
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Chem. , 29 November 2018
Sec. Green and Sustainable Chemistry
Volume 6 - 2018 | https://doi.org/10.3389/fchem.2018.00586
This article is part of the Research Topic Novel Approaches to Design Eco-friendly Materials Based on Natural Nanomaterials View all 17 articles
In this study, kaoline is incorporated to prepare CoAl2O4/kaoline hybrid pigments via traditional solid-state reaction, and the introduction of kaoline decreases the preparation temperature for formation of spinel CoAl2O4, and reduces the production cost of cobalt blue as well. More importantly, kaoline may participate in the crystallization of spinel CoAl2O4 during calcining process, and the hybrid pigments prepared using 8.1% Co3O4 and 81.5% kaoline features bright blue and good chemical resistance. Due to the synergistic effect between the sheet-like kaoline and the loaded CoAl2O4, the as-prepared CoAl2O4/kaoline hybrid pigments can be incorporated into epoxy paint system to obtain the high-performance blue anticorrosion coating, especially for acetic acid-salt fog corrosion.
It is well-known that corrosion of the metal materials is one of the common and key problems to industry applications, especially the high risk fields including aviation, ocean, and chemical industry, etc. (De Leon et al., 2012; Daham et al., 2014; Hao et al., 2017). The organic anticorrosion coating has been recognized as the most effective and economical method for metal protection (Vesely et al., 2010; Golru et al., 2015; Gu et al., 2015; Madhup et al., 2017). The anticorrosion mechanisms of the coatings usually include protection of barrier type, inhibition type protection and electrochemical protection (Al-Sabagh et al., 2017), and the organic anticorrosion coating is served as a physical barrier between corrosive electrolyte and steel substrate. However, the main disadvantage of the organic anticorrosion coating, especially epoxy system, is the creation of holes and defects over the film due to the high crosslink density.
Several approaches have been proposed to enhance the anticorrosion properties of organic anticorrosion coating. By contrast, incorporation of inorganic pigments is efficient and economical way (Kartsonakis et al., 2012; Montemor et al., 2012; Jeon et al., 2013; Naderi et al., 2014). It is conducive to improving the aesthetics (e.g., gloss, opacity, and color) and the mechanical properties of the film (El Saeed et al., 2012). However, the relevant application of some commercial available inorganic pigments is limited due to their disadvantages, such as poor adhesive force and optical transparency, weak abrasion and scratch resistance performance (Zhou et al., 2002; Cayton and Sawitowski, 2005; El-Wahab et al., 2009). Therefore, many attempts have been carried out to prepare high-performance pigments with good anticorrosion properties by designing lamellar-based nano-materials preventing water, oxygen, or ions from penetrating film (Kalendová et al., 2008; Ahmed et al., 2012; Ammar et al., 2016; Zhang et al., 2016).
Cobalt aluminate (cobalt blue, CoAl2O4) is a spinel type structure blue eco-friendly pigments with excellent thermal and chemical stability, and it has been widely used in the fields of ceramics, plastics, paint, rubber and glass (Jafari and Hassanzadeh-Tabriz, 2014; Álvarez-Docio et al., 2017; He et al., 2017; Yoneda et al., 2018). Therefore, cobalt blue may be expected to develop the high-performance anticorrosion coating, especially for acetic acid-salt fog corrosion. However, the agglomeration of CoAl2O4 nanoparticles is inevitable during preparation process, which goes against the dispersion of cobalt blue in coating substrate. In addition, the high-cost of cobalt blue also limits its wide application in anticorrosion coating (Tirsoaga et al., 2011; Dandapat and De, 2012; Zou and Zheng, 2016). In our previous work (Mu et al., 2015; Zhang A. J. et al., 2017), clay minerals were employed to fabricate the high-performance CoAl2O4 hybrid pigment with low-cost and prefect color properties. Kaoline (Kaol) is a 1:1 type layered silicate mineral with one tetrahedral sheet of silica (SiO4) linked through oxygen atoms to one octahedral sheet of alumina (AlO6) octahedral (Liu et al., 2016; Kloprogge, 2017; Zhang S. L. et al., 2017). Owing to the advantages of excellent mechanical properties, thermal stability, high whiteness and unique flake-like morphology (Vesely et al., 2010; Ahmed et al., 2012; Qu et al., 2017), it is a promising candidate for preparation of cobalt blue hybrid pigment to design the color high-performance anticorrosion coating (Zhang et al., 2018a,b). However, the preparation process of co-precipitation usually involves in the discharge of the wastewater and lengthy steps including washing, solid-liquid separation and drying.
In this study, CoAl2O4/Kaol hybrid pigments were prepared by the traditional solid-state reaction after grinding the mixture of γ-Al2O3, Co3O4, and Kaol. The preparation conditions were systematically investigated including the grinding time, the calcining temperature and the added amount of Kaol. Furthermore, the formation and coloring mechanisms of CoAl2O4/Kaol hybrid pigments were also studied and discussed. In order to evaluate the anticorrosion application of the as-prepared hybrid pigment, it was incorporated into the epoxy paint system to obtain the blue anticorrosion coating, and then placed in acetic acid-salt spray testing chamber.
Kaol was obtained from Longyan kaoline Development Co., Ltd., (FuJian, China), Kaol were firstly crushed and purified by 4% HCl (wt%), and then the solid were filtered by passing through a 200-mesh sieve to remove quartz sand, and the XRF (X-ray fluorescence) chemical compositions of Kaol were presented in Table S1 (see ESI) before and after being treated by HCl. γ-Al2O3 (purity > 99.9%, particle size = 20 ~ 50 nm) and Co3O4 (purity > 99.9%, D50: 4 ~ 6 μm) were obtained from Shanghai Reagent Factory (Shanghai, China).
8.00 g of Kaol, 0.80 g of Co3O4 and 1.02 g of Al2O3 with a Co to Al mole ratio of 2:1 were mixed and grinded in a mortar mill (CRINOER, MG100, China) for 2 h in anhydrous ethanol medium, and then the mixture was calcined to obtain CoAl2O4/Kaol hybrid pigments. The optimum preparation conditions were systematically investigated including the calcining temperatures (900, 1,000, 1,100, and 1,200°C), the grinding time (0.5, 1, 2, and 4 h) and the added amount of Kaol (0, 1, 2, 4, 6, and 8 g), as summarized in Table 1.
The Fourier Transform infrared (FTIR) spectra were collected on a Thermo Nicolet NEXUS TM spectro photometer using KBr pellets. The morphology was observed using transmission electron microscopy (TEM, JEM-1200EX/S, JEOL). XRD test was conducted on X'pert PRO diffractometer with a scan step size of 0.02° per second. Raman spectra were tested using a Labram HR Evolution Raman spectrometer (Horiba). The chemical compositions were measured on a MiniPal 4 XRF spectrometer (PANalytical Co., Netherland). The anticorrosion performance was evaluated on a salt spray testing chamber (YWX-750, Nanjing Huanke experimental equipment Co. Ltd, China). The colorimetric values and reflectance spectra were measured on a Color-Eye automatic differential colorimeter (X-Rite, Ci 7800).
In order to evaluate the environmental stability of CoAl2O4/Kaol hybrid pigment, it was sprayed onto glass substrate and dried in the open atmosphere after being ultrasonically dispersed into ethanol for 30 min. And then the glass plates were placed for 15 days in a UV Accelerated Weathering Tester (ZN-P, Xinlang, Shanghai, China) with eight UV-B (280-315 nm) bulbs (40 W) at 60°C under UV-B exposure with a radiation intensity of 320 W/m2. The color properties were measured before and after being exposed in a UV accelerated weathering tester to evaluate UV irradiation stability.
The obtained sample plates were also immersed into 3M HCl, 3M NaOH, and ethanol at room temperature for 72 h to study the chemical stability of CoAl2O4/Kaol hybrid pigment, respectively. Unlike with above glass substrate, the hybrid pigments were sprayed onto ceramic substrate and then placed in a muffle furnace to be calcined at 1,000°C for 2 h to evaluate the thermal stability.
The anticorrosion coating was prepared by following procedures: Firstly, epoxy resin, 5 wt% CoAl2O4/Kaol hybrid pigment, dispersing agent, flatting agent, auxiliary solvents, talcum powder, ethyl acetate, and some grading beads added in a 500 mL stainless steel under high shear for 30 min (2,000 rpm), and then the antifoaming agent, wetting agent were added into the above mill base and stirred under low shear for 60 min (500 rpm). If the maximum pigment size was lower than 30 μm, the grinding was considered for meet the requirements, and then the viscosity of the coating was adjusted by ethyl acetate to 20~30 s. And then it was sprayed onto steel plate substrate of size 15 cm × 10 cm × 0.1 cm and dried under the standard conditions (temperature: 25°C and humidity: 40%) for 7 days (the coating thickness: D = 50 ± 2 μm). The obtained sample plates were placed in salt spray testing chamber to evaluate the salt fog corrosion resistance performance (35°C, 100% humidity and 5.0 wt% NaCl solution). In addition, the obtained sample plates were also placed in acetic acid-salt spray testing chamber to evaluate the acetic acid-salt fog corrosion resistance performance (35°C, 100% humidity and 5.0 wt% NaCl solution, pH = 2.8 ~ 3.0).
The calcining temperature is an important factor to form spinel CoAl2O4 with prefect blue color (Yang et al., 2013), and thus the effect of calcining temperatures on the color parameters of hybrid pigment was studied. Figure 1a gives the color parameters of CoAl2O4/Kaol hybrid pigments after being calcined at 900~1,200°C. The b* value of hybrid pigments firstly decreases with the increase in the temperatures, and then it almost has no obvious change as the temperature is above 1,100°C. The values of L* and a* continuously increase with the increase in the calcining temperatures. With the increase in the calcining temperature from 900 to 1,100°C, the color of the hybrid pigments transforms from green to blue to bright blue, which is also consistent with the change of their color parameters. The blue color is derived from Co2+ in tetrahedral sites, while the green color is attributed to Co3+ in octahedral coordination (Álvarez-Docio et al., 2017). By contrast, the b* value of hybrid pigments reaches the minimum value after being calcined at 1,100°C, suggesting that the optimum calcining temperature is 1,100°C. As shown in Figure 1b, the reflection bands and absorption bands are observed at 440~500 nm and at 550~650 nm, respectively, except for hybrid pigment prepared at 900°C. These bands are associated with the blue color of CoAl2O4 due to 4A2 (F) → 4T1 (P) of the Co2+ d-d transition in tetrahedral coordination (Tielens et al., 2006; Kurajica et al., 2012; Gomesan et al., 2015; Tahereh et al., 2016). And for the hybrid pigment prepared at 900°C, the bands at 380 and 670 nm are indicative of the occurrence of octahedrally coordinated Co3+, and are ascribed to the 1A1g→1T2g and 1A1g→1T1g transitions of low spin Co3+ in octahedral symmetry (Brik et al., 2001; Herrero et al., 2007), the sample exhibits greenish hue annealed at low temperature, which is also in accordance with the digital photos of the hybrid pigment calcined at 900°C (Kurajica et al., 2012). In addition, the intensity of the reflection peaks also increases with the increase in the preparation temperatures until 1,100°C, and then it decreases as the calcining temperature reaches 1,200°C, which may be due to crystal phase and structural transformation of Kaol (Juneja et al., 2010; Yeo, 2011).
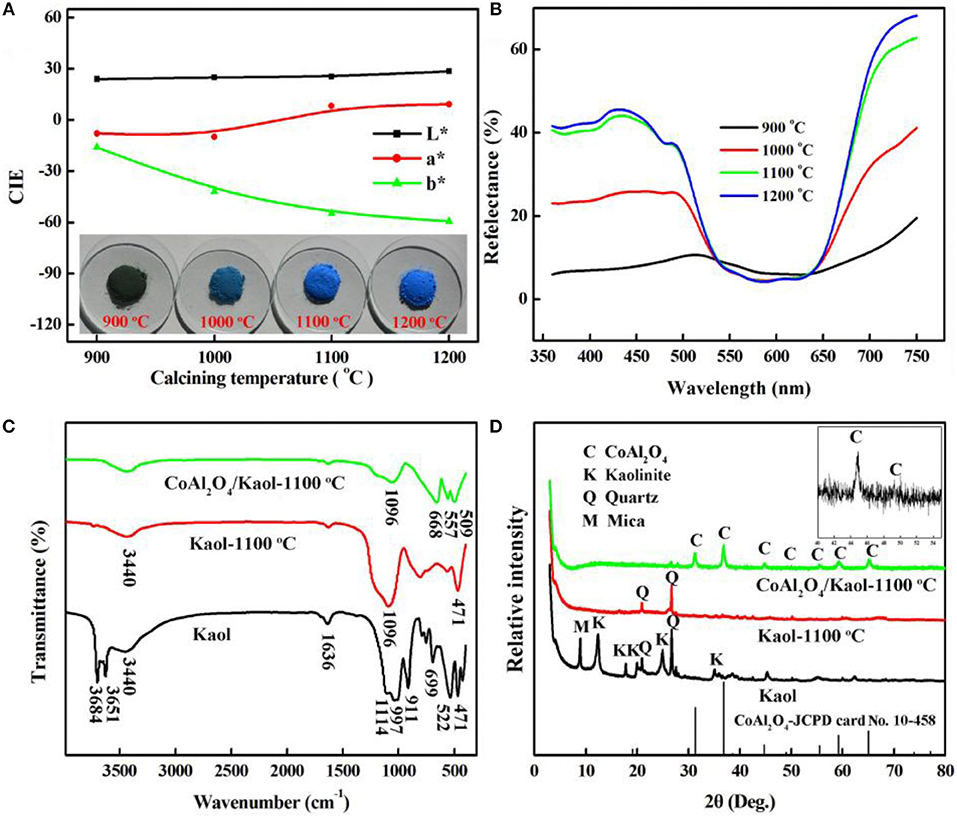
Figure 1. (A) CIE parameters and (B) reflectance spectra of CoAl2O4/Kaol hybrid pigments calcined at different temperatures, (C) FTIR, and (D) XRD patterns of Kaol, Kaol-1100°C, and CoAl2O4/Kaol-1100°C (the insert is the partial enlarged view of XRD patterns).
Figure S1a depicts the effect of grinding time on color parameters of hybrid pigment, the b* value of CoAl2O4/Kaol hybrid pigments firstly decreases with the increase in the grinding time from 0.5 to 1 h, and no obvious change is observed with the continuous increasing grinding time. In order to ensure the reaction uniformity, the grinding time is selected to 2 h. Figure S1b exhibits the effect of the added amount of Kaol on color parameters. At the same calcining temperature (1,100°C), the L* value of hybrid pigments gradually increases with increase in the added amount of Kaol, while the a* and b* values of the sample containing Kaol of 35.5 wt% (1 g Kaol) reach maximum. Interestingly, CoAl2O4/Kaol hybrid pigments still exhibits good color properties (L* = 29.9, a* = 2.01, b* = −54.93) as the added amount of Kaol is up to 81.5 wt% (8 g Kaol). However, the sample is atrovirens prepared without Kaol at 1,100°C, suggesting that the introduction the low-cost Kaol not only reduces the production cost of CoAl2O4, but also decreases the high temperature crystallization temperature for formation of spinel structure. During calcining process, Kaol (2SiO2·Al2O3·2H2O) transforms into metakaolinite (2SiO2·Al2O3) at 400~600°C, and then it turns into SiAl2O5 and amorphous SiO2 (>900°C), even amorphous SiO2 and low-order crystalline α-Al2O3 at above 1,100°C (Ribeiro et al., 2005; Gilkes and Prakongkep, 2016). Because the mass transfer process is the rate controlling step during CoAl2O4 preparation (He and Becker, 1997), the uniform distribution Co3O4 and Al2O3 on the surface of Kaol after being grinded is in favor of reducing the mass transfer resistance, thus the incorporation of Kaol decreases the calcining temperature for formation of spinel CoAl2O4 (Wang et al., 2006; Gabrovska et al., 2014). Based on above results, the optimum conditions for preparing hybrid pigment with the prefect color are 1,100°C and 81.5 wt% of Kaol.
Figure 1c presents FTIR spectra of Kaol, the calcined Kaol at 1,100°C and CoAl2O4/Kaol hybrid pigment, respectively. FTIR spectrum of Kaol exhibits the characteristic bands of Si-O-Si (at about 471, 522, 699, 1,032, and 1,114 cm−1), Si-O-Al (at around 525, 750, and 795 cm−1), Al-Al-OH (at around 522 and 911 cm−1), -OH (at about 3,684, 3,684, 3,651, and 3,440 cm−1), while the bending vibration of the inter-layer water is observed at 1,636 cm−1 (Rekik et al., 2017; Sreelekshmi et al., 2017). After being calcined, these typical absorption bands disappear compared with that of the raw Kaol, which is ascribed to the dehydroxylation and the crystal phase transition of Kaol during calcining process. In the case of FTIR spectrum of hybrid pigment, several new absorption bands appear at 668, 557, and 509 cm−1, which can be attributed to the stretching vibration of Al-O of AlO6 and Co-O of CoO4, respectively (Zayat and Levy, 2000; Zhang A. J. et al., 2017), indicating the formation of CoAl2O4.
Figure 1d shows the XRD patterns of Kaol, the calcined Kaol at 1,100°C and CoAl2O4/Kaol hybrid pigment, respectively. It is clear that the associated minerals are quartz (2θ = 20.8° and 26.7°) and mica (2θ = 8.9°) (Chhikara et al., 2015; Mymrin et al., 2017), and the typical characteristic peaks of kaolinite presents the well-defined reflections at 2θ = 12° and 25°. When Kaol was calcined at 1,100°C, only diffraction peaks of quartz are remained. For hybrid pigment, new diffraction peaks are observed at 2θ = 31.1°, 36.8°, 44.8°, 49.0°, 55.5°, 59.2° and 65.2°, which correspond to (220), (311), (400), (331), (422), (511), and (440) planes of CoAl2O4 according to JCPD card No. 10-458, respectively (Xi et al., 2012; Zhu et al., 2015). In fact, CoO also reacts with silica derived from Kaol to form cobaltous silicate, but this reaction is considered to be a minor reaction since most of CoO react with Al2O3 because CoO has high affinity to Al2O3 (Ahmed et al., 2012).
Figure S2 shows Raman spectra of CoAl2O4 pigment without Kaol and CoAl2O4/Kaol hybrid pigment. CoAl2O4 pigment with a spinel structure usually exhibits five Raman active modes: A1g (764 cm−1), F2g (644, 511, and 203 cm−1) and Eg (413 cm−1). Hybrid pigment also clearly presents the five expected Raman active modes at 760, 641, 510, 410, and 202 cm−1, indicating that hybrid pigments is assigned to the spinel structure (Zha et al., 2016; Liu et al., 2017). Compared with the Raman spectrum of CoAl2O4, all modes of hybrid pigment shift to lower frequencies, and this red shift suggests that primary coordinative environments of tetrahedron and octahedron positions may change after incorporation of Kaol.
The typical structures and the morphology evolution of the samples can be observed by TEM. As illustrated in Figure 2a, Kaol presents a typical lamellar structure with a smooth surface. After being calcined at 1,100°C (Figure S3a), the lamellar morphology remains well, which reveals that Kaol possesses the good thermal stability. With the introduction of CoAl2O4 nanoparticles, the surface of lamellar morphology becomes coarse (Figures 2b,c), and CoAl2O4 nanoparticles are uniformly distributed on the substrate surface with a diameter of 10~20 nm. Furthermore, no obvious aggregation is observed, thus it indicates that incorporation of Kaol effectively prevents from the aggregation and controls the size of CoAl2O4 nanoparticles. The selected area electron diffraction pattern of hybrid pigment also indicates the successful formation of spinel CoAl2O4 (Figure S3b) (Naderi et al., 2014; Zou and Zheng, 2016). Figure 2d gives an enlarged electron micrograph, and it provides a well-resolved lattice plane with an interplanar spacing of 0.244 nm, corresponding to (311) plane of the cubic Fd3m space group, which is identified on the basis of data of the standard CoAl2O4 database JCPD card No. 10-458 (Kim et al., 2012). Meanwhile, the micrograph also displays the coexistence of amorphous and crystalline phases, which is attributed to the amorphous SiO2 derived from Kaol and spinel CoAl2O4, respectively (Cho and Kakihana, 1999). In addition, Figure 3 illustrates the elemental mapping of hybrid pigment, it is clear that hybrid pigment is mainly composed of Co, Al, O, and Si elements. Co element is uniformly distributed on the surface of lamellar substrates, suggesting that the generated CoAl2O4 is uniformly anchored on the surface of substrate.
Based on our previous studies, Kaol is not merely a carrier for loading of CoAl2O4 nanoparticles, Al2O3 as one of the main compositions of Kaol might participate in the high-temperature crystallization to form clay mineral doped spinel CoAl2O4. In order to confirm above inference, the sample was prepared using Co3O4 and Kaol without Al2O3 by the same procedure with CoAl2O4/Kaol hybrid pigments. As shown in Figure 4 and Table S2, the color of the as-prepared pigment is blue, and the values of L* and a* decrease with the increase of the addition amount of Co3O4, but b* firstly decrease with the increase of the added amount of Co3O4 to 13%, and then it begins to increase as Co3O4 reaches 16%. At this moment, the obtained pigment is dark-green color, which is mainly ascribed to the fact that the excess Co3O4 is not fully involved in the reaction and anchors on the pigment surface (Aguilar-Elguézabal et al., 2017). At the low addition amount of Co3O4 (4.8%), CoO derived from the thermal reduction of Co3O4 can react with Al2O3 derived from Kaol to form spinel CoAl2O4 at 1,100°C, which can be confirmed by their XRD patterns and FTIR spectra (Figure S4). Therefore, it can be safely concluded that Kaol may be served as an aluminum source to take part in the crystallization process of CoAl2O4.
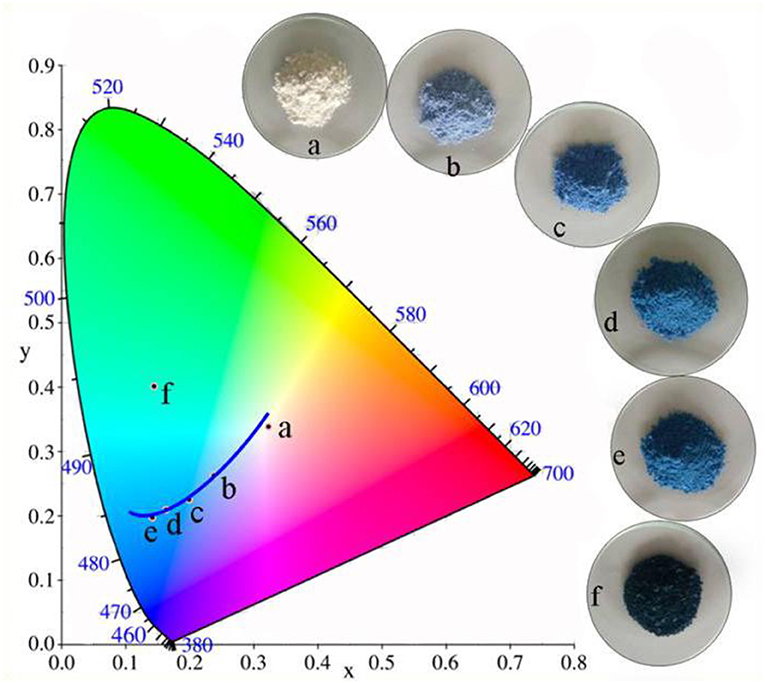
Figure 4. Color coordinates of pigment prepared using the different addition of Co3O4 in the presence of 2 g Kaol without Al2O3 (a-0%, b-2.4%, c-4.8%, d-9.1%, e-13%, f-16.7%).
According to the above results, the as-prepared hybrid pigment exhibit good color performances at a low Co content compared with the commercial CoAl2O4 pigments even 7 wt%. The possible formation mechanism of CoAl2O4/Kaol hybrid pigments can be described as follows: Co3O4 and Al2O3 can be uniformly distributed on the surface of Kaol during grinding. With the increase of the calcining temperature (400~600°C), Kaol (2SiO2·Al2O3·2H2O) is turned into metakaolinite (2SiO2·Al2O3). As the calcining temperature increases to 900°C, Co3O4 begins to thermally reduce into CoO, subsequently the increase in the calcining temperature results in the formation of spinel CoAl2O4 based on the reaction between CoO and Al2O3 on Kaol substrate accompanied with the crystal phase transition of Kaol to amorphous SiO2 and low-order crystalline α-Al2O3. In this process, the uniform distribution of Co3O4 and Al2O3 may be expected to decrease the mass transfer resistance for formation of spinel CoAl2O4, which reduces the processing time and calcining temperature, while the traditional solid-phase method for preparation of CoAl2O4 spends the long time ranging from several hours to days at high temperature (>1,200°C) (Wang et al., 2006). Furthermore, α-Al2O3 from Kaol also simultaneously reacts with CoO during high-temperature crystallization, thus the generated CoAl2O4 and the calcined production of Kaol finally form a solid solution. The possible formation mechanism of CoAl2O4/Kaol hybrid pigment can be illustrated in Scheme 1.
As shown in Figure S5a, the reflectance spectra of hybrid pigments are nearly same before and after being exposed under UV light for 15 days, indicating the excellent stability of hybrid pigment to UV light. In addition, the color of the sample plates has no obvious difference before and after being immersed into HCl, NaOH and ethanol, suggesting the excellent resistance of hybrid pigments to the corresponding reagents, which also can be confirmed from their color parameters (Table 2). Meanwhile, CoAl2O4/Kaol hybrid pigment presents the excellent thermal stability (Figures S5b,c).
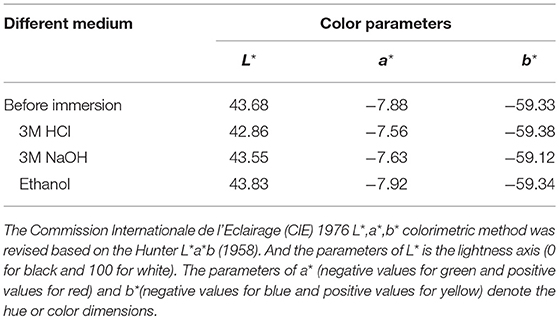
Table 2. Color parameters of the sample plates before and after being immersed into 3M HCl, 3M NaOH, and ethanol for 72 h, respectively.
In this study, the potential applications of the as-prepared hybrid pigment were also explored in this study. The thermal resistance coating was prepared by following procedures: Firstly, 5 wt% CoAl2O4/Kaol hybrid pigment was dispersed into ethyl acetate and stirred under high shear for 30 min (2,000 rpm), and the above obtained mill base were added into commercial thermal resistance coating and stirred under low shear for 60 min (500 rpm). The viscosity of the coating was adjusted by ethyl acetate to 20~30 s. And then it was sprayed onto steel plate substrate of size 15 cm × 10 cm × 0.1 cm and dried under the standard conditions (temperature: 25°C and humidity: 40%) for 7 days (the coating thickness: D = 50 ± 2 μm). The obtained sample plates were shown in Figure S6c, it is clear that the surface of the sample plate is smooth after hybrid pigment (5 wt%) is added into the commercial thermal resistance coating, indicating hybrid pigment disperses well in the painting. Furthermore, the film containing hybrid pigments exhibits the similar color parameters (L* = 24.88, a* = 13.54, b* = −57.80) (Figure S6d) after being calcined at 800°C for 2 h. However, the steel plate coated the commercial thermal resistance coating (Figure S6a) is damaged heavily (Figure S6b). It indicates that the film containing hybrid pigment has a good thermal stability, and it can effectively protect steel plate substrate to avoid excessive damage. Meanwhile, this film also exhibited excellent fireproof properties, which can be illustrated by its fire test using a flame gun (800~1,000°C) (Figure S6e). It is found that this film has no obvious change after being placed on the fire for 30 s (Movie S1 and Figure S6f), revealing that the hybrid pigment may be used as fireproof paint. In addition, the as-prepared hybrid pigment also can be used as a colored painting and artistic pigment (Figures S7a,b).
Corrosion is a natural and gradual destruction process of metal materials resulting from chemical and/or electrochemical reaction with their environment, which leads to enormous losses to our lives, finances, etc. Therefore, corrosion engineering is the field to control and stop corrosion using various methods, especially surface coating protection technology, in which inorganic pigments (e.g., ZnO and TiO2) are commonly incorporated to improve the anticorrosion properties of coating, and perhaps provide the color for coating (e.g., α-Fe2O3). Therefore, the as-prepared hybrid pigment is incorporated into the common epoxy anticorrosion coating to investigate its anticorrosion properties. As shown in Figure 5, the steel plate substrate suffers from an aggravating corrosion referred from the large-scale red rust without the epoxy anticorrosion coating (Figure 5e). After coating of epoxy anticorrosion coating, the corrosion of steel plate is slightly improved (Figure 5f). In particular, this anticorrosion effect is obvious after introducing the commercial CoAl2O4 pigments (Figure 5g). With the incorporation of the as-prepared hybrid pigment, no obvious rust was found on the surface of coating (Figure 5h). It suggests that the introduction of hybrid pigment obviously improves the corrosion resistance of coating. This is mainly ascribed to the synergistic effect between the lamellar layered structure of Kaol and the excellent weatherability of CoAl2O4, which can prevents small molecules (H2O and O2) or ions from penetrating into the coating film. Thus, the relevant mechanisms of anticorrosion can be illustrated in Scheme 2.
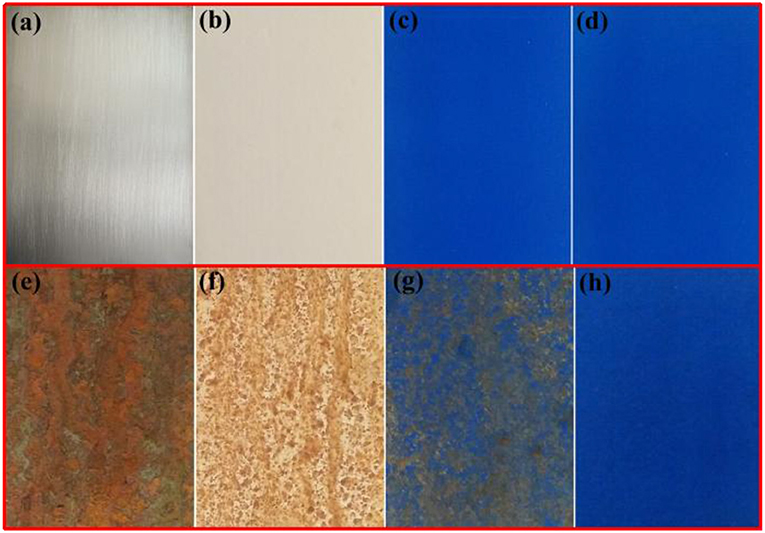
Figure 5. Photographs of (a) steel substrate, (b) steel coated the paint without pigment, (c) steel coated the paint containing commercial CoAl2O4, (d) steel coated the paint containing hybrid pigment. (e–h) Their corresponding images after being exposed to salt spray test for 60 days, respectively.
Furthermore, the chemical stability of the anticorrosion coating film was also evaluated by immersing into 3M HCl, 3M NaOH, and ethanol at room temperature for 72 h, respectively. The film has no obvious difference before and after being immersed, exhibiting the excellent resistance to HCl, NaOH, and ethanol (Figure S8). This is also confirmed by their color parameters before and after being immersed in the corresponding reagents (Table 3). More importantly, the obtained sample plates were also immersed into 3.5 wt% saline water to study the salted water resistance test for 60 days. As shown in Figure 6, the surface of coating film without pigment is smooth and dense before immersing (Figure 6a). After adding of CoAl2O4 without Kaol, the film color seems darker compared with that of containing CoAl2O4/Kaol hybrid pigment (Figures 6b,c). As expected, the film without pigment is swelling, blistering, and rusty after immersed into saline water for 60 days (Figure 6d). Furthermore, the same phenomenon also is observed from the one containing CoAl2O4 (Figure 6e), but the situation is better than the former due to the good weatherability of CoAl2O4 to acid, salt, etc. Surprisingly, the film incorporating of CoAl2O4/Kaol hybrid pigment was perfect without any defects (Figure 6f). It suggests that the coating film exhibited the excellent salted water resistance, which may be ascribed to the synergistic effect of the lamellar layered structure of Kaol and the excellent weatherability of CoAl2O4.
Table 3. Color parameters of the film before and after being immersed into 3M HCl, 3M NaOH and ethanol for 72 h, respectively.
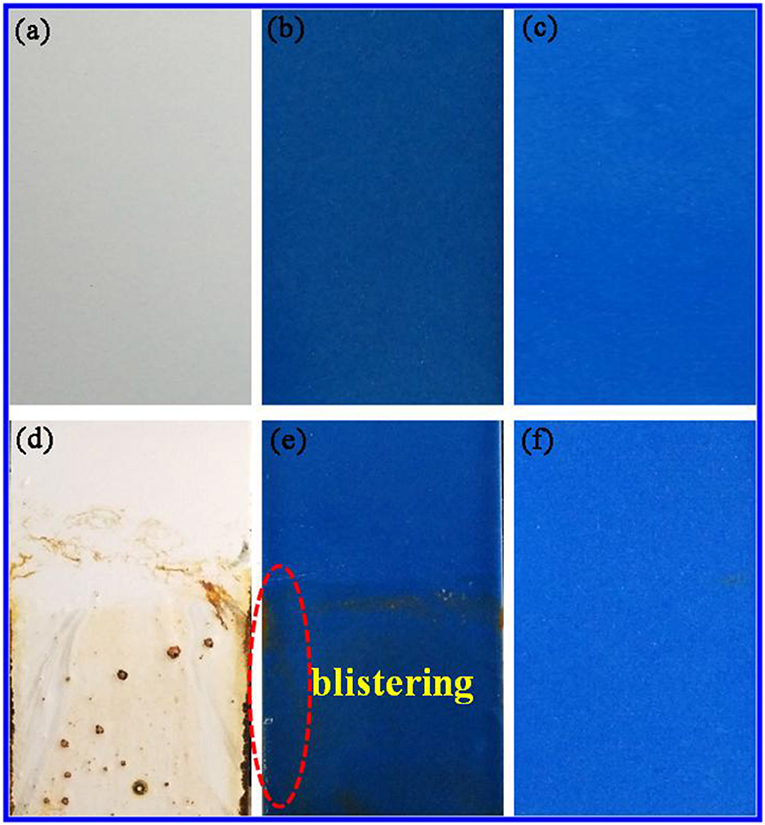
Figure 6. Photographs of the film (a) without pigment, (b) containing CoAl2O4, and (c) hybrid pigment. (d–f) Their corresponding images after being exposed to 3.5% NaCl for 60 days, respectively.
Furthermore, the obtained sample plate containing hybrid pigment presents the better acid resistance than that of incorporating of ultramarine after contacting with 36.5 wt% HCl, which changes from blue to white due to its instability to acid (Figure S9). Therefore, the obtained sample plate was also placed in acetic acid-salt spray testing chamber to evaluate the acetic acid-salt fog corrosion resistance of the anticorrosion coating. As a control, the coatings containing Kaol, ZnO, Fe2O3 and CoAl2O4 calcined at 1,100°C are also investigated, and the components of paints were provided in Table S3. Firstly, the different amounts of the CoAl2O4/Kaol hybrid pigment were added into epoxy resin to determine the amounts of the hybrid pigment, and the results are shown in Figure S10. It is clear that the sample that containing 5 wt% CoAl2O4/Kaol hybrid pigment is bright blue, so the added amounts of the hybrid pigment is 5 wt%. And then the sample containing Kaol, ZnO, Fe2O3, and CoAl2O4 calcined at 1,100°C are shown in Figure 7, the corrosion of the films containing Fe2O3 (Figure 7f), CoAl2O4 calcined at 1,100°C (Figure 7g), and ZnO (Figure 7j) are very serious after be exposed in salt spray testing chamber 60 days. Interestingly, the corrosion of the sample containing CoAl2O4/Kaol hybrid pigment (Figure 7h) and Kaol (Figure 7j) were negligible, especially the sample of hybrid pigment. It may be ascribed to the excellent acid resistance and lamellar layered structure of hybrid pigment and Kaol, respectively. In addition, Figure 8 gives the anticorrosion ability of the coatings containing CoAl2O4/Kaol hybrid pigments, ZnO, Fe2O3, CoAl2O4 calcined at 1,100°C and the one without pigment. Bode plots indicates that the addition of CoAl2O4/Kaol hybrid pigments in epoxy paint shows a significant improvement in the anticorrosion properties compared with the pure epoxy paint and others pigment. It also suggests the synergistic effect between the lamellar layered structure of Kaol and the excellent weatherability of CoAl2O4 is in favor of improving the resistance to salts, which also is superior to the reported works (Table 4).
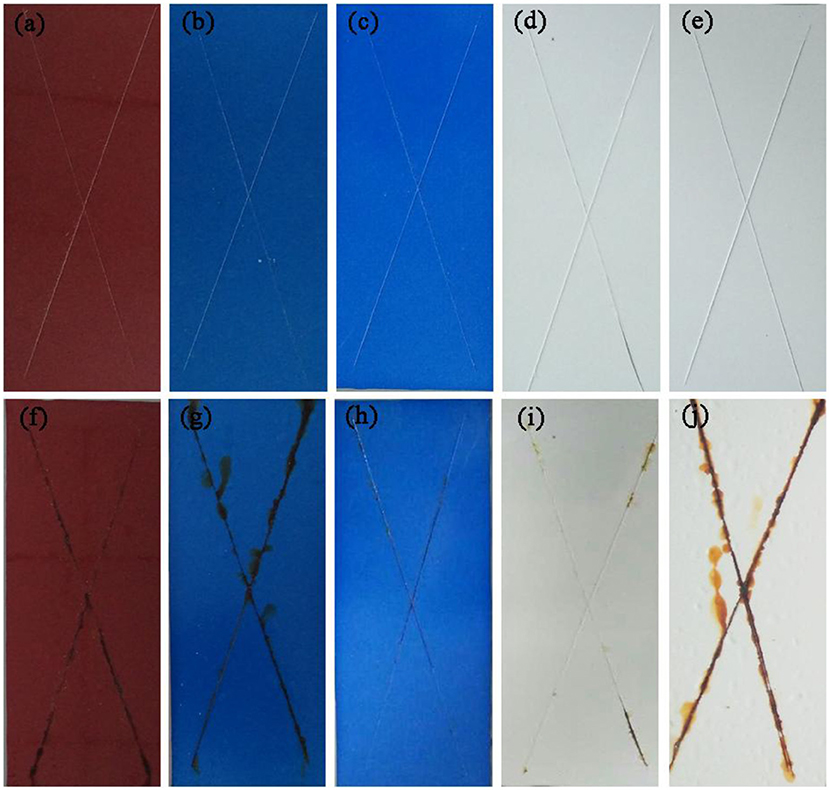
Figure 7. Photographs of the film containing (a) Fe2O3, (b) CoAl2O4, (c) CoAl2O4/Kaol hybrid pigment, (d) Kaol, and (e) ZnO. (f–j) The corresponding images of the film after being exposed to acetic acid-salt spray for 60 days, respectively.
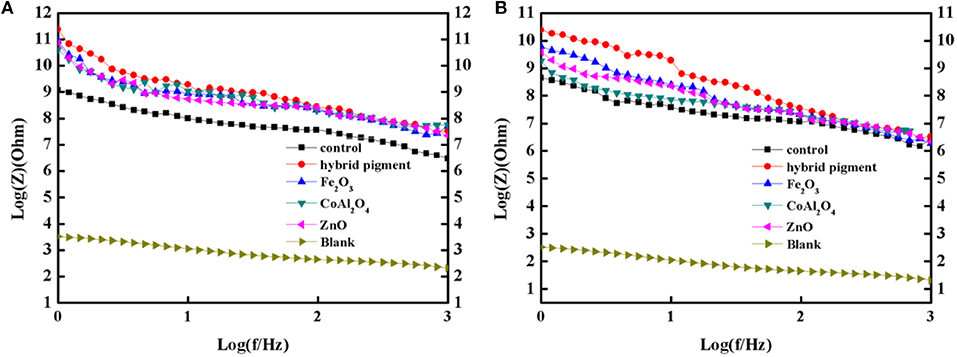
Figure 8. Bode plots of the coatings containing CoAl2O4/Kaol hybrid pigment, ZnO, Fe2O3, CoAl2O4, the control without pigment and steel before (A) and after (B) being immersed in the 3.5 wt% NaCl solution for 60 days.
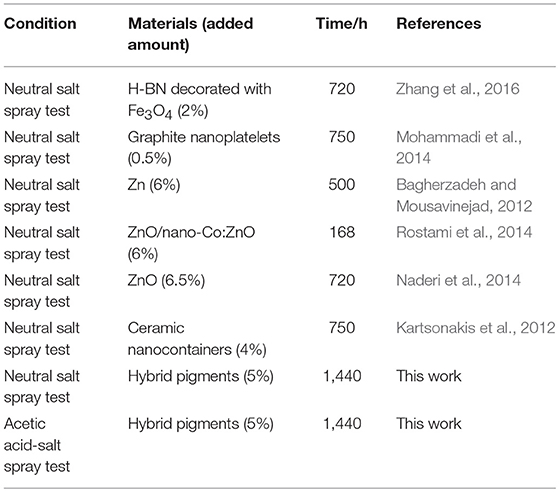
Table 4. Comparison with the anticorrosion performance of the reported additives and hybrid pigment in epoxy anticorrosive coating.
Anti-corrosion of the anticorrosive film is commonly determined from electrochemical measurements such as corrosion potential (Ecorr) and corrosion current (Icorr), and Electrochemical Impedance Spectroscopy (EIS) measurements were carried out with the use of an electrochemical working station (CHI660E) at open circuit potential. A three-electrode cell including the Ag/AgCl (3M KCl) reference electrode, the studied sample as working electrode and the platinum counter electrode was used to run EIS tests. EIS measurements are studied in the frequency range from 100 kHz to 0.005 Hz with a perturbation of 5 mV, and the working electrode area was 1 cm2. Figure 9 shows the Tafel plots generated for the anticorrosion coating containing CoAl2O4/hybrid pigment, ZnO, Fe2O3, CoAl2O4 calcined at 1,100°C, control (without pigment) and blank immersed in 3.5 wt% NaCl solution about 30 min. And the Tafel curve parameters are given in Table 5. As shown in Figure 9 and Table 5, the corrosion current of the control (without pigment) decreases from −1.52 × 10−7 A to −3.39 × 10−8 A compared with the blank, and the corrosion current obviously declines when the pigments were added into the anticorrosion coating. After incorporation of CoAl2O4/hybrid pigment, the corrosion current decreases from −3.39 × 10−8 A to −5.75 × 10−12 A, while the corrosion potential increases from −1.439 V to −0.443 V. In fact, the anticorrosion ability of the coating is related to the chemical composition and microstructure. The lower of the corrosion current or the higher corrosion potential suggests the better of the anticorrosion ability (Liang et al., 2007; Chang et al., 2012). Therefore, the anticorrosion coatings containing CoAl2O4/hybrid pigment, ZnO, Fe2O3 present good anticorrosion ability, and CoAl2O4/hybrid pigment exhibits superior anticorrosion ability compared with than that of ZnO and Fe2O3, which is consistent with that of the Bode plots (Figure 8), implying that the addition of CoAl2O4/Kaol hybrid pigments in epoxy paint shows a significant improvement in the electrochemical corrosion properties compared with the pure epoxy paint and other pigments.
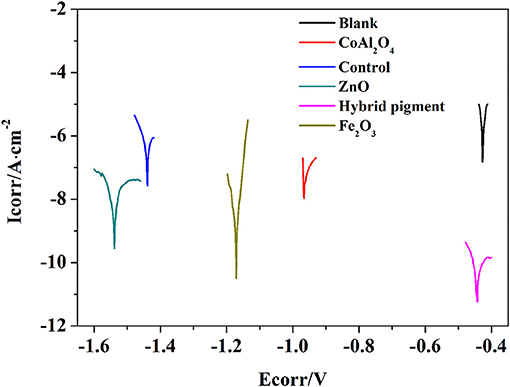
Figure 9. Tafel plots of blank and anticorrosion coatings containing CoAl2O4 calcined at 1,100°C, control without pigment, ZnO, CoAl2O4/hybrid pigment, and Fe2O3, respectively.
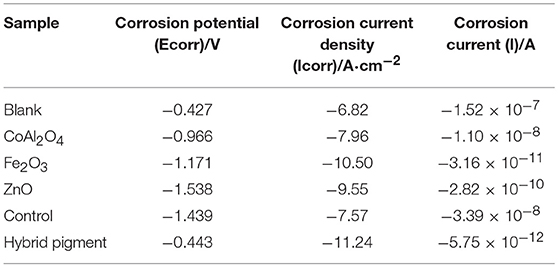
Table 5. Tafel curve parameters of the anticorrosion coatings containing different pigments and blank sample.
In conclusion, the low-cost bright blue CoAl2O4/Kaol hybrid pigments were successfully prepared by the traditional solid-state reaction. The introduction of Kaol not only reduces the Co consumption and the temperature for formation of spinel CoAl2O4, but also enhances the color properties of cobalt blue pigments with bright blue and high NIR reflectance, especially prepared using 8.1% Co3O4 and 81.5% Kaol. During preparation, Co3O4 and Al2O3 can be uniformly distributed on the surface of Kaol, which reduces the mass transfer resistance and prevents from the aggregation CoAl2O4 nanoparticles during high-temperature crystallization process. In addition, the Al2O3 originated from Kaol also may participate in the formation of spinel CoAl2O4 pigments. Due to the synergistic effect between the lamellar layered structure of Kaol and the excellent weatherability of CoAl2O4, the anticorrosion coating containing hybrid pigments exhibited the excellent corrosion resistance toward the salt and acetic acid-salt system. Furthermore, the CoAl2O4/Kaol hybrid pigments prepared by traditional solid-phase method can be applied in thermal resistance coating, wall painting and artistic pigment.
AZ and XW contribute to the experiment process, samples characterization, data analysis, and paper preparation. BM and AW are mainly responsible for the design of experiment, data analysis, and paper revision.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This work is supported by the Regional Key Project of the Science and Technology Service of the Chinese Academy of Sciences ([2016] No. 70), the Youth Innovation Promotion Association CAS (2017458), the Major Projects of the National Natural Science Foundation of Gansu, China (18JR4RA001), and The Funds for Creative Research Groups of Gansu, China (17JR5RA306).
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fchem.2018.00586/full#supplementary-material
Aguilar-Elguézabal, A., Román-Aguirre, M., De la Torre-Sáenz, L., Pizá-Ruiz, P., and Bocanegra-Bernal, M. (2017). Synthesis of CoAl2O4/Al2O3 nanoparticles for ceramic blue pigments. Ceram. Int. 43, 15254–15257. doi: 10.1016/j.ceramint.2017.08.062
Ahmed, N. A., Abdel-Fatah, H. T. M., and Youssef, E. A. (2012). Corrosion studies on tailored Zn·Co aluminate/kaolin core-shell pigments in alkyd based paints. Prog. Org. Coat. 73, 76–87. doi: 10.1016/j.porgcoat.2011.09.003
Al-Sabagh, A. M., Abdou, M. I., Migahed, M. A., Fadl, A. M., Farag, A. A., Mohammedy, M. M., et al. (2017). Influence of ilmenite ore particles as pigments on the anticorrosion and mechanical performance properties of polyamine cured epoxy for internal coating of gas transmission pipelines. Egypt. J. Pet. doi: 10.1016/j.ejpe.2017.07.005. [Epub ahead of print].
Álvarez-Docio, C. M., Reinosa, J. J., Del Campo, A., and Fernández, J. F. (2017). 2D particles forming a nanostructured shell: a step forward cool NIR reflectivity for CoAl2O4 pigments. Dyes Pigments 137, 1–11. doi: 10.1016/j.dyepig.2016.09.061
Ammar, S., Ramesh, K., Vengadaesvaran, B., Ramesh, S., and Arof, A. K. (2016). Amelioration of anticorrosion and hydrophobic properties of epoxy/pdms composite coatings containing Nano ZnO particles. Prog. Org. Coat. 92, 54–65. doi: 10.1016/j.porgcoat.2015.12.007
Bagherzadeh, M. R., and Mousavinejad, T. (2012) Preparation investigation of anticorrosion properties of the water-based epoxy-clay nanocoating modified by Na+-MMT Cloisite 30B. Prog. Org. Coat. 74, 589–595. doi: 10.1016/j.porgcoat.2012.02.006
Brik, Y., Kacimi, M., Ziyad, M., and Bozon-Verduraz, F. (2001). Titania-supported cobalt and cobalt–phosphorus catalysts: characterization and performances in ethane oxidative dehydrogenation. J. Catal. 202, 118–128. doi: 10.1006/jcat.2001.3262
Cayton, H. R., and Sawitowski, T. (2005). The impact of nano-materials on coating technologies. NSTI-Nanotech 2, 83–85.
Chang, C. H., Huang, T. C., Peng, C. W., Yeh, T. C., Lu, H. I., Hung, W. I., et al. (2012). Novel anticorrosion coatings prepared from polyaniline/graphene composites. Carbon 50, 5044–5051. doi: 10.1016/j.carbon.2012.06.043
Chhikara, D., Kumar, M. S., and Srivatsa, K. M. K. (2015). On the synthesis of Zn/ZnO core-shell solid microspheres on quartz substrate by thermal evaporation technique. Superlattice. Microstruct. 82, 368–377. doi: 10.1016/j.spmi.2015.02.036
Cho, W. S., and Kakihana, M. (1999). Crystallization of ceramic pigment CoAl2O4 nanocrystals from Co-Al metal organic precursor. J. Alloy. Compd. 287, 87–90. doi: 10.1016/S0925-8388(99)00059-6
Daham, P. V., Gunawardana, S., Nguyen, T. T. M., Walmsley, J. C., and Venvik, H. J. (2014). Initiation of metal dusting corrosion in conversion of natural gas to syngas studied under industrially relevant conditions. Ind. Eng. Chem. Res. 53, 1794–1803. doi: 10.1021/ie4024947
Dandapat, A., and De, G. (2012). Host-mediated synthesis of cobalt aluminate/γ-alumina nanoflakes: a dispersible composite pigment with high catalytic activities. ACS Appl. Mater. Inter. 4, 228–234. doi: 10.1021/am201283c
de Leon, A. C., Pernites, R. B., and Advincula, R. C. (2012). Superhydrophobic colloidally textured polythiophene film as superior anticorrosion coating. ACS Appl. Mater. Inter. 4, 3169–3176. doi: 10.1021/am300513e
El Saeed, A. M., El-Fattah, M. A., and Azzam, A. M. (2012). Synthesis of ZnO nanoparticles and studying its influence on the antimicrobial, anticorrosion and mechanical behavior of polyurethane composite for surface coating. Dyes Pigments 121, 282–289. doi: 10.1016/j.dyepig.2015.05.037
El-Wahab, H. A., EL-Fattah, M. A., Abdou, M. I., and El-Hai, F. A. (2009). New anticorrosive coating compositions incorporated ilmenite ore. Prog. Org. Coat. 66, 242–247. doi: 10.1016/j.porgcoat.2009.07.010
Gabrovska, M. V., Crisan, D., Stanica, N., and Kardjieva, R. (2014). Co-Al layered double hydroxides as precursors of ceramic pigment CoAl2O4. Part I: phase composition. Rev. Roum. Chim. 59, 445–450.
Gilkes, R. J., and Prakongkep, N. (2016). How the unique properties of soil kaolin affect the fertility of tropical soils. Appl. Clay Sci. 131, 100–106. doi: 10.1016/j.clay.2016.01.007
Golru, S. S., Attar, M. M., and Ramezanadeh, B. (2015). Effects of surface treatment of aluminium alloy 1050 on the adhesion and anticorrosion properties of the apoxy coating. Appl. Surf. Sci. 345, 360–368. doi: 10.1016/j.apsusc.2015.03.148
Gomesan, Y. F., Medeirosa, P. N., Bomioa, M. R. D., Santosb, I. M. G., Paskocimasa, C. A., and Nascimentoa, R. M. (2015). Optimizing the synthesis of cobalt aluminate pigment using fractional factorial design. Ceram. Int. 41, 699–706. doi: 10.1016/j.ceramint.2014.08.125
Gu, L., Liu, S., Zhao, H., and Yu, H. B. (2015). Facile preparation of water-dispersible graphene sheets stabilized by carboxylated oligoanilines and their anticorrosion coatings. ACS Appl. Mater. Inter. 7, 17641–17648. doi: 10.1021/acsami.5b05531
Hao, L., Yan, T. T., Zhang, Y. M., Zhao, X. H., Lei, X. D., Xu, S. L., et al. (2017). Fabrication and anticorrosion properties of composite films of silica/layered double hydroxide. Surf. Coat. Tech. 326, 200–206. doi: 10.1016/j.surfcoat.2017.06.024
He, T., and Becker, K. D. (1997). An optical in-situ study of a reacting spinel crystal. Solid State Ionics 101, 337–342. doi: 10.1016/S0167-2738(97)84050-7
He, Y., Cao, Y., Liao, H. L., and Wang, J. A. (2017). Preparation of porous cobalt aluminate and its chromogenic mechanism. Powder Technol. 324, 95–101. doi: 10.1016/j.powtec.2017.08.056
Herrero, M., Benito, P., Labajos, F. M., and Rives, V. (2007). Stabilization of Co2+ in layered double hydroxides (LDHs) by microwave-assisted ageing. J. Solid State Chem. 180, 873–884. doi: 10.1016/j.jssc.2006.12.011
Jafari, M., and Hassanzadeh-Tabriz, S. A. (2014). Preparation of CoAl2O4 nanoblue pigment via polyacrylamide gel method. Powder Technol. 266, 236–239. doi: 10.1016/j.powtec.2014.06.018
Jeon, H., Park, J., and Shon, M. (2013). Corrosion protection by epoxy coating containing multi-walled carbon nanotubes. J. Ind. Eng. Chem. 19, 849–853. doi: 10.1016/j.jiec.2012.10.030
Juneja, A., Hegde, A., Lee, F. H., and Yeo, C. H. (2010). Centrifuge modelling of tunnel face reinforcement using forepoling. Tunn. Undergr. Space Tech. 25, 377–381. doi: 10.1016/j.tust.2010.01.013
Kalendová, A., Sapurina, I., Stejskal, J., and Veselý, D. (2008). Anticorrosion properties of polyaniline-coated pigments in organic coatings. Corros. Sci. 50, 3549–3560. doi: 10.1016/j.corsci.2008.08.044
Kartsonakis, I. A., Balaskas, A. C., Koumoulos, E. P., Charitidis, C. A., and Kordas, G. C. (2012). Incorporation of ceramic nanocontainers into epoxy coatings for the corrosion protection of hot dip galvanized steel. Corros. Sci. 57, 30–41. doi: 10.1016/j.corsci.2011.12.037
Kim, J. H., Son, B. R., Yoon, D. H., Hwang, K. T., Noh, H. G., Cho, W. S., et al. (2012). Characterization of blue CoAl2O4 nano-pigment synthesized by ultrasonic hydrothermal method. Ceram. Int. 38, 5707–5712. doi: 10.1016/j.ceramint.2012.04.015
Kloprogge, J. T. (2017). Chapter 6 - Raman spectroscopy of clay minerals. Dev. Clay Sci. 8, 150–199. doi: 10.1016/B978-0-08-100355-8.00006-0
Kurajica, S., Popovic, J., Tkalcec, E., Grzeta, B., and Mandic, V. (2012). The effect of annealing temperature on the structure and optical properties of sol-gel derived nanocrystalline cobalt aluminate spinel. Mater. Chem. Phys. 135, 587–593. doi: 10.1016/j.matchemphys.2012.05.030
Liang, J., Hu, L. T., and Hao, J. C. (2007). Improvement of corrosion properties of microarc oxidation coating on magnesium alloy by optimizing current density parameters. Appl. Surf. Sci. 253, 6939–6945. doi: 10.1016/j.apsusc.2007.02.010
Liu, Q. F., Li, X. G., and Cheng, H. F. (2016). Insight into the self-adaptive deformation of kaolinite layers into nanoscrolls. Appl. Clay Sci. 124–125, 175–182. doi: 10.1016/j.clay.2016.02.015
Liu, Y., Jia, L. T., Hou, B., Sun, D. K., and Li, D. B. (2017). Cobalt aluminate-modified alumina as a carrier for cobalt in fischer-tropsch synthesis. Appl. Catal. A: Gen. 530, 30–36. doi: 10.1016/j.apcata.2016.11.014
Madhup, M. K., Shah, N. K., and Parekh, N. R. (2017). Investigation and improvement of abrasion resistance, water vapor barrier and anticorrosion properties of mixed clay epoxy nanocomposite coating. Prog. Org. Coat. 102, 186–193. doi: 10.1016/j.porgcoat.2016.10.012
Mohammadi, S., Taromi, F. A., Shariatpanahi, H., Neshati, J., and Hemmati, M. (2014). Electrochemical and anticorrosion behavior of functionalized graphite nanoplatelets epoxy coating. J. Ind. Eng. Chem. 20, 4124–4139. doi: 10.1016/j.jiec.2014.01.011
Montemor, M. F., Snihirov, D. V., Taryb, M. G., Lamaka, S. V., Kartsonakis, I. A., and Balaskas, A. C. (2012). Evaluation of self-healing ability in protective coatings modified with combinations of layered double hydroxides and cerium molibdate nanocontainers filled with corrosion inhibitors. Electrochim Acta 60, 31–40. doi: 10.1016/j.electacta.2011.10.078
Mu, B., Wang, Q., and Wang, A. Q. (2015). Effect of different clay minerals and calcination temperature on the morphology and color of clay/CoAl2O4 hybrid pigments. RSC Adv. 5, 102674–102681. doi: 10.1039/C5RA19955G
Mymrin, V., Pedroso, A. M., Ponte, H. A., Ponte, M. J. J., Alekseeva, K., Evaniki, D., et al. (2017). Thermal engineering method application for hazardous spent petrochemical catalyst neutralization. Appl. Therm. Eng. 110, 1428–1436. doi: 10.1016/j.applthermaleng.2016.09.077
Naderi, R., Arman, S. Y., and Fouladvand, S. (2014). Investigation on the inhibition synergism of new generations of phosphate-based anticorrosion pigments. Dyes Pigments 105, 23–33. doi: 10.1016/j.dyepig.2014.01.015
Qu, M. N., Liu, S. S., He, J. M., Feng, J., Yao, Y. L., Ma, X. R., et al. (2017). Fabrication of recyclable and durable superhydrophobic materials with wear/corrosion-resistance properties from kaolin and polyvinylchloride. Appl. Surf. Sci. 410, 299–307. doi: 10.1016/j.apsusc.2017.03.127
Rekik, S. B., Gassara, S., Bouaziz, J., Deratani, A., and Baklouti, S. (2017). Development and characterization of porous membranes based on kaolin/chitosan composite. Appl. Clay Sci. 143, 1–9. doi: 10.1016/j.clay.2017.03.008
Ribeiro, M. J., Tulyagavov, D. U., Ferreira, J. M., and Labrincha, J. A. (2005). High temperature mullite dissolution in ceramic bodies derived from al-rich sludge. J. Eur. Ceram. Soc. 25, 703–710. doi: 10.1016/j.jeurceramsoc.2004.03.028
Rostami, M., Rasouli, S., Ramezanzadeh, B., and Askari, A. (2014). Electrochemical investigation of the properties of Co doped ZnO nanoparticle as a corrosion inhibitive pigment for modifying corrosion resistance of the epoxy coating. Corros. Sci. 88, 387–399. doi: 10.1016/j.corsci.2014.07.056
Sreelekshmi, R. V., Brahmakumar, M., Sudha, J. D., and Menon, A. R. (2017). Studies on natural rubber containing kaolin modified with hexamethylenediamine derivative of phosphorylated cashew nut shell liquid prepolymer. Appl. Clay Sci. 141, 171–179. doi: 10.1016/j.clay.2017.02.034
Tahereh, G., Masoud, S. N., and Shokufeh, V. (2016). Investigation of the electrochemical hydrogen storage and photocatalytic properties of CoAl2O4 pigment: green synthesis and characterization. Int. J. Hydrogen Ener. 41, 9418–9426. doi: 10.1016/j.ijhydene.2016.03.144
Tielens, F., Calatayud, M., Franco, R., Recio, J. M., Pérez-Ramírez, J., and Minot, C. (2006). Periodic DFT study of the structural and electronic properties of bulk CoAl2O4 spinel. J. Phys. Chem. B 110, 988–995. doi: 10.1021/jp053375l
Tirsoaga, A., Visinescu, D., Jurca, B., Ianculescu, A., and Carp, O. (2011). Eco-friendly combustion-based synthesis of metal aluminates MAl2O4 (M = Ni, Co). J. Nanopart. Res. 13, 6397–6408. doi: 10.1007/s11051-011-0392-1
Vesely, D., Kalendov, A., and Kalend, P. (2010). A study of diatomite and calcined kaoline properties in anticorrosion protective coatings. Prog. Org. Coat. 68, 173–179. doi: 10.1016/j.porgcoat.2010.02.007
Wang, C., Liu, S. M., Liu, L. H., and Bai, X. (2006). Synthesis of cobalt-aluminate spinels via glycine chelated precursors. Mater. Chem. Phys. 96, 361–370. doi: 10.1016/j.matchemphys.2005.07.066
Xi, X. L., Nie, Z. R., Ma, L. W., Xu, X. Y., and Zuo, E. Y. (2012). Synthesis and characterization of ultrafine Co2AlO4 pigment by freeze-drying. Powder Technol. 226, 114–116. doi: 10.1016/j.powtec.2012.04.029
Yang, Y., Li, L. L., and Li, W. K. (2013). Plasmon absorption of Au-in-CoAl2O4 linear nanopeapod chains. J. Phys. Chem. C 117, 14142–14148. doi: 10.1021/jp403150h
Yeo, C. H. (2011). Stability and Collapse Mechanisms of Unreinforced and Forepole-Reinforced Tunnel Headings, Ph.D. thesis National university of Singapore, Singapore.
Yoneda, M., Gotoh, K., Nakanishi, M., Fujii, T., and Nomura, T. (2018). Influence of aluminum source on the color tone of cobalt blue pigment. Powder Technol. 323, 574–580. doi: 10.1016/j.powtec.2016.06.021
Zayat, M., and Levy, D. (2000). Blue CoAl2O4 particles prepared by the sol-gel and citrate-gel methods. Chem. Mater. 12, 2763–2769. doi: 10.1021/cm001061z
Zha, W. W., Zhou, Z. H., Zhao, D. L., and Feng, S. J. (2016). Positive effects of Al3+ partially substituted by Co2+ cations on the catalytic performance of Co1+xAl2−xO4 (x = 0-0.2) for methane combustion. J. Sol-Gel Sci. Techn. 78, 144–150. doi: 10.1007/s10971-015-3910-2
Zhang, A., Mu, B., Wang, X., Wen, L., and Wang, A. (2018b). Formation and coloring mechanism of typical aluminosilicate clay minerals for CoAl2O4 hybrid pigment preparation. Front. Chem. 6:125. doi: 10.3389/fchem.2018.00125
Zhang, A. J., Mu, B., Hui, A. P., and Wang, A. Q. (2018a). A facile approach to fabricate bright blue heat-resisting paint with self-cleaning ability based on CoAl2O4/kaoline hybrid pigment. Appl. Clay Sci. 160, 153–161. doi: 10.1016/j.clay.2017.12.004
Zhang, A. J., Mu, B., Luo, Z. H., and Wang, A. Q. (2017). Bright blue halloysite/CoAl2O4 hybrid pigments: preparation, characterization and application in water-based painting. Dyes Pigments 139, 473–481. doi: 10.1016/j.dyepig.2016.12.055
Zhang, C. L., He, Y., Li, F., Di, H. H., Zhang, L., and Zhan, Y. Q. (2016). h-BN decorated with Fe3O4 nanoparticles through mussel-inspired chemistry of dopamine for reinforcing anticorrosion performance of epoxy coatings. J. Alloy. Compd. 685, 743–751. doi: 10.1016/j.jallcom.2016.06.220
Zhang, S. L., Liu, Q. F., Yang, Y. J., Wang, D., He, J. K., and Sun, L. Y. (2017). Preparation, morphology, and structure of kaolinites with various aspect ratios. Appl. Clay Sci. 147, 117–122. doi: 10.1016/j.clay.2017.07.014
Zhou, S., Wu, L., Sun, J., and Shen, W. (2002). The change of the properties of acrylic-based polyurethane via addition of nano-silica. Prog. Org. Coat. 45, 33–42. doi: 10.1016/S0300-9440(02)00085-1
Zhu, X. H., Li, H., Zhou, H., and Zhong, S. A. (2015). Fabrication and evaluation of protein imprinted polymer based on magnetic halloysite nanotubes. RSC Adv. 5, 66147–66154. doi: 10.1039/C5RA09740A
Keywords: CoAl2O4, hybrid pigments, kaoline, anticorrosion coating, acetic acid-salt fog
Citation: Zhang A, Mu B, Wang X and Wang A (2018) CoAl2O4/Kaoline Hybrid Pigment Prepared via Solid-Phase Method for Anticorrosion Application. Front. Chem. 6:586. doi: 10.3389/fchem.2018.00586
Received: 31 July 2018; Accepted: 09 November 2018;
Published: 29 November 2018.
Edited by:
Pu-Xian Gao, University of Connecticut, United StatesCopyright © 2018 Zhang, Mu, Wang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bin Mu, bXViaW5AbGljcC5jYXMuY24=
Aiqin Wang, YXF3YW5nQGxpY3AuY2FzLmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.