- 1Department of Chemistry, Emory University, Atlanta, GA, United States
- 2Emerson Center for Scientific Computation, Emory University, Atlanta, GA, United States
Polyoxometalate (POM)-based materials of current interest are summarized, and specific types of POM-containing systems are described in which material facilitates multiple complex interactions or catalytic processes. We specifically highlight POM-containing multi-hydrogen-bonding polymers that form gels upon exposure to select organic liquids and simultaneously catalyze hydrolytic or oxidative decontamination, as well as water oxidation catalysts (WOCs) that can be interfaced with light-absorbing photoelectrode materials for photoelectrocatalytic water splitting.
POM Properties That Make Them Effective in Materials Applications
Materials chemistry has evolved to the point where rational design of multifunctional systems with synergistic capabilities is possible. POMs are very effective as components of materials owing to their great synthetic tunability, allowing for many physical and chemical properties to be tailored to specific applications. Incorporating POMs into heterogeneous systems therefore allows for a bottom-up approach to the development of multifunctional materials (Miras et al., 2012; He et al., 2014; Zhang et al., 2014).
Polyoxometalates are useful in catalysis and other applications owing to their redox potentials, acidities, polarities, negative charge densities on surface oxygens and other parameters (Hill, 1998; Wang and Yang, 2015). Several reviews can be consulted for early studies of fundamental structure and reactivity (Pope, 1983, 2004; Hill, 2004; Yamase and Pope, 2006; Wang and Yang, 2015). The polyanion can be extensively modified by substitution of surface metal-oxo units (addenda or surface metal-oxo units) with many first-row transition-metal and other redox-active metal ions as well as organometallic groups. Of the large number of polyanion structural families, derivatives of the two most common families, Keggin and Wells-Dawson, still dominate fundamental studies of the impact of polyanion substitution and modification on the chemistry of POMs (Coronado and Gómez-García, 1995; Hill, 1998; Borrás-Almenar et al., 2001; Long et al., 2010). The fact that POMs are polyoxoanions with several counterions balancing the charge allows this huge and growing class of inorganic cluster compounds to be highly versatile; both the polyanion and the counterions can be altered, impacting the factors that make POMs useful in catalysis and other applications. This variability allows for small changes to be made in the POM systematically, facilitating the study and optimization of the resulting material.
The redox potentials of POMs are key to the reactions that feature prominently in many applications. POM potentials are controlled by the redox-active metals in the POM framework, by transition metal substituted into addenda (outside) structural sites, and by the charge density and geometry of the POM. Different POM geometries have intrinsically different charge densities on the framework metals and the oxygens bridging these metals, thus both the framework and substituted transition metals have altered potentials when present in different POM structural families. In addition to the framework metal and/or substituted transition metal, the nature and type of the counterion can also impact POM potentials (Hill, 2004). Ever more studies have demonstrated that POM counterions impact nearly every property of POMs that feature in their applications (Grigoriev et al., 2000, 2001).
The relative ease of incorporating POMs into heterogeneous matrices is another reason they are particularly well-suited for construction of functional materials. Several strategies exist for the immobilization of POMs including electrostatic attraction, solvophobic interactions, or covalent linkages (Proust et al., 2012; Hill and Kholdeeva, 2013; Xiao et al., 2016). Immobilization of POMs through electrostatic and solvophobic interactions is the most common and simple method, owing to the high negative charge of the POMs and ease of counter cation exchange (to produce insoluble salts)(Proust et al., 2012). Attaching POMs to materials covalently leads to the most stable products, however relatively few methods exist to achieve this covalent linkage while retaining all of the desired properties of the POM. As such, the development of covalently functionalized materials remains a very active area of research. Many studies have focused on the development of POM-based polymers to enable easier processing and facilitate the creation of POM-based devices and advanced materials. The tuning of flexible organic ligands and polyanions in POM-hybrids also allows for the engineering of POM-based compounds and materials with specific desired topologies (Taleghani et al., 2016). Most commonly, covalent incorporation of POMs and other inorganic compounds into polymer matrices is achieved through side-chain functionalization either before or after the polymerization process (Hu et al., 2012; Rieger et al., 2012; Macdonell et al., 2015; Wu et al., 2016). Both the self-assembly of POM-organic hybrids and their incorporation into polymers have been reviewed recently (Carraro and Gross, 2014; Wu et al., 2016).
Survey of POM Materials Applications
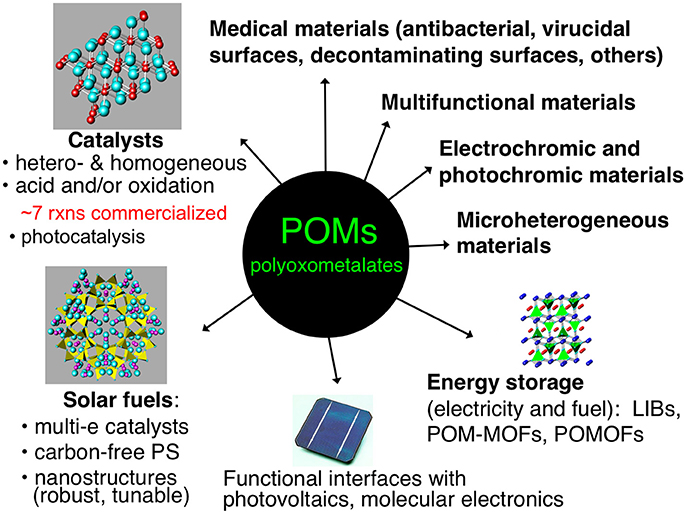
Figure 1 illustrates several applications of POMs in materials. Some of these topics, such as catalysis of organic transformations, are more mature than others, but substantial research continues in all these areas. Several heterogeneous and homogeneous processes catalyzed by POMs have had commercial applications, such as in the hydration of alkenes or polymerization of tetrahydrofuran (Kozhevnikov, 1998). These industrial processes primarily involve the use of heteropolyacids as acid catalysts or more complex, mixed-metal POM derivatives as oxidation catalysts (Hill and Prosser-McCartha, 1995; Okuhara et al., 1996; Kozhevnikov, 1998, 2002; Mizuno and Misono, 1998; Moffat, 2001).
Several recent reviews have been published covering applications of POMs in heterogeneous catalysis (Long et al., 2007; Nlate Jahier and Jahier, 2012; Ren et al., 2015; Wang and Yang, 2015; Patel et al., 2016). One area of significant interest in POM-based materials has been the incorporation of POMs into metal-organic frameworks (MOFs) (Du et al., 2014). MOFs have attained a very high profile in the field of heterogeneous materials, as their high surface area and modifiable topologies allow for the design of a variety of very active functional materials (Corma et al., 2010; Furukawa et al., 2013). Combining the versatility of function of POMs with the high surface area of MOFs has led to several interesting and highly active materials. Song et al. reported the synthesis and characterization of a MOF (MOF-199) containing the Keggin-type POM [CuPW11O39]5− within its pores. This POM-MOF exhibited substantially increased catalytic aerobic oxidation of sulfides and thiols to deodorized products compared to the POM or the MOF alone (Song et al., 2011). Ma et al. carried out a similar study in which a POM [PW12O40]3− was used as a template during the synthesis of the MOF (NENU-11), aiding in its formation during a simple one-step hydrothermal synthesis. The resulting POM-MOF was then shown to be active towards the adsorption and hydrolysis of dimethyl methylphosphonate, an analog of organophosphonate chemical warfare agents, demonstrating synergistic roles of both the POM and the MOF in the composite material (Ma et al., 2011).
As mentioned previously, POM organic/inorganic hybrids are highly attractive for developing processable materials that incorporate the functionality of POMs. Research on POM organic/inorganic hybrids has developed into an extensive field with applications in many disciplines (Zonnevijlle and Pope, 1979; Gouzerh and Proust, 1998; Qi and Wu, 2009; Dolbecq et al., 2010; Proust et al., 2012; Song and Tsunashima, 2012). These include POM-modified organic/inorganic nanocomposites for energy applications (which will be discussed in greater depth later in this article)(Genovese and Lian, 2015; Ji et al., 2015), green synthesis(Omwoma et al., 2015; Zhou et al., 2015), photochemical and electrochemical properties (Walsh et al., 2016), environmental remediation (Sivakumar et al., 2012), among others. These POM materials often exhibit multiple synergistic functions. For example, Haimov et al. reported the synthesis of a cross-linked polyethyleneimine polymer containing [ZnWZn2(H2O)2(ZnW9O34)2]12− POMs. The hydrophilic domains of the polymer contained both the POMs and the 2-alkanol substrates, and the lipophilic domain affects the solubility of the substrate in the hydrophilic domain. When the material was used to catalyze the hydrogen peroxide-based oxidation of the 2-alkanol substrates to the corresponding ketones, the material had the dual role of enhancing the reaction rate by bringing the POM and substrate in closer proximity and imparting a liposelectivity component to the reaction rate as a function of the hydrophobic nature of the substrate (Haimov and Neumann, 2006).
Another class of POM-based materials that warrant note are POMs intercalated into layered double hydroxides (LDHs). POM-LDH systems date back to Pinnavaia's early studies (Kwon et al., 1988), and have expanded to become an active field within POM materials chemistry (Omwoma et al., 2014; Li T. et al., 2017). An example by Zhao et al. demonstrates the synergistic effect of intercalating various sandwich-structure POMs into LDHs. They tested these materials on the mild and solvent-free oximation of aromatic aldehydes by the POM and observed substantial selectivity enhancements due to the ability of the LDH to suppress the formation of byproducts. Additionally, the heterogeneous support facilitated easy recovery and reuse of the material (Zhao S. et al., 2011).
Many types of POMs lend themselves to energy applications, such as energy storage and solar fuel generation. The ability of certain categories of POMs (most polytungstates, polymolybdates, and polyvanadates) to be reduced by many electrons, first dramatically noted in a study by Launay and co-workers (Launay, 1976) has led to intriguing studies of POM-based batteries and energy storage assemblies (Wang et al., 2012; Pratt et al., 2013; Genovese and Lian, 2015). One study published by Suárez-Guevara et al. uses electrodes made from activated carbon and the POM, H3PW12O40, in which the POM exhibits multiple functions: it increases the capacitance, operating voltage, and energy density of the battery. Additionally, the POM protects the activated carbon electrode from oxidation, allowing for the cell to have a high capacitance retention after a large number of charge-discharge cycles (Suárez-Guevara et al., 2014). An example of an energy application in which a POM has four planned and realized functions is a POM-Pt-MOF material that facilitates visible-light-driven catalytic H2 evolution and does this far more effectively than any of the 3 components alone. In this material, the POMs (1) catalyze reduction of platinum salts to Pt(0) nanoparticles, (2) stabilize the Pt nanoparticles and prevent them from aggregating, (3) induce a strong electrostatic association of the negatively charged Pt NPs with the protonated NH2-MIL-53 sites on the MOF particle surfaces, and (d) helps catalyze the H2 evolution reaction (Guo et al., 2016).
We now focus on two types of POM-based materials that illustrate the broad utility landscape of such materials. One involves the incorporation of a POM into the main chain of a polymer, resulting in a material which sequesters and decontaminates toxic or odorous compounds. The other involves immobilization of POM catalysts in photoelectrode assemblies in which the POM undergoes multiple photoinduced electron transfer processes and also carries the many steps involved in the oxidation of water molecules to O2.
Pom-Containing Network Materials That Physically Entrap and Catalyze Degradation
Very recently we reported the design and synthesis of a POM-based polymer that demonstrates the advantages of combining the rich chemistry of POMs with properties obtained from incorporating the POM unit into a material. We synthesized and characterized a polymer composed of hexavanadate (V6O19) POM units and 1,3,5-benzenetricarboxamide-based linkers (trisBTA), with the formula [(n-C4H9)4N]2n[(V6O13)n[((OCH2)3CNHCO)3C6H3]x[((OCH2)3- CNHCO)2((HOCH2)3CNHCO)C6H3]y[(OCH2)3CNHCO ((HOCH2)3CNHCO) 2C6H3] z] (TBA-polyV6, x = triply bound trisBTA, y = doubly-bound trisBTA, z = singly-bound trisBTA) (Figure 2)(Sullivan et al., 2017). This class of materials exhibits several functions: it forms gels within seconds after contact with polar aprotic organic liquids, catalyzes the oxidative or hydrolytic degradation of toxins and odorants under mild conditions, and exhibits color change during select oxidation reactions. This combination of properties was made possible through the incorporation of the POMs (capable of catalyzing oxidation and hydrolysis) and the organic linkers to form a polymeric material. The design of this polymer material from modular components allowed us to tailor the multiple functions to be useful for the entrapment and removal of toxic substances, including chemical warfare agent (CWA) analogs.
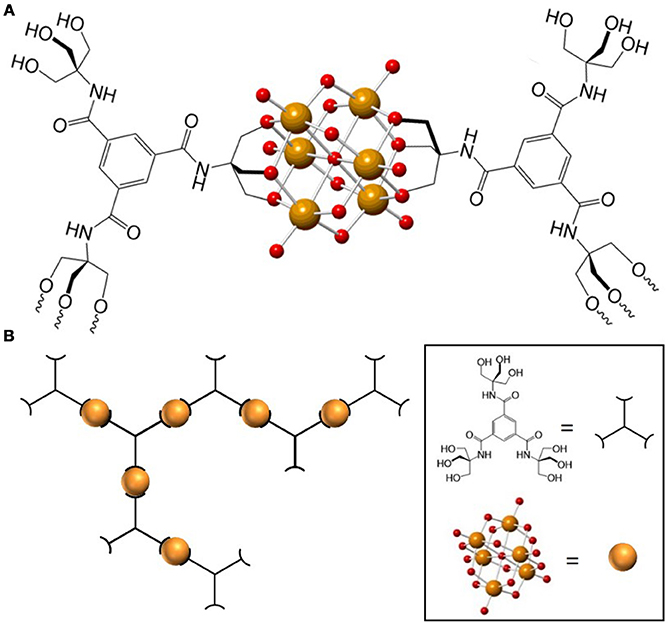
Figure 2. (A) Representation of a monomeric TBA-polyV6 unit. (B) Representation of the TBA-polyV6 polymer. Orange and red spheres represent V(V) and O2−, respectively. Reproduced from (Sullivan et al., 2017) with permission from the Royal Society of Chemistry.
Polyoxometalate-organic hybrid species can oxidize a variety of organic substrates (Dolbecq et al., 2010), with hexavanadates demonstrating particular activity for oxidation of sulfides (Hill et al., 2006; Han and Hill, 2007). Thus, redox-active TBA-polyV6 facilitates color-change detection and oxidative decontamination. To demonstrate the applicability of TBA-polyV6 for air-based oxidative removal reactions, we conducted studies on the catalytic oxidation of 1-propanethiol (PrSH), as thiols are a major class of odorants in human environments. This representative thiol is fully oxidized to the corresponding non-odorous disulfide, and oxygen reoxidizes the reduced POM units (Figure 3a). The material is red in its powder state and reddish-orange when dispersed in solvent, indicative of the oxygen-to-metal charge transfer absorption manifold of a fully-oxidized [O13(OR)6)]2− core (R = trisBTA linkers). Upon reduction of the POM, TBA-polyV6 becomes dark green as a broad peak between 600 and 900 nm increases, attributed to intervalence charge-transfer bands in the reduced POM (Chen et al., 1992). The persistent observation of a green reduced hexavanadate species during the reaction provides colorimetric detection capabilities for this polymeric material (Figure 3). In addition to aerobic oxidation of thiols, TBA-polyV6 catalyzes the oxidation of sulfides by hydrogen peroxide, including 2-chloroethyl ethyl sulfide, an analog of the CWA sulfur mustard. The sulfide is completely oxidized within 30 min after adding H2O2, whereas a reaction run without catalyst requires multiple hours to go to completion.
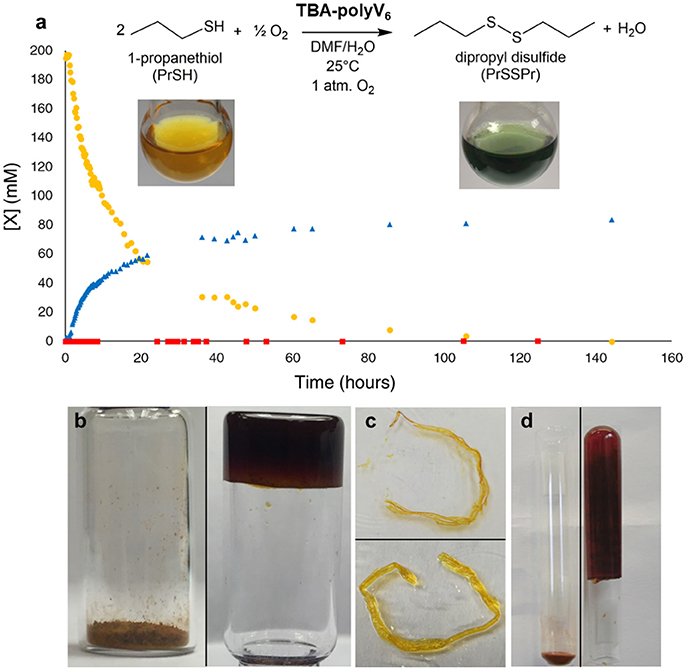
Figure 3. Aerobic oxidation catalyzed by TBA-polyV6. (a) Oxidation of 1-propanethiol () to dipropyl disulfide () catalyzed by TBA-polyV6. The molar ratio was 127 1-propanethiol: 1 V6. A control reaction () was run under identical conditions but without TBA-polyV6. Inset: A yellow dispersion of TBA-polyV6 in DMF before (left) and after (right) addition of 1-propanethiol (Figure 4.) (b) Swelling of TBA-polyV6 (left) in the presence of dimethyl methyl phosphonate (DMMP) (right). (c) A strand of TBA-polyV6 before (above) and after (below) addition of DMSO. (c) Swelling of TBA-polyV6 (left) in the presence of DMF (right). (d) Swelling behavior measured in mL of liquid per gram of material after 24-hour exposure. Reproduced from (Sullivan et al., 2017) with permission from the Royal Society of Chemistry.
Recent studies have demonstrated both polyoxometalate-catalyzed hydrolysis of phosphoester bonds as well as hydrogen bond donor-catalyzed hydrolysis of organophosphate (OP) CWAs (Steens et al., 2010; Barba-Bon et al., 2013; Sambrook and Notman, 2013; Kinnan et al., 2014). Additionally, Zr-based MOFs and POMs have received a great deal of attention for their high activities toward hydrolysis of organophosphates, including OP nerve agents (Mondloch et al., 2015; Luong et al., 2016; Collins-Wildman et al., 2018). To demonstrate that TBA-polyV6 is highly modular in nature, we synthesized a new polymer through simple cation exchange with zirconyl chloride, affording Zr-polyV6, which exhibits high activity for catalytic hydrolysis of dimethyl p-nitrophenylphosphate (DMNP). We again show how the chemical and physical properties of the multifunctional POM-based polymer can be readily tuned to achieve targeted applications.
After demonstrating that the catalytic capabilities of the POM units remain intact in the heterogeneous material, we examined the gelation capabilities of the polymers. Studies of compounds containing 1,3,5-benzenetricarboxamides have shown them to be capable of forming gels through the presence of extensive hydrogen-bonding networks through the amide units, as well as π-stacking between adjacent aromatic groups (Sambrook and Notman, 2013). This property is preserved in TBA-polyV6, demonstrated by organogelation resulting from the addition of polar aprotic liquids to the material (Figures 3b–d). Significantly, we observed that TBA-polyV6 can form gels when exposed to OP agent analogs. Addition of the nerve agent analog dimethyl methyl phosphonate DMMP results in immobilization of a substantial amount of the liquid with rapid swelling kinetics. We demonstrate, therefore, that incorporating multiple functionalities to this POM-based polymer has allowed us to develop the first examples of materials that are potentially capable of both immobilizing and decontaminating CWAs.
Heterogeneous Polyoxometalate Water Oxidation Catalysts (WOCs) and Their Use in Photoelectrocatalytic Water Splitting
The applications of POMs or POM-based materials to solar fuels (artificial photosynthesis) is now a substantial category by itself. POMs have been examined in several functional roles for solar fuel generation. The first of these is broad-spectrum and intense visible light absorption with charge separation. Some POM derivatives have high extinction coefficients for charge transfer absorption (Zhao C. et al., 2011; Zhao et al., 2013), and while the charge-transfer excited-state lifetimes are highly variable, most are too short to result in high-quantum-yield chemical capture. However, POMs are quite active as catalysts for multi-electron reduction (reduction of H2O to H2 or CO2 to carbon-based fuel molecules)(Wang et al., 2016; Gumerova and Rompel, 2018). Several POMs that are efficient water reduction catalysts under visible-light-driven or dark conditions have been reported (Lv et al., 2014, 2015, 2016), and some POMs facilitate CO2 reduction under appropriate conditions (Ettedgui et al., 2010; Wang et al., 2016). One of the primary applications that has been the subject of extensive research within the field of POMs has been the catalysis of water oxidation. Since the original reports of a POM water oxidation catalyst (WOC), namely the tetra-ruthenium sandwich polytungstate, [{Ru4O4(OH)2(H2O)4}(γ-SiW10O36)2]10− (Ru4Si2)(Geletii et al., 2008; Sartorel et al., 2008), and the first publication on a POM WOC of all earth-abundant elements (Yin et al., 2010), there have been scores of papers on POM WOCs (Lauinger et al., 2017b; Li J. et al., 2017).
The issue of the true active catalyst species is always one of great interest in any catalytic system (Vickers et al., 2013; Wu et al., 2015). Polyoxometalate WOCs have been of particular concern in this regard (Stracke and Finke, 2011, 2014; Vickers et al., 2013; Folkman and Finke, 2017). This is due to the fact that POMs are essentially molecular metal oxides and could potentially have extensive speciation equilibria in solution (Sumliner et al., 2014; Sara et al., 2015; Lauinger et al., 2017b; Nyman, 2017). Some of the species that could be present include the metal aqua complexes as well as the corresponding metal oxide nanoparticles, all of which could also be active as WOCs (Stracke and Finke, 2014; Sumliner et al., 2014; Folkman and Finke, 2017; Lauinger et al., 2017b; Suen et al., 2017). It is therefore essential that any POM species is demonstrated to be stable and active under experimental conditions. POMs have been immobilized on photoanodes to make photocatalytic water oxidizing electrodes, allowing careful tailoring of POMs during their synthesis to be combined with the stabilizing effects of a heterogeneous material. Heterogeneous systems involving POMs are generally more stable than their molecular counterparts due to the limiting of solution phase equilibria involving the POM, preventing speciation that could lead to a shift in the composition of the catalytic POM cluster (Lauinger et al., 2017b). Heterogeneous WOCs are also advantageous because both half reactions involved in water splitting are spatially separated, preventing product recombination or the collection of an explosive mixture of hydrogen and oxygen. In addition, heterogeneous architectures enable efficient charge transfer and separation between photosensitizer and catalyst. In a homogeneous setup, the two components must diffuse to one another leaving time for charge recombination or breakdown of unstable intermediates, lowering the overall system efficiency.
In general, studies of POMs immobilized on photoelectrodes are of three types: electrostatic binding to a surface-bound photosensitizer dye, electrostatic immobilization on semiconductor photoanodes, and partial encapsulation of POM WOCs on photoanodes via metal oxide nanofilm deposition. Studies done by Xiang et. al. used ultrafast transient absorption spectroscopy to probe electron transfer between the Ru4Si2 WOC, photosensitizer dye [Ru(bpy)2(dpbpy)]2+, and various metal oxide electrode surfaces (Figure 4) (Xiang et al., 2013). A similar study was also reported by Bonchio, Scandola and co-workers in which illumination of the POM-dye-metal oxide triad resulted in a rapid (10 ns) electron injection which occurs from the catalyst to sensitizer, generating a long lived (0.5 μs) charge-separated state (Orlandi et al., 2010). This proved to be an effective strategy for water oxidation as Fielden et. al. later demonstrated with oxygen measurements on a similar triad (Fielden et al., 2015), however, a limitation in both cases was the stability of the dye on the surface.
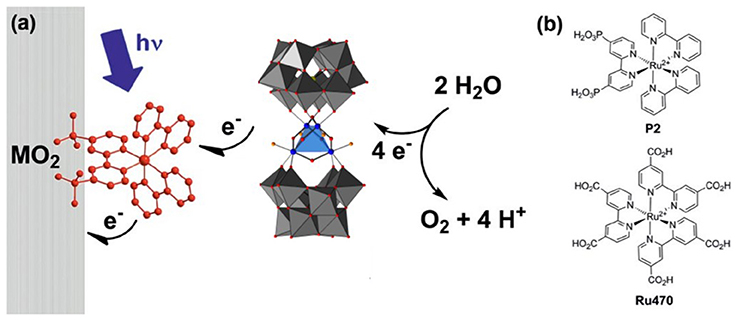
Figure 4. (a) Principle of operation of a triadic water-oxidizing photoanode incorporating both Ru4Si2 and a dye. (b) Structures of the dyes which have been used in triads with Ru4Si2 (Xiang et al., 2013). Adapted with permission from J. Phys Chem. C., 2013, 117 (2), pp 918–926. Copyright 2013, American Chemical Society.
Several studies have focused on direct binding of the POM to photoactive metal oxides, such as TiO2 and hematite. Recently, our group published a report in which we treated TiO2 with 3-aminopropyltrimethoxysilane (APS) to generate a cationic surface that strongly bound the highly negatively charged POM WOC, Ru4Si2 (Lauinger et al., 2015). This was effective for surface immobilization of the POM and light driven water oxidation. To improve this system by moving the observed catalysis from the UV into the visible range, we examined surface treatment of hematite with Ru4Si2 as the POM WOC to achieve visible-light-driven water oxidation (Lauinger et al., 2017a). In this system, atomic layer deposition (ALD) of Al2O3 was used to provide stabilization through partial encapsulation of the POM. The thickness of the ALD layer was optimized to prevent desorption of the catalyst without greatly reducing the efficiency of electron injection from Ru4Si2 into the hematite electrode (Figure 5).
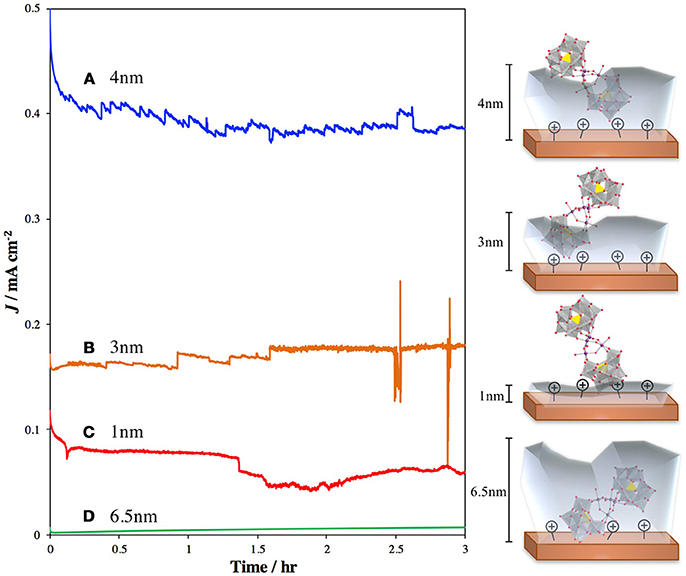
Figure 5. Current densities and schematic illustrations of photoanodes composed of hematite–APS–Ru4Si2-Al2O3 atomic layer deposition thickness of (A) 4 nm (blue line); (B) 3 nm Al2O3 (orange line); (C) 1 nm Al2O3 (red line); and (D) 6.5 nm Al2O3 (green line) (Lauinger et al., 2017a). The translucent gray layers represent the depth of the Al2O3 coating.Reprinted with permission from ACS Appl. Mater. Interfaces, 2017 9 (40), 35048–35056. Copyright 2017, American Chemical Society.
Perhaps most impressive of recent efforts to turn known homogeneous POM WOCs into functional heterogeneous electrocatalysts involves the immobilization of a septa-cobalt POM into a graphite matrix using a carbon paste electrode (Blasco-Ahicart et al., 2017). By formulating the POM cluster in combination with Ba2+ and a stable graphite matrix, Blasco-Ahicart et al. observed a dramatic synergistic effect of both increased stability and catalytic performance. This was the first reported example of a stable discrete complex with earth-abundant elements for water oxidation in strongly acidic media. At certain current densities, this heterogeneous composition even manages to have a lower overpotential than the long-running state-of-the-art iridium oxide and ruthenium oxide WOC films. Having a concrete understanding of how this type of synergistic effect between the hydrophobic carbon paste framework and the POM occurs could yield substantial improvements in the WOC field (Yin and Hill, 2017).
Conclusions
The field of POM-based materials has been expanding rapidly and is likely to remain fruitful as ever more applications are explored. The ease of tunability and high activity of POMs combined with the processability, stability, or other physical advantages of heterogeneous systems allows for extraordinary versatility in application of these materials. These efforts have led many scientists traditionally working on homogeneous systems towards materials chemistry, and vice versa. Thus, there is an imperative to combine the vast knowledge base of each of these fields and foster collaboration to aid in the production of high quality research towards the development and characterization of new hybrid POM systems.
Author Contributions
KS and CH oversaw all writing. QY, DC-W, MT, YG all contributed to the writing of the WOC section. DM and TL provided input.
Funding
The multi-functional POM-based polymer work was funded by the Defense Threat Reduction Agency (grant # HDTRA1-16-1-0029), and the catalytic water oxidation work was funded by the U.S. Department of Energy, Office of Basic Energy Sciencies, Solar Photochemistry program (grant # DE-FG02-07ER15906).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Barba-Bon, A., Costero, A. M., Parra, M., Gil, S., Martínez-Máñez, R., Sancenón, F., et al. (2013). Neutral 1,3-diindolylureas for nerve agent remediation. Chem. Eur. J. 19, 1586–1590. doi: 10.1002/chem.201202028
Blasco-Ahicart, M., Soriano-López, J., Carbó, J. J., Poblet, J. M., and Galan-Mascaros, J. R. (2017). Polyoxometalate electrocatalysts based on earth-abundant metals for efficient water oxidation in acidic media. Nat. Chem. 10, 24–30. doi: 10.1038/nchem.2874
Borrás-Almenar, J. J., Clemente-Juan, J. M., Clemente-Leon, M., Coronado, E., Galán-Mascarós, J. R., and Gómez-Garcia, C. J. (2001). “Molecular materials from polyoxometalates,” in Polyoxometalate Chemistry From Topology Via Self-Assembly to Applications, eds M. T. Pope and A. Müller (Dordrecht: Kluwer), 231.
Carraro, M., and Gross, S. (2014). Hybrid materials based on the embedding of organically modified transition metal oxoclusters or polyoxometalates into polymers for functional applications a review. Materials 7, 3956–3989. doi: 10.3390/ma7053956
Chen, Q., Goshorn, D. P., Scholes, C. P., Tan, X. L., and Zubieta, J. (1992). Coordination compounds of polyoxovanadates with a hexametalate core. Chemical and structural characterization of [O13{(OCH2)3CR}2]2−, [O11(OH)2{(OCH2)3CR}2], [O9(OH)4{(OCH2)3CR}2]2−, and [O7(OH)6{(OCH2)3CR}2]2−. J. Am. Chem. Soc. 114, 4667–4681. doi: 10.1021/ja00038a033
Collins-Wildman, D. L., Kim, M., Sullivan, K. P., Plonka, A. M., Frenkel, A. I., Musaev, D. G., et al. (2018). Buffer-induced acceleration and inhibition in polyoxometalate-catalyzed organophosphorus ester hydrolysis. ACS Catal. 8, 7068–7076. doi: 10.1021/acscatal.8b00394
Corma, A., García, H., and Llabrés i Xamena, F. X. (2010). Engineering metal organic frameworks for heterogeneous catalysis. Chem. Rev. 110, 4606–4655. doi: 10.1021/cr9003924
Coronado, E., and Gómez-García, C. J. (1995). Polyoxometalates From magnetic clusters to molecular materials. Comments Inorg. Chem. 17, 255–281. doi: 10.1080/02603599508032707
Dolbecq, A., Dumas, E., Mayer, C. R., and Mialane, P. (2010). Hybrid organic-inorganic polyoxometalate compounds from structural diversity to applications. Chem. Rev. 110, 6009–6048. doi: 10.1021/cr1000578
Du, D. Y., Qin, J. S., Li, S. L., Su, Z. M., and Lan, Y. Q. (2014). Recent advances in porous polyoxometalate-based metal–organic framework materials. Chem. Soc. Rev. 43, 4615–4632. doi: 10.1039/C3CS60404G
Ettedgui, J., Diskin-Posner, Y., Weiner, L., and Neumann, R. (2010). Photoreduction of carbon dioxide to carbon monoxide with hydrogen catalyzed by a rhenium(I) phenanthroline–polyoxometalate hybrid complex. J. Am. Chem. Soc. 133, 188–190. doi: 10.1021/ja1078199
Fielden, J., Sumliner, J. M., Han, N., Geletii, Y. V., Xiang, X., Musaev, D. G., et al. (2015). Water splitting with polyoxometalate-treated photoanodes enhancing performance through sensitizer design. Chem. Sci. 6, 5531–5543. doi: 10.1039/C5SC01439E
Folkman, S. J., and Finke, R. G. (2017). Electrochemical water oxidation catalysis beginning with Co(II) polyoxometalates the case of the precatalyst Co4V2W18O6810. ACS Catal. 7, 7–16. doi: 10.1021/acscatal.6b
Furukawa, H., Cordova, K. E., O'Keeffe, M., and Yaghi, O. M. (2013). The chemistry and applications of metal-organic frameworks. Science 341:1230444. doi: 10.1126/science.1230444
Geletii, Y. V., Botar, B., Kögerler, P., Hillesheim, D. A., Musaev, D. G., and Hill, C. L. (2008). An all-inorganic, stable, and highly active tetraruthenium homogeneous catalyst for water oxidation. Selected as the VIP Article by the reviewers and editor. Angew. Chem. Int. Ed. 47, 3896–3899. doi: 10.1002/anie.200705652
Genovese, M., and Lian, K. (2015). Polyoxometalate modified inorganic–organic nanocomposite materials for energy storage applications: a review. Curr. Opin. Solid State Mater. Sci. 19, 126–137. doi: 10.1016/j.cossms.2014.12.002
Gouzerh, P., and Proust, A. (1998). Main-group element, organic, and organometallic derivatives of polyoxometalates. Chem. Rev. 98, 77–111. doi: 10.1021/cr960393d
Grigoriev, V. A., Cheng, D., Hill, C. L., and Weinstock, I. (2001). A. role of alkali metal cation size in the energy and rate of electron transfer to solvent-separated 1:1 [(M+)(Acceptor)] (M+) Li+, Na+, K+) ion pairs. J. Am. Chem. Soc. 123, 5292–5307. doi: 10.1021/ja010074q
Grigoriev, V. A., Hill, C. L., and Weinstock, I. (2000). A. role of cation size in the energy of electron transfer to 1:1 polyoxometalate ion pairs {(M+)(Xn+VW11O40)}(8−n)−(M = Li, Na,K). J. Am. Chem. Soc. 122, 3544–3545. doi: 10.1021/ja993862c
Gumerova, N. I., and Rompel, A. (2018). Synthesis, structures and applications of electron-rich polyoxometalates. Nat. Rev. Chem. 2, 1–20. doi: 10.1038/s41570-018-0112
Guo, W., Lv, H., Chen, Z., Sullivan, K. P., Lauinger, S. M., Chi, Y., et al. (2016). Self-assembly of polyoxometalates, Pt nanoparticles and metal-organic frameworks into a hybrid material for synergistic hydrogen evolution. J. Mater. Chem. A 4, 5952–5957. doi: 10.1039/C6TA00011H
Haimov, A., and Neumann, R. (2006). An example of lipophiloselectivity the preferred oxidation, in water, of hydrophobic 2-Alkanols catalyzed by a cross-linked polyethyleneimine-polyoxometalate catalyst assembly. J. Am. Chem. Soc. 128, 15697–15700. doi: 10.1021/ja064294l
Han, J. W., and Hill, C. L. (2007). A coordination network that catalyzes O2-based oxidations. J. Am. Chem. Soc. 129, 15094–15095. doi: 10.1021/ja069319v
He, W. W., Li, S. L., Zang, H. Y., Yang, G. S., Zhang, S. R., Su, Z. M., et al. (2014). Entangled structures in polyoxometalate-based coordination polymers. Coord. Chem. Rev. 279, 141–160. doi: 10.1016/j.ccr.2014.03.022
Hill, C. L. (1998). Introduction polyoxometalates—multicomponent molecular vehicles to probe fundamental issues and practical problems. Chem. Rev. 98, 1–2. doi: 10.1021/cr960395y
Hill, C. L. (2004). “Polyoxometalates reactivity,” in Comprehensive Coordination Chemistry-II From Biology to Nanotechnology Comprehensive Coordination Chemistry II, eds A. G. Wedd (Oxford: Elsevier Ltd) 4, 679–759. doi: 10.1002/chin.200440228
Hill, C. L., Anderson, T. M., Han, J., Hillesheim, D. A., Geletii, Y. V., Okun, N. M., et al. (2006). New complexes and materials for O2-based oxidations. J. Mol. Catal. A Chem. 251, 234–238. doi: 10.1016/j.molcata.2006.02.046
Hill, C. L., and Kholdeeva, O. A. (2013). “Selective liquid phase oxidations in the presence of supported polyoxometalates,” in Liquid Phase Oxidation via Heterogeneous Catalysis Organic Synthesis and Industrial Applications, eds M. G. Clerici, and O. A. Kholdeeva (Hoboken, NJ: John Wiley and Sons, Inc), 263–319.
Hill, C. L., and Prosser-McCartha, C. M. (1995). Homogeneous catalysis by transition metal oxygen anion clusters. Coord. Chem. Rev. 143, 407–455.
Hu, M.-B., Xia, N., Yu, W., Ma, C., Tang, J., Hou, Z.-Y., et al. (2012). A click chemistry approach to the efficient synthesis of polyoxometalate–polymer hybrids with well-defined structures. Polym. Chem. 3:617. doi: 10.1039/c2py00546h
Ji, Y., Huang, L., Hu, J., Streb, C., and Song, Y.-F. (2015). Polyoxometalate-functionalized nanocarbon materials for energy conversion, energy storage and sensor systems. Energy Environ. Sci. 8, 776–189. doi: 10.1039/C4EE03749A
Kinnan, M. K., Creasy, W. R., Fullmer, L. B., Schreuder-Gibson, H. L., and Nyman, M. (2014). Nerve agent degradation with polyoxoniobates. Eur. J. Inorg. Chem. 2014, 2361–2367. doi: 10.1002/ejic.201400016
Kozhevnikov, I. V. (1998). Catalysis by heteropoly acids and multicomponent polyoxometalates in liquid-phase reactions. Chem. Rev. 98, 171–198. doi: 10.1021/cr960400y
Kwon, T., Tsigdinos, G. A., and Pinnavaia, T. J. (1988). Pillaring of layered double hydroxides (LDH's) by polyoxometalate anions. J. Am. Chem. Soc. 110, 3653–3654. doi: 10.1021/ja00219a048
Lauinger, S. M., Piercy, B. D., Li, W., Yin, Q., Collins-Wildman, D. L., Glass, E. N., et al. (2017a). Stabilization of polyoxometalate water oxidation catalysts on hematite by atomic layer deposition. ACS Appl. Mater. Interfaces 9, 35048–35056. doi: 10.1021/acsami.7b12168
Lauinger, S. M., Sumliner, J. M., Yin, Q., Xu, Z., Liang, G., Glass, E. N., et al. (2015). High stability of immobilized polyoxometalates on TiO2 nanoparticles and nanoporous films for robust, light-induced water oxidation. Chem. Mater. 27, 5886–5891. doi: 10.1021/acs.chemmater.5b01248
Lauinger, S. M., Yin, Q., Geletii, Y. V., and Hill, C. L. (2017b). “Polyoxometalate multielectron catalysts in solar fuel production,” in Advances in Inorganic Chemistry Polyoxometallate Chemistry, 1st Edn, eds L. Cronin and R. V. Eldik (Oxford: Elsevier), 69, 117–154.
Launay, J. P. (1976). Reduction of the metatungstate ion. high levels of reduction of H2W12, derivatives of the ion HW12, and general discussion. J. Inorg. Nucl. Chem. 38, 807–816. doi: 10.1016/0022-1902(76)80361-2
Li, J., Güttinger, R., Moré, R., Song, F., Wan, W., and Patzke, G. R. (2017). Frontiers of water oxidation the quest for true catalysts. Chem. Soc. Rev. 46, 6124–6147. doi: 10.1039/C7CS00306D
Li, T., Miras, H. N., and Song, Y.-F. (2017). Polyoxometalate (POM)-layered double hydroxides (LDH) composite materials design and catalytic applications. Catalysis 7:260. doi: 10.3390/catal7090260
Long, D. L., Burkholder, E., and Cronin, L. (2007). Polyoxometalate clusters, nanostructures and materials: from self assembly to designer materials and devices. Chem. Soc. Rev. 36, 105–121. doi: 10.1039/B502666K
Long, D. L., Tsunashima, R., and Cronin, L. (2010). Polyoxometalates building blocks for functional nanoscale systems. Angew. Chem. Int. Ed. 49, 1736–1758. doi: 10.1002/anie.200902483
Luong, T. K. N., Shestakova, P., Absillis, G., and Parac-Vogt, T. N. (2016). Detailed mechanism of phosphoanhydride bond hydrolysis promoted by a binuclear ZrIV-substituted keggin polyoxometalate elucidated by a combination of 31P, 31P DOSY, and 31P EXSY NMR spectroscopy. Inorg. Chem. 55, 4864–4873. doi: 10.1021/acs.inorgchem.6b00385
Lv, H., Chi, Y., Leusen, J. V., Kögerler, P., Chen, Z., Bacsa, J., et al. (2015). [{Ni4(OH)3AsO4}4(B-α-PW9O34)4]28−− A new polyoxometalate structural family with catalytic hydrogen evolution activity. Chem. Eur. J. 21, 17363–17370. doi: 10.1002/chem.201503010
Lv, H., Gao, Y., Guo, W., Lauinger, S. M., Chi, Y., Bacsa, J., et al. (2016). A Cu-based polyoxometalate catalyst for efficient catalytic hydrogen evolution. Inorg. Chem. 55, 6750–6758. doi: 10.1021/acs.inorgchem.6b01032
Lv, H., Guo, W., Wu, K., Chen, Z., Bacsa, J., Musaev, D. G., et al. (2014). A noble-metal-free, tetra-nickel polyoxotungstate catalyst for efficient photocatalytic hydrogen evolution. J. Am. Chem. Soc. 136, 14015–14018. doi: 10.1021/ja5084078
Ma, F. J., Liu, S. X., Sun, C. Y., Liang, D. D., Ren, G. J., Wei, F., et al. (2011). A sodalite-type porous metal-organic framework with polyoxometalate templates: adsorption and decomposition of dimethyl methylphosphonate. J. Am. Chem. Soc. 133, 4178–4181. doi: 10.1021/ja109659k
Macdonell, A., Johnson, N. A., Surman, A. J., and Cronin, L. (2015). Configurable nanosized metal oxide oligomers via precise “Click” coupling control of hybrid polyoxometalates. J. Am. Chem. Soc. 137, 5662–5665. doi: 10.1021/jacs.5b02466
Miras, H. N., Yan, J., Long, D. L., and Cronin, L. (2012). Engineering polyoxometalates with emergent properties. Chem. Soc. Rev. 41, 7403–7430. doi: 10.1039/c2cs35190k
Mizuno, N., and Misono, M. (1998). Heterogeneous catalysis. Chem. Rev. 98, 199–218. doi: 10.1021/cr960401q
Moffat, J. B. (2001). Metal-Oxygen Clusters The Surface and Catalytic Properties of Heteropoly Oxometalates. New York, NY: Kluwer Academic/Plenum Publishers.
Mondloch, J. E., Katz, M. J. III., William, C. I. III., Ghosh, P., Liao, P., Bury, W., et al. (2015). Destruction of chemical warfare agents using metal–organic frameworks. Nat. Mater. 14, 512–516. doi: 10.1038/nmat4238
Nlate, S., and Jahier, C. (2012). Dendritic polyoxometalate hybrids: efficient and recoverable catalysts for oxidation reactions. Eur. J. Inorg. Chem. 2013, 1606–1619. doi: 10.1002/ejic.201201129
Nyman, M. (2017). Small-angle X-ray scattering to determine solution speciation of metal-oxo clusters. Coord. Chem. Rev. 352, 461–472. doi: 10.1016/j.ccr.2016.11.014
Okuhara, T., Mizuno, N., and Misono, M. (1996). Catalytic chemistry of heteropoly compounds. Adv. Catal. 41, 113–252. doi: 10.1016/S0360-0564(08)60041-3
Omwoma, S., Chen, W., Tsunashima, R., and Song, Y.-F. (2014). Recent advances on polyoxometalates intercalated layered double hydroxides from synthetic approaches to functional material applications. Coord. Chem. Rev. 258–259, 58–71. doi: 10.1016/j.ccr.2013.08.039
Omwoma, S., Gore, C. T., Ji, Y., Hu, C., and Song, Y.-F. (2015). Environmentally benign polyoxometalate materials. Coord. Chem. Rev. 286, 17–29. doi: 10.1016/j.ccr.2014.11.013
Orlandi, M., Argazzi, R., Sartorel, A., Carraro, M., Scorrano, G., Bonchio, M., et al. (2010). Ruthenium polyoxometalate water splitting catalyst very fast hole scavenging from photogenerated oxidants. Chem. Commun. 46, 3152–3154. doi: 10.1039/b926823e
Patel, A., Narkhede, N., Singh, S., and Pathan, S. (2016). Keggin-type lacunary and transition metal substituted polyoxometalates as heterogeneous catalysts: a recent progress. Catal. Rev. 58, 337–370. doi: 10.1080/01614940.2016.1171606
Pope, M. T. (2004). “Polyoxo anions synthesis and structure,” in Comprehensive Coordination Chemistry II From Biology to Nanotechnology Comprehensive Coordination Chemistry II, ed A. G. Wedd (Oxford: Elsevier Ltd), 635–678.
Pratt, H. D. III., Hudak, N. S., Fang, X., and Anderson, T. M. (2013). A polyoxometalate flow battery. J. Pow. Sour. 236, 259–264. doi: 10.1016/j.jpowsour.2013.02.056
Proust, A., Matt, B., Villanneau, R., Guillemot, G., Gouzerh, P., and Izzet, G. (2012). Functionalization and post-functionalization a step towards polyoxometalate-based materials. Chem. Soc. Rev. 41, 7605–7622. doi: 10.1039/c2cs35119f
Qi, W., and Wu, L. (2009). Polyoxometalate / polymer hybridmaterials fabrication and properties. Polym. Int. 58, 1217–1225. doi: 10.1002/pi.2654
Ren, Y., Wang, M., Chen, X., Yue, B., and He, H. (2015). Heterogeneous catalysis of polyoxometalate based organic–inorganic hybrids. Materials 8, 1545–1567. doi: 10.3390/ma8041545
Rieger, J., Antoun, T., Lee, S. H., Chenal, M., Pembouong, G., de la Haye, J., et al. (2012). Synthesis and characterization of a thermorespons ive polyoxometalate–polymer hybrid. Chem. Eur. J. 18, 3355–3361. doi: 10.1002/chem.201101771
Sambrook, M. R., and Notman, S. (2013). Supramolecular chemistry and chemical warfare agents from fundamentals of recognition to catalysis and sensing. Chem. Soc. Rev. 42, 9251–9267. doi: 10.1039/c3cs60230c
Sara, G.-F., Joaquín, S.-L., Ramón, G.-M. J., and May, N. (2015). Solution speciation and stability of cobalt-polyoxometalate water oxidation catalysts by X-ray scattering. Eur. J. Inorg. Chem. 2015, 2833–2840. doi: 10.1002/ejic.201500404
Sartorel, A., Carraro, M., Scorrano, G., Zorzi, R. D., Geremia, S., McDaniel, N. D., et al. (2008). Polyoxometalate embedding of a tetraruthenium(IV)-oxo-core by template-directed metalation of [γ-SiW10O36]8− a totally inorganic oxygen-evolving catalyst. J. Am. Chem. Soc. 130, 5006–5007. doi: 10.1021/ja077837f
Sivakumar, R., Thomas, J., and Yoon, M. (2012). Polyoxometalate-based molecular/nano composites: advances in environmental remediation by photocatalysis and biomimetic approaches to solar energy conversion. J. Photochem. Photobiol. C Photochem. Rev. 13, 277–298. doi: 10.1016/j.jphotochemrev.2012.08.001
Song, J., Luo, Z., Britt, D., Furukawa, H., Yaghi, O. M., Hardcastle, K. I., et al. (2011). A multi-unit catalyst with synergistic stability and reactivity A polyoxometalate-metal organic framework for aerobic decontamination. J. Am. Chem. Soc. 133, 16839–16846. doi: 10.1021/ja203695h
Song, Y. F., and Tsunashima, R. (2012). Recent advances on polyoxometalate-based molecular and composite materials. Chem. Soc. Rev. 41, 7384–7402. doi: 10.1039/c2cs35143a
Steens, N., Ramadan, A. M., Absillis, G., and Parac-Vogt, T. N. (2010). Hydrolytic cleavage of DNA-model substrates promoted by polyoxovanadates. Dalton Trans. 39, 585–592. doi: 10.1039/B913471A
Stracke, J. J., and Finke, R. G. (2011). Electrocatalytic water oxidation beginning with the cobalt polyoxometalate [Co4(H2O)2(PW9O34)2]10– identification of heterogeneous CoOx as the dominant catalyst. J. Am. Chem. Soc. 133, 14872–14875. doi: 10.1021/ja205569j
Stracke, J. J., and Finke, R. G. (2014). Distinguishing homogeneous from heterogeneous water oxidation catalysis when beginning with polyoxometalates. ACS Catal. 4, 909–933. doi: 10.1021/cs4011716
Suárez-Guevara, J., Ruiz, V., and Gomez-Romero, P. (2014). Hybrid energy storage high voltage aqueous supercapacitors based on activated carbon-phosphotungstate hybrid materials. J. Mater. Chem. A 2, 1014–1021. doi: 10.1039/C3TA14455K
Suen, N.-T., Hung, S.-F., Quan, Q., Zhang, N., Xu, Y.-J., and Chen, H. (2017). M. Electrocatalysis for the oxygen evolution reaction recent development and future perspectives. Chem. Soc. Rev. 46, 337–365. doi: 10.1039/C6CS00328A
Sullivan, K. P., Neiwert, W. A., Zeng, H., Mehta, A. K., Yin, Q., Hillesheim, D. A., et al. (2017). Polyoxometalate-based gelating networks for entrapment and catalytic decontamination. Chem. Commun. 53, 11480–11483. doi: 10.1039/C7CC05657E
Sumliner, J. M., Lv, H., Fielden, J., Geletii, Y. V., and Hill, C. L. (2014). Polyoxometalate multi-electron-transfer catalytic systems for water splitting. Eur. J. Inorg. Chem. 2014, 635–644. doi: 10.1002/ejic.201301573
Taleghani, S., Mirzaei, M., Eshtiagh-Hosseini, H., and Frontera, A. (2016). Tuning the topology of hybrid inorganic–organic materials based on the study of flexible ligands and negative charge of polyoxometalates A crystal engineering perspective. Coord. Chem. Rev. 309, 84–106. doi: 10.1016/j.ccr.2015.10.004
Vickers, J. W., Lv, H., Sumliner, J. M., Zhu, G., Luo, Z., Musaev, D. G., et al. (2013). Differentiating homogeneous and heterogeneous water oxidation catalysis confirmation that [Co4(H2O)2(α-PW9O34)2]10– is a molecular water oxidation catalyst. J. Am. Chem. Soc. 135, 14110–14118. doi: 10.1021/ja4024868
Walsh, J. J., Bond, A. M., Forster, R. J., and Keyes, T. E. (2016). Hybrid polyoxometalate materials for photo(electro-) chemical applications. Coord. Chem. Rev. 306, 217–234. doi: 10.1016/j.ccr.2015.06.016
Wang, H., Hamanaka, S., Nishimoto, Y., Irle, S., Yokoyama, T., Yoshikawa, H., et al. (2012). In operando X-ray absorption fine structure studies of polyoxometalate molecular cluster batteries polyoxometalates as electron sponges. J. Am. Chem. Soc. 134, 4918–4924. doi: 10.1021/ja2117206
Wang, M.-Y., Ma, R., and He, L.-N. (2016). Polyoxometalate-based ionic liquids-promoted CO2 conversion. Sci. China Chem. 59, 507–516. doi: 10.1007/s11426-016-5560-9
Wang, S. S., and Yang, G. Y. (2015). Recent advances in polyoxometalate-catalyzed reactions. Chem. Rev. 115, 4893–4962. doi: 10.1021/cr500390v
Wu, H., Yang, H.-K., and Wang, W. (2016). Covalently-linked polyoxometalate–polymer hybrids optimizing synthesis, appealing structures and prospective applications. New J. Chem. 40, 886–897. doi: 10.1039/C5NJ01257K
Wu, X., Li, F., Zhang, B., and Sun, L. (2015). Molecular complexes in water oxidation Pre-catalysts or real catalysts. J. Photochem. Photobiol. C Photochem. Rev. 25, 71–89. doi: 10.1016/j.jphotochemrev.2015.07.002
Xiang, X., Fielden, J., Rodríguez-Córdoba, W., Huang, Z., Zhang, N., Luo, Z., et al. (2013). Electron transfer dynamics in semiconductor–chromophore–polyoxometalate catalyst photoanodes. J. Phys. Chem. C 117, 918–926. doi: 10.1021/jp312092u
Xiao, Z., Chen, K., Wu, B., Li, W., Wu, P., and Wei, Y. (2016). An easy way to construct polyoxovanadate-based organic–inorganic hybrids by stepwise functionalization. Eur. J. Chem. 2016, 808–811. doi: 10.1002/ejic.201501297
Yamase, T., and Pope, M. T. (2006). Polyoxometalate Chemistry for Nano-Composite Design New York, NY: Kluwer Academic Publishers.
Yin, Q., Tan, J. M., Besson, C., Geletii, Y. V., Musaev, D. G., Kuznetsov, A. E., et al. (2010). A fast soluble carbon-free molecular water oxidation catalyst based on abundant metals. Science 328, 342–345. doi: 10.1126/science.1185372
Zhang, B., Yin, P., Haso, F., Hu, L., and Liu, T. (2014). Soft matter approaches for enhancing the catalytic capabilities of polyoxometalate clusters. J. Cluster Sci. 25, 695–710. doi: 10.1007/s10876-013-0643-7
Zhao, C., Huang, Z., Rodríguez-Córdoba, W., Kambara, C. S., O'Halloran, K. P., Hardcastle, K. I., et al. (2011). Synthesis and characterization of a metal-to-polyoxometalate charge transfer molecular chromophore. J. Am. Chem. Soc. 133, 20134–20137. doi: 10.1021/ja209360x
Zhao, C., Rodríguez-Córdoba, W., Kaledin, A. L., Yang, Y., Geletii, Y. V., Lian, T., et al. (2013). An inorganic chromophore based on a molecular oxide supported metal carbonyl cluster [P2W17O61{Re(CO)3}3{ORb(H2O)}(μ3-OH)]9−−. Inorg. Chem. 52, 13490–13495. doi: 10.1021/ic4018823
Zhao, S., Xu, J., Wei, M., and Song, Y.-F. (2011). Synergistic catalysis by polyoxometalate-intercalated layered double hydroxides oximation of aromatic aldehydes with large enhancement of selectivity. Green Chem. 13, 384–389. doi: 10.1039/c0gc00664e
Zhou, Y., Guo, Z., Hou, W., Wang, Q., and Wang, J. (2015). Polyoxometalate-based phase transfer catalysis for liquid–solid organic reactions: a review. Catal. Sci. Technol. 5, 4324–4335. doi: 10.1039/C5CY00674K
Keywords: Polyoxometalates (POMs), heterogeneous catalysis, multi-functional polymers, catalytic water oxidation, photoelectrochemical water splitting
Citation: Sullivan KP, Yin Q, Collins-Wildman DL, Tao M, Geletii YV, Musaev DG, Lian T and Hill CL (2018) Multi-Tasking POM Systems. Front. Chem. 6:365. doi: 10.3389/fchem.2018.00365
Received: 29 June 2018; Accepted: 30 July 2018;
Published: 21 August 2018.
Edited by:
Debbie C. Crans, Colorado State University, United StatesReviewed by:
Enrique González-Vergara, Benemérita Universidad Autónoma de Puebla, MexicoYoshihito Hayashi, Kanazawa University, Japan
Copyright © 2018 Sullivan, Yin, Collins-Wildman, Tao, Geletii, Musaev, Lian and Hill. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Craig L. Hill, Y2hpbGxAZW1vcnkuZWR1
 Kevin P. Sullivan
Kevin P. Sullivan Qiushi Yin1
Qiushi Yin1 Craig L. Hill
Craig L. Hill