
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Chem., 08 February 2019
Sec. Green and Sustainable Chemistry
Volume 7 - 2019 | https://doi.org/10.3389/fchem.2019.00062
This article is part of the Research TopicGreen Synthesis of HeterocyclesView all 9 articles
A Brønsted acid catalyzed intramolecular cyclization of N-Cbz-protected diazoketones, derived from α-amino acids, is described. The reaction proceeds under metal-free conditions and is promoted by ecofriendly silica-supported HClO4 as the catalyst and methanol as the solvent. This transformation enables the short synthesis of various 1,3-oxazinane-2,5-diones under mild reaction conditions and in good yields (up to 90%). The set-up is very simple; by just mixing all reagents together with no work-up necessary before purification, this protocol takes a greener approach.
Oxazinanones (six-membered cyclic urethanes) are an important class of heterocycles, which have been found to be key structural units in bioactive natural products and pharmaceutically important molecules. Some important examples are the anti-HIV drug Efavirenz (Staszewski et al., 1999) and the potent anticancer agent Maytansine (Rao et al., 1979) and its synthetic derivatives Maytansinoid (Blanc et al., 2011) and Ansamitocin P3 (Taft et al., 2012). Other significant biological activities described for this class of compounds are: antibacterial (Zanatta et al., 2006; Wang, 2008), anti-influenza (Kuznetsov et al., 2017), anti-inflammatory (Ullrich et al., 2004), antidiabetes [11β HSD1 inhibitor (BI 135585)] (Zhuang et al., 2017), antithrombotic (Jin and Confalone, 2000), antialzheimer (Fuchs et al., 2007), and enzyme inhibiting [(Latli et al., 2017); Figure 1]. Furthermore, they are extensively used as valuable synthetic intermediates in fine chemicals (Woodward et al., 1981; Wang et al., 1982; Hilborn et al., 2001; Takahata et al., 2002; Wang and Tunge, 2006), cosmetics (Zofchak, 2003), and pesticides (Hino et al., 2008). They have also showed wide applications as ligands, auxiliaries and as phase transfer catalysts in organic synthesis (Davies et al., 2006; Lait et al., 2007).
Thus, it is therefore not surprising that various synthetic methods for the construction of 1,3-oxazinan-2-one rings have been reported in literature. Among many current methodologies, reactions of CO2 (Kubota et al., 1993) or Urea (Bhanage et al., 2004) with amino alcohols, cycloaddition of isocyanates to oxetanes (Fujiwara et al., 1989), coupling of the adducts from the reaction between (Bu3Sn)2O and haloalkyl isocyanate with alkyl halides (Shibata et al., 1989), iodine-mediated (Fujita et al., 1997; Quinodoz et al., 2017), gold-catalyzed (Robles-Machín et al., 2006; Alcaide et al., 2013), Pd/sulfoxide-catalyzed C-H amination (Rice and White, 2009), intramolecular Michael addition reactions (Hirama et al., 1985) of appropriately functionalized allylic/homoallylic/homopropargyl/allenic carbamates, tethered aminohydroxylation (Donohoe et al., 2007), Brønsted base catalyzed Michael addition of α-isocyanoacetates to phenyl vinyl selenones (followed by domino oxidative cyclization) (Buyck et al., 2014) and Brønsted acid catalyzed elimination-cycloaddition reaction of Boc-imines (Uddin et al., 2011) are the most interesting ones (Figure 2).
All the methodologies described above are dedicated to 1,3-oxazinan-2-one skeletons, whereas only a few are reported for the preparation of 1,3-oxazinane-2,5-dione rings. Hanessian and Fu (2001) described the synthesis of this class of compound as a by-product, during a rhodium catalyzed N-H insertion reaction of a diazoketone (synthesis of 3-azetidinones) (Figure 3A). Pansare et al. (1999) treated a diazoketone derived from N-Cbz-phenylalanine with scandium triflate (Sc(OTf)3) as the catalyst in methanol, to obtain the oxazinanedione moiety (Figure 3B). Similarly, Jung and Avery (2006) successfully demonstrated the synthesis of cyclic urethanes from Boc-protected diazocarbonyl substrates through an indium triflate [In(OTf)3] catalyzed intramolecular cyclization reaction (Figure 3C).
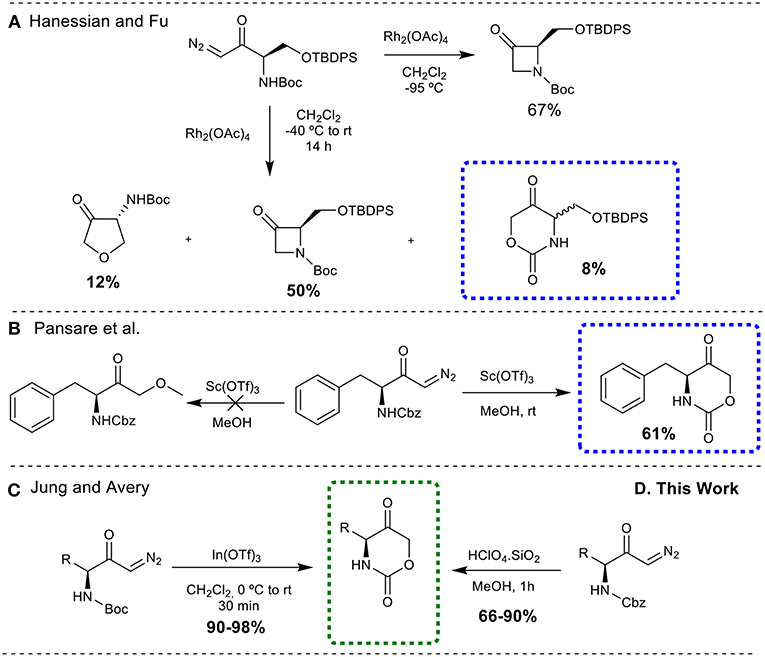
Figure 3. Synthesis of 1,3-oxazinane-2,5-diones from diazo carbonyl moieties. (A) Through rhodium catalyzed N-H insertion reaction; (B) through scandium triflate (Sc(OTf)3) catalyzed insertion reaction; (C) through an indium triflate [In(OTf)3] catalyzed intramolecular cyclization reaction; (D) through silica-supported HClO4 catalyzed intramolecular cyclization reaction.
Despite the fact that numerous modern, scalable and greener methods to obtain diazo carbonyl compounds were described over the past few years (Maas, 2009; Burtoloso et al., 2018), the development of greener, metal free, cheap and easily available catalysts for the efficient synthesis of cyclic urethanes is still highly desirable. With these demands in mind, efforts have been made to use Brønsted acid catalyst as a potential substitute to perform the desired transformation. It is also important to mention here that our group has developed an O-H insertion reaction into diazo carbonyl compounds employing Bronsted acid catalyst (Gallo and Burtoloso, 2018). Herein, we report the operationally simple and greener synthesis of 1,3-oxazinane-2,5-diones via silica-supported HClO4 catalyzed cyclization of N-Cbz-protected diazoketones, offering an interesting alternative to the existing synthetic methods (Figure 3D). Although a single example for a N-Boc-protected diazoketone was described by Jung and Avery with HClO4, the conditions employed (solution in CH2Cl2) and the use of Boc protecting group makes this method less interesting when compared to the present protocol.
We initiated our screening by selecting phenylalanine-derived N-Cbz-protected diazoketone 1 as the model substrate and investigated its behavior under different reaction conditions (Table 1). Based on our previous work (Gallo and Burtoloso, 2018), compound 1 was simply mixed with 10 mol% of H2SO4 (pKa = −3.0) as the BrØnsted acid in benzyl alcohol (BnOH) as the solvent for 24 h at room temperature. To our delight, we isolated the intramolecular cyclization product, oxazinanone 2, in 12% yield instead of getting O-H insertion product (entry 1). A slight improvement in the yield was observed while using stronger acid HClO4 (pKa = −10) as the catalyst under similar reaction conditions (entry 2). In order to minimize side product formation, as well as ease in acid handling, H2SO4 was immobilized on silica gel (230–400 mesh) (Chakraborti and Gulhane, 2003; Chakraborti and Chankeshwara, 2006; Rudrawar et al., 2006). This manipulation proved to be useful, providing target molecule 2 with 35% yield (entry 3). Encouraged by this outcome, we used silica-supported HClO4 which led to a further increase in the yield of the reaction (entry 4). Significant increase in the yield (up to 62%) was noticed when EtOH was employed as a reaction medium (entry 5). Using MeOH as the solvent improved the reaction efficiency and the desired product 2 was isolated in 71% yield in shorter reaction times (12 h) (entry 6). Poor yield or no product formation was observed in the presence of non-nucleophilic solvents such as DCE, THF, and toluene (entries 7–9). Increasing catalyst loading to 20 and 30 mol % provided product 2 in 75 and 83% yield, respectively (entries 10 and 11). Further increasing the catalyst loading (40 mol %) did not affect the reaction yield (entry 12). Under similar conditions of entry 11, comparable yield (81%) of compound 2 was obtained when the reaction was carried-out during 1 h. Thus, conditions in entry 13 were chosen as the optimal to explore the scope of the reaction.
To explore the scope and generality of the reaction, we prepared several N-Cbz-protected diazoketones (1 and 3–11) with different substituents (Scheme 1, for detail procedure see Supplementary Material for the synthesis of diazoketones). In our approach, diazoketones 1 and 3–11 were accessed in excellent yields by protection of the respective amino acids with benzyl chloroformate in aqueous NaHCO3, followed by reaction with isobutyl chloroformate (to activate the carboxylic acid as a mixed anhydride) and freshly prepared diazomethane.
Employing the conditions from entry 13 (Table 1), the substrate scope was investigated (Scheme 2). The HClO4-SiO2 catalyst smoothly converted 2-phenylglycine derived diazo carbonyl 3 into cyclic urethane 12 in 84% yield. Similarly, for leucine-, alanine-, and valine-derived substrates 3–6, the corresponding oxazinanones 13–15 were obtained in good yields. Surprisingly, no product formation was observed with glycine-derived diazo compound 7 under the standard reaction conditions (complex mixture) and this result is under investigation for a better understanding. Diazoketone 8, possessing terminal ester functionality, also render no product. In the case of bicyclic oxazinanones 18 and 19, derived from 9 and 10, high yields were obtained. Finally, diazoketone 11, with terminal Cbz-protected amine chain, did not provide the desired product.
Although merely speculative (studies are being carried-out), the two cbz groups in compound 11 can compete against each other for attack in the protonated diazo carbon (in an inter- or intramolecular fashion). In diazoketone 8, the ester functionality can also compete with the cbz group during the insertion in the protonated diazo carbon. Moreover, the formation of the enol ether from 8 in acidic medium can furnish by-products through competing reactions.
Based on the above experimental results, a proposed mechanism to rationalize the formation of the 1,3-oxazinano-2,5-diones skeleton is shown in Figure 4. Protonation of diazo compound 21 by the Brønsted acid generates diazonium intermediate 22. Next, the intramolecular nucleophilic attack from the Cbz carboxyl group at C1 releases molecular nitrogen and furnishes ammonium intermediated 23. Finally, intermediate 23 is converted into the desired oxazinanone 24 after the nucleophilic attack of MeOH to the benzyl group. Hydrogen abstraction from 25 by the conjugate base of the catalyst regenerates the catalyst and provides (methoxymethyl)benzene 26 (detected by 1H NMR) as a byproduct.
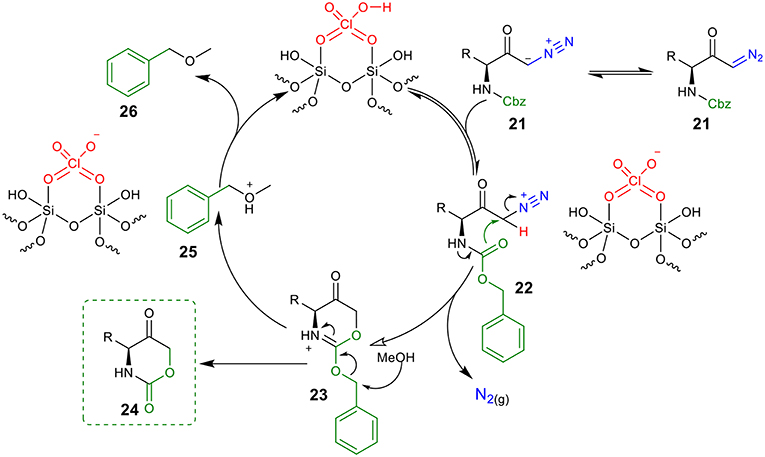
Figure 4. A proposed mechanism for the synthesis of oxazinanones from diazo carbonyls through silica-supported HClO4 catalysis.
In conclusion, we have disclosed a direct cyclization of amino acid-derived diazoketones via a silica-supported HClO4 catalysis, offering a practical and efficient route for the construction of several 1,3-oxazinane-2,5-diones in 66–90% yield. This protocol features a metal free, inexpensive, stable, and easy to handle catalyst, simple reaction set-up, short reaction time, non-chlorinated solvent and broad substrate scope.
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We would like to acknowledge Fundacão de Amparo à Pesquisa do Estado de Sao Paulo (FAPESP) for research financial support (2017/23329-9) (2013/18009-4) (2015/20084-0). We also thank CAPES and CNPq for fellowships.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fchem.2019.00062/full#supplementary-material
Alcaide, B., Almendros, P., Quirós, M. T., and Fernández, I. (2013). Gold-catalyzed oxycyclization of allenic carbamates: expeditious synthesis of 1,3-oxazin-2-ones. Beilstein J. Org. Chem. 9, 818–826. doi: 10.3762/bjoc.9.93
Bhanage, B. M., Fujita, S., Ikushima, Y., and Arai, M. (2004). Non-catalytic clean synthesis route using urea to cyclic urea and cyclic urethane compounds. Green Chem. 6:78. doi: 10.1039/b310115k
Blanc, V., Bousseau, A., Caron, A., Carrez, C., Lutz, R. J., and Lambert, J. M. (2011). SAR3419: an anti-CD19-maytansinoid immunoconjugate for the treatment of B-cell malignancies. Clin. Cancer Res. 17, 6448–6458. doi: 10.1158/1078-0432.CCR-11-0485
Burtoloso, A. C. B., Momo, P. B., and Novais, G. L. (2018). Traditional and new methods for the preparation of diazocarbonyl compounds. An. Acad. Bras. Cienc. 90, 859–893. doi: 10.1590/0001-3765201820170768
Buyck, T., Wang, Q., and Zhu, J. (2014). Triple role of phenylselenonyl group enabled a one-pot synthesis of 1,3-oxazinan-2-ones from α-isocyanoacetates, phenyl vinyl selenones, and water. J. Am. Chem. Soc. 136, 11524–11528. doi: 10.1021/ja506031h
Chakraborti, A. K., and Chankeshwara, S. V. (2006). HClO4-SiO2 as a new, highly efficient, inexpensive and reusable catalyst for N-tert-butoxycarbonylation of amines. Org. Biomol. Chem. 4, 2769–2771. doi: 10.1039/B605074C
Chakraborti, A. K., and Gulhane, R. (2003). Perchloric acid adsorbed on silica gel as a new, highly efficient, and versatile catalyst for acetylation of phenols, thiols, alcohols, and amines. Chem. Commun. 1896–1897. doi: 10.1039/B304178F
Davies, S. G., Garner, A. C., Roberts, P. M., Smith, A. D., Sweet, M. J., and Thomson, J. E. (2006). Oxazinanones as chiral auxiliaries: synthesis and evaluation in enolate alkylations and aldol reactions. Org. Biomol. Chem. 4:2753. doi: 10.1039/b604073j
Donohoe, T. J., Bataille, C. J., Gattrell, W., Kloesges, J., and Rossignol, E. (2007). Tethered Aminohydroxylation: Dramatic Improvements to the Process. Org. Lett. 9, 1725–1728. doi: 10.1021/ol070430v
Fuchs, K., Eickmeier, C., Heine, N., Peters, S., Dorner-Ciossek, C., Handschuh, S., et al. (2007). Preparation of Isophthalamides for the Treatment of Alzheimer's Disease. Ingelheim Am Rhein: Boehringer, IngelheimInternational GmbHand Boehringer Ingelheim Pharma Gmbh and Co. KG. Available online at: https://patents.google.com/patent/WO2007017511A2/en?oq=WO2007017511A2%2c+(2007)
Fujita, M., Kitagawa, O., Suzuki, T., and Taguchi, T. (1997). Regiocontrolled iodoaminocyclization reaction of an ambident nucleophile mediated by basic metallic reagent. J. Org. Chem. 62, 7330–7335. doi: 10.1021/jo970898j
Fujiwara, M., Baba, A., and Matsuda, H. (1989). The cycloaddition of heterocumulenes to oxetanes in the presence of catalytic amounts of tetraphenylstibonium iodide. J. Heterocycl. Chem. 26, 1659–1663. doi: 10.1002/jhet.5570260628
Gallo, R. D. C., and Burtoloso, A. C. B. (2018). Silica-supported HClO4 promotes catalytic solvent- and metal-free O–H insertion reactions with diazo compounds. Green Chem. 20, 4547–4556. doi: 10.1039/C8GC02574F
Hanessian, S., and Fu, J. (2001). Total synthesis of polyoximic acid. Can. J. Chem. 79, 1812–1826. doi: 10.1139/v01-171
Hilborn, J. W., Lu, Z.-H., Jurgens, A. R., Fang, Q. K., Byers, P., Wald, S. A., et al. (2001). A practical asymmetric synthesis of (R)-fluoxetine and its major metabolite (R)-norfluoxetine. Tetrahedron Lett. 42, 8919–8921. doi: 10.1016/S0040-4039(01)01877-9
Hino, T., Yamada, Y., Shigenari, T., and Mametsuk, K. A. (2008). Preparation of HaloalkylsulfonylaminobenzylMoiety-Containing Oxazinane and Oxazepane Derivatives as Herbicides. Osaka: Nihon Nohyaku, Co. Ltd. Available online at: https://patents.google.com/patent/WO2008059948A1/en?oq=WO2008059948A1%2c+(2008)
Hirama, M., Shigemoto, T., Yamazaki, Y., and Ito, S. (1985). Carbamate-mediated functionalization of unsaturated alcohols. 3. Intramolecular Michael addition of O-carbamates to .alpha.,.beta.-unsaturated esters. A new diastereoselective amination in an acyclic system. J. Am. Chem. Soc. 107, 1797–1798. doi: 10.1021/ja00292a077
Jin, F., and Confalone, P. N. (2000). Cyclic Carbamates and Isoxazolidines as Glycoprotein IIb/IIIa Fibrinogen Receptor Antagonists. Willington, DE: Du Pont Pharmaceuticals Company. Available online at: https://patents.google.com/patent/WO2000000481A1/en?oq=WO2000000481A1+119+pp.+p.+2000
Jung, J.-C., and Avery, M. A. (2006). An efficient synthesis of cyclic urethanes from Boc-protected amino acids through a metal triflate-catalyzed intramolecular diazocarbonyl insertion reaction. Tetrahedron Lett. 47, 7969–7972. doi: 10.1016/j.tetlet.2006.08.111
Kubota, Y., Kodaka, M., Tomohiro, T., and Okuno, H. Y. (1993). Formation of cyclic urethanes from amino alcohols and carbon dioxide using phosphorus(III) reagents and halogenoalkanes. J. Chem. Soc. Perkin Trans. 1:5. doi: 10.1039/p19930000005
Kuznetsov, N. Y., Tikhov, R. M., Godovikov, I. A., Medvedev, M. G., Lyssenko, K. A., Burtseva, E. I., et al. (2017). Stereoselective synthesis of novel adamantane derivatives with high potency against rimantadine-resistant influenza A virus strains. Org. Biomol. Chem. 15, 3152–3157. doi: 10.1039/C7OB00331E
Lait, S. M., Rankic, D. A., and Keay, B. A. (2007). 1,3-Aminoalcohols and their derivatives in asymmetric organic synthesis. Chem. Rev. 107, 767–796. doi: 10.1021/cr050065q
Latli, B., Hrapchak, M., Savoie, J., Zhang, Y., Busacca, C. A., and Senanayake, C. H. (2017). Potent and selective inhibitors of 11β-hydroxysteroid dehydrogenase type 1 labeled with carbon-13 and carbon-14. J. Label. Compd. Radiopharm. 60, 420–430. doi: 10.1002/jlcr.3518
Maas, G. (2009). New syntheses of diazo compounds. Angew. Chemie Int. Ed. 48, 8186–8195. doi: 10.1002/anie.200902785
Pansare, S. V., Jain, R. P., and Bhattacharyya, A. (1999). Scandium triflate catalyzed diazocarbonyl insertions into heteroatom- hydrogen bonds. Tetrahedron Lett. 40, 5255–5258. doi: 10.1016/S0040-4039(99)00995-8
Quinodoz, P., Quelhas, A., Wright, K., Drouillat, B., Marrot, J., and Couty, F. (2017). Iodocarbamation of N -homopropargyl carbamates: mild and stereoselective entry to functionalized oxazinan-2-ones. Eur. J. Org. Chem. 2017, 2621–2626. doi: 10.1002/ejoc.201700231
Rao, P. N., Freireich, E. J., Smith, M. L., and Loo, T. L. (1979). Cell cycle phase-specific cytotoxicity of the antitumor agent maytansine. Cancer Res. 39, 3152–3155. doi: 10.1158/0008-5472.can-05-3973
Rice, G. T., and White, M. C. (2009). Allylic C–H amination for the preparation of syn−1,3-amino alcohol motifs. J. Am. Chem. Soc. 131, 11707–11711. doi: 10.1021/ja9054959
Robles-Machín, R., Adrio, J., and Carretero, J. C. (2006). Gold-catalyzed synthesis of alkylidene 2-oxazolidinones and 1,3-oxazin-2-ones. J. Org. Chem. 71, 5023–5026. doi: 10.1021/jo060520y
Rudrawar, S., Besra, R. C., and Chakraborti, A. K. (2006). Perchloric acid adsorbed on silica gel (HClO4-SiO2) as an extremely efficient and reusable catalyst for 1,3-dithiolane/dithiane formation. Synthesis 2006, 2767–2771. doi: 10.1055/s-2006-942474
Shibata, I., Nakamura, K., Baba, A., and Matsuda, H. (1989). Formation of N -tributylstannyl heterocycle from bis(tributyltin) oxide and ω-haloalkyl isocyanate. one-pot convenient synthesis of 2-oxazolidinones and tetrahydro-2 h−1,3-oxazin-2-one. Bull. Chem. Soc. Jpn. 62, 853–859. doi: 10.1246/bcsj.62.853
Staszewski, S., Morales-Ramirez, J., Tashima, K. T., Rachlis, A., Skiest, D., Stanford, J., et al. (1999). Efavirenz plus zidovudine and lamivudine, efavirenz plus indinavir, and indinavir plus zidovudine and lamivudine in the treatment of hiv-1 infection in adults. N. Engl. J. Med. 341, 1865–1873. doi: 10.1056/NEJM199912163412501
Taft, F., Harmrolfs, K., Nickeleit, I., Heutling, A., Kiene, M., Malek, N., et al. (2012). Combined muta- and semisynthesis: a powerful synthetic hybrid approach to access target specific antitumor agents based on ansamitocin P3. Chem. A Eur. J. 18, 880–886. doi: 10.1002/chem.201101640
Takahata, H., Ouchi, H., Ichinose, M., and Nemoto, H. (2002). A novel C2-symmetric 2,6-diallylpiperidine carboxylic acid methyl ester as a promising chiral building block for piperidine-related alkaloids. Org. Lett. 4, 3459–3462. doi: 10.1021/ol0265620
Uddin, N., Ulicki, J. S., Foersterling, F. H., and Hossain, M. M. (2011). Brønsted acid-catalyzed C–C bond forming reaction for one step synthesis of oxazinanones. Tetrahedron Lett. 52, 4353–4356. doi: 10.1016/j.tetlet.2011.06.054
Ullrich, T., Baumann, K., Welzenbach, K., Schmutz, S., Camenisch, G., Meingassner, J. G., et al. (2004). Statin-derived 1,3-oxazinan-2-ones as submicromolar inhibitors of LFA-1/ICAM-1 interaction: stabilization of the metabolically labile vanillyl side chain. Bioorg. Med. Chem. Lett. 14, 2483–2487. doi: 10.1016/j.bmcl.2004.03.006
Wang, C., and Tunge, J. A. (2006). Decarboxylative ring contractions and olefin insertions of vinyl oxazinanones. Org. Lett. 8, 3211–3214. doi: 10.1021/ol0610744
Wang, G. (2008). Synthesis and antibacterial properties of oxazolidinones and oxazinanones. Antiinfect. Agents Med. Chem. 7, 32–49. doi: 10.2174/187152108783329771
Wang, Y. F., Izawa, T., Kobayashi, S., and Ohno, M. (1982). Stereocontrolled synthesis of (+)-negamycin from an acyclic homoallylamine by 1,3-asymmetric induction. J. Am. Chem. Soc. 104, 6465–6466. doi: 10.1021/ja00387a060
Woodward, R. B., Au-Yeung, B. W., Balaram, P., Browne, L. J., Ward, D. E., Au-Yeung, B. W., et al. (1981). Asymmetric total synthesis of erythromycin. 2. Synthesis of an erythronolide A lactone system. J. Am. Chem. Soc. 103, 3213–3215. doi: 10.1021/ja00401a050
Zanatta, N., Borchhardt, D. M., Alves, S. H., Coelho, H. S., Squizani, A. M., Marchi, T. M., et al. (2006). Synthesis and antimicrobial activity of new (4,4,4-trihalo-3-oxo-but-1-enyl)-carbamic acid ethyl esters, (4,4,4-trihalo-3-hydroxy-butyl)-carbamic acid ethyl esters, and 2-oxo-6-trihalomethyl-[1,3]oxazinane-3-carboxylic acid ethyl esters. Bioorg. Med. Chem. 14, 3174–3184. doi: 10.1016/j.bmc.2005.12.031
Zhuang, L., Tice, C. M., Xu, Z., Zhao, W., Cacatian, S., Ye, Y.-J., et al. (2017). Discovery of BI 135585, an in vivo efficacious oxazinanone-based 11β hydroxysteroid dehydrogenase type 1 inhibitor. Bioorg. Med. Chem. 25, 3649–3657. doi: 10.1016/j.bmc.2017.04.033
Zofchak, A. (2003). Monohydric Alcohol Derived Urethanes and their Use in Cosmetic Formulations. Bridgeport, CT: Alzo International Inc. Available online at: https://patents.google.com/patent/US20030065213A1/en?oq=US20030065213A1%2c+(2003)
Keywords: heterocycles, oxazinanones, intramolecular cyclization, Brønsted acid, diazo carbonyls
Citation: Gallo RDC, Campovilla OC Jr, Ahmad A and Burtoloso ACB (2019) Synthesis of Oxazinanones: Intramolecular Cyclization of Amino Acid-Derived Diazoketones via Silica-Supported HClO4 Catalysis. Front. Chem. 7:62. doi: 10.3389/fchem.2019.00062
Received: 31 October 2018; Accepted: 22 January 2019;
Published: 08 February 2019.
Edited by:
Svetlana Ivanova, Universidad de Sevilla, SpainReviewed by:
Oliver Reiser, University of Regensburg, GermanyCopyright © 2019 Gallo, Campovilla, Ahmad and Burtoloso. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Antonio C. B. Burtoloso, YW50b25pb0BpcXNjLnVzcC5icg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.