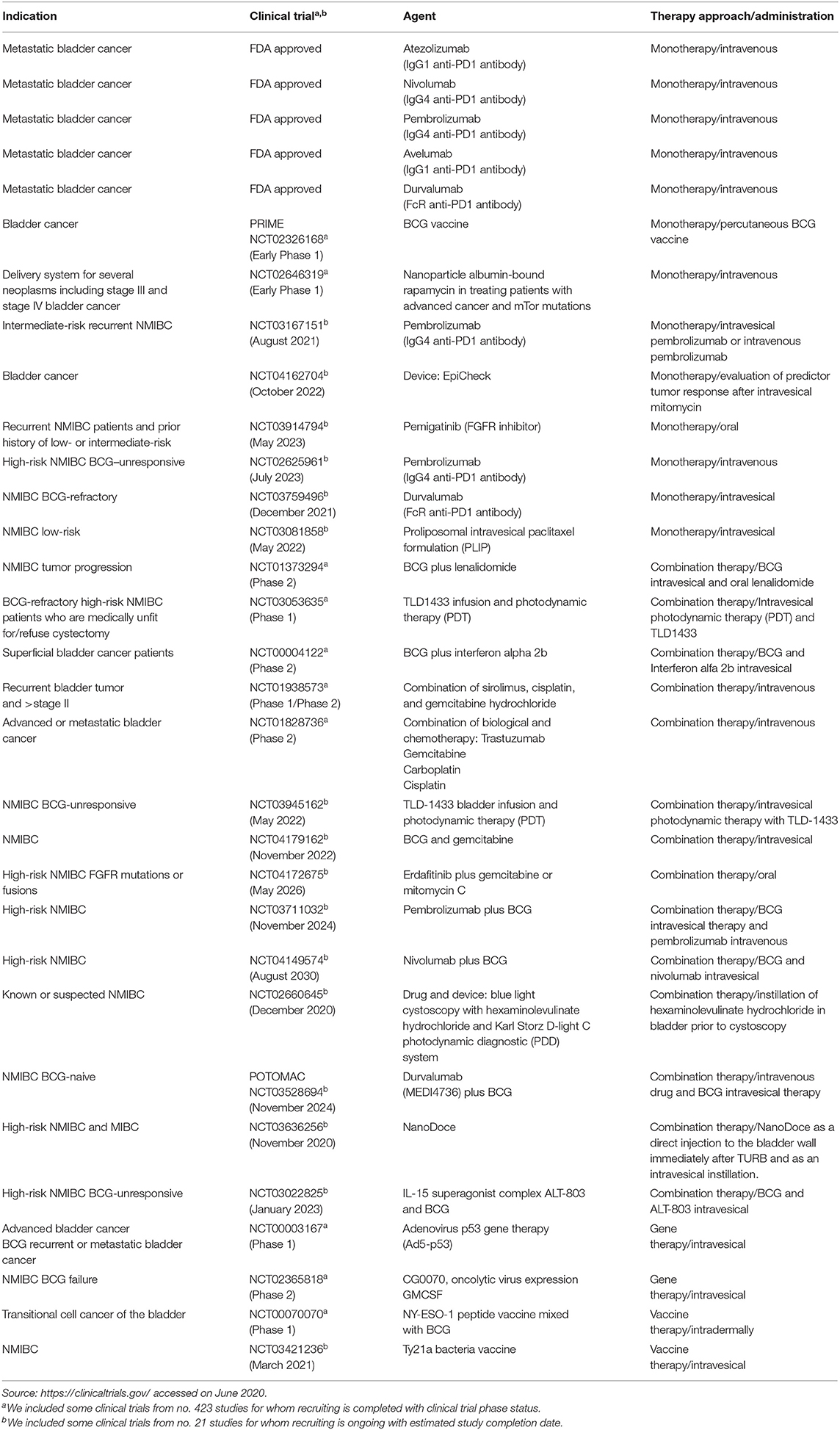
Table 2. Modern therapeutic approaches for bladder cancer based on intravesical, intravenous, or subcutaneous applications and some clinical trials completed or with ongoing recruitment.
Immunotherapy of Bladder Cancer: New Frontiers
During the 1990s, the immune checkpoint key proteins, cytotoxic T lymphocyte-associated protein 4 (CTLA-4), programmed cell death protein (PD-1), and programmed death-ligand 1 (PD-L1), revolutionized cancer therapy (Korman et al., 2006; Okazaki and Honjo, 2007). Currently, immunotherapy, together with radiotherapy and chemotherapy, represents standard clinical care for cancer including BC (Butt and Malik, 2018). Immunotherapeutic drugs inhibit immune checkpoint pathways, enabling the immune system to fend off cancer cells through cytotoxic T killer cells (Korman et al., 2006). BC can cause inflammation by attracting inflammatory cells to the cancer cell site which led to the activation of different components of the immune system such as tumor-associated macrophages (TAMs), neutrophils, and granulocytes. Nevertheless, a crucial role is played by T lymphocytes; this immune system cell component has an important role in tumor development and progression; regulatory T cells have been shown to be involved in maintaining self-tolerance modulating the antitumor immune response and therefore potential cancer growth and spread. These evidences have highlighted that lymphocytes are intrinsically linked to tumorigenesis in the bladder, acting as target for BC immunomodulatory therapy. To date, five FDA-approved immunotherapy agents are commonly used for metastatic BC treatment (Table 2). Nivolumab and pembrolizumab are anti-PD1 drugs, tremilimumab and ipilimumab are anti-CTLA-4 drugs; furthermore, MBG453 is a TIM3 inhibitor. Through the inhibition of PD-L1, the tumor cells are targeted; for this approach, the molecules used are durvalumab, avelumab, and atezolizumab. Further strategies include a modulation of regulatory T cells by targeting CD25 (daclizumab) or alternatively CCR4 (mogamulizumab). Genitourinary cancers are most likely responsive to immune checkpoint inhibitors such as PD-1 and PD-L1 antibodies (Lalani and Sonpavde, 2019). The promising POTOMAC study compares the effect of combining durvalumab plus BCG vs. BCG alone both in high-risk NMIBC patients; the BMS-986205 study compares the administration of nivolumab alone or in combination with BCG in BCG-unresponsive patients. The goal of both studies has been to evaluate the safety and effectiveness and a possible decrease of costs for patient management compared to standard therapy. As shown in Table 2, several clinical trials are designed to investigate different therapeutic approaches in BC. Combination therapies, based on BCG immunotherapy and different chemical (NCT01240824) or biological compounds (NCT00004122) or vaccines (NCT00070070), or combination therapies encompass also combination chemotherapies (Steinberg et al., 2018) especially in recurrent and advanced BC (NCT01938573 and NCT01828736, respectively). In addition, Table 2 shows ongoing clinical trials that might have clinical implications on the use of these drugs in the near future. Many BC trials are exploring immunotherapy, vaccines, chemotherapy, or gene therapy efficacy for BCG-unresponsive disease, advanced, recurrent, and metastatic BC. For a comprehensive list of drugs for several BC phenotypes, see Butt and Malik (2018), Rouanne et al. (2018), and Soria et al. (2019), while for specific NMIBCs, BCG-unresponsive, see Tse et al. (2019).
Bacillus Calmette-Guérin Intravesical Therapy
According to the EAU guidelines, the gold standard immunotherapy for intermediate- and high-risk NMIBC is intravesical full-dose BCG instillation (instillations 3, 6, 12, 18, 24, 30, and 36 months), for 1 year (intermediate-risk) or for 1–3 years (high-risk), respectively (Butt and Malik, 2018; Babjuk et al., 2019; Bajic et al., 2019a). In highest-risk tumors, e.g., associated with concurrent bladder carcinoma in situ (CIS) and BCG failure, radical cystectomy is recommended (Babjuk et al., 2019).
BCG immunotherapy ensures NMIBC treatment also in the elderly, not eligible for cisplatin systemic chemotherapy (Soria et al., 2019). Although BCG mode of action is still not completely understood, its efficacy in superficial bladder carcinoma might be achieved through the local enhancement of the immune response, recruitment of inflammatory cells, and release of cytokines (Lawrence et al., 2013; Song et al., 2019). Moreover, BCG therapy has been shown to reduce tumor progression and recurrence rate in NMIBC treated patients compared with NMIBC who underwent TURB alone or TURB plus chemotherapy (Lawrence et al., 2013; Song et al., 2019).
A debated point which requires intense further investigation is to understand if BCG substrains, generated over time, could alter the host response. The main substrains used are Russia, Moreau, Japan, Sweden, and Birkhaug (elimination of region of differentiation 1) and BCG Prague, Glaxo, Danish, Tice, Frappier, and others (deletion region of differentiation 2) (Hayashi et al., 2010; Kasempimolporn et al., 2018). However, findings available so far are not sufficient to support more effectiveness of specific BCG strain over another.
Bacillus Calmette-Guérin Unresponsive Patients: New Treatments
According to established EAU guidelines, in NMIBC intermediate-risk, 1-year full-dose intravesical BCG treatment or full-dose intravesical BCG for 1–3 years for high-risk NMIBC is recommended; however, a subset of NMIBC patients do not respond to BCG treatment (BCG failure) and may recur or progress with the neoplasm (Packiam et al., 2019). In BCG-unresponsive patients, poor therapeutic alternatives are available so far; radical cystectomy is the standard of care for the majority of BCG-refractory; intravesical valrubicin remains the only agent that is FDA approved in BCG-refractory patients with CIS (Cookson et al., 2014; Babjuk et al., 2019). Nevertheless, NMIBCs include disease entities with distinct prognoses. In 2020, EAU made some changes to the guidelines on NMIBC BCG failure treatments. The EAU recommends, in addition to radical cystectomy, bladder-preserving strategies with new treatment options or enrollment in clinical trials, although the strength rating for this latter chance is weak. Table 2 shows some clinical trials designed for BCG-refractory high-risk NMIBCs such as NCT03053635 and NCT00003167.
Innovative strategies deal with the improvement of BCG efficacy through recombinant BCG and priming–boosting strategy or the use of alternative systems such as (i) viruses, (ii) bacteria, and (iii) chemotherapeutic drugs. In regard to the therapeutic enhancement of the BCG immunotherapy, initial stimulation by subcutaneous BCG vaccination allowed to develop a more effective immune response following intravesical BCG bladder instillation (Svatek et al., 2018). Furthermore, biotechnologically advanced strategies have allowed the intravesical BCG to acquire characteristics such as to develop a beneficial immune response through the expression of specific bacterial antigens or through the modification of the genetic patrimony for the expression of molecules (cytokines or chemokines) with immunomodulatory properties (Begnini et al., 2015; Burggraaf et al., 2019).
Innovative strategies other than BCG therapy involve:
- Viruses can be delivered into the bladder through the current procedures and can have higher effectivity than BCG; by targeting specifically cancer cells, viruses could reduce adverse events compared with the use of the bacillus (Taguchi et al., 2017). Several ongoing clinical trials are testing this promising new strategy (Tse et al., 2019).
- Bacteria utilization could be another possible strategy in the unresponsive NMIBC or in NMIBC patients who have developed side effects. The use of other Mycobacteria substrains is being studied for BC treatment. Mycobacterium phlei represents an alternative in the treatment of unresponsive BCG patients; in addition, in preclinical studies, Mycobacterium brumae was evaluated as a safe and efficacious candidate for NMIBC (Morales and Cohen, 2016; Noguera-Ortega et al., 2016)
- Intravesical chemotherapeutic drugs, alone or in combination, are used for high-risk NMIBC such as pirarubicin, gemcitabine, and epirubicin (Kang et al., 2016); intravesical application of mitomycin C is a chemotherapeutic agent most used in patients who do not respond to BCG (Fankhauser et al., 2020). The latter approach is also useful for patients who have developed significant side effects; the combination of BCG treatment with chemotherapeutic agents has demonstrated the reduction of side effects and an improvement of tolerability to BCG (Huang et al., 2019).
- Cancer vaccines, novel biological drugs such as BCG vaccines have been evaluated to elicit an immune response and modulate side effects using recombinant BCG strains able to stimulate Th1 immune cells as well as induce cytokine release (Cho et al., 2019; Rodriguez et al., 2019).
Monotherapy, Combination Therapy, and Multi-Target Therapy
Combination therapies based on immunomodulators such as checkpoint inhibitors have shown a synergistic effect to augment the immune response (Marshall and Djamgoz, 2018).
A discrete amount of studies are based on combination therapy with chemotherapeutic drugs, intravesical BCG, and immune checkpoint inhibitors, as some trials reported in Table 1 (Aggen and Drake, 2017). Currently, an FDA-approved combination therapy is based on intravesical gemcitabine and cisplatin for NMIBCs (Rayn et al., 2018).
As shown in Table 2, several clinical trials are designed to investigate combination therapies based on BCG immunotherapy and different chemical (NCT01240824) or biological compounds (NCT00004122) or vaccines (NCT00070070). Combination therapies encompass also combination chemotherapies (Steinberg et al., 2018) especially in recurrent and advanced BC including (NCT01938573 and NCT01828736, respectively). Furthermore, photodynamic immunotherapy emerged recently to stimulate the immune response in NMIBC BCG-refractory or intolerant to BCG treatment as well (NCT03053635) (Lee et al., 2013).
One frontier of medicinal chemistry is polypharmacology (Proschak et al., 2019). Benedetti et al. (2015) reviewed the immuno-oncological dynamic interactions to design multi-target modulators.
A multitarget drug can be considered as a key drug that opens multiple locks, able to inhibit multiple molecules within cascade signaling or within crosstalk pathways (de Oliveira Viana et al., 2018). Among multitarget modulators, epi-enzymes, histone deacetylases (HDACs), and DNA methyltransferase (DNMT) families represent the most studied drug targets for several cancer types (Benedetti et al., 2015; Lu et al., 2018). One promising compound achieved by this design approach is levosimendan, which proved efficacy on several cancer cell lines including BC urothelial carcinoma (Lim et al., 2019).
Concluding Remarks
As opposed to other tumors, to date, there is not a real “routine” tumor prognostic molecular marker in BC clinical practice (Koncina et al., 2020). At diagnosis, most BCs are non-muscle-invasive and can be curable according to risk stratification into low, intermediate, and high risk; however, the minority of patients who do not respond to BCG immunotherapy poses a great challenge for the clinical decision-making. How to predict in advance patients with intermediate- or high-risk NMIBC, who will respond to BCG immunotherapy, or who will progress or recur into muscle-invasive phenotype is an open question. Furthermore, recurrence after BCG therapy for the primary tumor is still a challenge in the management of BC.
A better assessment of the NMIBC risk stratification and prognosis will provide significant medical, economic, and societal benefits. To overcome the high disease recurrence rate of NMIBCs and BCG failure, novel NGS techniques explore the association between bladder microbiome and therapy response and could provide BCG response biomarker in advance. To date, the possibility of using urobiome signatures as non-invasive biomarkers is still unlikely especially because contamination from other body districts is very high. For this reason, clinical studies focusing on patients catheterized urine from surveillance cystoscopy, rather than voided urine, are largely encouraged. Microbiome composition may be helpful to understand why some patients with NMIBC after BCG therapy have disease recurrences or progressions and others remain cured over time. New treatment options for bladder-preserving strategies are under evaluation to improve therapy efficacy in support of precision medicine also for bladder cancer. Urologists have to face the lack of prognosis accuracy for providing information on treatment options to intermediate- or high-risk NMIBCs patients. This has strong consequences also on patient counseling. In this concern, molecular nomograms for predicting prognosis and treatment response in NMIBCs will be very helpful.
Collectively, advances in multi-omic studies suggest that continuous efforts from wet-lab might provide shortly reliable molecular prognostic biomarkers.
Author Contributions
KP conceived and wrote the draft. All authors revised the manuscript and approved the final version and contributed to the conception of this work.
Funding
This work was supported by Progetti di Ricerca Corrente funded by the Italian Ministry of Health and in part under contract by “5 per mille” of IRCCS-SDN.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
ASMA, Alpha smooth muscle actin; BC, Bladder cancer; BCG, Bacillus Calmette-Guérin; CIS, Carcinoma in situ; CTLA-4, Cytotoxic T-lymphocyte associated protein 4; DNMT, DNA methyltransferase; EAU, European Association of Urology; EQUC, Expanded quantitative urine culture; FAP, Fibroblast activation protein alpha; FGFR3, Fibroblast growth factor receptor 3; HDACs, Histone deacetylases; IDO1, Indoleamine 2,3-dioxgenase 1; lncRNA, Long noncoding RNA; MIBCs, Muscle-invasive bladder cancers; NGS, Next-generation sequencing; NMIBCS, Non-muscle-invasive bladder cancers; OTUs, Operational taxonomic units; PD-1, Programmed cell death protein; PDGFRa-b, Platelet-derived growth factor receptor alpha and beta; PD-L1, Programmed death-ligand 1; TAMs, Tumor associated macrophages; TERTp, Telomerase reverse transcriptase gene promoter; TILs, Tumor infiltrating lymphocytes; TURB, Transurethral Resection of the Bladder.
References
Aggen, D. H., and Drake, C. G. (2017). Biomarkers for immunotherapy in bladder cancer: a moving target. J. Immunother. Cancer 5:94. doi: 10.1186/s40425-017-0299-1
Babjuk, M., Burger, M., Compérat, E., Gontero, P., Mostafid, A. H., Palou J., et al. (2020). “EAU guidelines on non-muscle-invasive bladder cancer (TaT1 and CIS)” in European Association of Urology Guidelines presented at the EAU Annual Congress Amsterdam 2020 (Arnhem: EAU Guidelines Office). Available online at: http://uroweb.org/guidelines/compilations-of-all-guidelines/
Babjuk, M., Burger, M., Compérat, E. M., Gontero, P., Mostafid, A. H., Palou, J., et al. (2019). European Association of Urology guidelines on non-muscle-invasive bladder cancer (TaT1 and Carcinoma In Situ)-−2019 update. Eur. Urol. 76, 639–657. doi: 10.1016/j.eururo.2019.08.016
Bajic, P., Wolfe, A. J., and Gupta, G. N. (2019a). Old instillations and new implications for bladder cancer: the urinary microbiome and intravesical BCG. BJU Int. 124, 7–8. doi: 10.1111/bju.14683
Bajic, P., Wolfe, A. J., and Gupta, G. N. (2019b). The urinary microbiome: implications in bladder cancer pathogenesis and therapeutics. Urology 126, 10–15. doi: 10.1016/j.urology.2018.12.034
Batista, R., Lima, L., Vinagre, J., Pinto, V., Lyra, J., Máximo, V., et al. (2020). TERT promoter mutation as a potential predictive biomarker in BCG-treated bladder cancer patients. Int. J. Mol. Sci. 21:947. doi: 10.3390/ijms21030947
Begnini, K. R., Buss, J. H., Collares, T., and Seixas, F. K. (2015). Recombinant mycobacterium bovis BCG for immunotherapy in nonmuscle invasive bladder cancer. Appl. Microbiol. Biotechnol. 99, 3741–3754. doi: 10.1007/s00253-015-6495-3
Benedetti, R., Conte, M., Iside, C., and Altucci, L. (2015). Epigenetic-based therapy: from single- to multi-target approaches. Int. J. Biochem. Cell Biol. 69, 121–131. doi: 10.1016/j.biocel.2015.10.016
Bersanelli, M., Santoni, M., Ticinesi, A., and Buti, S. (2019). The urinary microbiome and anticancer immunotherapy: the potentially hidden role of unculturable microbes. Target. Oncol. 14, 247–252. doi: 10.1007/s11523-019-00643-7
Bučević Popović, V., Šitum, M., Chow, C. E. T., Chan, L. S., Roje, B., and Terzić, J. (2018). The urinary microbiome associated with bladder cancer. Sci. Rep. 8:12157. doi: 10.1038/s41598-018-29054-w
Burggraaf, M. J., Ates, L. S., Speer, A., Van Der Kuij, K., Kuijl, C., and Bitter, W. (2019). Optimization of secretion and surface localization of heterologous OVA protein in mycobacteria by using LipY as a carrier. Microb. Cell Fact. 18:44. doi: 10.1186/s12934-019-1093-1
Butt, S.-R., and Malik, L. (2018). Role of immunotherapy in bladder cancer: past, present and future. Cancer Chemother. Pharmacol. 81, 629–645. doi: 10.1007/s00280-018-3518-7
Castaldo, R., Pane, K., Nicolai, E., Salvatore, M., and Franzese, M. (2020). The impact of normalization approaches to automatically detect radiogenomic phenotypes characterizing breast cancer receptors status. Cancers. 12:518. doi: 10.3390/cancers12020518
Chakraborty, S., Hosen, M. I., Ahmed, M., and Shekhar, H. U. (2018). Onco-Multi-OMICS approach: a new frontier in cancer research. BioMed Res. Int. 2018:9836256. doi: 10.1155/2018/9836256
Cho, M. J., Kim, M. J., Kim, K., Choi, Y. W., Lee, S. J., Whang, Y. M., et al. (2019). The immunotherapeutic effects of recombinant Bacillus Calmette-Guérin resistant to antimicrobial peptides on bladder cancer cells. Biochem. Biophys. Res. Commun. 509, 167–174. doi: 10.1016/j.bbrc.2018.12.097
Chu, C. E., Porten, S. P., Grossfeld, G. D., and Meng, M. V. (2020). Role of indoleamine-2,3-dioxygenase inhibitors in salvage therapy for non-muscle invasive bladder cancer. Urol. Clin. North Am. 47, 111–118. doi: 10.1016/j.ucl.2019.09.013
Cookson, M. S., Chang, S. S., Lihou, C., Li, T., Harper, S. Q., Lang, Z., et al. (2014). Use of intravesical valrubicin in clinical practice for treatment of nonmuscle-invasive bladder cancer, including carcinoma in situ of the bladder. Ther. Adv. Urol. 6, 181–191. doi: 10.1177/1756287214541798
Cooley, L. F., McLaughlin, K. A., and Meeks, J. J. (2020). Genomic and therapeutic landscape of non-muscle-invasive bladder cancer. Urol. Clin. North Am. 47, 35–46. doi: 10.1016/j.ucl.2019.09.006
Coppola, L., Cianflone, A., Grimaldi, A. M., Incoronato, M., Bevilacqua, P., Messina, F., et al. (2019). Biobanking in health care: evolution and future directions. J. Transl. Med. 17:172. doi: 10.1186/s12967-019-1922-3
de la Fuente-Nunez, C., Torres, M. D., Mojica, F. J., and Lu, T. K. (2017). Next-generation precision antimicrobials: towards personalized treatment of infectious diseases. Curr. Opin. Microbiol. 37, 95–102. doi: 10.1016/j.mib.2017.05.014
de Oliveira Viana, J., Barbalho Félix, M., dos Santos Maia, M., de Lima Serafim, V., Scotti, L., and Tullius Scotti, M. (2018). Drug discovery and computational strategies in the multitarget drugs era. Braz. J. Pharm. Sci. 54:e01010. doi: 10.1590/s2175-97902018000001010
Fankhauser, C. D., Teoh, J. Y. C., and Mostafid, H. (2020). Treatment options and results of adjuvant treatment in nonmuscle-invasive bladder cancer (NMIBC) during the Bacillus Calmette-Guérin shortage. Curr. Opin. Urol. 30, 365–369. doi: 10.1097/MOU.0000000000000739
Ferreira, L., Sánchez-Juanes, F., González-Ávila, M., Cembrero-Fuciños, D., Herrero-Hernández, A., González-Buitrago, J. M., et al. (2010). Direct identification of urinary tract pathogens from urine samples by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J. Clin. Microbiol. 48, 2110–2115. doi: 10.1128/JCM.02215-09
Forkosh, E., and Ilan, Y. (2019). The heart-gut axis: new target for atherosclerosis and congestive heart failure therapy. Open Heart 6:e000993. doi: 10.1136/openhrt-2018-000993
Gaglione, R., Pane, K., Dell'Olmo, E., Cafaro, V., Pizzo, E., Olivieri, G., et al. (2019). Cost-effective production of recombinant peptides in Escherichia coli. N. Biotechnol. 51, 39–48. doi: 10.1016/j.nbt.2019.02.004
Govender, Y., Gabriel, I., Minassian, V., and Fichorova, R. (2019). The current evidence on the association between the urinary microbiome and urinary incontinence in women. Front. Cell. Infect. Microbiol. 9:133. doi: 10.3389/fcimb.2019.00133
Guillamón, C. F., Gimeno, L., Server, G., Martínez-Sánchez, M. V., Escudero, J. F., López-Cubillana, P., et al. (2019). Immunological risk stratification of bladder cancer based on peripheral blood natural killer cell biomarkers. Eur. Urol. Oncol. (Elsevier). doi: 10.1016/j.euo.2019.04.009. [Epub ahead of print].
Hayashi, D., Takii, T., Mukai, T., Makino, M., Yasuda, E., Horita, Y., et al. (2010). Biochemical characteristics among Mycobacterium bovis BCG substrains. FEMS Microbiol. Lett. 306, 103–109. doi: 10.1111/j.1574-6968.2010.01947.x
Huang, D., Jin, Y. H., Weng, H., Huang, Q., Zeng, X. T., and Wang, X. H. (2019). Combination of intravesical Bacille Calmette-Guérin and chemotherapy vs. Bacille Calmette-Guérin alone in non-muscle invasive bladder cancer: a meta-analysis. Front. Oncol. 9:121. doi: 10.3389/fonc.2019.00121
Incoronato, M., Aiello, M., Infante, T., Cavaliere, C., Grimaldi, A. M., Mirabelli, P., et al. (2017). Radiogenomic analysis of oncological data: a technical survey. Int. J. Mol. Sci. 18:805. doi: 10.3390/ijms18040805
Kamat, A. M., Hahn, N. M., Efstathiou, J. A., Lerner, S. P., Malmström, P. U., Choi, W., et al. (2016). Bladder cancer. Lancet 388, 2796–2810. doi: 10.1016/S0140-6736(16)30512-8
Kang, M., Jeong, C. W., Kwak, C., Kim, H. H., and Ku, J. H. (2016). Single, immediate postoperative instillation of chemotherapy in non-muscle invasive bladder cancer: a systematic review and network meta-analysis of randomized clinical trials using different drugs. Oncotarget 7, 45479–45488. doi: 10.18632/oncotarget.9991
Karstens, L., Asquith, M., Caruso, V., Rosenbaum, J. T., Fair, D. A., Braun, J., et al. (2018). Community profiling of the urinary microbiota: considerations for low-biomass samples. Nat. Rev. Urol. 15, 735–749. doi: 10.1038/s41585-018-0104-z
Kasempimolporn, S., Premchaiporn, P., Thaveekarn, W., Boonchang, S., and Sitprija, V. (2018). Comparative proteomic profiling of Mycobacterium tuberculosis and the Thai vaccine strain Mycobacterium bovis Bacille Calmette-Guerin Tokyo172: diverse biomarker candidates for species differentiation. J. Glob. Infect. Dis. 10, 196–200. doi: 10.4103/jgid.jgid_149_17
Koncina, E., Haan, S., Rauh, S., and Letellier, E. (2020). Prognostic and predictive molecular biomarkers for colorectal cancer: udates and challenges. Cancers 12:319. doi: 10.3390/cancers12020319
Korman, A. J., Peggs, K. S., and Allison, J. P. (2006). Checkpoint blockade in cancer immunotherapy. Adv. Immunol. 90, 297–339. doi: 10.1016/S0065-2776(06)90008-X
Krämer, A., Green, J., Pollard, J., and Tugendreich, S. (2014). Causal analysis approaches in ingenuity pathway analysis. Bioinformatics. 30, 523–530. doi: 10.1093/bioinformatics/btt703
Lalani, A. K. A., and Sonpavde, G. P. (2019). Systemic treatments for metastatic urothelial carcinoma. Expert Opin. Pharmacother. 20, 201–208. doi: 10.1080/14656566.2018.1544242
Lawrence, M. S., Stojanov, P., Polak, P., Kryukov, G. V., Cibulskis, K., Sivachenko, A., et al. (2013). Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature 499, 214–218. doi: 10.1038/nature12213
Lee, J. Y., Diaz, R. R., Cho, K. S., Lim, M. S., Chung, J. S., Kim, W. T., et al. (2013). Efficacy and safety of photodynamic therapy for recurrent, high grade nonmuscle invasive bladder cancer refractory or intolerant to Bacille Calmette-Guérin immunotherapy. J. Urol. 190, 1192–1199. doi: 10.1016/j.juro.2013.04.077
Lim, H., He, D., Qiu, Y., Krawczuk, P., Sun, X., and Xie, L. (2019). Rational discovery of dual-indication multi-target PDE/Kinase inhibitor for precision anti-cancer therapy using structural systems pharmacology. PLoS Comput. Biol. 15:e1006619. doi: 10.1371/journal.pcbi.1006619
Lu, W., Zhang, R., Jiang, H., Zhang, H., and Luo, C. (2018). Computer-aided drug design in epigenetics. Front. Chem. 6:57. doi: 10.3389/fchem.2018.00057
Marchesi, J. R., Adams, D. H., Fava, F., Hermes, G. D. A., Hirschfield, G. M., Hold, G., et al. (2016). The gut microbiota and host health: a new clinical frontier. Gut 65, 330–339. doi: 10.1136/gutjnl-2015-309990
Marshall, H. T., and Djamgoz, M. B. A. (2018). Immuno-oncology: emerging targets and combination therapies. Front. Oncol. 8:315. doi: 10.3389/fonc.2018.00315
McConkey, D. J., and Lerner, S. P. (2019). SIU–ICUD consultation on bladder cancer: basic science. World J. Urol. 37, 15–29. doi: 10.1007/s00345-018-2594-y
Mezheyeuski, A., Segersten, U., Leiss, L. W., Malmström, P. U., Hatina, J., Östman, A., et al. (2020). Fibroblasts in urothelial bladder cancer define stroma phenotypes that are associated with clinical outcome. Sci. Rep. 10:281. doi: 10.1038/s41598-019-55013-0
Morales, A., and Cohen, Z. (2016). Mycobacterium phlei cell wall-nucleic acid complex in the treatment of nonmuscle invasive bladder cancer unresponsive to Bacillus Calmette-Guerin. Expert Opin. Biol. Ther. 16, 273–283. doi: 10.1517/14712598.2016.1134483
Noguera-Ortega, E., Secanella-Fandos, S., Eraña, H., Gasión, J., Rabanal, R. M., Luquin, M., et al. (2016). Nonpathogenic Mycobacterium brumae inhibits bladder cancer growth in vitro, ex vivo, and in vivo. Eur. Urol. Focus 2, 67–76. doi: 10.1016/j.euf.2015.03.003
Okazaki, T., and Honjo, T. (2007). PD-1 and PD-1 ligands: from discovery to clinical application. Int. Immunol. 19, 813–824. doi: 10.1093/intimm/dxm057
Packiam, V. T., Werntz, R. P., and Steinberg, G. D. (2019). Current clinical trials in non-muscle-invasive bladder cancer: heightened need in an era of chronic BCG shortage. Curr. Urol. Rep. 20:84. doi: 10.1007/s11934-019-0952-y
Proschak, E., Stark, H., and Merk, D. (2019). Polypharmacology by design: a medicinal chemist's perspective on multitargeting compounds. J. Med. Chem. 62, 420–444. doi: 10.1021/acs.jmedchem.8b00760
Rayn, K. N., Hale, G. R., Grave, G. P.-L., and Agarwal, P. K. (2018). New therapies in nonmuscle invasive bladder cancer treatment. Indian J. Urol. 34, 11–19. doi: 10.4103/iju.IJU_296_17
Rodriguez, D., Goulart, C., Pagliarone, A. C., Silva, E. P., Cunegundes, P. S., Nascimento, I. P., et al. (2019). In vitro evidence of human immune responsiveness shows the improved potential of a recombinant BCG strain for bladder cancer treatment. Front. Immunol. 10:1460. doi: 10.3389/fimmu.2019.01460
Rouanne, M., Betari, R., Radulescu, C., Goubar, A., Signolle, N., Neuzillet, Y., et al. (2019). Stromal lymphocyte infiltration is associated with tumour invasion depth but is not prognostic in high-grade T1 bladder cancer. Eur. J. Cancer 108, 111–119. doi: 10.1016/j.ejca.2018.12.010
Rouanne, M., Roumiguié, M., Houédé, N., Masson-Lecomte, A., Colin, P., Pignot, G., et al. (2018). Development of immunotherapy in bladder cancer: present and future on targeting PD(L)1 and CTLA-4 pathways. World J. Urol. 36, 1727–1740. doi: 10.1007/s00345-018-2332-5
Siegel, R. L., Miller, K. D., and Jemal, A. (2020). Cancer statistics, 2020. CA Cancer J. Clin. 70, 7–30. doi: 10.3322/caac.21590
Song, D., Powles, T., Shi, L., Zhang, L., Ingersoll, M. A., and Lu, Y.-J. (2019). Bladder cancer, a unique model to understand cancer immunity and develop immunotherapy approaches. J. Pathol. 249, 151–165. doi: 10.1002/path.5306
Soria, F., Mosca, A., and Gontero, P. (2019). Drug strategies for bladder cancer in the elderly: is there promise for the future? Expert Opin. Pharmacother. 20, 1387–1396. doi: 10.1080/14656566.2019.1615055
Soukup, V., Capoun, O., Cohen, D., Hernández, V., Burger, M., Compérat, E., et al. (2020). Risk stratification tools and prognostic models in non–muscle-invasive bladder cancer: a critical assessment from the European Association of Urology non-muscle-invasive bladder cancer guidelines panel. Eur. Urol. Focus 6, 479–489. doi: 10.1016/j.euf.2018.11.005
Steinberg, R. L., Thomas, L. J., and O'Donnell, M. A. (2018). Combination intravesical chemotherapy for non–muscle-invasive bladder cancer. Eur. Urol. Focus 4, 503–505. doi: 10.1016/j.euf.2018.07.005
Svatek, R. S., Tangen, C., Delacroix, S., Lowrance, W., and Lerner, S. P. (2018). Background and update for S1602 “a phase iii randomized trial to evaluate the influence of BCG strain differences and T cell priming with intradermal BCG before intravesical therapy for BCG-naïve high-grade non-muscle-invasive bladder cancer. Eur. Urol. Focus 4, 522–524. doi: 10.1016/j.euf.2018.08.015
Taguchi, S., Fukuhara, H., Homma, Y., and Todo, T. (2017). Current status of clinical trials assessing oncolytic virus therapy for urological cancers. Int. J. Urol. 24, 342–351. doi: 10.1111/iju.13325
Tang, Z. Z., Chen, G., and Alekseyenko, A. V. (2016). PERMANOVA-S: Association test for microbial community composition that accommodates confounders and multiple distances. Bioinformatics. 32, 2618–2625. doi: 10.1093/bioinformatics/btw311
Tse, J., Singla, N., Ghandour, R., Lotan, Y., and Margulis, V. (2019). Current advances in BCG-unresponsive non-muscle invasive bladder cancer. Expert Opin. Investig. Drugs 28, 757–770. doi: 10.1080/13543784.2019.1655730
Wolfe, A. J., and Brubaker, L. (2019). Urobiome updates: advances in urinary microbiome research. Nat. Rev. Urol. 16, 73–74. doi: 10.1038/s41585-018-0127-5
Wu, Z., Zhang, Z., Xia, W., Cai, J., Li, Y., and Wu, S. (2019). Extracellular vesicles in urologic malignancies—implementations for future cancer care. Cell Prolif. 52:e12659. doi: 10.1111/cpr.12659
Xu, W., Yang, L., Lee, P., Huang, W. C., Nossa, C., Ma, Y., et al. (2014). Mini-review: perspective of the microbiome in the pathogenesis of urothelial carcinoma. Am. J. Clin. Exp. Urol. 2, 57–61.
Yoshida, N., Yamashita, T., and Hirata, K. (2018). Gut microbiome and cardiovascular diseases. Diseases 6:56. doi: 10.3390/diseases6030056
Zanfardino, M., Franzese, M., Pane, K., Cavaliere, C., Monti, S., Esposito, G., et al. (2019a). Bringing radiomics into a multi-omics framework for a comprehensive genotype-phenotype characterization of oncological diseases. J. Transl. Med. 17:337. doi: 10.1186/s12967-019-2073-2
Zanfardino, M., Pane, K., Mirabelli, P., Salvatore, M., and Franzese, M. (2019b). TCGA-TCIA impact on radiogenomics cancer research: a systematic review. Int. J. Mol. Sci. 20:6033. doi: 10.3390/ijms20236033
Keywords: bladder cancer, Bacillus Calmette-Guérin, omics, biomarker, prognostic factor, microbiome, immunotherapy, non-muscle-invasive bladder cancer
Citation: Pane K, Mirabelli P, Coppola L, Illiano E, Salvatore M and Franzese M (2020) New Roadmaps for Non-muscle-invasive Bladder Cancer With Unfavorable Prognosis. Front. Chem. 8:600. doi: 10.3389/fchem.2020.00600
Received: 14 February 2020; Accepted: 09 June 2020;
Published: 31 July 2020.
Edited by:
Mariarosaria Conte, University of Campania Luigi Vanvitelli, ItalyReviewed by:
Bernd Wullich, University Hospital Erlangen, GermanyUlrike Zwergel, Saarland University, Germany
Copyright © 2020 Pane, Mirabelli, Coppola, Illiano, Salvatore and Franzese. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Katia Pane, a2F0aWEucGFuZUBzeW5sYWIuaXQ=
 Katia Pane
Katia Pane Peppino Mirabelli
Peppino Mirabelli Luigi Coppola
Luigi Coppola Ester Illiano2
Ester Illiano2 Marco Salvatore
Marco Salvatore Monica Franzese
Monica Franzese