
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Chem. , 28 May 2019
Sec. Electrochemistry
Volume 7 - 2019 | https://doi.org/10.3389/fchem.2019.00379
This article is part of the Research Topic Conversion Materials for Rechargeable Batteries View all 16 articles
LiFeBO3/C cathode material with hollow sphere architecture is successfully synthesized by a spray-drying method. SEM and TEM results demonstrate that the micro-sized LiFeBO3/C hollow spheres consist of LiFeBO3@C particles and the average size of LiFeBO3@C particles is around 50–100 nm. The thickness of the amorphous carbon layer which is coated on the surface of LiFeBO3 nanoparticles is about 2.5 nm. LiFeBO3@C particles are connected by carbon layers and formed conductive network in the LiFeBO3/C hollow spheres, leading to improved electrical conductivity. Meanwhile, the hollow structure boosts the Li+ diffusion and the carbon layers of LiFeBO3@C particles protect LiFeBO3 from moisture corrosion. Consequently, synthesized LiFeBO3/C sample exhibits good electrochemical properties and storage performance.
With the development of electric vehicles in the twenty-first century, Li-ion batteries (LIBs) play an increasingly important role in modern society (Nayak et al., 2018; Wu et al., 2019) and various cathode materials are developed (Ma et al., 2015; Zheng et al., 2018a,b; Yang et al., 2019). Recently, borate-based materials (LiMBO3, M = Fe, Mn, and Co) have received wide attention in the field of LIBs (Lin et al., 2012; Tao et al., 2013). Compared with the commonly used phosphate-based materials (LiMPO4, M = Fe, Mn, and Co), borate groups (LiMBO3, M = Fe, Mn, and Co) with a low specific weight show higher capacities. For instance, the theoretical capacity of LiMnBO3 is 222 mAh g−1, whereas the phosphate counterpart, LiMnPO4, is limited to 170 mAh g−1 (Moskon et al., 2016; Zhang et al., 2017). Moreover, the volume changes of borate-based materials during charging-discharging process are less than those of phosphate-based materials so that they usually show good structural stability (Loftager et al., 2017). Among various borate-based cathode materials, LiFeBO3 with the theoretical capacity of 220 mAh g−1 presents moderate working voltage, relatively high intrinsic conductivity, and good structural stability (Michalski et al., 2017). Therefore, LiFeBO3 is considered as a potential cathode material for LIBs. However, there is only one-dimensional (1D) pathway for Li+ diffusing in the LiFeBO3 crystal, which leading to low electronic and ionic conductivity (Dong et al., 2008; Kalantarian et al., 2013). Furthermore, LiFeBO3 is highly sensitive to moisture so that surface poisoning by H2O molecules of air would seriously disrupt its electrochemical properties (Gu et al., 2017). These problems make it challenging to achieve LiFeBO3-based materials with high reversible specific capacity and good storage performance. In order to improve the electrochemical performance of LiFeBO3-based materials, various strategies have been introduced, such as metal-ions doping, conductive carbon coating, and nano-architecturing (Aravindan and Umadevi, 2012; Afyon et al., 2013; Le Roux et al., 2015; Sin et al., 2015). Among these methods, carbon coating is a promising and effective way to improve the properties of LiFeBO3, which attributes to the improved conductivity and the prevention of moisture corrosion. For example, Li et al. reported that the carbon coated LiFeBO3 nanoparticles exhibit the improved first discharge capacities of 190.4 and 106.6 mAh g−1 at 0.1 and 1°C rate, respectively (Li et al., 2015). The LiFeBO3/C prepared by Zhang et al. also indicated carbon coating is an effective way to improve the properties of LiFeBO3. However, the rate capability and storage performance of LiFeBO3 are still not satisfied (Zhang et al., 2014). Therefore, achieving homogeneous carbon coated LiFeBO3 cathode materials with high electrochemical properties and good storage performance is still a challenge right now.
Herein, LiFeBO3/C with hollow porous sphere architecture is successfully synthesized by spray-drying method at the temperature as low as 450°C (Li et al., 2015). Amorphous carbon layer which was produced by polyethylene glycol 6,000 (PEG-6000) decomposition is coated on the surface of LiFeBO3 nanoparticles. The LiFeBO3@C particles are connected together and formed LiFeBO3/C hollow porous spheres. The obtained LiFeBO3/C sample with distinctive architecture shows the improved electrochemical and storage performances as cathode for LIBs.
Firstly, LiNO3, 1.38g LiNO3, 8.08g Fe(NO3)3·9H2O, and 1.24g H3BO3 were weighed and dissolved gradually in deionized water under stirring, and the mixed solution was named solution A. Meanwhile, 1.22 g PEG-6000 was dropped and dissolved in deionized water under stirring to form solution B. Then the solution B was added into the solution A and stirred carefully. A homogeneous sol was obtained when the mixed solution has been stirred at 80°C for 1 h. The precursor of LiFeBO3/C was synthesized using the obtained sol as raw material via a spray drying process. The inlet and outlet air temperatures are 200 and 100°C during the spray drying process with the air pressure of 0.25 MPa. The as-obtained precursor was calcined at 350°C (3 h), followed by crystallized at 450°C for 10 h in an argon atmosphere, and the final LiFeBO3/C was obtained. For contrast, the LiFeBO3 sample was also prepared by the similar synthetic process but without PEG-6000. All the chemical regents employed in this work were of analytic grade.
The structure of LiFeBO3/C and LiFeBO3 samples was investigated by XRD (Rigaku/Ultima-IV) and Raman micro-spectroscopy. And the range of XRD and Raman spectroscopic analysis is 2θ = 10 ~ 80° and 600–2,000 cm−1, respectively. Morphology and microstructure of LiFeBO3/C and LiFeBO3 samples were investigated by SEM (JSM/6380LV) and TEM (TecnaiG220). C-S analysis (Eltar) was used to determine the carbon content of samples.
The electrochemical measurements were performed in coin-type cells (CR2025) with lithium metal as the negative electrode. The positive electrodes were prepared by mixing as-prepared LiFeBO3/C or LiFeBO3 powder, acetylene black (99.9%, Sinopharm Chemial Reagent Co., Ltd.) and polyvinylidene fluoride (PVDF, 99.9%, Sinopharm Chemial Reagent Co., Ltd. the weight ratio is 80:10:10) in N-methylpyrolline onto an Al foil and dried at 120°C for 4 h in vacuum oven. Then the coin-type cells were assembled in a glove box filled with high purity argon. 1M LiPF6 solution in a mixture of ethylene carbonate and dimethyl carbonate with 1:1 volumetric ratio was used as electrolyte (Sinopharm Chemial Reagent Co., Ltd.). The cells were tested in the voltage range of 1.5–4.5 V under various charge/discharge current densities from 5 to 100 mA g−1 at room temperature. The electrochemical impedance spectroscopy (EIS) and the cyclic voltammetry (CV) was both measured by a CHI 660D workstation.
XRD patterns of the synthesized LiFeBO3/C and LiFeBO3 samples are shown in Figure 1A. Obviously, the LiFeBO3 with monoclinic structure is well-crystallized. XRD patterns of two samples are similar, indicating the combination of LiFeBO3 and carbon has little influence on the crystal structure. Although the amount of carbon in the LiFeBO3/C composites is 4.9 wt. % (proved by C-S analysis), the diffraction peak of carbon is not detected from the XRD patterns, implying the carbon in the LiFeBO3/C composite is amorphous.
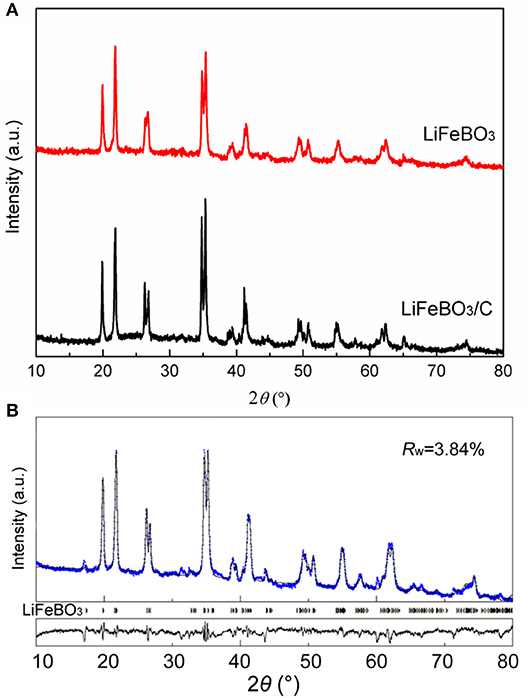
Figure 1. (A) XRD patterns of the as-prepared LiFeBO3/C and LiFeBO3; (B) Rietveld refinement XRD pattern of the as-prepared LiFeBO3/C.
In order to further clarify the crystal structure and lattice parameters of the synthesized LiFeBO3/C, XRD data was refined by Rietveld method (Rietveld, 1967) using Maud software (Lutterotti, 2011), and the Rietveld refinement XRD pattern and atoms positions and occupancy for Li and Fe atoms in LiFeBO3/C investigated. As can be seen from Figure 1B and Table 1, the sharp peaks of the sample can be identified as monoclinic LiFeBO3 with space group of C2/c and the structure is crystallized well. Meanwhile, the observed and calculated patterns match well, and the reliability factors are good. In accordance with the refinement results, the lattice parameters of LiFeBO3 are a = 5.1662 Å, b = 8.9141 Å, c = 10.1700 Å, β = 91.25°, agreeing well with the reported literature (Zhang et al., 2014).
Figure 2 represents the Raman spectroscopy of the as-prepared LiFeBO3/C sample. As can be seen from Figure 2, the broad peak observed at 1,354 cm−1 correspond to the D (disordered) band of carbon while the broad peak located at 1,590 cm−1 agreeing well with G (graphitized) band of sp2 type carbon (Liu et al., 2015; Zhu et al., 2017). The strong D-peak further reveals that the carbon is amorphous, which is in agreement with the XRD result.
SEM and TEM were employed to analyze morphology and micro-structure of the synthesized LiFeBO3 and LiFeBO3/C. As shown in Figure 3a, the LiFeBO3 sample displays a spherical shape with a size distribution in the range of 1–4 μm and the surface is compact. While, the LiFeBO3/C sample exhibits hollow spherical shape with a rough and porous surface layer (Figures 3b,c). The comparison of the darker outer shell and the lighter central regions indicates that the LiFeBO3/C spheres present porous and hollow internal structure (Figure 3d), which is consistent with the SEM results. As shown in Figure 3c, it is obvious that the spherical LiFeBO3/C are actually consist of numerous primary nanoparticles. Figure 3e shows that nano-sized primary LiFeBO3 particles are well wrapped by thick carbon layer. This carbon-coated layer is benefit to prevent LiFeBO3 from moisture corrosion. As we can see from Figure 3f, the lattice fringe with an interior planar distance of 0.4472 nm is correspond well to (1 1 0) crystal planes of LiFeBO3. The coating layer with the thickness of 2.5 nm could be assigned to the amorphous carbon, so that LiFeBO3@C shows a core-shell structure. In addition, the carbon-coated layer and conductive carbon network can improve the conductivity of LiFeBO3-based cathode material, which is also benefit to the electrochemical performances.
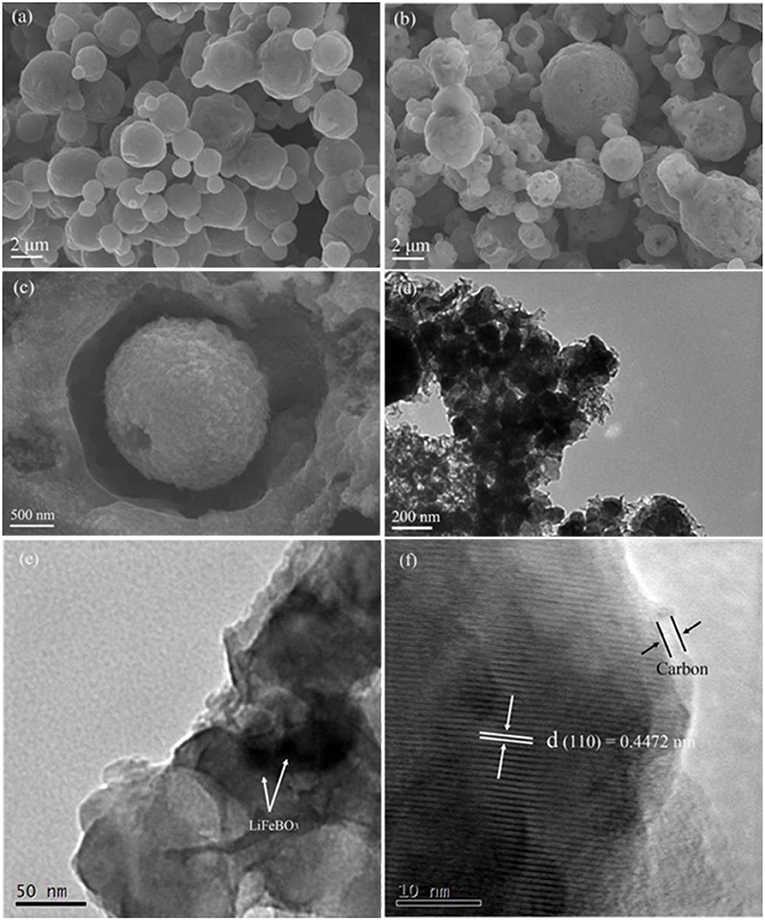
Figure 3. SEM images of the as-prepared LiFeBO3 (a) and LiFeBO3/C (b,c); TEM images of the as-prepared LiFeBO3/C (d–f).
In the potential (vs. Li/Li+) range of 1.5–4.5 V, the cells were charged/discharged at 10 mA g−1, and the curves of the as-synthesized LiFeBO3/C and LiFeBO3 samples are exhibited in Figures 4A,B, respectively. It is noted that the initial charge curves of both samples show obviously higher voltage plateau than the subsequent cycles, which mainly due to the high polarization before cycling. This phenomenon is very similar to cases of LiMnBO3 and LiCoBO3 (Afyon et al., 2014; Tang et al., 2015). The charge plateau (~3.2 V) and the discharge plateau (~2.8 V) in the voltage profiles correspond to the plateaus of LiFeBO3. There is a reduced thermodynamic potential for the degraded LiFeBO3 phase so that a short discharge plateau ~1.8 V appeared in the discharge profiles (Bo et al., 2012).
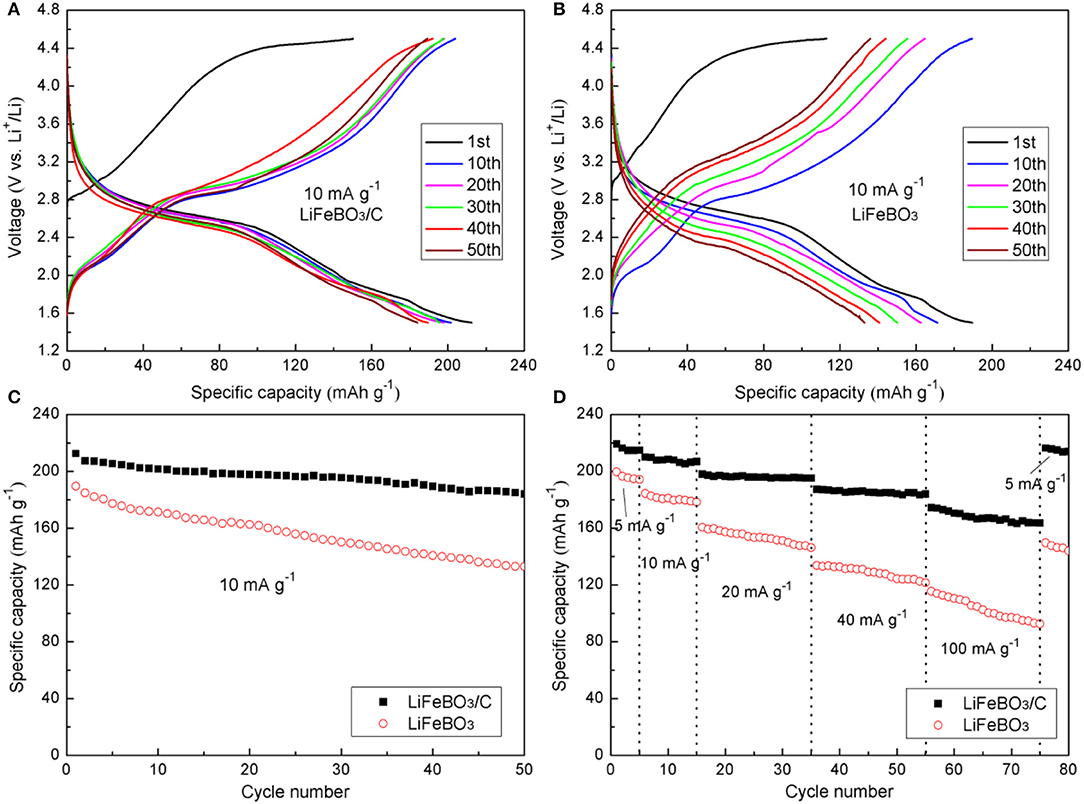
Figure 4. Charge-discharge curves of the LiFeBO3/C (A) and LiFeBO3 (B) at the current density of 10 mA g−1; (C) Cycle performance of LiFeBO3/C and LiFeBO3 at the current density of 10 mA g−1; (D) Rate capability of LiFeBO3/C and LiFeBO3 at various current densities.
The cycling performances of the as-prepared LiFeBO3/C and LiFeBO3 samples at 10 mA g−1 are shown in Figure 4C. The initial discharge specific capacities of LiFeBO3/C and LiFeBO3 are 212.4 and 189.5 mAh g−1, respectively. The specific capacity of LiFeBO3/C is as high as 184.1 mAh g−1 while the specific capacity of LiFeBO3 is only 132.9 mAh g−1 after 50 cycles, indicating the LiFeBO3/C sample has a better cyclic stability. Figure 4D displays the rate capability of the as-prepared LiFeBO3/C and LiFeBO3 samples under different current densities. The initial capacity of the as-prepared LiFeBO3/C is 219.2 mAh g−1 at 5 mA g−1, which is approximately 98.7% of the theoretical capacity. When the current density is increased up to 40 and 100 mA g−1, the LiFeBO3/C sample holds a stable reversible capacity of 187.3 and 175.8 mAh g−1, respectively. However, the discharge capacity of LiFeBO3 at 40 and 100 mA g−1 are only 144.5 and 115.5 mAh g−1, respectively. If the current density returns back to 5 mA g−1 after 75 cycles test, and 98.6% of the initial discharge specific capacity (216.3 mAh g−1) can be recovered for LiFeBO3/C but only 75.1% was recovered for LiFeBO3 electrode (149.6 mAh g−1), implying the LiFeBO3/C sample has a better capacity recovery ability than LiFeBO3. The improved electrochemical performances can be attributed to the special hollow sphere structure and network of amorphous carbon layer. The conductivity is improved by the network of amorphous carbon layer and the volume expansion during the charge and discharge progresses is againsted due to the special hollow sphere architecture provides a flexible structure, so that the electrochemical performance of the synthesized cathode is improved (Li et al., 2013).
Figure 5A shows the cyclic voltammetry (CV) of the as-prepared LiFeBO3/C and LiFeBO3 samples. The oxidation/reduction peaks located at ~2.95/2.35 V correspond to the phase transition between LiFeBO3 and FeBO3. It is clearly observed that LiFeBO3/C sample shows sharper peaks and larger areas than LiFeBO3, implying the synthesized LiFeBO3/C has a better reversibility. In addition, a couple of oxidation/reduction peaks are located at ~2.09/1.85 V, and these patterns can be ascribed to the degradation of LiFeBO3 (Afyon et al., 2014).
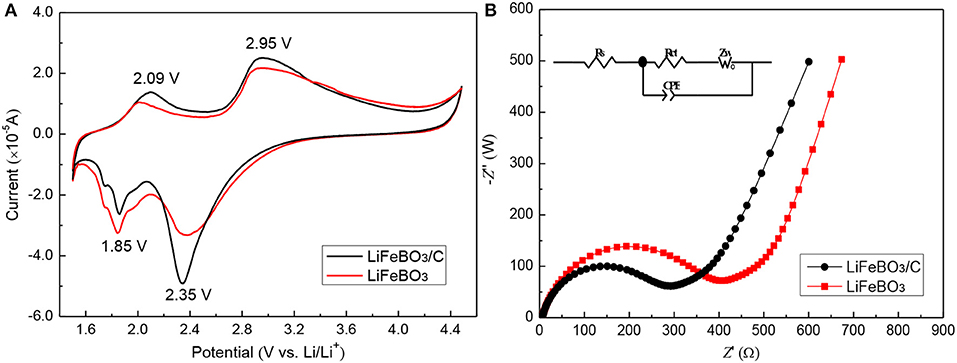
Figure 5. (A) Cyclic voltammetry profiles of LiFeBO3/C and LiFeBO3 at the scan rate of 0.1 mV s−1; (B) Electrochemical impedance spectra of as-prepared LiFeBO3/C and LiFeBO3.
In order to analyze the conductivity of the as-prepared LiFeBO3/C and LiFeBO3 samples, the EIS tests (Figure 5B) are measured in our study. Then the EIS data is fitted with Z-view software using an equivalent circuit, and the related parameters are listed in Table 2. Rs, Zw, and Rct in the equivalent circuit (insert Figure 5B) represent the resistance of the electrolyte, Warburg impedance and charge transfer resistance, respectively. The Rct of LiFeBO3/C (238.6 Ω) is lower than that of LiFeBO3 (354.1 Ω), implying the charge transfer speed during the charge and discharge processes of electrode is significantly improved. The exchange current density (j0) of synthesized LiFeBO3/C and LiFeBO3 is calculated by the following equation, respectively.
Where T and n refer to the temperature and the electrons number, respectively. The exchange current density (j0) of LiFeBO3/C (10.78 × 10−4 mA cm−2) is higher than that of LiFeBO3 (7.26 × 10−5 mA cm−2), which indicates that LiFeBO3/C has a better electrode reaction reversibility than LiFeBO3.
To evaluate the storage stability, LiFeBO3/C and LiFeBO3 samples were stored in air exposure for 6 months. XRD patterns of the LiFeBO3/C and LiFeBO3 samples before and after storage are shown in Figure 6. Gratifyingly, the stored LiFeBO3/C sample also remains its original crystal structure, verifying high storage stability of LiFeBO3/C, as shown in Figure 6A. However, the XRD pattern of LiFeBO3 changed after storing 6 months. As can be seen from Figure 6B, the intensity of (1 1 2) peak tends to increase while the intensity of (1 1 2) peak tends to decrease, and the two peaks prone to overlap each other. This phenomenon can be attributed to phase changing of LiFeBO3 from monoclinic to orthorhombic structure with air corrosion (Bo et al., 2012; Chen et al., 2015).
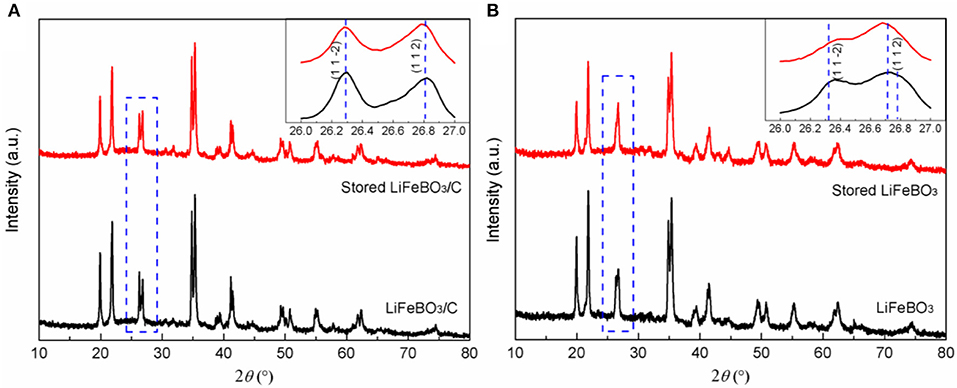
Figure 6. XRD patterns of LiFeBO3/C (A) and LiFeBO3 (B) before and after storing for 6 months (air exposure at room temperature).
After 6 months of storage, the cycling performances of LiFeBO3/C and LiFeBO3 samples are investigated, and the results are shown in Figure 7. The capacity of the stored LiFeBO3/C is as high as 198.9 mAh g−1 and there is 76.9% capacity retention after 50 cycles. However, the stored LiFeBO3 delivers a capacity of 191.7 mAh g−1 and only 56.1% capacity retention after 50 cycles. Obviously, the stored LiFeBO3/C shows higher stability and cycling performance than the stored LiFeBO3. In summary, carbon coating and structural modification improve the conductivity of LiFeBO3, shorten the Li+ diffusion/conduction path and protect LiFeBO3 from moisture corrosion, thus leading to good electrochemical performance.
LiFeBO3/C hollow sphere is successfully synthesized by a facile spray-drying method. Primary LiFeBO3@C particles are connected by carbon layers and formed LiFeBO3/C hollow spheres with improved electrical conductivity. Meanwhile, the hollow porous structure boosts the Li+ diffusion and the carbon layers of LiFeBO3@C particles protect LiFeBO3 from moisture corrosion. Therefore, the synthesized LiFeBO3/C sample shows good electrochemical performances and improved storage performance. This work is conducive to obtaining promising and high-performance cathode materials for LIBs.
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.
YS, ST, and WC did the main experiment and write the manuscript. LW and BW envolved the discussion of the experiment and revised the manuscript. HL and WL assisted the material synthesis. LW and SZ made the research plan. SZ and LW also provided the financial support.
This study was supported by the National Natural Science Foundation of China (51574168, 51574170, and 51774207), Guangxi Key Laboratory of Electrochemical and Magnetochemical Functional Materials (EMFM20182202), and Major Projects for Science & Technology Development of Guangxi Province, China (AA16380043).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Afyon, S., Kundu, D., Krumeich, F., and Nesper, R. (2013). Nano-LiMnBO3, a high-capacity cathode material for Li-ion batteries. J. Power Sources 224, 145–151. doi: 10.1016/j.jpowsour.2012.09.099
Afyon, S., Mensing, C., Krumeich, F., and Nesper, R. (2014). The electrochemical activity for nano-LiCoBO3 as a cathode material for Li-ion batteries. Solid State Ionics 256, 103–108. doi: 10.1016/j.ssi.2014.01.010
Aravindan, V., and Umadevi, M. (2012). Synthesis and characterization of novel LiFeBO3/C cathodes for lithium batteries. Ionics 18, 27–30. doi: 10.1007/s11581-011-0594-7
Bo, S. H., Wang, F., Janssen, Y., Zeng, D. L., Nam, K. W., Xu, W. Q., et al. (2012). Degradation and (de)lithiation processes in the high capacity battery material LiFeBO3. J. Mater. Chem. 22, 8799–8809. doi: 10.1039/c2jm16436a
Chen, Z. X., Cao, L. F., Chen, L., Zhou, H. H., Zheng, C. M., Xie, K., et al. (2015). Mesoporous LiFeBO3/C hollow spheres for improved stability lithium ion battery cathodes. J. Power Sources 298, 355–362. doi: 10.1016/j.jpowsour.2015.08.073
Dong, Y. Z., Zhao, Y. M., Shi, Z. D., An, X. N., Fu, P., and Chen, L. (2008). The structure and electrochemical performance of LiFeBO3 as a novel Li-battery cathode material. Electrochim. Acta. 53, 2339–2345. doi: 10.1016/j.electacta.2007.09.050
Gu, X. F., Ting, M., and Zhi, S. (2017). Sol-gel synthesis of nanostructure LiFeBO3/C lithium-ion cathode materials with high storage capacity. J. Sol-Gel. Sci. Techn. 81, 362–366. doi: 10.1007/s10971-016-4264-0
Kalantarian, M. M., Asgari, S., and Mustarelli, P. (2013). A theoretical approach to evaluate the rate capability of Li-ion battery cathode materials. J. Mater. Chem. A 2, 107–115. doi: 10.1039/C3TA13387G
Le Roux, B., Bourbon, C., Lebedev, O. I., Colin, J. F., and Pralong, V. (2015). Synthesis and characterization of the LiMnBO3-LiCoBO3 solid solution and its use as a lithium-ion cathode material. Inorg. Chem. 54, 5273–5279. doi: 10.1021/acs.inorgchem.5b00260
Li, S. L., Xu, L. Q., Li, G. D., Wang, M., and Zhai, Y. J. (2013). In-situ controllable synthesis and performance investigation of carbon-coated monoclinic and hexagonal LiMnBO3 composites as cathode materials in lithium-ion batteries. J. Power Sources 236, 54–60. doi: 10.1016/j.jpowsour.2013.02.027
Li, Z., Wang, Y., Hu, Q., Yang, Y., Wu, Z., and Ban, C. (2015). Improved electrochemical performance of carbon-coated LiFeBO3 nanoparticles for lithium-ion batteries. J. Nanosci. Nanotechno. 15, 7186–7190. doi: 10.1166/jnn.2015.10555
Lin, Z. P., Zhao, Y. J., and Zhao, Y. M. (2012). First-principles study of the structural, magnetic, and electronic properties of LiMBO3 (M=Mn, Fe, Co). Phys. Lett. A 376, 179–184. doi: 10.1016/j.physleta.2011.08.018
Liu, Y., Xu, X., Wang, M., Lu, T., Sun, Z., and Pan, L. (2015). Nitrogen-doped carbon nanorods with excellent capacitive deionization ability. J. Mater. Chem. A 3, 17304–17311. doi: 10.1039/C5TA03663A
Loftager, S., Garcialastra, J. M., and Vegge, T. (2017). A density functional theory study of the carbon-coating effects on lithium iron borate battery electrodes. Phys. Chem. Chem. Phys. 19, 2087–2094. doi: 10.1039/C6CP06312H
Lutterotti, L. (2011). Maud verxsion 2.91. Avliable online at: https://www.softpedia.com/get/Science-CAD/Maud.shtml (accessed March, 2019)
Ma, T., Muslim, A., and Su, Z. (2015). Microwave synthesis and electrochemical properties of lithium manganese borate as cathode for lithium ion batteries. J. Power Sources 282, 95–99. doi: 10.1016/j.jpowsour.2015.02.013
Michalski, P. P., Pietrzak, T. K., Nowinski, J. L., Wasiucionek, M., and Garbarczyk, J. E. (2017). Novel nanocrystalline mixed conductors based on LiFeBO3 glass. Solid State Ionics 302, 40–44. doi: 10.1016/j.ssi.2016.12.002
Moskon, J., Pivko, M., Jerman, I., Tchernychova, E., Zabukovec Logar, N., Selih, V. S., et al. (2016). Cycling stability and degradation mechanism of LiMnPO4 based electrodes. J. Power Sources 303, 97–108. doi: 10.1016/j.jpowsour.2015.10.094
Nayak, P. K., Yang, L., Brehm, W., and Adelhelm, P. (2018). From Lithium-ion to sodium-ion batteries: advantages, challenges, and surprises. Angew. Chem. Int. Edit. 57, 102–120. doi: 10.1002/anie.201703772
Rietveld, H. M. (1967). Line profiles of neutron powder-diffraction peaks for structure refinement. Acta Cryst. 22, 151–152. doi: 10.1107/S0365110X67000234
Sin, B. C., Singh, L., Lee, K. E., Kim, M., Cho, M., Yarger, J. L., et al. (2015). Enhanced electrochemical performance of LiFe0.4Mn0.6(PO4)1−x(BO3)x as cathode material for lithium ion batteries. J. Electroanal. Chem. 756, 56–60. doi: 10.1016/j.jelechem.2015.08.012
Tang, A. P., He, D. H., He, Z. Q., Xu, G. R., Song, H. S., and Peng, R. H. (2015). Electrochemical performance of LiMnBO3/C composite synthesized by a combination of impregnation and precipitation followed by annealing. J. Power Sources 275, 888–892. doi: 10.1016/j.jpowsour.2014.11.087
Tao, L., Neilson, J. R., Melot, B. C., Mcqueen, T. M., Masquelier, C., and Rousse, G. (2013). Magnetic structures of LiMBO3 (M=Mn, Fe, Co) lithiated transition metal borates. Inorg. Chem. 52, 11966–11974. doi: 10.1021/ic401671m
Wu, L., Zheng, J., Wang, L., Xiong, X., Shao, Y., Wang, G., et al. (2019). PPy-encapsulated SnS2 nanosheets stabilized by defects on a TiO2 support as a durable anode material for lithium-ion batteries. Angew. Chem. Int. Edit. 58, 811–815. doi: 10.1002/anie.201811784
Yang, H., Wu, H. H., Ge, M., Li, L., Yuan, Y., Yao, Y., et al. (2019). Simultaneously dual modification of Ni-rich layered oxide cathode for high-energy lithium-ion batteries. Adv. Funct. Mater. 29:1808825. doi: 10.1002/adfm.201808825
Zhang, B., Ming, L., Zheng, J. C., Zhang, J. F., Shen, C., Han, Y. D., et al. (2014). Synthesis and characterization of multi-layer core-shell structural LiFeBO3/C as a novel Li-battery cathode material. J. Power Sources 261, 249–254. doi: 10.1016/j.jpowsour.2014.03.082
Zhang, B., Zhu, Y. S., Yu, W. J., Zhang, J. F., and An, C. S. (2017). Facile synthesis of carbon-encapsulated LiMnBO3 composite by the sol-gel method as a lithium-ion battery cathode material. J. Alloys. Compd. 704, 343–347. doi: 10.1016/j.jallcom.2017.02.058
Zheng, J. C., Yang, Z., He, Z. J., Tong, H., Yu, W. J., and Zhang, J. F. (2018a). In situ formed LiNi0.8Co0.15Al0.05O2@Li4SiO4 composite cathod material with high rate capability and long cycling stability for lithium-ion batteries. Nano Energy 53, 613–621. doi: 10.1016/j.nanoen.2018.09.014
Zheng, J. C., Yang, Z., Wang, P. B., Tang, L. B., An, C. S., and He, Z. J. (2018b). Multiple linkage modification of lithium-rich layered oxide Li1.2Mn0.54Ni0.13Co0.13O2 for lithium ion battery. ACS Appl. Mater. Inter. 10, 31324–31329. doi: 10.1021/acsami.8b09256
Keywords: Li-ion batteries, LiFeBO3, hollow sphere, cathode materials, spray drying
Citation: Sui Y, Chen W, Tang S, Wu L, Wang B, Li H, Li W and Zhong S (2019) Spray-Drying Synthesis of LiFeBO3/C Hollow Spheres With Improved Electrochemical and Storage Performances for Li-Ion Batteries. Front. Chem. 7:379. doi: 10.3389/fchem.2019.00379
Received: 29 March 2019; Accepted: 09 May 2019;
Published: 28 May 2019.
Edited by:
Juchen Guo, University of California, Riverside, United StatesReviewed by:
Qiaobao Zhang, Xiamen University, ChinaCopyright © 2019 Sui, Chen, Tang, Wu, Wang, Li, Li and Zhong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ling Wu, bHd1QHN1ZGEuZWR1LmNu; Shengkui Zhong, emhvbmdzaGVuZ2t1aUBzdWRhLmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.