
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
PERSPECTIVE article
Front. Chem. , 30 June 2020
Sec. Chemical Physics and Physical Chemistry
Volume 8 - 2020 | https://doi.org/10.3389/fchem.2020.00541
This article is part of the Research Topic Advances in Oscillating Reactions View all 11 articles
We present an overview of our studies on the hampering effect of heavy water (D2O) on spontaneous oscillatory peptidization of selected proteinogenic α-amino acids. The investigated set of compounds included three endogenous and two exogenous species. The experiments were carried out with use of high-performance liquid chromatography (HPLC), mass spectrometry (MS) and scanning electron microscopy (SEM). These techniques were chosen to demonstrate spontaneous oscillatory peptidization of α-amino acids in an absence of D2O (HPLC) and the hampering effect of D2O on peptidization (HPLC, MS and SEM). The HPLC analyses were carried out at 21 ± 0.5°C with each α-amino acid freshly dissolved in the binary liquid mixture of organic solvent + H2O, 70:30 (v/v) or in pure D2O for several dozen hours or several hours, respectively. The analyses with use of MS and SEM were carried out, respectively, after 7 days and 1 month of sample storage period in the darkness at 21 ± 0.5°C and for these experiments, each α-amino acid was dissolved in the liquid mixture of organic solvent + X, 70:30 (v/v), where X: H2O + D2O in volume proportions from 30:0 to 0:30. The results obtained with use of HPLC, MS and SEM point out to the strong hampering effect of D2O on the oscillations and peptidization yields, yet the dynamics of these processes significantly depends on chemical structure of a given α-amino acid.
Our research on spontaneous oscillatory processes in organic chemistry has started with an observation that the thin-layer chromatographic (TLC) runs of ibuprofen resulted in an irreproducible retardation factor (RF) which was, however, confined to the two borderlines (Sajewicz et al., 2005a). Although ibuprofen is regarded as structurally stable and indestructible in the binary organic-aqueous solvents, irreproducibility of retardation factor (RF) was clearly related to the length of its storage period in solution. Later, an analogical phenomenon was observed with other profen drugs (Sajewicz et al., 2005b, 2006a,b, 2007; Marczak et al., 2006), α-hydroxy acids (Sajewicz et al., 2008a, 2009) and α-amino acids (Sajewicz et al., 2008b,c). Common structural denominator was that all these compounds were chiral low molecular weight carboxylic acids. Soon it became evident that the borderlines of the retardation factor (RF) values were those characterizing pure (+)- and pure (–)-enantiomer, and the phenomenon was the oscillatory chiral inversion (Sajewicz et al., 2008a,c, 2009).
Based on general knowledge of reaction mechanisms, it was understood that the elementary steps of chiral inversion proceed via the non-chiral intermediary products and their presence was demonstrated in the experiment performed with use of the high-performance liquid chromatography (HPLC) employing chiral stationary phase dedicated to the enantioseparation of α-amino acids (Sajewicz et al., 2014b). As the test analytes, two enantiomers of phenylglycine (Phg) were used and separation thereof was carried out for the period of 44 h. After 6 h, two separate chromatographic peaks of L-PhG and D-Phg coalesced into one and the coalescence period lasted 12 h. After that time, the single coalesced peak split to again give two peaks of L-PhG and D-Phg. This process was monitored with use of the DAD detector and a clear difference was observed between the identical UV spectra valid for L-PhG and D-Phg on the one hand, and the UV spectrum of the coalesced peak on the other. The latter one was ascribed to the non-chiral intermediary product(s) and its relative longevity (12 h) was regarded as striking and noteworthy. Our discovery of spontaneous oscillatory chiral inversion was approved by some other researchers as a reasonable justification of their own striking and quite unexpected results (e.g., Rincon et al., 2009; Stich et al., 2013).
Proteinogenic α-amino acids as the smallest “bricks” of all living matter seem the most important species undergoing oscillatory chiral inversion. Soon it became clear that α-amino acids undergo oscillatory peptidization as well. The main analytical tool to monitor this phenomenon was the non-chiral HPLC system and we focused on signals originating from the monomeric (i.e., non-peptidized) α-amino acids and their oscillatory concentration changes in the function of time, due to the peptidization–depeptidization process. We published a number of reports on this phenomenon which was also theoretically modeled, and the most important papers are (Sajewicz et al., 2010, 2014a; Godziek et al., 2016; Maciejowska et al., 2016). Up to our best knowledge, there had never been any earlier report neither on spontaneous peptidization of α-amino acids under such mild external conditions (dissolution at ambient temperature in an organic aqueous solvent), nor on oscillatory nature of this process. All our investigations were inspired by an interest in chemical evolution preceding biological one, in which spontaneous peptidization of α-amino acids might have played a significant role.
With an improved availability of D2O in the thirties of the twentieth century, its influence on animals and plants has become investigated and a slowdown effect on life processes was recognized, in extreme cases resulting in an organism's death (Harvey, 1934; Lewis, 1934; Katz et al., 1962). Current experiments with D2O largely focus on its apoptotic effect on cancer cells (Takeda et al., 1998; Hartmann et al., 2005). All these results instigated our interest in an impact of D2O on the dynamics of spontaneous oscillatory peptidization and peptidization yields with selected endogenous (L-Cys, L-Pro, and L-Ala) and exogenous (L-Met and L-Hyp) α-amino acids as the smallest building blocks of all living matter.
Five α-amino acids (L-Cys, L-Met, L-Pro, L-Hyp, and L-Ala) were dissolved in the binary liquid mixture of the organic solvent + H2O, 70:30 (v/v) (where organic solvent: acetonitrile or methanol) and due to oscillatory peptidization-depeptidization process, the monomer concentrations of these compounds underwent spontaneous oscillatory changes. These changes were recorded with use of the non-chiral HPLC and graphically presented in supplementary materials of papers (Fulczyk et al., 2018, 2019a,b, 2020a,b) as time series, together with the corresponding plots of the Fourier-transformed data. From Fourier transformation it came out that with two endogenous species (L-Cys and L-Pro) the circadian rhythm of the oscillatory concentration changes occurs as equal to ca. 24 and ca. 20.8 h, respectively, and with two exogenous species (L-Met and L-Hyp) no periodicity of oscillations is observed. With the third endogenous species (L-Ala), the oscillatory pattern is still different. After two initial and not periodic oscillations lasting for ca.10 h, the investigated system reaches a steady state.
Dissolution of the investigated α-amino acids in pure D2O results in full inhibition of oscillatory peptidization, as confirmed by HPLC. Differentiation of peptidization dynamics and peptide yields becomes visible only, when proportions of D2O in solution stepwise change. Mass spectrometry (MS) and scanning electron microscopy (SEM) were used to record differences in the hampering effect of D2O on dynamics of peptidization and peptide yields depending of proportions of heavy water in solution.
Apart from considering the investigated compounds as belonging to two groups of endogenous and exogenous α-amino acids, they can also be viewed as three structural entities: two sulfur atom containing species (L-Cys, L-Met), two pyrrolidine ring and secondary amino group containing species (L-Pro, L-Hyp), and the simplest endogenous α-amino acid, L-Ala. First, let us reflect on the mass spectrometric results. As mass spectrometric technique used in this study was applicable to monitoring liquid samples alone, the obtained mass spectra provided information on changing contents of soluble lower molecular weight peptides depending on proportions of D2O in solution. For each α-amino acid considered, mass spectra were recorded after 7 days sample storage period in the darkness at 21 ± 0.5°C, in presence of 0, 5, 10, 20, 30, and 100% D2O. The obtained MS results were originally published in papers (Fulczyk et al., 2018, 2019a,b, 2020a,b). It was shown that with increasing proportions of D2O in solution, L-Cys and L-Met produce growing yields of soluble lower peptides. With L-Pro, the yields of soluble lower peptides are decreasing up to 10% D2O, but then with higher amounts of D2O these yields start gradually growing. With L-Hyp and L-Ala, the decreasing yields of soluble lower peptides are observed in pace with growing proportions of D2O in solution. It can be concluded that the response of five investigated α-amino acids to changing quantitative proportions of D2O in solution depends on chemical structure of individual species rather than on belongingness to the endogenous/exogenous category, or on structural similarity of species (although a similar trend is observed with two sulfur atom containing α-amino acids, i.e., L-Cys and L-Met). Figure 1 briefly illustrates an impact of selected proportions of D2O on the yields of soluble lower peptides upon the examples of L-Cys, L-Pro, and L-Ala.
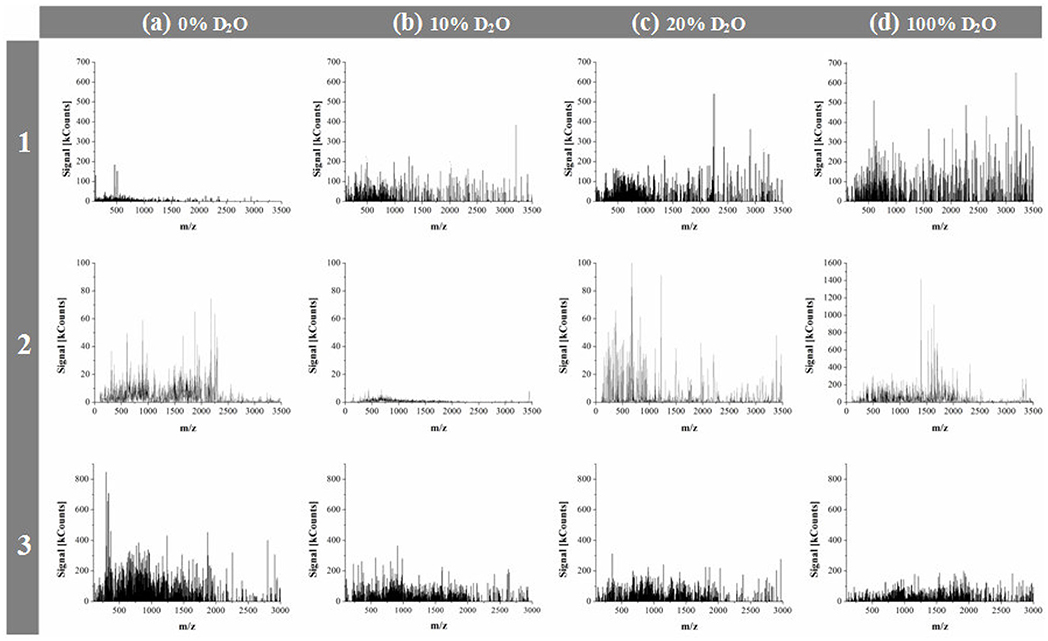
Figure 1. Mass spectra recorded for L-Cys (1), L-Pro (2), and L-Ala (3), respectively, dissolved in organic solvent + X, 70:30 (v/v), where X: the binary mixture of H2O + D2O in the changing volume proportions; (a) 0% D2O; (b) 10% D2O; (c) 20% D2O, and (d) 100% D2O. Organic solvent: ACN (L-Cys), or MeOH (L-Pro and L-Ala); [adapted from Figures in Fulczyk et al. (2018, 2019a, 2020a)].
Now let us consider the results originating from the scanning electron microscopy. For each α-amino acid under the discussion, scanning electron micrographs were recorded after 1 month sample storage period in the darkness at 21 ± 0.5°C, in presence of 0, 5, 10, 20, 30, and 100% D2O. As this technique was applicable to solid samples alone, basic information which can be derived from the obtained micrographs focuses on changing yields of insoluble higher peptides, depending on proportions of D2O in solution. The obtained results unequivocally demonstrate that irrespective of the α-amino acid considered, in the presence of D2O in solution the hampering effect on peptidization process is observed and consequently, the yields of higher insoluble peptide drastically lower. Apparently, the higher is the D2O proportion in solution, the stronger pronounced is the hampering effect. For each species, a series of photographs was taken at different magnifications to best illustrate regularities and trends of this inhibitory effect and they were published in papers (Fulczyk et al., 2018, 2019a,b, 2020a,b). In Figure 2, we present selected micrographs valid for L-Cys, L-Met, and L-Pro, which illustrate an impact of the growing proportions of D2O on formation of insoluble higher peptides. They also allow a comparison of different structural forms of peptides derived from each individual α-amino acid. Peptides derived from L-Cys are spherical and gather in greater and spongy-looking structures. Peptides derived from L-Met are the flat, elongated laminas with sharp edges, and peptides derived from L-Pro resemble the 3D starry-looking objects of different sizes. All these micrograph series collected in Figure 2 provide convincing evidence that the increasing proportions of D2O in solutions really result in diminishing yields of higher insoluble peptides.
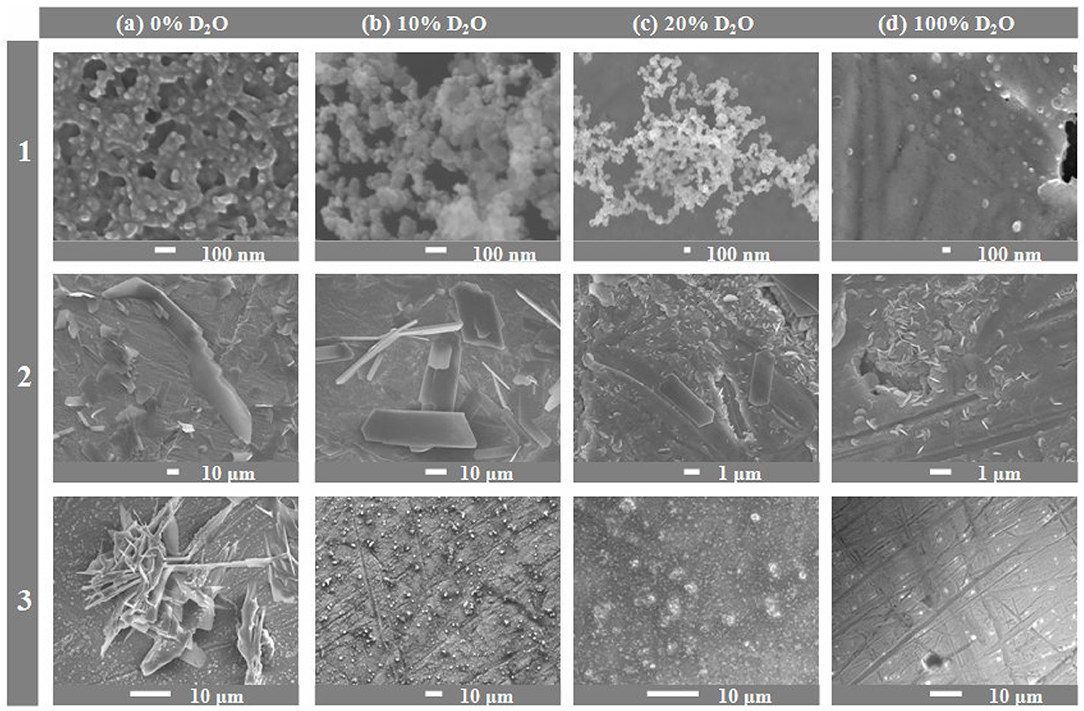
Figure 2. Scanning electron micrographs recorded for L-Cys (1), L-Met (2), and L-Pro (3), respectively, dissolved in organic solvent + X, 70:30 (v/v), where X: the binary mixture of H2O + D2O in the changing volume proportions; (a) 0% D2O; (b) 10% D2O; (c) 20% D2O, and (d) 100% D2O. Organic solvent: ACN (L-Cys and L-Met), or MeOH (L-Pro). Magnifications of individual micrographs: (a1) × 100,000; (a2) × 550; (a3) × 1,900; (b1) × 100,000; (b2) × 1,000; (b3) × 750; (c1) × 30,000; (c2) × 7,500; (c3) × 2,500, (d1) × 37,000; (d2) × 10,000; (d3) × 1,500 [partially adapted from Figures in Fulczyk et al. (2018, 2019a,b)].
The starting point of our research project was demonstration with use of the non-chiral HPLC that all investigated proteinogenic (endogenous and exogenous) α-amino acids dissolved in an organic-aqueous solvent yet in an absence of D2O undergo spontaneous oscillatory peptidization. Then we focused on the impact of D2O on spontaneous oscillatory peptidization process carried out either in pure heavy water, or in the mixture of organic solvent, H2O and D2O. With use of scanning electron microscopy (SEM), it was shown that D2O hampers formation of insoluble higher peptides with all investigated species and this effect is monotonously dependent on proportions of D2O in solution. With use of mass spectrometry (MS), it was shown though that D2O affects formation of the soluble lower peptides in a less straightforward manner. With L-Cys and L-Met, growing proportions of D2O in solution result in growing yields of respective lower soluble peptides. With L-Hyp and L-Ala, growing proportions of D2O in solution result in declining yields of respective peptides. Response of L-Pro to proportions of D2O in solution is non-monotonous. Initially, growing proportions of D2O in solution result in the declining yields of the lower soluble peptides, but for the D2O proportions above 10% these yields rather unexpectedly start growing. Within the framework of the adopted research project, we disclosed individual patterns of spontaneous oscillatory peptidization in an absence of heavy water and also inhibition of this process with D2O under the assumed working conditions for three endogenous and two exogenous α-amino acids.
Summing up, even if the research perspective outlined in this study is currently absent from consciousness of scientific community worldwide or underestimated by many (partially due to serious limitations of effective analytical tools), we firmly believe in purposefulness of carrying out similar research even on a much larger scale, simply because we can see its relevance to a wide spectrum of diverse scientific disciplines spanned between evolutionary history of life and contemporary medicine.
The original contributions presented in the study are included in the article/supplementary materials, further inquiries can be directed to the corresponding author/s.
All authors equally contributed to preparation of the submitted manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Fulczyk, A., Łata, E., Dolnik, M., Talik, E., Kowalska, T., and Sajewicz, M. (2018). Impact of D2O on peptidization of L-cysteine. React. Kinet. Mech. Catal. 125, 555–565. doi: 10.1007/s11144-018-1469-y
Fulczyk, A., Łata, E., Talik, E., Dolnik, M., Kowalska, T., and Sajewicz, M. (2020a). Impact of D2O on peptidization of L-alanine. React. Kinet. Mech. Catal. 43, 745–750. doi: 10.1007/s11144-020-01783-y
Fulczyk, A., Łata, E., Talik, E., Kowalska, T., and Sajewicz, M. (2019a). Impact of D2O on peptidization of L-proline. React. Kinet. Mech. Catal. 128, 599–610. doi: 10.1007/s11144-019-01681-y
Fulczyk, A., Łata, E., Talik, E., Kowalska, T., and Sajewicz, M. (2019b). Impact of D2O on peptidization of L-methionine. React. Kinet. Mech. Catal. 126, 939–949. doi: 10.1007/s11144-019-01538-4
Fulczyk, A., Łata, E., Talik, E., Kowalska, T., and Sajewicz, M. (2020b). Impact of D2O on peptidization of L-hydroxyproline. React. Kinet. Mech. Catal. 129, 17–28. doi: 10.1007/s11144-019-01711-9
Godziek, A., Maciejowska, A., Talik, E., Wrzalik, R., Sajewicz, M., and Kowalska, T. (2016). On spontaneously pulsating proline-phenylalanine peptide microfibers. Curr. Protein Pept. Sci. 17, 106–116. doi: 10.2174/138920371702160209121513
Hartmann, J., Bader, Y., Horvath, Z., Saiko, P., Grusch, M., Illmer, C., et al. (2005). Effects of heavy water (D2O) on human pancreatic tumor cells. Anticancer Res. 25, 3407–3411.
Harvey, E. N. (1934). Biological effects of heavy water. Biol. Bull. 66, 91–96. doi: 10.2307/1537322
Katz, J. J., Crespi, H. L., Czajka, D. M., and Finkel, A. J. (1962). Course of deuteriation and some physiological effects of deuterium in mice. Am. J. Physiol. 203, 907–913. doi: 10.1152/ajplegacy.1962.203.5.907
Lewis, G. N. (1934). The biology of heavy water. Science 79, 151–153. doi: 10.1126/science.79.2042.151
Maciejowska, A., Godziek, A., Talik, E., Sajewicz, M., Kowalska, T., and Epstein, I. R. (2016). Spontaneous pulsation of peptide microstructures in an abiotic liquid system. J. Chromatogr. Sci. 54, 1301–1309. doi: 10.1093/chromsci/bmw073
Marczak, W., Kowalska, T., Bucek, M., Piotrowski, D., and Sajewicz, M. (2006). Effect of dilution on compressibility of naproxen in acetonitrile studied by ultrasonic method. J. Phys. 137, 219–222. doi: 10.1051/jp4:2006137045
Rincon, A. G., Guzman, M. I., Hoffmann, M. R., and Colussi, A. J. (2009). Optical absorptivity versus molecular composition of model organic aerosol matter. J. Phys. Chem. A 113, 10512–10520. doi: 10.1021/jp904644n
Sajewicz, M., Dolnik, M., Kowalska, T., and Epstein, I. R. (2014a). Condensation dynamics of L-proline and L-hydroxyproline in solution. RCS Adv. 4, 7330–7339. doi: 10.1039/C3RA46921B
Sajewicz, M., Gontarska, M., and Kowalska, T. (2014b). HPLC/DAD evidence of the oscillatory chiral conversion of phenylglycine. J. Chromatogr. Sci. 52, 329–333. doi: 10.1093/chromsci/bmt033
Sajewicz, M., Gontarska, M., Kronenbach, D., and Kowalska, T. (2008a). Investigation of the spontaneous oscillatory in vitro chiral conversion of L-(+)-lactic acid. Acta Chromatogr. 20, 209–225. doi: 10.1556/AChrom.20.2008.2.6
Sajewicz, M., Gontarska, M., Kronenbach, D., Leda, M., Kowalska, T., and Epstein, I. R. (2010). Condensation oscillations in the peptidization of phenylglycine. J. Syst. Chem. 1:7. doi: 10.1186/1759-2208-1-7
Sajewicz, M., Gontarska, M., Wojtal, Ł., Kronenbach, D., Leda, M., Epstein, I. R., et al. (2008c). Experimental and model investigation of the oscillatory transenantiomerization of L-α-phenylalanine. J. Liq. Chromatogr. Relat. Technol. 31, 1986–2005. doi: 10.1080/10826070802197578
Sajewicz, M., Kronenbach, D., Gontarska, M., Wróbel, M., Pietka, R., and Kowalska, T. (2009). TLC in search for structural limitations of spontaneous oscillatory in-vitro chiral conversion. α-hydroxybutyric and mandelic acids. J. Planar. Chromatogr. Modern TLC 22, 241–248. doi: 10.1556/JPC.2009.1001
Sajewicz, M., Kronenbach, D., Staszek, D., Wróbel, M., Grygierczyk, G., and Kowalska, T. (2008b). Experimental investigation of the oscillatory transenantiomerization of L-tyrosine. J. Liq. Chromatogr. Relat. Technol. 31, 2006–2018. doi: 10.1080/10826070802197693
Sajewicz, M., Pietka, R., Drabik, G., and Kowalska, T. (2006a). On the mechanism of oscillatory changes of the retardation factor (RF) and the specific rotation [α]D with selected solutions of S-(+)-naproxen. J. Liq. Chromatogr. Relat. Technol. 29, 2071–2082. doi: 10.1080/10826070600759934
Sajewicz, M., Pietka, R., Pieniak, A., and Kowalska, T. (2005b). Application of thin-layer chromatography (TLC) to investigate oscillatory instability of the selected profen enantiomers in dichloromethane. J. Chromatogr. Sci. 43, 542–548. doi: 10.1093/chromsci/43.10.542
Sajewicz, M., Gontarska, M., Kronenbach, D., Wojtal, Ł., Grygierczyk, G., and Kowalska, T. (2007). Study of the oscillatory in vitro transenantiomerization of the antimers of flurbiprofen and their enantioseparation by thin-layer chromatography (TLC). Acta Chromatogr. 18, 226–237.
Sajewicz, M., Pietka, R., Kuś, P., and Kowalska, T. (2006b). On the gelation of profens as a property causing their oscillatory transenantiomerization. Acta Chromatogr. 16, 181–191.
Sajewicz, M., Pietka, R., Pieniak, A., and Kowalska, T. (2005a). Application of thin-layer chromatography (TLC) to investigating oscillatory instability of the selected profen enantiomers. Acta Chromatogr. 15, 131–149.
Stich, M., Blanco, C., and Hochberg, D. (2013). Chiral and chemical oscillations in a simple dimerization model. Phys. Chem. Chem. Phys. 15, 255–261. doi: 10.1039/C2CP42620J
Keywords: spontaneous oscillatory peptidization, proteinogenic α-amino acids, soluble peptides, insoluble peptides, D2O
Citation: Fulczyk A, Łata E, Talik E, Kowalska T and Sajewicz M (2020) The Hampering Effect of Heavy Water (D2O) on Oscillatory Peptidization of Selected Proteinogenic α-Amino Acids. Front. Chem. 8:541. doi: 10.3389/fchem.2020.00541
Received: 14 April 2020; Accepted: 26 May 2020;
Published: 30 June 2020.
Edited by:
Zeljko Dimitrije Cupic, University of Belgrade, SerbiaReviewed by:
Katarina Novakovic, Newcastle University, United KingdomCopyright © 2020 Fulczyk, Łata, Talik, Kowalska and Sajewicz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Teresa Kowalska, dGVyZXNhLmtvd2Fsc2thQHVzLmVkdS5wbA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.