
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Chem., 18 December 2019
Sec. Chemical and Process Engineering
Volume 7 - 2019 | https://doi.org/10.3389/fchem.2019.00874
This article is part of the Research TopicFrom Biomass to Advanced Bio-Based Chemicals & Materials: A Multidisciplinary Perspective View all 17 articles
Lignocellulosic biomass (LB) is an abundant and renewable resource from plants mainly composed of polysaccharides (cellulose and hemicelluloses) and an aromatic polymer (lignin). LB has a high potential as an alternative to fossil resources to produce second-generation biofuels and biosourced chemicals and materials without compromising global food security. One of the major limitations to LB valorisation is its recalcitrance to enzymatic hydrolysis caused by the heterogeneous multi-scale structure of plant cell walls. Factors affecting LB recalcitrance are strongly interconnected and difficult to dissociate. They can be divided into structural factors (cellulose specific surface area, cellulose crystallinity, degree of polymerization, pore size and volume) and chemical factors (composition and content in lignin, hemicelluloses, acetyl groups). Goal of this review is to propose an up-to-date survey of the relative impact of chemical and structural factors on biomass recalcitrance and of the most advanced techniques to evaluate these factors. Also, recent spectral and water-related measurements accurately predicting hydrolysis are presented. Overall, combination of relevant factors and specific measurements gathering simultaneously structural and chemical information should help to develop robust and efficient LB conversion processes into bioproducts.
The environment is suffering from climate change, worsened by over-exploitation of resources thus increasing global greenhouse gas emission (Anderson et al., 2019; Hassan et al., 2019). Sustainable and environmentally friendly energy based on renewable resources are required in order to meet the world's future energy needs. Lignocellulosic biomass (LB) continues to attract global interest as a sustainable alternative to fossil carbon resources to produce second-generation biofuels and other biobased chemicals without compromising global food security (Menon and Rao, 2012; Chandel et al., 2018). These include agricultural wastes such as cereal straw (Yuan et al., 2018) and bagasse (Dias et al., 2009), forest residues such as pine (Cotana et al., 2014) and dedicated crops and short rotation coppices such as miscanthus (Lewandowski et al., 2000), switchgrass (Schmer et al., 2008), and poplar (Sannigrahi et al., 2010). LB is mainly composed of cellulose, hemicelluloses and lignin, making a complex assembly of polymers naturally recalcitrant to enzymatic conversion. That is why some pre-treatment steps are mandatory to make cellulose more accessible by changing the physical and/or the chemical structure of LB and facilitating the conversion of polysaccharides into fermentable sugars (Zhao et al., 2012a; Kumar and Sharma, 2017). Factors affecting LB recalcitrance are strongly interconnected and difficult to dissociate (Zhao et al., 2012b; Bichot et al., 2018). They can be divided into structural factors, which mainly refer to cellulose specific surface area, cellulose crystallinity, degree of polymerization, pore size and volume; chemical factors, related to composition and content in lignin, hemicelluloses and acetyl groups. Although many studies have investigated the impact of these factors on recalcitrance by examining different LB feedstocks and operating process conditions, conclusions obtained are not always obvious and even sometimes contradictory.
This review aims to propose an up-to-date survey of the role of chemical and structural factors on biomass recalcitrance and of the most advanced techniques to evaluate these factors. Resulting from the assessment of these factors, some promising methods aimed at predicting hydrolysis are presented and discussed, so that they should help to develop robust LB conversion processes into biofuels and biobased chemicals.
LB is naturally recalcitrant to microbial and enzymatic degradation, which constitutes a real obstacle to its industrial valorisation into bioenergy and biomaterials. To optimize deconstruction, it is necessary to understand and overcome the chemical and the structural factors conferring the recalcitrance property to lignocellulose in plant cell walls.
Cellulose, the most abundant LB polymer, representing 40–60% in weight (Sharma et al., 2019), consists of ß-D-glucopyranose units linked via ß-(1,4) glycosidic bonds, with cellobiose as the fundamental repeating unit. The cellulose chains made up of 500–1400 D-glucose units are arranged together to form microfibrils, which are packed together to form cellulose fibrils (McKendry, 2002; Robak and Balcerek, 2018). Cellulose fibrils are embedded in a lignocellulosic matrix that makes it very resistant to enzymatic hydrolysis. Yoo et al. reported that the cellulose content was positively correlated with the glucose release (Yoo et al., 2017a). The degree of polymerization (DP) of cellulose which is the number of glucose units in the polymer playing a crucial role on LB recalcitrance. But its exact role is still not quite clear and difficult to investigate individually with the current knowledge. Indeed, altering DP is always accompanied by changes in structural parameters such as crystallinity and porosity. For example, Sinitsyn et al. (1991) found that reduction in DP of cotton linters by γ-irradiation had a minor effect on the saccharification rate. Ioelovich et al. got similar conclusion (Ioelovich and Morag, 2011). However, Lu et al. (2019) reported that the cellulose DP was negatively correlated to the cellulose hydrolysis. It is assumed that long cellulose chains contain more hydrogen bonds and are difficult to hydrolyze, whereas shorter cellulose chains contain a weaker hydrogen-bonding system and therefore are believed to facilitate enzyme accessibility (Hallac and Ragauskas, 2011; Meng et al., 2017).
Hemicelluloses are heterogeneous groups of biopolymers, representing 20–35% of the biomass weight (Chandel et al., 2018). It contains various monosaccharide subunits to form xylans, xyloglucan, mannans and glucomannans, and others (McKendry, 2002). The DP of hemicelluloses is in the range of 100–200 units (Mota et al., 2018), which is much lower than that of cellulose, but it can present a high degree of more or less complex substitutions. Hemicellulose is amorphous, with little physical strength. It is readily hydrolysed by dilute acids or bases, as well as hemicellulase enzymes (Isikgor and Becer, 2015). Hemicelluloses act as a physical barrier limiting the accessibility of enzymes. It has been reported that removal of hemicelluloses by dilute acid or steam explosion pre-treatment could increase cellulose conversion by improving the accessibility of enzymes to cellulose (Auxenfans et al., 2017a; Herbaut et al., 2018; Santos et al., 2018). Kruyeniski et al. (2019) reported that the removal of hemicelluloses on pre-treated pine improved the fibers porosity and the area available for enzymes. The impact of hemicelluloses on LB recalcitrance still not quite clear as some lignin is often removed with hemicelluloses. Some studies have reported that hemicelluloses removal was more efficient than lignin removal for improving enzymatic hydrolysis rate (Yoshida et al., 2008; Leu and Zhu, 2013; Lv et al., 2013), whereas others indicated that lignin removal was much more important (Gao et al., 2013; Kruyeniski et al., 2019).
LB hemicelluloses can be extensively acetylated with acetyl groups (OAc). OAc may restrict cellulose accessibility by interfering with enzyme recognition (Pan et al., 2006). It also might hinder the formation of productive binding between cellulose and the catalytic domain of cellulases through increasing the diameter of cellulose chain or changing its hydrophobicity (Zhao et al., 2012a). Previous studies on corn stover reported that reducing the acetyl content improved enzyme effectiveness (Kumar and Wyman, 2009a,b). Whereas, other studies on poplar wood, wheat straw, switchgrass and bagasse pointed out that the effect of deacetylation was more significant on hemicellulose digestibility than on cellulose digestibility (Grohmann et al., 1989; Chang and Holtzapple, 2000; Liu et al., 2014). Chang and Holtzapple (2000) and Zhu et al. (2008) showed that the impact of OAc depends on the lignin and cellulose content and biomass crystallinity.
Lignin is the second most abundant polymer in LB after cellulose, corresponding to 15–40% of dry weight (Ragauskas et al., 2014). It is a very complex amorphous heteropolymer of phenylpropanoid building units (p-coumaryl, coniferyl, and sinapyl alcohol) (Agbor et al., 2011). Lignin is responsible for hydrophobicity and structural rigidity. It binds hemicelluloses to cellulose in the cell wall. It is well-known that lignin plays a negative role in the conversion of cellulose influenced by several factors such as total lignin content, lignin composition/structure (in particular hydroxyl groups content and S and G units content) (Santos et al., 2012). First of all, lignin can physically limit polysaccharide accessibility: it plays a role as physical barrier that blocks the access of enzymes to cellulose. Also, it can irreversibly adsorb cellulases and other enzymes during enzymatic hydrolysis due to its hydrophobic structural features including hydrogen bonding, methoxy groups, and polyaromatic structures (Kumar and Wyman, 2009b; Zeng et al., 2014). Previous studies showed that the lignin content was negatively correlated with enzymatic digestibility in poplar (Meng et al., 2017; Yoo et al., 2017a), also in miscanthus, in wheat straw (Herbaut et al., 2018) and in transgenic rice (Huang et al., 2017). The removal of lignin generally disrupts the lignin-carbohydrates matrix, increases the porosity and reduces non-productive adsorption sites for enzymes (Pihlajaniemi et al., 2016; Kruyeniski et al., 2019). It has been reported that phenolic hydroxyl groups (lignin-derived compounds) cause reversible inhibition of cellulases (Yu et al., 2014; Yang and Pan, 2016; Yao et al., 2018). Blocking free phenolic hydroxyl groups by chemical reaction such as hydroxypropylation significantly reduced (by 65–91%) the inhibitory effect of lignin (Yang and Pan, 2016). Yoo et al. showed that lignin S/G ratio is important as an independent recalcitrance factor (Yoo et al., 2017a). However, the correlation between lignin S/G ratio and recalcitrance is still not obvious. For example, Herbaut et al. and Yu et al. showed a positive correlation between the S/G ratio and the hydrolysis yields for miscanthus and woody chips (Yu et al., 2014; Herbaut et al., 2018) because of the higher binding capacity of G (with branched structure) over S (with linear structure and low degree of polymerisation) to cellulase (Guo et al., 2014; Yoo et al., 2017b). By contrast, others found a negative correlation between S/G ratio and the enzymatic hydrolysis in woody chips (Papa et al., 2012), in pre-treated miscanthus (Xu et al., 2012; Li et al., 2014), in pre-treated wheat straw (Jiang et al., 2016) and in genetically engineering poplar (Escamez et al., 2017). On the other hand, previous studies showed that changes in S/G ratio of untreated LB did not influence the enzymatic hydrolysis: for untreated poplar with S/G ratio between 1.0 and 3.0 (Studer et al., 2011), for Arabidopsis stems containing G- and S-rich lignin (Li et al., 2010) and for transgenic alfalfa (Chen and Dixon, 2007). Overall, lignin contributes strongly to LB recalcitrance influenced by its chemical composition and its structure, limiting the accessibility of enzymes to cellulose.
We detailed the impact of each polymer on LB recalcitrance above. There is a need to find out how interactions between them increase the recalcitrance of the cell walls to the enzymatic hydrolysis. Cellulose and hemicelluloses are intimately associated together through hydrogen bonds (Lee et al., 2014), meanwhile lignin are covalently linked to hemicelluloses to form lignin-carbohydrate complex (LCC) (Tarasov et al., 2018; Giummarella and Lawoko, 2019). There are five different types of lignin-carbohydrate bonds, phenyl glycosides (PG), benzyl ethers (BE), γ-esters esters (GE), ferulate/coumarate esters (FE/CE) and hemiacetal/acetal linkages that are linked to lignin at 4-OH and 4-O positions (Giummarella and Lawoko, 2019). It has been suggested that the interactions between the microfibers from cellulose and hemicelluloses, as well as the LCC linkage plays a significant role in wood structure and affects significantly its enzymatic hydrolysis by reducing the area of cellulose accessible for enzymes (Balan et al., 2009; Du et al., 2014). Until now, the study of LCC is still a controversial topic in lignocellulosic chemistry, due to difficulties in the characterization of heterogeneous biomass substrate in addition to the low concentration of LCC (Obst, 1982). That is why there is a need to develop efficient methods to enrich the LCC such as mild chemical methods or the use of pure enzymes in order to achieve a quantitative analysis of LCC using NMR (Giummarella and Lawoko, 2019).
Crystallinity has been identified as one of the most extensively studied supramolecular properties of cellulose. It represents the proportion of crystalline regions to amorphous regions. Crystalline cellulose fibers are closely related to each other by non-covalent hydrogen bonds that make their enzymatic hydrolysis 3–30 times lower than in amorphous zones (Zhao et al., 2012b). But the impact of crystallinity on hydrolysis differs. Some studies reported that crystallinity correlated negatively with enzymatic hydrolysis especially at the initial hydrolysis rate on pre-treated wheat straw (Pihlajaniemi et al., 2016), on pre-treated corn stover (Liu et al., 2014; Xu et al., 2019) and on hybrid polar, switchgrass, and bagasse (Chang and Holtzapple, 2000). Others showed that crystallinity was less critical in limiting hydrolysis than other physical features such as DP, pore volume, accessible surface area, and particle size (Mansfield et al., 1999; Ioelovich and Morag, 2011; Aldaeus et al., 2015; Auxenfans et al., 2017a; Meng et al., 2017; Zhang et al., 2018). Another piece of evidence is that in the majority of cases, pure cellulose is used as a substrate to correlate the crystallinity to the saccharification yield, which is not representative of the heterogeneous of LB substrate.
Particle size was identified as a key parameter affecting cellulose hydrolysis potential (Barakat et al., 2014; Vaidya et al., 2016). The reduction of particle size through milling, grinding, and extrusion could enhance the affinity between cellulose and enzymes, deconstruct lignocellulose compact structure and thus increase the rate of hydrolysis (Silva et al., 2012; Pang et al., 2018; Yu et al., 2019). Studies have demonstrated that mechanical deconstruction facilitates enzymatic hydrolysis of various feedstocks such as wood chips (Jiang et al., 2017), miscanthus and wheat straw (Kim et al., 2018) and corn stover (Yu et al., 2019). However, some researchers pointed out that there is a size threshold depending on the lignocellulosic feedstocks. Chang and Holtzapple (2000), observed that a particle size reduction below 400 μm has a negligible effect on the hydrolysis yield of poplar. Whereas, Silva et al. (2012) reported that the size threshold was 270 μm for wheat straw.
Accessible surface area (ASA) of LB is a critical factor for enzymatic hydrolysis, highly related to porosity structure properties, such as specific surface area (SSA) and pore volume (Liu et al., 2014). Reduction in the particle size or increase in pore volume causes an increase of ASA. It has been shown that theenzymatic conversion of pre-treated pine wood is increased with ASA (Torr et al., 2016). Also Goshadrou et al. (2013) reported that ASA could enhance the fiber accessibility of aspen wood to the hydrolytic enzymes. However, ASA is difficult to estimate, SSA is often used to measure the real surface that is available to enzymes (Silvi Octavia et al., 2017). Moreover, just like crystallinity, it is impossible to consider only SSA (Karimi and Taherzadeh, 2016a). The smaller the particle is, the higher the SSA is (Silvi Octavia et al., 2017). Zhang et al. (2018) reported that the hydrothermal pre-treated corn stover increased the SSA by 2-fold, resulting in 138% enhancement of enzymatic digestibility. Lu et al. (2019) also found that ball milling increased the SAA of cellulose by a factor of two due to the reduction in particle size; these changes made cellulose more accessible and more reactive and resulted in higher glucose yield. However, Peciulyte et al. (2015) pointed out the absence of significant correlation between the yield of cellulose conversion of cellulosic substrates and the SSA.
Accessible volume of cellulose in LB is considered as an important factor influencing enzymatic deconstruction (Jeoh et al., 2007). According to their sizes or their shapes, pore volumes are more or less accessible to enzymes. The size of a cellulase is typically around 5.1 nm, and so, only the pores larger than 5.1 nm are supposed to be accessible to enzyme (Grethlein, 1985). Some authors found that there is a strong correlation between pore size of the biomass and the enzymatic conversion yield for dilute acid pre-treated poplar (Meng et al., 2013) and cellulosic substrates (Peciulyte et al., 2015). Herbaut et al. demonstrated that correlations with specific porosity ranges are biomass specific and pre-treatment dependent. For example, hydrolysis yield correlated strongly to pore size range 15–30 nm for wheat straw, whereas it correlated to pore size range 10–15 nm for poplar. For miscanthus, only pores below 10 nm correlated strongly with hydrolysis, which proved that there is no generic pore size allowing an enhancement of hydrolysis yield and that diffusion of enzymes within the plant cell wall is specific to each biomass species (Herbaut et al., 2018). Other reports found that there is no correlation between pore size and hydrolysis yield, for dilute acid pre-treated corn stover (Ishizawa et al., 2007), pre-treated pine (Kruyeniski et al., 2019), and dilute acid pre-treated and delignified sugarcane (Santos et al., 2018). Moreover, Stoffel et al. (2014) and Vaidya et al. (2016) also showed that the increase of pore volume when lignin contents does not exceed 15% has a negligible effect on enzymatic digestibility of pre-treated pine.
LB recalcitrance to enzymatic degradation was found to be a multi-variant and multi-scale phenomenon, affected by several physical and chemical factors such as hemicelluloses and lignin content, DP of cellulose and accessible surface area and volume (Figure 1). However, various studies have demonstrated opposing trends in the effects of these factors due to the complexity of LB and the unknown interactions between these factors.
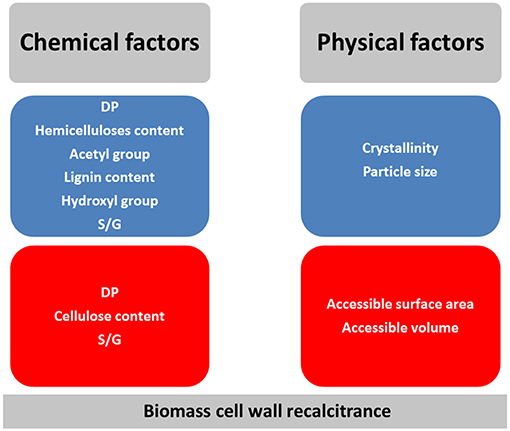
Figure 1. Factors influencing LB recalcitrance. In blue box: the increase in value of the factor increases LB recalcitrance; in red box: the increase in value of the factor decreases LB recalcitrance. When factors are in two boxes, their effect is variable.
Several studies have developed high throughput methodologies to characterize the composition and the structure of large sets of LB samples using wet chemistry and spectroscopy (Studer et al., 2010; Krasznai et al., 2018). These methods have the advantages of being fast and automatic with a very low sample mass and minimal sample preparation (Decker et al., 2018). For instance, Selig et al. developed a high throughput method to determine glucan and xylan content in poplar, pine, wheat stover and pine using a glucose oxidase and a xylose dehydrogenase-based assays instead of HPLC analysis which reduced remarkably the duration of the analysis from 48 to 5-6 h (Selig et al., 2011). Pyrolysis-molecular beam mass spectrometry was used as a high throughput technique to analyse lignin content and structure and polysaccharides content in LB feedstocks (Penning et al., 2014; Decker et al., 2015; Sykes et al., 2015; Harman-Ware et al., 2017). Decker et al. (2012) developed a high throughput method to investigate the effect of the starch content in a set of 250 switchgrass variants on the recalcitrance after hydrothermal pre-treatment and enzymatic hydrolysis (5 days).
High throughput techniques provide useful information to characterize LB while saving time and effort. However, those techniques are not fully automatic (some manual transfers between operations is often necessary), also specialized costly robots, reactors, and sophisticated computational tools are required. More importantly, conducting enzymatic hydrolysis takes several days, whereas there is a need to predict from initial properties of biomass how it will behave during biotechnological transformation in order to adapt the process conditions.
As detailed in the previous section, measuring conventional factors such as lignin content (for example using standard wet-chemistry analysis), cellulose crystallinity or porosity (for example using Simons's staining or thermoporosimetry) provide reliable information about composition, accessibility and structure and can be carried out by several techniques with their advantages and drawbacks (Table 1).
In many cases, they are very useful to understand the relationship between assayed factors and hydrolysis. But these parameters are far from being universal to predict hydrolysis, for several reasons:
– Hydrolysis conditions depend on biomass species, pre-treatments, and enzymatic cocktails, which are not standard from one research report to another;
– Measurements of the chemical and structural parameters depend on the instrument, methods and conditions of analysis which are difficult to compare (for example, there are at least 6 different techniques to evaluate porosity, Table 1);
– Most importantly, due to the multi-scale architecture of LB, not a single chemical or structural parameter can so far explain hydrolysis.
Therefore, some other methods are required to evaluate, as a single measurement, the interactions existing between several parameters which are responsible for recalcitrance, in order to predict enzymatic hydrolysis. Among them, spectral and water-related properties of LB appear as relevant measurements to be correlated to hydrolysis.
Quantitative spectroscopy is a fast, relatively low-cost, non-destructive alternative to classic analytical methods for the chemical analysis of biomass. Several spectroscopic techniques have been applied to analyse biomass properties, including fast-Fourier Transform InfraRed (FT-IR) spectroscopy, near-infrared (NIR) spectroscopy, and Raman scattering spectroscopy.
NIR is a good method for qualitative and quantitative screening of large population of samples. It has also been used to predict LB composition and enzymatic digestibility (Hou and Li, 2011; Huang et al., 2012, 2017).
FTIR spectroscopy is a reliable technique used to determine the effect of pre-treatment (monitor the crystallinity changes), and the degradation of cellulose during the enzymatic saccharification. However, it provides especially qualitative structural information rather than quantitative information. Bekiaris et al. (2015) demonstrated that FTIR spectroscopy combined with chemometric techniques can be used to predict sugar conversions and yields from enzymatic saccharification of pre-treated wheat straw. Earlier study on untreated and pre-treated switchgrass and corn stover got similar conclusion (Sills and Gossett, 2012). Raman spectroscopy is also a robust analytical technique coupled to FTIR spectroscopy allowed the prediction of lignin syringyl/guaiacyl content in diverse lignocellulosic feedstocks (Lupoi et al., 2014).
Overall, even if infrared spectroscopy is very powerful to predict the composition and the saccharification of LB, it requires the creation of mathematical models which needs hundreds of samples, which represents an intensive work. Also, models are specific to biomass species and hydrolysis conditions, which can limit their use.
Plant cell walls are autofluorescent materials, containing some endogenous fluorophores, especially aromatic molecules: monolignols in lignin, ferulic, acid and cinnamic acids in hemicellulose (Auxenfans et al., 2017b). Fluorescence can be easily and fastly measured on lignocellulosic samples through spectrofluorimetry. Auxenfans et al. reported that fluorescence intensity correlated strongly with the glucose released from untreated and steam exploded lignocellulosic feedstocks (miscanthus, poplar, and wheat straw) and concluded that fluorescence can predict the LB saccharification.
Fluorescence lifetime as a rapid method can also be used to explain and even predict saccharification with efficiency: Chabbert et al. (2018) reported a strong positive correlation between lifetime fluorescence and saccharification yields. Fluorescence Recovery After Photobleaching (FRAP) technique allowed to explore LB accessibility. Herbaut et al., studied the mobility of PEG-rhodamine probes in cell walls of poplar samples and demonstrated a strong correlation between the accessibility of probes and the saccharification yields (Herbaut et al., 2018). Overall, even if fluorescence by itself cannot be related to a single parameter (lignin content, polymer interactions,…), this can be turned into an advantage since it provides a fingerprint of lignin organization and architecture in LB, which is likely directly related to cellulose accessibility and thus to hydrolysis potential.
Water acts as a swelling agent allowing enzymes diffusion toward plant cell wall. It has been reported that water retention value (WRV) can serve as predictor of hydrolysis rate (Noori and Karimi, 2016; Crowe et al., 2017; Williams et al., 2017; Paës et al., 2019). WRV is a complex function related to chemical and structural properties of the cell wall, such as accessible surface area and particle size. Several works reported a positive correlation between WRV and cellulose conversion rate for various lignocellulosic feedstocks: untreated maize (Li et al., 2015), pre-treated poplar, pine and miscanthus with dilute sulfuric acid (Weiss et al., 2018) and pre-treated corn stover and switchgrass with liquid hot water (Williams and Hodge, 2014). This method is rapid, simple, inexpensive, and provides results comparable to the results of more advanced methods, e.g., NMR and Simons' staining (Karimi and Taherzadeh, 2016a).
Another experimental parameter related to water that can be easily and fastly measured is the contact angle value that indirectly quantifies the hydrophobicity of lignin. This parameter could predict the inhibitory effect of lignin. Yang et al. reported that softwood lignin more hydrophobic than the hardwood lignin, was more able to absorb cellulase and inhibit enzymatic cellulose hydrolysis than the hardwood lignin (Yang and Pan, 2016).
Biomass recalcitrance is a multi-variant and multi-scale phenomenon, and thus cannot simply be assayed by one single chemical or structural factor due to the complex and still unknown interactions between these parameters (Figure 1). Nonetheless, spectral analysis based on infrared and fluorescence properties of LB together with water-related characteristics seem to be able to represent chemical and structural properties of LB, thus relating nano- and macro-scale properties. Regarding future developments, the integration of large amount of data (chemical properties, images, spectra) by the means of machine learning approaches should help devising more complex models predicting not only composition but also dynamical behavior of LB over transformation such as hydrolysis. In this context, imaging and quantification of structural features at the cellular/tissular scale (by fluorescence confocal microscopy based on previous reports studying for example plant morphogenesis) or at nano-scale (by atomic force microscopy) might be relevant paths to follow.
AZ and GP discussed the outline, content of the article, and approved the content of the manuscript. AZ drafted the manuscript. GP finalized the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Ph.D. studentship of AZ was granted by Grand Est Region and FEDER (TECMI-4D project).
Agarwal, U. P., Ralph, S. A., Reiner, R. S., and Baez, C. (2016). Probing crystallinity of never-dried wood cellulose with Raman spectroscopy. Cellulose 23, 125–144. doi: 10.1007/s10570-015-0788-7
Agbor, V. B., Cicek, N., Sparling, R., Berlin, A., and Levin, D. B. (2011). Biomass pretreatment: fundamentals toward application. Biotechnol. Adv. 29, 675–685. doi: 10.1016/j.biotechadv.2011.05.005
Aldaeus, F., Larsson, K., Srndovic, J. S., Kubat, M., Karlström, K., Peciulyte, A., et al. (2015). The supramolecular structure of cellulose-rich wood pulps can be a determinative factor for enzymatic hydrolysability. Cellulose 22, 3991–4002. doi: 10.1007/s10570-015-0766-0
An, S., Li, W., Liu, Q., Xia, Y., Zhang, T., Huang, F., et al. (2019). Combined dilute hydrochloric acid and alkaline wet oxidation pretreatment to improve sugar recovery of corn stover. Bioresour. Technol. 271, 283–288. doi: 10.1016/j.biortech.2018.09.126
Anderson, E. M., Stone, M. L., Katahira, R., Reed, M., Muchero, W., Ramirez, K. J., et al. (2019). Differences in S/G ratio in natural poplar variants do not predict catalytic depolymerization monomer yields. Nat. Commun. 10, 2033–2043. doi: 10.1038/s41467-019-09986-1
Arai, T., Biely, P., Uhliariková, I., Sato, N., Makishima, S., Mizuno, M., et al. (2019). Structural characterization of hemicellulose released from corn cob in continuous flow type hydrothermal reactor. J. Biosci. Bioeng. 127, 222–230. doi: 10.1016/j.jbiosc.2018.07.016
Auxenfans, T., Crônier, D., Chabbert, B., and Paës, G. (2017a). Understanding the structural and chemical changes of plant biomass following steam explosion pretreatment. Biotechnol. Biofuels 10, 36–52. doi: 10.1186/s13068-017-0718-z
Auxenfans, T., Terryn, C., and Paës, G. (2017b). Seeing biomass recalcitrance through fluorescence. Sci. Rep. 7, 8838–8846. doi: 10.1038/s41598-017-08740-1
Balan, V., Sousa, L. C., Chundawat, S. P., Marshall, D., Sharma, L. N., Chambliss, C. K., et al. (2009). Enzymatic digestibility and pretreatment degradation products of AFEX-treated hardwoods (Populus nigra). Biotechnol. Progr. 25, 365–375. doi: 10.1002/btpr.160
Barakat, A., Mayer-Laigle, C., Solhy, A., Arancon, R. A., De Vries, H., Luque., et al. (2014). Mechanical pretreatments of lignocellulosic biomass: towards facile and environmentally sound technologies for biofuels production. RSC Adv. 4, 48109–48127. doi: 10.1039/C4RA07568D
Barnette, A. L., Bradley, L. C., Veres, B. D., Schreiner, E. P., Park, Y. B., Park, J., et al. (2011). Selective detection of crystalline cellulose in plant cell walls with sum-frequency-generation (SFG) vibration spectroscopy. Biomacromolecules 12, 2434–2439. doi: 10.1021/bm200518n
Bekiaris, G., Lindedam, J., Peltre, C., Decker, S. R., Turner, G. B., Magid, J., et al. (2015). Rapid estimation of sugar release from winter wheat straw during bioethanol production using FTIR-photoacoustic spectroscopy. Biotechnol. Biofuels 8, 85–97. doi: 10.1186/s13068-015-0267-2
Benouadah, N., Aliouche, D., Pranovich, A., and Willför, S. (2019). Chemical characterization of Pinus halepensis sapwood and heartwood. Wood Mater. Sci. Eng. 14, 157–164. doi: 10.1080/17480272.2018.1448436
Bichot, A., Delgenès, J.-P., Méchin, V. H. C., Bernet, N., and Garcia-Bernet, D. (2018). Understanding biomass recalcitrance in grasses for their efficient utilization as biorefinery feedstock. Rev. Environ. Sci. Biotechnol. 17, 707–748. doi: 10.1007/s11157-018-9485-y
Brewer, C. E., Chuang, V. J., Masiello, C. A., Gonnermann, H., Gao, X., Dugan, B., et al. (2014). New approaches to measuring biochar density and porosity. Biomass Bioenergy 66, 176–185. doi: 10.1016/j.biombioe.2014.03.059
Chabbert, B., Terryn, C., Herbaut, M., Vaidya, A., Habrant, A., Paës, G., et al. (2018). Fluorescence techniques can reveal cell wall organization and predict saccharification in pretreated wood biomass. Ind. Crops Prod. 123, 84–92. doi: 10.1016/j.indcrop.2018.06.058
Chandel, A. K., Garlapati, V. K., Singh, A. K., Antunes, F. A. F., and da Silva, S. S. (2018). The path forward for lignocellulose biorefineries: bottlenecks, solutions, and perspective on commercialization. Bioresour. Technol. 264, 370–381 doi: 10.1016/j.biortech.2018.06.004
Chang, V. S., and Holtzapple, M. T. (2000). Fundamental factors affecting biomass enzymatic reactivity. Appl. Biochem. Biotechnol. 84, 5–37. doi: 10.1007/978-1-4612-1392-5_1
Chen, F., and Dixon, R. A. (2007). Lignin modification improves fermentable sugar yields for biofuel production. Nat. Biotechnol. 25, 759. doi: 10.1038/nbt1316
Chen, S., Ling, Z., Zhang, X., Kim, Y. S., and Xu, F. (2018). Towards a multi-scale understanding of dilute hydrochloric acid and mild 1-ethyl-3-methylimidazolium acetate pretreatment for improving enzymatic hydrolysis of poplar wood. Ind. Crops Prod. 114, 123–131. doi: 10.1016/j.indcrop.2018.02.007
Cotana, F., Cavalaglio, G., Gelosia, M., Nicolini, A., Coccia, V., Petrozzi., et al. (2014). Production of bioethanol in a second generation prototype from pine wood chips. Energy Proc. 45, 42–51. doi: 10.1016/j.egypro.2014.01.006
Crowe, J. D., Zarger, R. A., and Hodge, D. B. (2017). Relating nanoscale accessibility within plant cell walls to improved enzyme hydrolysis yields in corn stover subjected to diverse pretreatments. J. Agric. Food Chem. 65, 8652–8662. doi: 10.1021/acs.jafc.7b03240
Decker, S. R., Carlile, M., Selig, M. J., Doeppke, C., Davis, M., Sykes, R., et al. (2012). Reducing the effect of variable starch levels in biomass recalcitrance screening. Methods Mol. Biol. 908, 181–95, doi: 10.1007/978-1-61779-956-3_17
Decker, S. R., Harman-Ware, A. E., Happs, R. M., Wolfrum, E. J., Tuskan, G. A., Kainer, D., et al. (2018). High throughput screening technologies in biomass characterization. Front. Energy Res. 6:120. doi: 10.3389/fenrg.2018.00120
Decker, S. R., Sykes, R. W., Turner, G. B., Lupoi, J. S., Doepkke, C., Tucker, M. P., et al. (2015). High-throughput screening of recalcitrance variations in lignocellulosic biomass: total lignin, lignin monomers, and enzymatic sugar release. JoVE 103:e53163. doi: 10.3791/53163
Dias, M. O., Ensinas, A. V., Nebra, S. A., Maciel Filho, R., Rossell, C. E., and Maciel, M. R. W. (2009). Production of bioethanol and other bio-based materials from sugarcane bagasse: integration to conventional bioethanol production process. Chem. Eng. Res Des. 87, 1206–1216. doi: 10.1016/j.cherd.2009.06.020
Du, X., Pérez-Boada, M., Fernández, C., Rencoret, J., del Río, J. C., Jiménez-Barbero, J., et al. (2014). Analysis of lignin–carbohydrate and lignin–lignin linkages after hydrolase treatment of xylan–lignin, glucomannan–lignin and glucan–lignin complexes from spruce wood. Planta 239, 1079–1090. doi: 10.1007/s00425-014-2037-y
Engel, P., Hein, L., and Spiess, A. C. (2012). Derivatization-free gel permeation chromatography elucidates enzymatic cellulose hydrolysis. Biotechnol. Biofuels 5:77. doi: 10.1186/1754-6834-5-77
Escamez, S., Latha Gandla, M., Derba-Maceluch, M., Lundqvist, S. -O., Mellerowicz, E. J., Jönsson, L. J., et al. (2017). A collection of genetically engineered Populus trees reveals wood biomass traits that predict glucose yield from enzymatic hydrolysis. Sci. Rep. 7:15798. doi: 10.1038/s41598-017-16013-0
Gao, Y., Xu, J., Zhang, Y., Yu, Q., Yuan, Z., Liu., et al. (2013). Effects of different pretreatment methods on chemical composition of sugarcane bagasse and enzymatic hydrolysis. Bioresour. Technol. 144, 396–400. doi: 10.1016/j.biortech.2013.06.036
Giummarella, N., and Lawoko, Y. P. A. J. R. a. M. (2019). A critical review on the analysis of lignin carbohydrate bonds in plants. Green Chem. 21, 1573–1595. doi: 10.1039/C8GC03606C
Goshadrou, A., Karimi, K., and Lefsrud, M. (2013). Characterization of ionic liquid pretreated aspen wood using semi-quantitative methods for ethanol production. Carbohydr. Polym. 96, 440–449. doi: 10.1016/j.carbpol.2013.04.017
Grethlein, H. E. (1985). The effect of pore size distribution on the rate of enzymatic hydrolysis of cellulosic substrates. Biotechnology 3:155. doi: 10.1038/nbt0285-155
Grigsby, W. J., Kroese, H., and Dunningham, E. A. (2013). Characterisation of pore size distributions in variously dried Pinus radiata: analysis by thermoporosimetry. Wood Sci. Technol. 47, 737–747. doi: 10.1007/s00226-013-0537-8
Grohmann, K., Mitchell, D., Himmel, M., Dale, B., and Schroeder, H. (1989). The role of ester groups in resistance of plant cell wall polysaccharides to enzymatic hydrolysis. Appl. Biochem. Biotech. 20:45. doi: 10.1007/BF02936472
Guo, F., Shi, W., Sun, W., Li, X., Wang, F., Zhao, J., et al. (2014). Differences in the adsorption of enzymes onto lignins from diverse types of lignocellulosic biomass and the underlying mechanism. Biotechnol. Biofuels 7:38. doi: 10.1186/1754-6834-7-38
Gustafsson, S., Westermann, F., Hanrieder, T., Jung, L., Ruppach, H., Mihranyan., et al. (2019). Comparative analysis of dry and wet porometry methods for characterization of regular and cross-linked virus removal filter papers. Membranes 9:1. doi: 10.3390/membranes9010001
Hallac, B. B., and Ragauskas, A. J. (2011). Analyzing cellulose degree of polymerization and its relevancy to cellulosic ethanol. Biofuels, Bioprod. Bioref. 5, 215–225. doi: 10.1002/bbb.269
Harman-Ware, A. E., Davis, M. F., Peter, G. F., Wang, Y., and Sykes, R. W. (2017). Estimation of terpene content in loblolly pine biomass using a hybrid fast-GC and pyrolysis-molecular beam mass spectrometry method. J. Anal. Appl. Pyrolysis. 124, 343–348. doi: 10.1016/j.jaap.2017.01.011
Hassan, S. S., Williams, G. A., and Jaiswal, A. K. (2019). Moving towards the second generation of lignocellulosic biorefineries in the EU: drivers, challenges, and opportunities. Renew. Sust. Energ. Rev. 101, 590–599. doi: 10.1016/j.rser.2018.11.041
Hatfield, R., and Fukushima, R. S. (2005). Can lignin be accurately measured? Crop Sci. 45, 832–839. doi: 10.2135/cropsci2004.0238
Herbaut, M., Zoghlami, A., Habrant, A., Falourd, X., Foucat, L., Chabbert, B., et al. (2018). Multimodal analysis of pretreated biomass species highlights generic markers of lignocellulose recalcitrance. Biotechnol. Biofuels 11:52. doi: 10.1186/s13068-018-1053-8
Hou, S., and Li, L. (2011). Rapid characterization of woody biomass digestibility and chemical composition using near-infrared spectroscopy free access. J. Integr. Plant Biol. 53, 166–175. doi: 10.1111/j.1744-7909.2010.01003.x
Huang, J., Li, Y., Wang, Y., Chen, Y., Liu, M., Wang, Y., et al. (2017). A precise and consistent assay for major wall polymer features that distinctively determine biomass saccharification in transgenic rice by near-infrared spectroscopy. Biotechnol. Biofuels 10:294. doi: 10.1186/s13068-017-0983-x
Huang, J., Xia, T., Li, A., Yu, B., Li, Q., Tu, Y., et al. (2012). A rapid and consistent near infrared spectroscopic assay for biomass enzymatic digestibility upon various physical and chemical pretreatments in Miscanthus. Bioresour. Technol. 121, 274–281. doi: 10.1016/j.biortech.2012.06.015
Ioelovich, M., and Morag, E. (2011). Effect of cellulose structure on enzymatic hydrolysis. BioResources 6, 2818–2835. doi: 10.15376/biores.6.3.2818_2835
Isaac, A., Antunes, F. A., Conti, R., Montoro, L. A., Malachias, A., Massara, P., et al. (2018). Unveiling 3D physicochemical changes of sugarcane bagasse during sequential acid/alkali pretreatments by synchrotron phase-contrast imaging. Ind. Crops Prod. 114, 19–27. doi: 10.1016/j.indcrop.2018.01.028
Ishizawa, C. I., Davis, M. F., Schell, D. F., and Johnson, D. K. (2007). Porosity and its effect on the digestibility of dilute sulfuric acid pretreated corn stover. J. Agric. Food Chem. 55, 2575–2581. doi: 10.1021/jf062131a
Isikgor, F. H., and Becer, C. R. (2015). Lignocellulosic biomass: a sustainable platform for the production of bio-based chemicals and polymers. Polym. Chem. 6, 4497–4559. doi: 10.1039/C5PY00263J
Jeoh, T., Ishizawa, C. I., Davis, M. F., Himmel, M. E., Adney, W. S., and Johnson, D. K. (2007). Cellulase digestibility of pretreated biomass is limited by cellulose accessibility. Biotechnol. Bioeng. 98, 112–122. doi: 10.1002/bit.21408
Jiang, B., Wang, W., Gu, F., Cao, T., and Jin, Y. (2016). Comparison of the substrate enzymatic digestibility and lignin structure of wheat straw stems and leaves pretreated by green liquor. Bioresour. Technol. 199, 181–187. doi: 10.1016/j.biortech.2015.08.104
Jiang, J., Wang, J., Zhang, X., and Wolcott, M. (2017). Assessing multi-scale deconstruction of wood cell wall subjected to mechanical milling for enhancing enzymatic hydrolysis. Ind. Crops Prod. 109, 498–508. doi: 10.1016/j.indcrop.2017.09.009
Karimi, K., and Taherzadeh, M. J. (2016a). A critical review on analysis in pretreatment of lignocelluloses: degree of polymerization, adsorption/desorption, and accessibility. Bioresour. Technol. 203, 348–356. doi: 10.1016/j.biortech.2015.12.035
Karimi, K., and Taherzadeh, M. J. (2016b). A critical review of analytical methods in pretreatment of lignocelluloses: composition, imaging, and crystallinity. Bioresour. Technol. 200, 1008–18. doi: 10.1016/j.biortech.2015.11.022
Kim, S., Um, B., Im, D., Lee, J., and Oh, K. (2018). Combined ball milling and ethanol organosolv pretreatment to improve the enzymatic digestibility of three types of herbaceous biomass. Energies 11:2457. doi: 10.3390/en11092457
Krasznai, D. J., Champagne Hartley, R., Roy, H. M., Champagne, P., and Cunningham, M. F. (2018). Compositional analysis of lignocellulosic biomass: conventional methodologies and future outlook. Crit. Rev. Biotechnol. 38, 199–217. doi: 10.1080/07388551.2017.1331336
Kruyeniski, J., Ferreira, P. J., Carvalho, M. G. V. S., Vallejos, M. E., Felissia, F. E., Area, M. C., et al. (2019). Physical and chemical characteristics of pretreated slash pine sawdust influence its enzymatic hydrolysis. Ind. Crops Prod. 130, 528–536. doi: 10.1016/j.indcrop.2018.12.075
Kumar, A. K., and Sharma, S. (2017). Recent updates on different methods of pretreatment of lignocellulosic feedstocks: a review. Bioresour. Bioprocess 4:7. doi: 10.1186/s40643-017-0137-9
Kumar, R., and Wyman, C. (2009a). Effect of enzyme supplementation at moderate cellulase loadings on initial glucose and xylose release from corn stover solids pretreated by leading technologies. Biotechnol. Bioeng. 102, 457–467. doi: 10.1002/bit.22068
Kumar, R., and Wyman, C. E. (2009b). Cellulase adsorption and relationship to features of corn stover solids produced by leading pretreatments. Biotechnol. Bioeng. 103, 252–267. doi: 10.1002/bit.22258
Lee, C., Dazen, K., Kafle, K., Moore, A., Johnson, D. K., Park, S., et al. (2015). Correlations of apparent cellulose crystallinity determined by XRD, NMR, IR, Raman, and SFG methods. Cell. Chem. Prop. 115–1311. doi: 10.1007/12_2015_320
Lee, H. V., Hamid, S. B., and Zain, S. K. (2014). Conversion of lignocellulosic biomass to nanocellulose: structure and chemical process. ScientificWorldJournal. 2014:631013. doi: 10.1155/2014/631013
Leu, S.-Y., and Zhu, J. (2013). Substrate-related factors affecting enzymatic saccharification of lignocelluloses: our recent understanding. Bioenerg. Res. 6, 405–415. doi: 10.1007/s12155-012-9276-1
Lewandowski, I., Clifton-Brown, J., Scurlock, J., and Huisman, W. (2000). Miscanthus: European experience with a novel energy crop. Biomass Bioenergy 19, 209–227. doi: 10.1016/S0961-9534(00)00032-5
Li, J., Lu, M., Guo, X., Zhang, H., Li, Y., Han., et al. (2018). Insights into the improvement of alkaline hydrogen peroxide (AHP) pretreatment on the enzymatic hydrolysis of corn stover: chemical and microstructural analyses. Bioresour. Technol. 265, 1–7. doi: 10.1016/j.biortech.2018.05.082
Li, M., Heckwolf, M., Crowe, J. D., Williams, D. L., Magee, T. D., Kaeppler, S. M., et al. (2015). Cell-wall properties contributing to improved deconstruction by alkaline pre-treatment and enzymatic hydrolysis in diverse maize (Zea mays L.) lines. J. Exp. Bot. 66, 4305–4315. doi: 10.1093/jxb/erv016
Li, M., Si, S., Hao, B., Zha, Y., Wan, C., Hong, S., et al. (2014). Mild alkali-pretreatment effectively extracts guaiacyl-rich lignin for high lignocellulose digestibility coupled with largely diminishing yeast fermentation inhibitors in Miscanthus. Bioresour. Technol. 169, 447–454. doi: 10.1016/j.biortech.2014.07.017
Li, X., Ximenes, E., Kim, Y., Slininger, M., Meilan, R., Ladisch, M., et al. (2010). Lignin monomer composition affects Arabidopsis cell-wall degradability after liquid hot water pretreatment. Biotechnol. Biofuels 3:27. doi: 10.1186/1754-6834-3-27
Liu, M., Wang, L., Si, M., Wang, Z., Zhang, T., Cheng, X., et al. (2019). New insight into enzymatic hydrolysis of the rice straw and poplar: an in-depth statistical analysis on the multiscale recalcitrance. Bioenerg. Res. 12, 1–13. doi: 10.1007/s12155-019-9959-y
Liu, Z.-H., Qin, L., Li, B.-Z., and Yuan, Y.-J. (2014). Physical and chemical characterizations of corn stover from leading pretreatment methods and effects on enzymatic hydrolysis. ACS Sustain. Chem. Eng. 3, 140–146. doi: 10.1021/sc500637c
Lu, M., Li, J., Han, L., and Xiao, W. (2019). An aggregated understanding of cellulase adsorption and hydrolysis for ball-milled cellulose. Bioresour. Technol. 273, 1–7. doi: 10.1016/j.biortech.2018.10.037
Lupoi, J. S., Singh, S., Davis, M., Lee, D. J., Shepherd, M., Simmons, B. A., et al. (2014). High-throughput prediction of eucalypt lignin syringyl/guaiacyl content using multivariate analysis: a comparison between mid-infrared, near-infrared, and Raman spectroscopies for model development. Biotechnol. Biofuels 7:93. doi: 10.1186/1754-6834-7-93
Lv, S., Yu, Q., Zhuang, X., Yuan, Z., Wang, W., Wang, Q., et al. (2013). The influence of hemicellulose and lignin removal on the enzymatic digestibility from sugarcane bagasse. Bioenerg. Res. 6, 1128–1134. doi: 10.1007/s12155-013-9297-4
Mansfield, S. D., Mooney, C., and Saddler, J. N. (1999). Substrate and enzyme characteristics that limit cellulose hydrolysis. Biotechnol. Progr. 15, 804–816. doi: 10.1021/bp9900864
McKendry, P. (2002). Energy production from biomass (part 1): overview of biomass. Bioresour. Technol. 83, 37–46. doi: 10.1016/S0960-8524(01)00118-3
Meng, X., Foston, M., Leisen, J., DeMartini, J., Wyman, C. E., and Ragauskas, A. J. (2013). Determination of porosity of lignocellulosic biomass before and after pretreatment by using Simons' stain and NMR techniques. Bioresour. Technol. 144, 467–476. doi: 10.1016/j.biortech.2013.06.091
Meng, X., Pu, Y., Yoo, C. G., Li, M., Bali, G., Park, D. Y., et al. (2017). An in-depth understanding of biomass recalcitrance using natural poplar variants as the feedstock. ChemSusChem 10, 139–150. doi: 10.1002/cssc.201601303
Meng, X., and Ragauskas, A. J. (2014). Recent advances in understanding the role of cellulose accessibility in enzymatic hydrolysis of lignocellulosic substrates. Curr. Opin. Biotechnol. 27, 150–158. doi: 10.1016/j.copbio.2014.01.014
Meng, X., Wells, T., Sun, Q., Huang, F., and Ragauskas, A. (2015). Insights into the effect of dilute acid, hot water or alkaline pretreatment on the cellulose accessible surface area and the overall porosity of Populus. Green Chem. 17, 4239–4246. doi: 10.1039/C5GC00689A
Menon, V., and Rao, M. (2012). Trends in bioconversion of lignocellulose: biofuels, platform chemicals and biorefinery concept. Prog. Energy Combust. Sci. 38, 522–550. doi: 10.1016/j.pecs.2012.02.002
Monrroy, M., Garcia, J. R., Troncoso, E., and Freer, J. (2015). Fourier transformed near infrared (FT-NIR) spectroscopy for the estimation of parameters in pretreated lignocellulosic materials for bioethanol production. J. Chem. Technol. Biot. 90, 1281–1289. doi: 10.1002/jctb.4427
Mota, T. R., Oliveira, D. M., Rogério Marchiosi, O., and Ferrarese-Filho Santos, W. D. (2018). Plant cell wall composition and enzymatic deconstruction. Bioengineering. 5, 63–77. doi: 10.3934/bioeng.2018.1.63
Neto, W. P. F., Putaux, J.-L., Mariano, M., Ogawa, Y., Otaguro, H., Pasquini, D., et al. (2016). Comprehensive morphological and structural investigation of cellulose I and II nanocrystals prepared by sulphuric acid hydrolysis. RSC Adv. 6, 76017–76027. doi: 10.1039/C6RA16295A
Noori, M. S., and Karimi, K. (2016). Detailed study of efficient ethanol production from elmwood by alkali pretreatment. Biochem. Eng. J. 105, 197–204. doi: 10.1016/j.bej.2015.09.019
Obst, J. R. (1982). Frequency and alkali resistance of wood lignin-carbohydrate bonds in wood. Tappi 65, 109–112. Available online at: https://www.fpl.fs.fed.us/products/publications/specific_pub.php?posting_id=17447
Over, L. C., Grau, E., Grelier, S., Meier, M. A., and Cramail, H. (2017). Synthesis and characterization of epoxy thermosetting polymers from glycidylated organosolv lignin and Bisphenol a. Macromol. Chem. Phys. 218:1600411. doi: 10.1002/macp.201600411
Paës, G., Navarro, D., Benoit, Y., Blanquet, S., Chabbert, B., Chaussepied, B., et al. (2019). Tracking of enzymatic biomass deconstruction by fungal secretomes highlights markers of lignocellulose recalcitrance. Biotechnol. Biofuels 12:76. doi: 10.1186/s13068-019-1417-8
Pan, X., Gilkes, N., and Saddler, J. N. (2006). Effect of acetyl groups on enzymatic hydrolysis of cellulosic substrates. Holzforschung 60, 398–401. doi: 10.1515/HF.2006.062
Pang, J., Zheng, M., Li, X., Sebastian, J., Jiang, Y., Zhao, Y., et al. (2018). Unlock the compact structure of lignocellulosic biomass by mild ball milling for ethylene glycol production. ACS Sustain. Chem. Eng. 7, 679–687. doi: 10.1021/acssuschemeng.8b04262
Papa, G., Varanasi, P., Sun, L., Cheng, G., Stavila, V., Holmes, B., et al. (2012). Exploring the effect of different plant lignin content and composition on ionic liquid pretreatment efficiency and enzymatic saccharification of Eucalyptus globulus L. mutants. Bioresour. Technol. 117, 352–359. doi: 10.1016/j.biortech.2012.04.065
Peciulyte, A., Karlström, K., Larsson, P. T., and Olsson, L. (2015). Impact of the supramolecular structure of cellulose on the efficiency of enzymatic hydrolysis. Biotechnol. Biofuels 8:56. doi: 10.1186/s13068-015-0236-9
Penning, B. W., Sykes, R. W., Babcock, N. C., Dugard, C. K., Held, M. A., Klimek, J. F., et al. (2014). Genetic determinants for enzymatic digestion of lignocellulosic biomass are independent of those for lignin abundance in a maize recombinant inbred population. Plant Physiol. 165, 1475–1487. doi: 10.1104/pp.114.242446
Pihlajaniemi, V., Sipponen, M. H., Liimatainen, H., Sirviö, J. A., Nyyssölä, A., Laakso., et al. (2016). Weighing the factors behind enzymatic hydrolyzability of pretreated lignocellulose. Green Chem. 18, 1295–1305. doi: 10.1039/C5GC01861G
Ragauskas, A. J., Beckham, G. T., Biddy, M. J., Chandra, R., Chen, F., Davis, M. F., et al. (2014). Lignin valorization: improving lignin processing in the biorefinery. Science 344:1246843. doi: 10.1126/science.1246843
Robak, K., and Balcerek, M. (2018). Review of second generation bioethanol production from residual biomass. Food Technol. Biotech. 56, 174–187. doi: 10.17113/ftb.56.02.18.5428
Sannigrahi, P., Ragauskas, A. J., and Tuskan, G. A. (2010). Poplar as a feedstock for biofuels: a review of compositional characteristics. Biofuels Bioprod. Bioref. 4, 209–226. doi: 10.1002/bbb.206
Santos, R. B., Lee, J. M., Jameel, H., Chang, H. -M., and Lucia, L. A. (2012). Effects of hardwood structural and chemical characteristics on enzymatic hydrolysis for biofuel production. Bioresour. Technol. 110, 232–238. doi: 10.1016/j.biortech.2012.01.085
Santos, V. T. O., Siqueira, G., Milagres, A. M. F., and Ferraz, A. (2018). Role of hemicellulose removal during dilute acid pretreatment on the cellulose accessibility and enzymatic hydrolysis of compositionally diverse sugarcane hybrids. Ind. Crops Prod. 111, 722–730. doi: 10.1016/j.indcrop.2017.11.053
Schmer, M. R., Vogel, K. P., Mitchell, R. B., and Perrin, R. K. (2008). Net energy of cellulosic ethanol from switchgrass. Proc. Natl. Acad. Sci. U.S.A. 105, 464–469. doi: 10.1073/pnas.0704767105
Selig, M. J., Tucker, M. P., Law, C., Doeppke, C., Himmel, M. E., and Decker, S. R. (2011). High throughput determination of glucan and xylan fractions in lignocelluloses. Biotechnol. Lett. 33, 961–967. doi: 10.1007/s10529-011-0526-7
Sharma, H. K., Xu, C., and Qin, W. (2019). Biological pretreatment of lignocellulosic biomass for biofuels and bioproducts: an overview. Waste Biomass Valori. 10, 235–251. doi: 10.1007/s12649-017-0059-y
Sills, D. L., and Gossett, J. M. (2012). Using FTIR to predict saccharification from enzymatic hydrolysis of alkali-pretreated biomasses. Biotechnol. Bioeng. 109, 353–62. doi: 10.1002/bit.23314
Silva, G. G., Couturier, M., Berrin, J. -G., Buléon, A., and Rouau, X. (2012). Effects of grinding processes on enzymatic degradation of wheat straw. Bioresour. Technol. 103, 192–200. doi: 10.1016/j.biortech.2011.09.073
Silvi Octavia, R. P., Arsa, P. I. D. G., and Tatang, H. (2017). Soerawidjaja: determining the enzyme accessibility of ammonia pretreated lignocellulosic substrates by Simon's Stain method. J. Eng. Appl. Sci. 12.
Sinitsyn, A., Gusakov, A., and Vlasenko, E. Y. (1991). Effect of structural and physico-chemical features of cellulosic substrates on the efficiency of enzymatic hydrolysis. Appl. Biochem. Biotech. 30, 43–59. doi: 10.1007/BF02922023
Stoffel, R. B., Felissia, F. E., Curvelo, A. A. S., Gassa, L. M., and Area, M. C. (2014). Optimization of sequential alkaline–acid fractionation of pine sawdust for a biorefinery. Ind. Crops Prod. 61, 160–168. doi: 10.1016/j.indcrop.2014.06.047
Studer, M. H., DeMartini, J. D., Brethauer, S., McKenzie, H. L., and Wyman, C. E. (2010). Engineering of a high-throughput screening system to identify cellulosic biomass, pretreatments, and enzyme formulations that enhance sugar release. Biotechnol. Bioeng. 105, 231–238. doi: 10.1002/bit.22527
Studer, M. H., DeMartini, J. D., Davis, M. F., Sykes, R. W., Davison, B., Keller, M., et al. (2011). Lignin content in natural Populus variants affects sugar release. Proc. Natl. Acad. Sci. U.S.A. 108, 6300–6305. doi: 10.1073/pnas.1009252108
Sykes, R. W., Gjersing, E. L., Doeppke, C. L., and Davis, M. F. (2015). High-throughput method for determining the sugar content in biomass with pyrolysis molecular beam mass spectrometry. Bioenerg. Res. 8, 964–972. doi: 10.1007/s12155-015-9610-5
Tarasov, D., Leitch, M., and Fatehi, P. (2018). Lignin-carbohydrate complexes: properties, applications, analyses, and methods of extraction: a review. Biotechnol. Biofuels 11:269. doi: 10.1186/s13068-018-1262-1
Torr, K. M., Love, K. T., Simmons, B. A., and Hill, S. J. (2016). Structural features affecting the enzymatic digestibility of pine wood pretreated with ionic liquids. Biotechnol. Bioeng. 113, 540–549. doi: 10.1002/bit.25831
Vaidya, A. A., Donaldson, L. A., Newman, R. H., Suckling, I. D., Campion, S. H., Lloyd, J. A., et al. (2016). Micromorphological changes and mechanism associated with wet ball milling of Pinus radiata substrate and consequences for saccharification at low enzyme loading. Bioresour. Technol. 214, 132–137. doi: 10.1016/j.biortech.2016.04.084
Weiss, N. D., Felby, C., and Thygesen, L. G. (2018). Water retention value predicts biomass recalcitrance for pretreated lignocellulosic materials across feedstocks and pretreatment methods. Cellulose 25, 3423–3434. doi: 10.1007/s10570-018-1798-z
Weiss, N. D., Thygesen, L. G., Felby, C., Roslander, C., and Gourlay, K. (2017). Biomass-water interactions correlate to recalcitrance and are intensified by pretreatment: an investigation of water constraint and retention in pretreated spruce using low field NMR and water retention value techniques. Biotechnol. Progr. 33, 146–153. doi: 10.1002/btpr.2398
Williams, D. L., Crowe, J. D., Ong, R. G., and Hodge, D. B. (2017). Water sorption in pretreated grasses as a predictor of enzymatic hydrolysis yields. Bioresour. Technol. 245, 242–249. doi: 10.1016/j.biortech.2017.08.200
Williams, D. L., and Hodge, D. B. (2014). Impacts of delignification and hot water pretreatment on the water induced cell wall swelling behavior of grasses and its relation to cellulolytic enzyme hydrolysis and binding. Cellulose 21, 221–235. doi: 10.1007/s10570-013-0149-3
Xu, H., Che, X., Ding, Y., Kong, Y., Li, B., Tian., et al. (2019). Effect of crystallinity on pretreatment and enzymatic hydrolysis of lignocellulosic biomass based on multivariate analysis. Bioresour. Technol. 279, 271–280. doi: 10.1016/j.biortech.2018.12.096
Xu, N., Zhang, W., Ren, S., Liu, F., Zhao, C., Liao, H., et al. (2012). Hemicelluloses negatively affect lignocellulose crystallinity for high biomass digestibility under NaOH and H 2 SO 4 pretreatments in Miscanthus. Biotechnol. Biofuels 5:58. doi: 10.1186/1754-6834-5-58
Yang, Q., and Pan, X. (2016). Correlation between lignin physicochemical properties and inhibition to enzymatic hydrolysis of cellulose. Biotechnol. Bioeng. 113, 1213–1224. doi: 10.1002/bit.25903
Yao, L., Yoo, C. G., Meng, X., Li, M., Pu, Y., Ragauskas, A. J., et al. (2018). A structured understanding of cellobiohydrolase I binding to poplar lignin fractions after dilute acid pretreatment. Biotechnol. Biofuels 11:96. doi: 10.1186/s13068-018-1087-y
Yoo, C. G., Dumitrache, A., Muchero, W., Natzke, J., Akinosho, H., Li, M., et al. (2017b). Significance of lignin S/G ratio in biomass recalcitrance of populus trichocarpa variants for bioethanol production. ACS Sustain. Chem. Eng. 6, 2162–2168. doi: 10.1021/acssuschemeng.7b03586
Yoo, C. G., Yang, Y., Pu, Y., Meng, X., Muchero, W., Yee, K. L., et al. (2017a). Insights of biomass recalcitrance in natural Populus trichocarpa variants for biomass conversion. Green Chem. 19, 5467–5478. doi: 10.1039/C7GC02219K
Yoshida, M., Liu, Y., Uchida, S., Kawarada, K., Ukagami, Y., Ichinose, H., et al. (2008). Effects of cellulose crystallinity, hemicellulose, and lignin on the enzymatic hydrolysis of Miscanthus sinensis to monosaccharides. Biosci. Biotechnol. Biochem. 72, 805–810. doi: 10.1271/bbb.70689
Yu, H., Xiao, W., Han, L., and Huang, G. (2019). Characterization of mechanical pulverization/phosphoric acid pretreatment of corn stover for enzymatic hydrolysis. Bioresour. Technol. 282, 69–74. doi: 10.1016/j.biortech.2019.02.104
Yu, Z., Gwak, K. S., Treasure, T., Jameel, H., Chang, H. M., and Park, S. (2014). Effect of lignin chemistry on the enzymatic hydrolysis of woody biomass. ChemSusChem 7, 1942–1950. doi: 10.1002/cssc.201400042
Yuan, Z., Wen, Y., and Li, G. (2018). Production of bioethanol and value added compounds from wheat straw through combined alkaline/alkaline-peroxide pretreatment. Bioresour. Technol. 259, 228–236 doi: 10.1016/j.biortech.2018.03.044
Zeng, Y., Zhao, S., Yang, S., and Ding, S.-Y. (2014). Lignin plays a negative role in the biochemical process for producing lignocellulosic biofuels. Curr. Opin. Biotechnol. 27, 38–45. doi: 10.1016/j.copbio.2013.09.008
Zhang, H., Li, J., Huang, G., Yang, Z., and Han, L. (2018). Understanding the synergistic effect and the main factors influencing the enzymatic hydrolyzability of corn stover at low enzyme loading by hydrothermal and/or ultrafine grinding pretreatment. Bioresour. Technol. 264, 327–334. doi: 10.1016/j.biortech.2018.05.090
Zhao, X., Zhang, L., and Liu, D. (2012a). Biomass recalcitrance. Part II: fundamentals of different pre-treatments to increase the enzymatic digestibility of lignocellulose. Biofuels Bioprod. Bioref. 6, 561–579. doi: 10.1002/bbb.1350
Zhao, X., Zhang, L., and Liu, D. (2012b). Biomass recalcitrance. Part I: the chemical compositions and physical structures affecting the enzymatic hydrolysis of lignocellulose. Biofuels, Bioprod. Bioref. 6, 465–482. doi: 10.1002/bbb.1331
Keywords: lignocellulose, recalcitrance, chemical composition, structure, enzymatic hydrolysis
Citation: Zoghlami A and Paës G (2019) Lignocellulosic Biomass: Understanding Recalcitrance and Predicting Hydrolysis. Front. Chem. 7:874. doi: 10.3389/fchem.2019.00874
Received: 14 June 2019; Accepted: 04 December 2019;
Published: 18 December 2019.
Edited by:
Jose Luis Sanchez, University of Zaragoza, SpainReviewed by:
David B. Hodge, Montana State University, United StatesCopyright © 2019 Zoghlami and Paës. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gabriel Paës, Z2FicmllbC5wYWVzQGlucmEuZnI=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.