
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Chem. , 21 August 2018
Sec. Green and Sustainable Chemistry
Volume 6 - 2018 | https://doi.org/10.3389/fchem.2018.00367
This article is part of the Research Topic Novel Approaches to Design Eco-friendly Materials Based on Natural Nanomaterials View all 17 articles
Hexavalent chromium species, Cr(VI), which can activate teratogenic processes, disturb DNA synthesis and induce mutagenic changes resulting in malignant tumors. The detection and quantification of Cr(VI) is very necessary. One of the rapid and simple methods for contaminant analysis is fluorescence detection using organic dye molecules. Its application is limited owing to concentration quenching due to aggregation of fluorescent molecules. In this study, we successfully intercalated 7-amino-4-methylcoumarin (AMC) into the interlayer space of montmorillonite (MMT), significantly inhibited fluorescence quenching. Due to enhanced fluorescence property, the composite was fabricated into a film with chitosan to detect Cr(VI) in water. Cr(VI) can be detected in aqueous solution by instruments excellent, ranging from 0.005 to 100 mM with a detection limit of 5 μM.
Due to the high toxicity of heavy metals and bioaccumulation in human body through the food chain, will bring human health and environmental great issue. Cr is one of the most serious contaminants among various heavy metals (Soewu et al., 2014; Wu et al., 2015a; Širić et al., 2017). While Cr(III) is an essential micronutrient, Cr(VI) is toxic (Weibel et al., 2016). Cr(VI) is mainly produced by electroplating, leather tanning, and textile dyeing (Dehghani et al., 2016; Omorogie et al., 2016). Cr(VI) is the most virulent form (Miretzky and Cirelli, 2010) of Cr, which is a highly toxic agent and act as carcinogens, mutagens, and teratogens in biological systems (Hlihor et al., 2017; Mullick et al., 2017). The carcinogenic and toxicity of Cr(VI) is based on its oxidation states like the most transition metals (Yusof and Malek, 2009; Kumar et al., 2017). An effective, simple and low-cost method for detecting Cr(VI) is needed (Babu and Gupta, 2008; Wang G. et al., 2017). The classic methods for detecting Cr(VI) include electrochemical method, atomic absorption method and liquid chromatography (Anthemidis et al., 2002; De Ruiter et al., 2008). These methods are credible. However, the processes are complicated. Due to its sensitivity, fluorescence spectroscopy has become one of the most increasingly used techniques (Wang et al., 2015; Li and Wei, 2017). The sensors made of organic or inorganic materials show great promise in detection, lighting, and other application (Rao et al., 2014).
Montmorillonite is a typical phyllosilicate of the smectite group of clay minerals (Ismadji et al., 2016; Xu et al., 2016). It is consists of two layers of silicon tetrahedron (T) and the middle layer of aluminum octahedron (O) (Bekri-Abbes and Srasra, 2016; Scholtzová et al., 2016; Wang W. et al., 2017). Because of the isomorphous replacement of Al3+ by Mg2+ and Fe2+ in the octahedral sites and Si4+ by Al3+ in the tetrahedral sites, TOT layers are negative electric charge, balanced by the cations such as Ca2+ and Na+ intercalate into the layers (Dominijanni and Manassero, 2012; Wu et al., 2016; Zhou et al., 2016). Luminescent materials of small organic molecules are easily modified and chemically purified to have wider emission spectrum coverage (Wu et al., 2015b; Tian et al., 2017). However, when they are applied in solid devices, stabilize and enhance their luminous intensity is crucial. Although several methods of assembling materials have been developed (Zhang et al., 2005), they often suffer from phase separation, agminated organic small molecules (Wei et al., 2016; De Sa et al., 2017). Clay minerals were rarely used as base materials for the organic-inorganic composite preparation for luminescence and fluorescence applications.
This research focused on the fabrication of a fluorescence material for Cr(VI) detection. To prevent quenching, the photoactive organic dye AMC was interposed into the interlining of MMT for maximal dye separation and minimum agminated (Yu et al., 2013, 2014). The AMC/MMT was fabricated in a film with chitosan. Quenching of fluorescence was used various solutions to evaluated. The response of AMC/MMT composites in Cr(VI) was excellent, ranging from 0.005 to 100 mM.
The montmorillonite was bought from Ningcheng, Inner Mongolia, China. Its CEC (Cation Exchange Capacity) was 9.8 mmolc/10 g which used Na+ as the staple commutable cationan exchange. The specific external surface area was 78 m2/g. AMC was purchased from Aladdin. The molecular structure (Nowakowska et al., 2001) of AMC is presented in Figure 1. The potassium dichromate Cr(VI), chitosan, and other reagents were purchased from various manufactures that were all of analytical grade. Deionized water was used as a solvent.
The effect of initial AMC concentrations on a performance of florescence were tested, 50 mL the original concentrations of AMC in water for 20, 50, 100, 200, 500, and 800 mg/L were blended with 0.25 g of MMT in every 100 mL centrifugal tube after shaking at 200 rotations per minute at indoor temperature for 8 h, respectively. The admixtures were centrifuged at 7,500 rotations per minute for 2 min. Afterwards, removing the supernatant, then at 60°C were dried the residues and pulverized the residues to powder as raw materials. This type of product was used for the AMC/MMT.
The chitosan film was prepared as follows: 0.25 g chitosan, 8 mL 0.1 M NaOH solution, 0.1 g AMC/MMT powder and two drops of 1 g/mL Polyvinyl Alcohol (PVA) solution were appended. The admixture was whipped for 30 min and then poured into a mold and air dried.
The followed solutions at a concentration of 0.1 M were originally screened for quenching of fluorescence in AMF: Al3+, Ca2+, Ba2+, Cr(VI), CTAB, K+, Na+, Ni3+, Pb2+, Fe3+, Imidazole, C2H5OH. Afterwards, they were dropped onto the AMF. Then, the fluorescence degrees were measured using a fluorescence spectrophotometer (Hitachi, F4600).
In order to estimate the Cr(VI) range of response, different concentrations of Cr(VI) solution were dropped onto the AMF. The Cr(VI) of original concentrations were the ranged of 5 μM to 100 mM.
Pictures of the AMF after in contact with Cr(VI) solutions were already obvious demonstration quenching of fluorescence effect by Cr(VI) and then measure the florescence intensions of AMF by fluorescence spectrophotometer (Hitachi, F4600).
Powder X-Ray Diffraction (XRD) was performed by a Rigaku D/max-IIIa diffractometer (Tokyo, Japan) with a Ni-filtered Cu Ka radiation at 40 kV and 40 mA. Prototypes were scanned the range of 3° to 70° at 8° per minute with a pace of 0.01° to research the particular changes in d001 separation distance of MMT as a function of original AMC concentrations. The changing of peak position proved the intercalation of AMC into the MMT was succeeded. The gallery heights were deduced from the (001) reflection of the composites using the Bragg's equation.
Fourier Transform Infrared Spectroscopy (FTIR) spectra were acquired on a Perkin Elmer Spectrum 100 Spectrometer. The spectra were obtained from 4,000 to 500 cm−1 by adding 256 scans in the resolution ratio of 4 cm−1.
Photoluminescence emission (PL) spectrum was obtained on a fluorescence spectrophotometer (Hitachi, F4600) at the scope of 400–600 nm with a photomultiplier tube handled at 800 volt. A 150 watt xenon lamp was served as the pumping source, at an excitation wavelength of 340 nm (Zamojć et al., 2015). The emission and excitation slits size were 5 nm and scan speed was setting at 240 nm per min.
Molecular mimicry was performed at the standard module “Forcite” of Materials Studio 6.0 software to research the structure of AMC in the interval of MMT. The unit lattice parameters were setted at a = 1.55 nm, b = 1.79 nm, c = 1.25 nm, α = γ = 90°, and β = 99°. A suite of 2 × 2 × 1 supercells were constructed of the interlayer spacing setting at 1.63 nm. Every circulation consisted of 106 steps, repeating three cycles.
The idea that interlayer cation was Na+ with a monolayer hydration was confirmed because the d001 value of pure MMT was 12.6 Å. The d001 spacing progressively increased from 12.6 to 16.3 Å with the initial AMC concentration increased (Figure 2), suggesting AMC molecules insert the portion between the interlayer space of MMT.
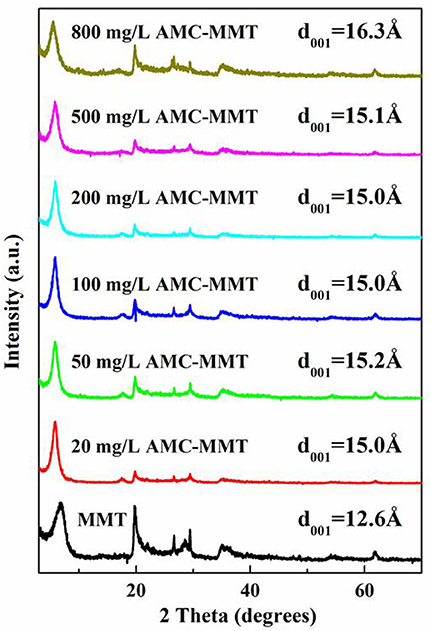
Figure 2. X-ray diffraction patterns of MMT was inserted with AMC in the interlayer, which was affected by initial AMC concentrations (mg/L).
FTIR spectra of MMT adsorbed with different amounts of AMC displayed both bands of raw MMT and AMC (Figure 3). The bands between 1,400 and 1,650 cm−1 were allocated to C-C stretching vibrations in aromatic and hetero aromatic compounds (Talbi et al., 1998; Krishnakumar and Xavier, 2005). Therefore, the bands observed at 1,727, 1,695, 1,625, 1,566, 1,525, 1,475, 1,446, 1,417, 1,398, 1,390 cm−1 in FTIR spectra were allocated to C-C stretching vibrations of AMC (Arivazhagana et al., 2010). The characteristic peaks of AMC appeared in FTIR spectra of AMC/MMT, as shown in the dotted line (Figure 3), indicating that AMC had been loaded onto MMT.
AMC powder was hardly used as a luminescent material just because the lack of strong luminescence (Wang et al., 2012), causing by concentration quenching (Wu et al., 2015b). The luminous intensity increased significantly after the intercalation of AMC into the interlayer of MMT, which reason was AMC was uniformly dispersed on MMT, inhibiting its aggregation. The highest spectral intensity was found by AMC/MMT at 100 mg/L, which was an initial concentration (Figure 4).
The AMF was screened for response to various solutes (Figure 5). The fluorescence response to Cr(VI) was very significant when the test solute with an initial concentration of 0.1 M, while others were not obvious, indicating the high selectivity of AMF for Cr(VI).
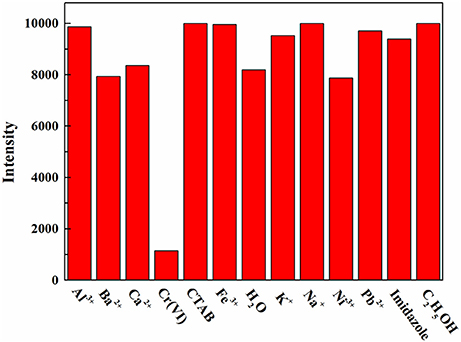
Figure 5. Fluorescence intensity of the AMF in response to a variety of aqueous solutions at a concentration of 0.1 M.
The mechanism of photoluminescence (PL) quenching of AFM was further investigated when the aqueous solution contains Cr(VI). Most importantly, an apparent decrease in fluorescence intensity was observed while the Cr(VI) concentration was increasing over the whole concentration ranges from 0.005 to 100 mM (Figure 6a). A full PL quenching process can be observed visually at a Cr(VI) concentration of 1 mM. The images of AMF with addition of Cr(VI) that exposed ultraviolet light showed a consistent tendency (Figure 6b).
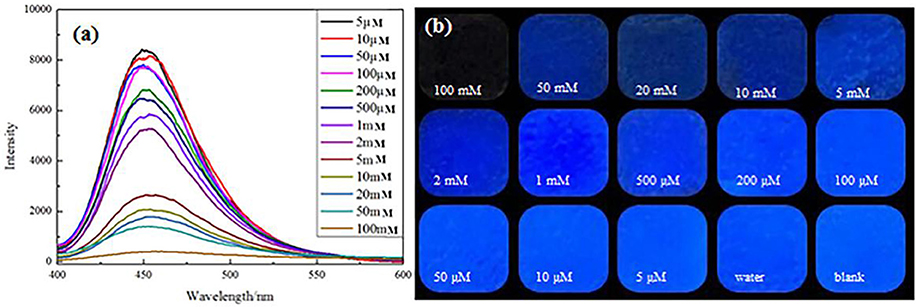
Figure 6. Fluorescence spectra of the AMF in the presence of multifarious concentrations of Cr(VI) (a). Pictures of the AMF with disparate concentrations of Cr(VI) exposed UV lamp (b).
Molecular mimicry was performed at the standard module “Forcite” of Materials Studio 6.0 software to research the structure of AMC between the interval of MMT. The interlayer spacing of MMT was directly influenced by the interlayer arrangement of AMC and the amount of AMC intercalation, this, on the other hand, would play an significant role in understanding the system structure and interaction forces (Krauss et al., 2011; Wang et al., 2011; Tournassat et al., 2016). The interlayer condition of AMC interpolation MMT with different quantities was qualitatively simulated, and the initial states were represented by a, c, and e; and b, d, and f represent the states after simulation (a stands for low concentration; c stands for medium concentration; and e stands for higher concentration; Figure 7). The concentration quenching could be effectively avoid by AMC intercalation MMT and it could also significantly improve luminous intensity, lifespan, and stability notably owing to the stronger interaction forces between AMC molecules and MMT sheet (Yan et al., 2010a,b; Liu et al., 2014).
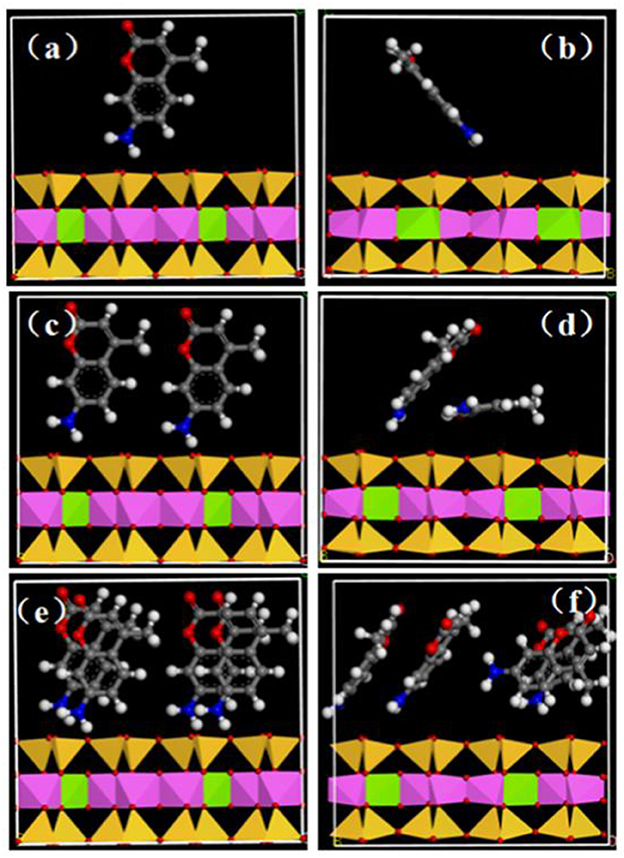
Figure 7. Dynamic simulation of molecular interposition AMC into MMT at a different angle on account of the electric density and AMC loading distinction. The initial states were represented by a, c, and e; and b, d, and f represent the states after simulation: a stands for low concentration, c stands for medium concentration and e stands for higher concentration. The all strains, Mg, green; Al, pink; Si, yellow; C, gray; N, blue; O, red; H, white.
In this research, an organic dye (AMC) was resoundingly intercalated into the interlayer space of MMT, resulting in observably inhibition in quenching of fluorescence. The composite was fabricated into a film with chitosan to detect Cr(VI) in water with its enhanced fluorescent property. Cr(VI) can be detected in aqueous solution by instruments ranging from 0.005 to 100 mM with a detection limit of 5 μM.
YW and LM conceived the project. LM, GL, and LibL designed and performed the experiments. RL, ML, JW, and LinL analyzed the data. YW, ZL, and LM wrote the manuscript.
This research was supported by National Natural Science Foundation of China (grant number: 51604248), the National Key R&D Program of China (grant number: 2017YFB0310704), and Fundamental Research Funds for the Central Universities (grant numbers: 2652017335, 2652017338 and 2652017366).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Anthemidis, A. N., Zachariadis, G. A., Kougoulis, J. S., and Stratis, J. A. (2002). Flame atomic absorption spectrometric determination of chromium (VI) by on-line preconcentration system using a PTFE packed column. Talanta 57, 15–22. doi: 10.1016/S0039-9140(01)00676-2
Arivazhagana, M., Sambathkumarb, K., and Jeyavijayanc, S. (2010). Density functional theory study of FTIR and FT-Raman spectra of 7-acetoxy-4-methyl coumarin. Indian J. Pure Appl. Phys. 48, 716–722. doi: 10.13140/RG.2.1.4538.7361
Babu, B. V., and Gupta, S. (2008). Adsorption of Cr (VI) using activated neem leaves: kinetic studies. Adsorption 14, 85–92. doi: 10.1007/s10450-007-9057-x
Bekri-Abbes, I., and Srasra, E. (2016). Effect of mechanochemical treatment on structure and electrical properties of montmorillonite. J. Alloys Compd. 671, 34–42. doi: 10.1016/j.jallcom.2016.02.048
De Ruiter, G., Gupta, T., and Van Der Boom, M. E. (2008). Selective optical recognition and quantification of parts per million levels of Cr6+ in aqueous and organic media by immobilized polypyridyl complexes on glass. J. Am. Chem. Soc. 130, 2744–2745. doi: 10.1021/ja7110527
De Sa, A., Moura, I., Abreu, A. S., Oliveira, M., Ferreira, M. F., and Machado, A. V. (2017). High fluorescent water soluble CdTe quantum dots—a promising system for light harvesting applications. J.Nanopart. Res. 19:180. doi: 10.1007/s11051-017-3872-0
Dehghani, M. H., Sanaei, D., Ali, I., and Bhatnagar, A. (2016). Removal of chromium (VI) from aqueous solution using treated waste newspaper as a low-cost adsorbent: kinetic modeling and isotherm studies. J. Mol. Liq. 215, 671–679. doi: 10.1016/j.molliq.2015.12.057
Dominijanni, A., and Manassero, M. (2012). Modelling the swelling and osmotic properties of clay soils. Part II: the physical approach. Int. J. Eng. Sci. 51, 51–73. doi: 10.1016/j.ijengsci.2011.11.001
Hlihor, R. M., Figueiredo, H., Tavares, T., and Gavrilescu, M. (2017). Biosorption potential of dead and living Arthrobacter viscosus biomass in the removal of Cr (VI): batch and column studies. Process Safe. Environ. Prot. 108, 44–56. doi: 10.1016/j.psep.2016.06.016
Ismadji, S., Tong, D. S., Soetaredjo, F. E., Ayucitra, A., Yu, W. H., and Zhou, C. H. (2016). Bentonite hydrochar composite for removal of ammonium from Koi fish tank. Appl. Clay Sci. 119, 146–154. doi: 10.1016/j.clay.2015.08.022
Krauss, T. N., Barrena, E., Lohmüller, T., Spatz, J. P., and Dosch, H. (2011). Growth mechanisms of phthalocyanine nanowires induced by Au nanoparticle templates. Phys. Chem. Chem. Phys. 13, 5940–5944. doi: 10.1039/C0CP02191A
Krishnakumar, V., and Xavier, R. J. (2005). Density functional theory calculations and vibrational spectra of 3, 5-dibromopyridine and 3, 5-dichloro-2, 4, 6-trifluoropyridine. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 61, 253–260. doi: 10.1016/j.saa.2004.03.038
Kumar, R., Kim, S. J., Kim, K. H., Lee, S. H., Park, H. S., and Jeon, B. H. (2017). Removal of hazardous hexavalent chromium from aqueous phase using zirconium oxide-immobilized alginate beads. Appl. Geochem. 88, 113–121. doi: 10.1016/j.apgeochem.2017.04.002
Li, C., and Wei, C. (2017). DNA-templated silver nanocluster as a label-free fluorescent probe for the highly sensitive and selective detection of mercury ions. Sens. Actuat. B Chem. 242, 563–568. doi: 10.1016/j.snb.2016.11.091
Liu, M., Wang, T., Ma, H., Fu, Y., Hu, K., and Guan, C. (2014). Assembly of luminescent ordered multilayer thin-films based on oppositely-charged MMT and magnetic NiFe-LDHs nanosheets with ultra-long lifetimes. Sci. Rep. 4:7147. doi: 10.1038/srep07147
Miretzky, P., and Cirelli, A. F. (2010). Cr (VI) and Cr (III) removal from aqueous solution by raw and modified lignocellulosic materials: a review. J. Hazard. Mater. 180, 1–19. doi: 10.1016/j.jhazmat.2010.04.060
Mullick, A., Moulik, S., and Bhattacharjee, S. (2017). Removal of hexavalent chromium from aqueous solutions by low-cost rice husk-based activated carbon: kinetic and thermodynamic studies. Indian Chem. Eng. 60, 58–71. doi: 10.1080/00194506.2017.1288173
Nowakowska, M., Smoluch, M., and Sendor, D. (2001). The effect of cyclodextrins on the photochemical stability of 7-amino-4-methylcoumarin in aqueous solution. J. Incl. Phenom. Macrocycl. Chem. 40, 213–219. doi: 10.1023/A:1011820513256
Omorogie, M. O., Babalola, J. O., Unuabonah, E. I., Song, W., and Gong, J. R. (2016). Efficient chromium abstraction from aqueous solution using a low-cost biosorbent: nauclea diderrichii seed biomass waste. J. Saudi Chem. Soc. 20, 49–57. doi: 10.1016/j.jscs.2012.09.017
Rao, K. V., Jain, A., and George, S. J. (2014). Organic–inorganic light-harvesting scaffolds for luminescent hybrids. J. Mater. Chem. C 2, 3055–3064. doi: 10.1039/C3TC31729C
Scholtzová, E., Madejová, J., and Tunega, D. (2016). Structural and spectroscopic characterization of montmorillonite intercalated with n-butylammonium cations (n = 1–4)–Modeling and experimental study. Clays Clay Miner. 64, 401–412. doi: 10.1346/CCMN.2016.0640404
Širić, I., Kasap, A., Bedeković, D., and Falandysz, J. (2017). Lead, cadmium and mercury contents and bioaccumulation potential of wild edible saprophytic and ectomycorrhizal mushrooms, Croatia. J. Environ.l Sci. Health Part B 52, 156–165. doi: 10.1080/03601234.2017.1261538
Soewu, D. A., Agbolade, O. M., Oladunjoye, R. Y., and Ayodele, I. A. (2014). Bioaccumulation of heavy metals in cane rat (Thryonomys swinderianus) in Ogun State, Nigeria. J. Toxicol. Environ. Health Sci. 6, 154–160. doi: 10.5897/JTEHS2014.0310
Talbi, H., Humbert, B., and Billaud, D. (1998). FTIR and Raman spectroscopic investigations on the redox behaviour of poly (5-cyanoindole) in acidic aqueous solutions. Spectrochim. Acta A Mol. Biomol. Spectr. 54, 1879–1893. doi: 10.1016/S1386-1425(98)00146-2
Tian, G., Wang, W., Wang, D., Wang, Q., and Wang, A. (2017). Novel environment friendly inorganic red pigments based on attapulgite. Powder Technol. 315, 60–67. doi: 10.1016/j.powtec.2017.03.044
Tournassat, C., Bourg, I. C., Holmboe, M., Sposito, G., and Steefel, C. I. (2016). Molecular dynamics simulations of anion exclusion in clay interlayer nanopores. Clays Clay Miner. 64, 374–388. doi: 10.1346/CCMN.2016.0640403
Wang, F. X., Liu, Y. D., and Pan, G. B. (2011). Vapor growth and photoconductivity of single-crystal nickel-phthalocyanine nanorods. Mater. Lett. 65, 933–936. doi: 10.1016/j.matlet.2010.12.012
Wang, G., Wang, S., Sun, W., Sun, Z., and Zheng, S. (2017). Synthesis of a novel illite@ carbon nanocomposite adsorbent for removal of Cr (VI) from wastewater. J. Environ. Sci. 57, 62–71. doi: 10.1016/j.jes.2016.10.017
Wang, J., Hu, Y., Deng, R., Xu, W., Liu, S., Liang, R., et al. (2012). Construction of multifunctional photonic crystal microcapsules with tunable shell structures by combining microfluidic and controlled photopolymerization. Lab. Chip 12, 2795–2798. doi: 10.1039/C2LC40419B
Wang, M., He, L., Xu, W., Wang, X., and Yin, Y. (2015). Magnetic assembly and field- tuning of ellipsoidal-nanoparticle-based colloidal photonic crystals. Angew. Chem. Int. Edn. 54, 7077–7081. doi: 10.1002/anie.201501782
Wang, W., Tian, G., Zong, L., Zhou, Y., Kang, Y., Wang, Q., et al. (2017). From illite/smectite clay to mesoporous silicate adsorbent for efficient removal of chlortetracycline from water. J. Environ. Sci. 51, 31–43. doi: 10.1016/j.jes.2016.09.008
Wei, X., Hao, T., Xu, Y., Lu, K., Li, H., Yan, Y., et al. (2016). Facile polymerizable surfactant inspired synthesis of fluorescent molecularly imprinted composite sensor via aqueous CdTe quantum dots for highly selective detection of λ-cyhalothrin. Sens. Actuat. B Chem. 224, 315–324. doi: 10.1016/j.snb.2015.10.048
Weibel, G., Waber, H. N., Eggenberger, U., and Mäder, U. K. (2016). Influence of sample matrix on the alkaline extraction of Cr (VI) in soils and industrial materials. Environ. Earth Sci. 75:548. doi: 10.1007/s12665-015-5236-3
Wu, L., Liao, L., Lv, G., and Qin, F. (2015a). Stability and pH-independence of nano-zero-valent iron intercalated montmorillonite and its application on Cr (VI) removal. J. Contam. Hydrol. 179, 1–9. doi: 10.1016/j.jconhyd.2015.05.001
Wu, L., Lv, G., Liu, M., Li, Z., Liao, L., and Pan, C. (2015b). Adjusting the layer charges of host phyllosilicates to prevent luminescence quenching of fluorescence dyes. J. Phys. Chem. C. 119, 22625–22631. doi: 10.1021/acs.jpcc.5b07243
Wu, L. M., Tong, D. S., Li, C. S., Ji, S. F., Lin, C. X., Yang, H. M., et al. (2016). Insight into formation of montmorillonite-hydrochar nanocomposite under hydrothermal conditions. Appl. Clay Sci. 119, 116–125. doi: 10.1016/j.clay.2015.06.015
Xu, W., Mu, B., and Wang, A. (2016). From adsorbents to electrode materials: facile hydrothermal synthesis of montmorillonite/polyaniline/metal oxide (hydroxide) composites. New J. Chem. 40, 2687–2695. doi: 10.1039/C5NJ03734D
Yan, D., Lu, J., Chen, L., Qin, S., Ma, J., Wei, M., et al. (2010a). A strategy to the ordered assembly of functional small cations with layered double hydroxides for luminescent ultra-thin films. Chem. Commun. 46, 5912–5914. doi: 10.1039/C0CC00522C
Yan, D., Lu, J., Ma, J., Wei, M., Wang, X., Evans, D. G., et al. (2010b). Anionic poly (p-phenylenevinylene)/layered double hydroxide ordered ultrathin films with multiple quantum well structure: a combined experimental and theoretical study. Langmuir 26, 7007–7014. doi: 10.1021/la904228b
Yu, W. H., Li, N., Tong, D. S., Zhou, C. H., Lin, C. X. C., and Xu, C. Y. (2013). Adsorption of proteins and nucleic acids on clay minerals and their interactions: a review. Appl. Clay Sci. 80–81, 443–452. doi: 10.1016/j.clay.2013.06.003
Yu, W. H., Ren, Q. Q., Tong, D. S., Zhou, C. H., and Wang, H. (2014). Clean production of CTAB-montmorillonite: formation mechanism and swelling behavior in xylene. Appl. Clay Sci. 97–98, 222–234. doi: 10.1016/j.clay.2014.06.007
Yusof, A. M., and Malek, N. A. (2009). Removal of Cr (VI) and As (V) from aqueous solutions by HDTMA-modified zeolite Y. J. Hazard. Mater. 162, 1019–1024. doi: 10.1016/j.jhazmat.2008.05.134
Zamojć, K., Wiczk, W., Zaborowski, B., Jacewicz, D., and Chmurzynski, L. (2015). Fluorescence quenching of 7-amino-4-methylcoumarin by different TEMPO derivatives. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 136, 1875–1880. doi: 10.1016/j.saa.2014.10.102
Zhang, H., Wang, C., Li, M., Ji, X., Zhang, J., and Yang, B. (2005). Fluorescent nanocrystal– polymer composites from aqueous nanocrystals: methods without ligand exchange. Chem. Mater. 17, 4783–4788. doi: 10.1021/cm050260l
Keywords: AMC/MMT composite, fluorescence, quenching, detection, hexavalent chromium
Citation: Wei Y, Mei L, Li R, Liu M, Lv G, Weng J, Liao L, Li Z and Lu L (2018) Fabrication of an AMC/MMT Fluorescence Composite for its Detection of Cr(VI) in Water. Front. Chem. 6:367. doi: 10.3389/fchem.2018.00367
Received: 07 June 2018; Accepted: 30 July 2018;
Published: 21 August 2018.
Edited by:
Wenbo Wang, Lanzhou Institute of Chemical Physics (CAS), ChinaReviewed by:
Liang Bian, Southwest University of Science and Technology, ChinaCopyright © 2018 Wei, Mei, Li, Liu, Lv, Weng, Liao, Li and Lu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lefu Mei, bWxmQGN1Z2IuZWR1LmNu
Guocheng Lv, Z3VvY2hlbmdsdkBjdWdiLmVkdS5jbg==
Libing Liao, Y2xheWxAY3VnYi5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.