- Department of Electrical and Electronic Information Engineering, Toyohashi University of Technology, Toyohashi, Japan
Metal nanowires (NWs) have attracted much attention because of their high electron conductivity, optical transmittance, and tunable magnetic properties. Metal NWs have been synthesized using soft templates such as surface stabilizing molecules and polymers, and hard templates such as anodic aluminum oxide, mesoporous oxide, carbon nanotubes. NWs prepared from hard templates are composites of metals and the oxide/carbon matrix. Thus, selecting appropriate elements can simplify the production of composite devices. The resulting NWs are immobilized and spatially arranged, as dictated by the ordered porous structure of the template. This avoids the NWs from aggregating, which is common for NWs prepared with soft templates in solution. Herein, the hard template synthesis of metal NWs is reviewed, and the resulting structures, properties and potential applications are discussed.
Introduction
Metal nanowires (NWs) are typically prepared using templates, with the exception of those grown using nanoparticulate catalysts (Choi et al., 2008; Bashouti et al., 2012). Surface stabilizing molecules or polymers can be used as soft templates (Tang and Tsuji, 2010). Porous solid materials such as anodic aluminum oxide (AAO), mesoporous oxides (MOs), and carbon nanotubes (CNTs) can be used as hard templates. Metal NWs formed from soft templates are dispersed in solution, and require subsequent immobilization on matrices for many devices. NWs prepared with hard templates can be spontaneously immobilized in an ordered arrangement, because of the ordered porous structure of the template. Thus, metal NWs formed from hard templates can simplify device production. Metal NWs and the composites of which with hard templates have a wide range of applications, from transparent electrodes, metamaterials to biosensors (Hu et al., 2011; Shin et al., 2012; Proenca et al., 2013; Wang et al., 2013; Ye et al., 2014). Some of the applications take advantages of high periodicity and chemical stability of the template. Thus, synthesizing metal NWs using various templates has been widely investigated. In this mini-review, we discuss the use of AAO, MO, CNTs, and other materials as hard templates for producing metal NWs. The possible applications of metal NWs prepared by these methods are also discussed with comparison highlighting the advantages and disadvantages of various composite structures.
AAO Templates
AAO contains two-dimensional hexagonal pores (Keller et al., 1953). The diameter and length of these pores can be precisely controlled by the anodization conditions, including the voltage, temperature, time and electrolyte composition (Sarkar et al., 2007; Lu and Chen, 2011; Poinern et al., 2011). The development of AAO synthesis techniques has led to the preparation of NWs with precisely controlled structures in AAO templates (Huber et al., 1994; Lu and Chen, 2011). Metal NWs have been prepared in AAO templates by casting, and vapor, supercritical fluid, chemical, electro-, electrochemical, and photochemical depositions. Electrodeposition has been most commonly employed, because of its simplicity and resulting dense NWs in high yield. Cu, Fe (Thongmee et al., 2008), Ni (Byrne et al., 2009), Co (Vivas et al., 2012a; Proenca et al., 2013), and CoNi (Vivas et al., 2012b) NWs have been prepared by electrodeposition, and their biocompatibilities and magnetic properties were investigated. All NWs except for Fe were single crystalline, without post-treatment after electrodeposition. NWs are typically synthesized in acidic solution (pH 2-5) containing H3BO3 buffer. The equipment required for electrodeposition is similar to that for anodization to prepare AAO. The AAO acts as the cathode when the metal NWs are deposited. Figure 1 shows horizontal (A) and vertical (B) cross-sectional SEM images of deposited Co NWs, with diameters of ~40 nm and lengths of ~3 μm. The horizontal cross-sectional image (Figure 1A) was captured after ion milling to a depth of 200 nm, to remove unfilled AAO template from the top of the sample. The SEM images demonstrated the homogeneity of the pore filling, and uniformity of the NWs along the pore walls. This NW uniformity allowed the detailed investigation of the effect of the NWs dimensions on their magnetic properties. In other words, the magnetic properties of metal NWs can be tailored by modifying the AAO structure.
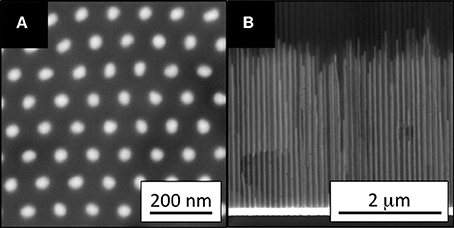
Figure 1. (A) Bottom (after milling to a depth of 200 nm) and (B) cross-sectional SEM images of Co NW arrays in an AAO template. The NW diameters are ~40 nm, and the interpore distances are ~100 nm. Reproduced with permission from AIP Publishing LLC (Proenca et al., 2013).
Casting is also often used to prepare metal NWs in AAO templates. Molten metals are cast into AAO pores, without complex chemical or electrochemical processes. Thus, casting is simpler than electrodeposition. However, the high gas pressure or hydraulic force required to fill the pores with metal usually increases the production cost. The aluminum layer underlying the AAO template melts at 660°C, so the filler metal must have a lower melting point. Thus, casting is usually reported for low melting point metals such as Bi (Heremans et al., 2000), and Pd (Kuo et al., 2013). Fabricating multilayered NWs consisting of different metals, alloys, or metal compounds with distinct borders by casting is very difficult.
MO Templates
MOs, especially mesoporous silica (SBA-15 and MCM-41), have been used as hard templates for preparing metal NWs. The MO pore size is normally smaller than that of AAO, so noble metals such as Au (Kanno et al., 2012; Kawamura et al., 2012b), Pt, Ag (Han et al., 2000; Takai et al., 2010; Kim et al., 2012), and Cu (Zhang et al., 2007) have more often been used to fabricate NWs in the tubular pores of MOs. This is because of their low sensitivity to oxygen, and possible application as catalysts and components of electronic devices. The methods used to deposit the metal NWs are similar to those for AAO templates. However, the smaller pore size results in incomplete filling of template pores by vapor-phase epitaxy, chemical vapor deposition, and photochemical deposition (Leon et al., 1995; Kawamura et al., 2012a). The supercritical fluid synthesis using MOs has also been applied to base metals such as Co, and Ni NWs (Coleman et al., 2001; Holmes et al., 2003). Metal chelate precursors, M(hfa)2 · × H2O (M: metal, hfa: hexafluoroacetylacetonate) were used in this method. Supercritical CO2 and H2O have been attracting more attention as an alternative to conventional organic solvents since they are nontoxic and nonflammable.
CNT Templates
In contrast to metal NW arrays prepared from AAO and MO templates, isolated NWs can be obtained using CNT templates. Metal filling in CNTs is typically achieved by chemical vapor deposition or wet chemical processes (Tsang et al., 1994; Li et al., 1998; Govindaraj et al., 2000). One problem with this method is the low percentage of filled CNTs caused by their small inner diameter, high aspect ratio and high curvature. Metal impregnation into CNT interiors can also be achieved by incorporating metals and metal precursors with the carbon source during CNT growth. Pd, Pb, Bi, Y, Mg, Gd, Ti, Cr, Fe, Co, Zn, Mo, Ta, W, Dy, and Yb have all been trapped inside CNTs by this method (Guerret-Piecourt et al., 1994; Sen et al., 1997; Liu et al., 2000). Impurities are often produced, including encapsulated carbon clusters and soot. CNTs are not wetted by liquids with surface tensions higher than 100–200 mM M−1, so there have been limited reports of molten metals penetrating into CNTs by capillary forces (Ajayan and Iijima, 1993; Ugarte et al., 1996). The rapid filling of Pd, Ni, and Cu into multiwalled CNTs has reportedly produced metal/multiwalled CNT composites. Supercritical CO2 was used as the reaction medium, and long metal NWs were prepared (Ye et al., 2003). Au, Pt, and Pd NWs were fabricated by the templated electrodeposition of metals on the outer surface of CNTs (Dudin et al., 2011). Although the application of metal NW/CNT composites has not become real yet, they can be used as catalysts, sensors, phtothermal nanomaterials, and in electrochemical energy storage and production by taking advantage of CNT characteristics including high chemical stability (Che et al., 1999; Weissker et al., 2010; Rossella et al., 2012).
Other Templates
Au NW arrays have been prepared by electro-, chemical, electrochemical, and photochemical deposition on track-etched porous polycarbonate (TEPP) membranes, which were used as hard templates. The dimensions of this template are much larger than those of AAO and MO, so it is better suited to biosensor applications (Cusma et al., 2007; Lu et al., 2007; Yang et al., 2007).
Si is the most commonly used material in microelectronics, photonics, and sensing, so composites of metal NWs and porous Si (PS) templates are of interest (Bell et al., 1996; Seals et al., 2002; Aravamudhan et al., 2007). PS with specific dimensions is easily prepared by anodizing Si wafers with or without the assistance of a magnetic field. Au, Cu, Fe, Ni, and Co NWs have been deposited into PS templates via electrochemical routes, or chemical vapor deposition. These composites have potential in magnetic sensors and magneto-optic devices in integrated Si-based circuits, and detectors of spin-injection from ferromagnetic metals to Si. (Granitzer and Rumpf, 2010, 2011; Granitzer et al., 2013).
Conclusion and Outlook
The synthesis of metal NWs using hard templates was reviewed. AAO, MOs, and CNTs are often employed as hard templates, because their pore dimensions are controllable at the nanoscale. NWs prepared using hard templates do not require subsequent immobilizing or aligning, in contrast to those prepared using soft templates in solution. Table 1 shows the comparison of NWs obtained using the various hard templates. Commonly, AAO is employed to prepare NW arrays with high periodicity. MO produces NW arrays with smaller dimensions than ones produced with AAO. CNT brings about formation of isolated NWs covered with the CNT. TEPP and PS are sometimes used when the dimensions or composition of the templates are appropriate for specific applications. The NWs can be used without removing the template before application. For example, Co NW arrays in AAO are expected to be used as metamaterials due to their long-range high periodicity (Ye et al., 2003), and Fe, Co, or Ni NWs in CNT can be applied to medicine owing to the magnetic anisotropy and anti-corrosivity resulting from the protection of the NWs by a layer of graphite (Weissker et al., 2010). Metal NWs prepared using hard templates are compatible with large-scale production, so are suitable for industrial application.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by Japan Society for the Promotion of Science (JSPS) KAKENHI (Grant no. 22760539).
References
Ajayan, P. M., and Iijima, S. (1993). Capillarity-induced filling of carbon nanotubes. Nature 361, 333–334. doi: 10.1038/361333a0
Aravamudhan, S., Luongo, K., Poddar, P., Srikanth, H., and Bhansali, S. (2007). Porous silicon templates for electrodeposition of nanostructures. Appl. Phys. A 87, 773–780. doi: 10.1007/s00339-007-3901-4
Bashouti, M. Y., Pietsch, M., Sardashti, K., Brönstrup, G., Schmitt, S. W., Srivastava, S. K., et al. (2012). “Hybrid silicon nanowires: from basic research to applied nanotechnology,” in Nanowires–Recent Advances, Chapter 9, ed X. Peng (Rijeka: InTech.), 177–210.
Bell, T. E., Gennissen, P. T. J., DeMunter, D., and Kuhl, M. (1996). Porous silicon as a sacrificial material. J. Micromech. Microeng. 6, 361–369. doi: 10.1088/0960-1317/6/4/002
Byrne, F., Prina-Mello, A., Whelan, A., Mohamed, B. M., Davies, A., Gunko, Y. K., et al. (2009). High content analysis of the biocompatibility of nickel nanowires. J. Magn. Magn. Mater. 321, 1341–1345. doi: 10.1016/j.jmmm.2009.02.035
Che, G., Lakshmi, B. B., Martin, C. R., and Fisher, E. R. (1999). Metal-nanocluster-filled carbon nanotubes: catalytic properties and possible applications in electrochemical energy storage and production. Langmuir 15, 750–758. doi: 10.1021/la980663i
Choi, W. K., Liew, T. H., Dawood, M. K., Smith, H. I., Thompson, C. V., and Hong, M. H. (2008). Synthesis of silicon nanowires and nanofin arrays using interference lithography and catalytic etching. Nano Lett. 8, 3799–3802. doi: 10.1021/nl802129f
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Coleman, N. R. B., Ryan, K. M., Spalding, T. R., Holmes, J. D., and Morris, M. A. (2001). The formation of dimensionally ordered germanium nanowires within mesoporous silica. Chem. Phys. Lett. 343, 1–6. doi: 10.1016/S0009-2614(01)00647-9
Cusma, A., Curulli, A., Zane, D., Kaciulis, S., and Padeletti, G. (2007). Feasibility of enzyme biosensors based on gold nanowires. Mater. Sci. Eng. C 27, 1158–1161. doi: 10.1016/j.msec.2006.09.035
Dudin, P. V., Snowden, M. E., Macpherson, J. V., and Unwin, P. R. (2011). Electrochemistry at nanoscale electrodes: individual single-walled carbon nanotubes (SWNTs) and SWNT-templated metal nanowires. ACS Nano 5, 10017–10025. doi: 10.1021/nn203823f
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Govindaraj, A., Satishkumar, B. C., Nath, M., and Rao, C. N. R. (2000). Metal nanowires and intercalated metal layers in single-walled carbon nanotube bundles. Chem. Mater. 12, 202–205. doi: 10.1021/cm990546o
Granitzer, P., and Rumpf, K. (2010). Porous silicon–a versatile host material. Materials 3, 943–998. doi: 10.3390/ma3020943
Granitzer, P., and Rumpf, K. (2011). Magnetic nanoparticles embedded in a silicon matrix. Materials 4, 908–928. doi: 10.3390/ma4050908
Granitzer, P., Rumpf, K., Ohta, T., Koshida, N., Poelt, P., and Reissner, M. (2013). Magnetic field assisted etching of porous silicon as a tool to enhance magnetic characteristics. ECS Trans. 50, 55–59. doi: 10.1149/05037.0055ecst
Guerret-Piecourt, C., Le Bouar, Y., Lolseau, A., and Pascard, H. (1994). Relation between metal electronic structure and morphology of metal compounds inside carbon nanotubes. Nature 372, 761–765. doi: 10.1038/372761a0
Han, Y.-J., Kim, J. M., and Stucky, G. D. (2000). Preparation of noble metal nanowires using hexagonal mesoporous silica SBA-15, Chem. Mater. 12, 2068–2069. doi: 10.1021/cm0010553
Heremans, J., Thrush, C. M., Lin, Y.-M., Cronin, S., Zhang, Z., Dresselhaus, M. S., et al. (2000). Bismuth nanowire arrays: synthesis and galvanomagnetic properties, Phys. Rev. B 61, 2921–2930. doi: 10.1103/PhysRevB.61.2921
Holmes, J. D., Lyons, D. M., and Ziegler, K. J. (2003). Supercritical fluid synthesis of metal and semiconductor nanomaterials. Chem. Eur. J. 9, 2144–2150. doi: 10.1002/chem.200204521
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Hu, L., Wu, H., and Cui, Y. (2011). Metal nanogrids, nanowires, and nanofibers for transparent electrodes. MRS Bull. 36, 760–765. doi: 10.1557/mrs.2011.234
Huber, C. A., Huber, T. E., Sadoqi, M., Lubin, J. A., Manalis, S., and Prater, C. B. (1994). Nanowire array composites. Science 263, 800–802. doi: 10.1126/science.263.5148.800
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Kanno, Y., Suzuki, T., Yamauchi, Y., and Kuroda, K. (2012). Preparation of Au nanowire films by electrodeposition using mesoporous silica films as a template: vital effect of vertically oriented mesopores on a substrate. J. Phys. Chem. C 116, 24672–24680. doi: 10.1021/jp308772b
Kawamura, G., Hayashi, I., Muto, H., and Matsuda, A. (2012a). Anisotropically assembled gold nanoparticles prepared using unidirectionally aligned mesochannels of silica film. Scripta Mater. 66, 479–482. doi: 10.1016/j.scriptamat.2011.12.023
Kawamura, G., Okuno, T., Muto, H., and Matsuda, A. (2012b). Selective preparation of zero- and one-dimensional gold nanostructures in a TiO2 nanocrystal-containing photoactive mesoporous template. Nanoscale Res. Lett. 7, 27. doi: 10.1186/1556-276X-7-27
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Keller, F., Hunter, M. S., and Robinson, D. L. (1953). Structural features of oxide coatings on aluminum. J. Electrochem. Soc. 100, 411–419. doi: 10.1149/1.2781142
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Kim, K.-J., Lee, E.-S., and Kwon, Y.-U. (2012). Syntheses of micrometer-long Pt and Ag nanowires through SBA-15 templating. J. Nanopart. Res. 14:1270. doi: 10.1007/s11051-012-1270-1
Kuo, C. G., Chang, H., Hwang, L.-R., Hor, S., Chen, J.-S., Liu, G.-Y., et al. (2013). Fabrication of a Pb-Sn nanowire array gas sensor using a novel high vacuum die casting technique. Electron. Mater. Lett. 9, 481–484. doi: 10.1007/s13391-013-0037-x
Leon, R., Margolese, D., Stucky, G., and Petroff, P. M. (1995). Nanocrystalline Ge filaments in the pores of a mesosilicate. Phys. Rev. B 52, R2285–R2288. doi: 10.1103/PhysRevB.52.R2285
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Li, J., Moskovits, M., and Haslett, T. L. (1998). Nanoscale electroless metal deposition in aligned carbon nanotubes. Chem. Mater. 10, 1963–1967. doi: 10.1021/cm980122e
Liu, S., Zhu, J., Mastai, Y., Felner, I., and Gedanken, A. (2000). Preparation and characteristics of carbon nanotubes filled with cobalt. Chem. Mater. 12, 2205–2211. doi: 10.1021/cm000062o
Lu, C., and Chen, Z. (2011). “Anodic aluminum oxide templates,” in Encyclopedia of Nanoscience and Nanotechnology, Vol. 11, ed H. S. Nalwa (Valencia, CA: American Scientigic Publishers), 235–259.
Lu, Y., Yang, M., Qu, F., Shen, G., and Yu, R. (2007). Enzyme-functionalized gold nanowires for the fabrication of biosensors. Bioelectrochemistry 71, 211–216. doi: 10.1016/j.bioelechem.2007.05.003
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Poinern, G. E. J., Ali, N., and Fawcett, D. (2011). Progress in nano-engineered anodic aluminum oxide membrane development. Materials 4, 487–526. doi: 10.3390/ma4030487
Proenca, M. P., Sousa, C. T., Escrig, J., Ventura, J., Vazquez, M., and Araujo, J. P. (2013). Magnetic interactions and reversal mechanisms in Co nanowire and nanotube arrays. J. Appl. Phys. 113, 093907. doi: 10.1063/1.4794335
Rossella, F., Soldano, C., Bellani, V., and Tommasini, M. (2012). Metal-filled carbon nanotubes as a novel class of photothermal nanomaterials. Adv. Mater. 24, 2453–2458. doi: 10.1002/adma.201104393
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Sarkar, J., Khan, G. G., and Basumallick, A. (2007). Nanowires: properties applications and synthesis via porous anodic aluminium oxide template. Bull. Mater. Sci. 30, 271–290. doi: 10.1007/s12034-007-0047-0
Seals, L., Gole, J. L., Tse, L. A., and Hesketh, P. J. (2002). Rapid, reversible, sensitive porous silicon gas sensor. J. Appl. Phys. 91, 2519. doi: 10.1063/1.1436556
Sen, R., Govindaraj, A., and Rao, C. N. R. (1997). Metal-filled and hollow carbon nanotubes obtained by the decomposition of metal-containing free precursor molecules. Chem. Mater. 9, 2078–2081. doi: 10.1021/cm9700965
Shin, S.-H., Kim, G.-Y., Shim, J., Kim, J., Hur, H.-G., Lee, D.-J., et al. (2012). Use of biologically designed gold nanowire for biosensor application. Korean J. Chem. Eng. 29, 1666–1669. doi: 10.1007/s11814-012-0140-y
Takai, A., Doi, Y., Yamauchi, Y., and Kuroda, K. (2010). Soft-chemical approach of noble metal nanowires templated from mesoporous silica (SBA-15) through vapor infiltration of a reducing agent. J. Phys. Chem. C 114, 7586–7593. doi: 10.1021/jp910288x
Tang, X., and Tsuji, M. (2010). “Syntheses of silver nanowires in liquid phase” in Nanowires Science and Technology, Chapter 2, ed Nicoleta Lupu (Rijeka: InTech.), 25–42.
Thongmee, S., Pang, H. L., Ding, J., Yi, J. B., and Lin, J. Y. (2008). “Fabrication and magnetic properties of metal nanowires via AAO templates,” in Nanoelectronics Conference, 2008 (INEC 2008), 2nd IEEE International (Shanghai), 1116–1120.
Tsang, S. C., Chen, Y. K., Harris, P. J. F., and Green, M. L. H. (1994). A simple chemical method of opening and filling carbon nanotubes, Nature 372, 159–162. doi: 10.1038/372159a0
Ugarte, U., Chatelain, A., and de Heer, W. A. (1996). Nanocapillarity and chemistry in carbon nanotubes. Science 274, 1897–1899. doi: 10.1126/science.274.5294.1897
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Vivas, L. G., Escrig, J., Trabada, D. G., Badini-Confalonieri, G. A., and Vázquez, M. (2012a). Magnetic anisotropy in ordered textured Co nanowires, Appl. Phys. Lett. 100, 252405. doi: 10.1063/1.4729782
Vivas, L. G., Vazquez, M., Escrig, J., Allende, S., Altbir, D., Leitao, D. C., et al. (2012b). Magnetic anisotropy in CoNi nanowire arrays: analytical calculations and experiments. Phys. Rev. B 85:035439. doi: 10.1103/PhysRevB.85.035439
Wang, Q., Min, F., and Zhu, J. (2013). Preparation of gold nanowires and its application in glucose biosensing. Mater. Lett. 91, 9–11. doi: 10.1016/j.matlet.2012.09.080
Weissker, U., Hampel, S., Leonhardt, A., and Buchner, B. (2010). Carbon nanotubes filled with ferromagnetic materials. Materials 3, 4387–4427. doi: 10.3390/ma3084387
Yang, M., Qu, F., Li, Y., He, Y., Shen, G., and Yu, R. (2007). Direct electrochemistry of hemoglobin in gold nanowire array. Biosens. Bioelectron. 23, 414–420. doi: 10.1016/j.bios.2007.05.003
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Ye, S., Rathmell, A. R., Stewart, I. E., Ha, Y.-C., Wilson, A. R., Chen, Z., et al. (2014). A rapid synthesis of high aspect ratio copper nanowires for high-performance transparent conducting films. Chem. Commun. 50, 2562–2564. doi: 10.1039/c3cc48561g
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Ye, X. R., Lin, Y., Wang, C., and Wai, C. M. (2003). Supercritical fluid fabrication of metal nanowires and nanorods templated by multiwalled carbon nanotubes. Adv. Mater. 15, 316–319. doi: 10.1002/adma.200390077
Keywords: metal deposition, anodic aluminum oxide, mesoporous oxide, carbon nanotube, tubular pore
Citation: Kawamura G, Muto H and Matsuda A (2014) Hard template synthesis of metal nanowires. Front. Chem. 2:104. doi: 10.3389/fchem.2014.00104
Received: 03 October 2014; Accepted: 31 October 2014;
Published online: 17 November 2014.
Edited by:
Shengrong Ye, Duke University, USAReviewed by:
Joan J. Carvajal Marti, Universitat Rovira i Virgili, SpainGuo-Hong Tao, Sichuan University, China
Copyright © 2014 Kawamura, Muto and Matsuda. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Go Kawamura, Department of Electrical and Electronic Information Engineering, Toyohashi University of Technology, 1-1 Hibarigaoka, Tempaku-cho, Toyohashi 441-8580, Japan e-mail:Z29rYXdhbXVyYUBlZS50dXQuYWMuanA=
 Go Kawamura
Go Kawamura Hiroyuki Muto
Hiroyuki Muto Atsunori Matsuda
Atsunori Matsuda