
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Chem. Eng. , 12 March 2025
Sec. Biochemical Engineering
Volume 7 - 2025 | https://doi.org/10.3389/fceng.2025.1518165
Parts of this article's content have been modified or rectified in:
Erratum: Effect of nitrogen sources on the yield and quality attributes of capsular polysaccharides in Streptococcus pneumoniae
 Yuelong Li1,2
Yuelong Li1,2 Yanli Liu1
Yanli Liu1 Hantian Yao3
Hantian Yao3 Yanyan Wang1
Yanyan Wang1 Yechi Zhou1
Yechi Zhou1 Hao Zheng4
Hao Zheng4 Yanbin Liu1
Yanbin Liu1 Xinyan You1
Xinyan You1 Xin Cao1*
Xin Cao1* Jiankai Liu1*
Jiankai Liu1*Streptococcus pneumoniae, a pathogenic bacterium, is responsible for a range of infections. With the rise in antibiotic resistance, vaccination against pneumococcal disease has become increasingly critical. Pneumococcal capsular polysaccharides (CPSs) serve as potent vaccine antigens, triggering the host’s production of protective antibodies. The immunogenicity of CPS antigens in pneumococcal vaccines is significantly influenced by the chain length, the content of functional chemical groups and additional chemical modifications. S. pneumoniae has stringent nutritional requirements for culture medium. One crucial aspect of fermentation medium development is the selection of nitrogen sources. These sources supply the essential nutrients for the synthesis of vital biomolecules and secondary metabolites, including the CPSs. Therefore, comprehending the impact of organic nitrogen sources on the yield and quality of CPSs is crucial for optimizing manufacturing processes for pneumococcal vaccines. In our study, we evaluated the effects of peptones from various sources on the growth profiles and CPS yields, as well as quality attributes related to CPS immunogenicity. We found that while CPS productivity was slightly impacted by peptone selection, the chain length and functional group content of CPSs were markedly influenced by the peptone source. Notably, using the non-animal HY-SOY 4D soy peptone as a nitrogen source in the fermentation medium led to CPSs with long chains and a high content of functional chemical groups. The structural identity and correctness of pure CPSs were verified by 1H nuclear magnetic resonance (NMR) spectroscopy. The findings offer insights into how the composition of the fermentation medium affects both the yield and quality of pneumococcal CPSs, aiming at improving vaccine production against pneumococcal infections.
Streptococcus pneumoniae, a lancet-shaped, Gram-positive, facultative anaerobic bacterium, is responsible for diseases such as pneumonia, meningitis, otitis media, and bacteremia, with children under five being particularly susceptible (Paton and Trappetti, 2019; Weiser, Ferreira, and Paton, 2018; Al-Jumaili et al., 2023). Amidst rising antibiotic resistance, vaccination is becoming a critical strategy for preventing pneumococcal infections (Lees et al., 2017; Weiser, Ferreira, and Paton, 2018; Fitzgerald and Waterer, 2019; Morais, Texeira, and Suarez, 2019; Guo and Qiao, 2021). Key virulence factors of S. pneumoniae include CPSs, hemolysin, and surface proteins, with CPSs being the primary factor (Kadioglu et al., 2008). The CPS protects bacteria from clearance by nasal mucus and shields them from immune system attacks, including neutrophils, macrophages, and antibodies (Hyams et al., 2010; Hamaguchi et al., 2018). Importantly, CPSs stimulate the host to produce protective antibodies, making them effective antigens for pneumococcal vaccines (Avery and Heidelberger, 1925; Avery, Heidelberger, and Goebel, 1925). Therefore, CPSs from highly virulent serotypes are incorporated into vaccines widely administered today (Jones and Currie, 1991; Talaga, Vialle, and Moreau, 2002).
The CPSs are synthesized extensively during the logarithmic phase of bacterial growth. Initiated by the formation of repetitive units on a carrier lipid within the cell membrane, these units are then exported to the membrane surface for polymerization into CPSs (Aanensen et al., 2007; Kolkman, van der Zeijst, and Nuijten, 1998; Morais, Dee, and Suárez, 2018). CPSs are classified as T-cell independent (TI) antigens (Mond J. J. et al., 1995; Vos et al., 2000). For effective antibody production, pneumococcal CPS must engage at least 10–20 repeating epitopes on the B cell receptor (BCR), with fewer epitopes potentially failing to initiate activation (Defrance, Taillardet, and Genestier, 2011; Jha and Janoff, 2019; Mond J. J. et al., 1995; Mond JamesJ. et al., 1995). This mechanism elucidates the close relationship between the chain lengths and immunogenicity of polysaccharide vaccines, and pneumococcal CPSs with long chain lengths are considered to possess increased immunogenicity (Qiong et al., 2022; Kabat and Bezer, 1958; Howard et al., 1971; Hefti et al., 2003; Xiao et al., 2006; Zhao, Zhao, and Xie, 2012; Soubal et al., 2013). The immunogenicity of CPSs is also influenced by content of functional chemical groups, along with other chemical modifications, as they may constitute important immunological epitopes (Fusco et al., 2007; McNeely et al., 1998; Szu et al., 1991; Werz and Seeberger, 2005; Berti, De Ricco, and Rappuoli, 2018; Anish et al., 2014; Bröker, Berti, and Costantino, 2016; Micoli, Stefanetti, and MacLennan, 2023; Anish, Beurret, and Poolman, 2021; Adamo et al., 2013). Quality control tests for CPSs used in vaccines involve molecular size, molecular weight, content of characteristic group and chemical modifications (EDQM, 2023; Qiong et al., 2022).
Microbial fermentation is a complex process influenced by multiple factors such as strain selection, medium composition, feed medium, fermentation conditions (pH and temperature), aeration, stirring, and operational modes like batch, fed-batch, or continuous culture (Grobben et al., 1997; Degeest and De Vuyst, 1999; Jain and Maithal, 2011; Zeidan et al., 2017; Gonçalves et al., 2014; Gururao et al., 2018). S. pneumoniae, having stringent nutritional needs, is particularly sensitive to culture medium composition, which greatly affects its growth and the production of key metabolic products, especially the CPSs (Texeira et al., 2015; Restrepo et al., 2005; Gonçalves et al., 2002). Under the premise of complying with current good manufacturing practice (cGMP), the development of S. pneumoniae cultivation medium focuses on developing animal-free medium (Texeira et al., 2015; Restrepo et al., 2005; Gonçalves et al., 2002), as the use of blood components or animal extracts in the culture medium may pose a serious health hazard due to the probable existence of contaminants like adventitious viruses, prions and mycoplasma (Jain and Maithal, 2011). Organic nitrogen sources are crucial components in culture medium, as they supply the essential nutrients for the synthesis of essential biomolecules. Multiple studies have shown that certain peptones as nitrogen source in fermentation medium can replace animal-derived components, and effectively enhance the growth of S. pneumoniae and the synthesis of CPSs (Liberman et al., 2008; Jain and Maithal, 2011; Gururao et al., 2018). Therefore, comprehending the impact of organic nitrogen sources on the yield and quality of CPSs is crucial for optimizing manufacturing processes for pneumococcal vaccines.
In this study, we aimed to assess the impact of different peptone sources on pneumococcal growth, CPS yield, and the quality attributes of the purified CPSs, such as chain length, content of functional chemical groups and modifications. To verify the structural identity and correctness of the pure CPSs, 1H NMR spectroscopy was employed. Additionally, we conducted tests to evaluate the quality of the purified CPSs in accordance with the European Pharmacopoeia (11th edition).
The strains of S. pneumoniae serotype 6A (CMCC 31476), 6B (CMCC 31490), 11A (CMCC 31572), and 33F (CMCC 31847) were purchased from the National Center for Medical Culture Collections. These lyophilized strains were used to establish both master seed lots and working seed lots. Tests were conducted to ascertain the characteristics of the seed lots by the National Institutes for Food and Drug Control of China according to Pharmacopeia, the purity of bacterial cultures was verified by methods of suitable sensitivity. These include inoculation into the solid medium, examination of colony morphology, microscopic examination of Gram-stained smears and culture agglutination with specific antisera obtained from Statens Serum Institute (Copenhagen, Denmark), following the immunochemical method (2.7.1) detailed in the European Pharmacopoeia (11th edition).
The composition of the solid medium utilized in the study includes the following ingredients: Difco soy peptone (Thermo Fisher), L-cysteine hydrochloride and tyrosine (Tianjin Pharmaceutical Group Co., Ltd.), tryptophan and dipotassium hydrogen phosphate (Sinopharm Chemical Reagent Co., Ltd.), hydrochloric acid (Hunan Erkang Pharmaceutical Co., Ltd.), and agar powder (Beijing Aoboxing Biotechnology Co., Ltd.).
The liquid medium was formulated with a diverse array of peptones including Difco soy peptone (Thermo Fisher), A3X soy peptone (ORGANOTECHINE), HY-SOY 4D soy peptone (KERRY) and casein tryptone (Chengdu Changshou Co., Ltd.), glucose (Shandong Saint-Show Technology & Pharmaceutical Co., Ltd.), as well as other supplements including: biotin, vitamin B2, vitamin B6, vitamin B1, adenine, uracil and catalase (SIGMA-ALDRICH), glutamine (Hebei Bailingwei Ultrafine Materials Co., Ltd.), L-asparagine, choline chloride, magnesium sulfate heptahydrate, ferrous sulfate heptahydrate, zinc sulfate heptahydrate and thioglycolic acid (Sinopharm Chemical Reagent Co., Ltd.), sodium hydroxide (Chengdu Huayu Pharmaceutical Excipients Co., Ltd.). The liquid medium was sterilized through filter sterilization before use, and the formulation of liquid medium is listed in Table 1.
Inactivation: DOC (SIGMA-ALDRICH).
Purification: acetic acid (Chengdu Huayu Pharmaceutical Excipients Co., Ltd.), disodium hydrogen phosphate and sodium dihydrogen phosphate (Hunan Jiudian Hongyang Pharmaceutical Co., Ltd.), sodium chloride (Hubei Wuhuan Salt Industry Group Co., Ltd.), anhydrous sodium acetate (Nanjing Chemical Reagent Co., Ltd.), calcium chloride (Beijing Yanjing Pharmaceutical Co., Ltd.), sodium hydroxide (Chengdu Huayu Pharmaceutical Excipients Co., Ltd.), and anhydrous ethanol (Nanjing Chemical Reagent Co., Ltd.).
Fermentation: 15-L microbial bioreactor (Shanghai Bailun Biotechnology), CO2 incubator (Thermo Fisher), UV-visible spectrophotometer (Beijing Puxi Technology), biological safety cabinet (Suzhou Antai Air Technology), XB43 microscope (Olympus), bioprocess analyzer (Shenzhen Hillman Biotechnology), vacuum freeze dryer (Shanghai Dongfulong), biochemical incubator (Qingdao Haier Biomedical Co., Ltd.), and high-speed centrifuge (Thermo Fisher).
Purification: magnetic stirrer (Shanghai Meiyingpu Instrument Manufacturing Co., Ltd.), electronic balance (Mettler Toledo), electronic platform scale (Mettler Toledo), multiparameter tester (Mettler Toledo), high-speed centrifuges (Thermo fisher), and ultrafiltration membranes (Millipore).
A 1 mL aliquot from the working seed lots was used to inoculate solid culture medium and incubated in a CO2 incubator for 9–15 h (with a CO2 concentration of 10% and a cultivation temperature of 36°C); subsequently, the strain was transferred to a 3 L flask containing 1 L of liquid medium and incubated at 36°C to achieve an optical density (OD) of 0.4–0.6 at 600 nm. Once the OD600 reached 0.4∼0.6, the culture was inoculated into bioreactors containing fermentation medium at a volume of 5%–10%.
The lab-scale fed-batch fermentations were performed in a 15 L bioreactor (Shanghai Bailun Biotechnology, China) with a working volume of 10 L. The bioreactor cultivation was conducted under a 100% CO2 atmosphere at a flow rate of 0.1 vvm. The pH was controlled by the addition of 5 M NaOH, maintaining a pH level of 7.2. The temperature was kept constant at 37°C as this culture temperature can potentially increase CPS yield according to previous study (Li et al., 2024), and the agitation was provided by a mechanically driven impeller at 90 rpm. Throughout the fermentation process, a continuous glucose medium was employed to ensure that the glucose concentration remained above 5 g/L within the fermentation system. Samples were collected at various time intervals for the measurement of optical density at 600 nm and glucose concentration analysis. At the final stage of fermentation, inactivation reagents were introduced for cellular lysis and sterilization. The fermentation broth was treated with 0.1% DOC, stirred for 40 min, and then incubated at 4°C for 12 h to achieve sterilization. After cell lysis using DOC, the samples were centrifuged to separate the pellets and supernatants, the polysaccharide content in the supernatant was determined.
Three fermentation replicates were conducted for each type of peptone. In these replicates, the fermentation medium formulation and process parameters were kept identical to assess the process variation in the growth curves. The average and standard deviation values were then plotted on the growth curves using Excel. Due to the fact that optical density of medium containing different peptones is quite low, all falling below 0.1. Therefore, their contribution to the total optical density is relatively minor.
A streamlined purification process was developed, which includes the following steps:
• Ultrafiltration and Diafiltration: After sterilization, the fermentation broth was centrifuged at 8000 rpm for 30 min to separate the supernatant. This supernatant was then subjected to ultrafiltration using a 100 kDa membrane to concentrate the solution. Subsequent diafiltration was performed to eliminate small molecular impurities.
• Acid Precipitation: The concentrated solution was adjusted to a pH of 4.00 with acetic acid, mixed thoroughly, and allowed to stand at 2°C–8°C for 2 h. Afterward, the supernatant was collected by centrifugation.
• Precipitation with alcohol: The supernatant was neutralized back to a pH range of 6.0–7.0 using a 5 M NaOH solution. Buffers and salts were added to specific concentrations: disodium hydrogen phosphate and monosodium dihydrogen phosphate to 10 mM each, sodium chloride to 0.15–1.0 M, anhydrous sodium acetate to 0.3–1.5 M, and calcium chloride to 0.25–0.5 M. The pH was readjusted to 5.40 with acetic acid. Then, 20%–30% (v/v) anhydrous ethanol was added, mixed well, and the solution was left to stand at 2°C–8°C for 3–24 h before centrifugation to collect the supernatant.
• Final Ultrafiltration and Lyophilization: The supernatant obtained from the previous step was ultrafiltered again using a 100 kDa membrane to enrich the polysaccharide content. The resulting ultrafiltrate was then lyophilized to obtain the purified polysaccharide.
The content of total polysaccharide was determined based on the rate nephelometric method using specific antisera obtained from Statens Serum Institute (Copenhagen, Denmark), following the immunochemical method (2.7.1) detailed in the European Pharmacopoeia (11th edition). The purified CPSs were subjected to a series of tests according to European Pharmacopoeia including: Protein (2.5.16), Nucleic acids (2.5.17), Total nitrogen (2.5.9), Phosphorus (2.5.18), Molecular size (2.2.30), Uronic acids (2.5.22), Hexosamines (2.5.20), Methyl pentoses (2.5.21), O-Acetyl groups (2.5.19).
Molecular-size and molecular-mass distribution were determined by size-exclusion chromatography (2.2.30) combined with an appropriate detection system. The size exclusion chromatography (SEC) liquid chromatography separation was conducted using a Shimadzu high performance liquid chromatography (HPLC) system (Shimadzu, Japan). The separation was conducted in a TOSOH TSKgel G5000PWxL column (7.8 mm × 30 cm, 13 µm particle size, Tosoh Bioscience, Tokyo, Japan). Both multi-angle light scattering (MALS) detector (DAWN HELEOS-II) and differential refractometers (Optilab T-rEX) (Wyatt Technology Corp., Santa Barbara, CA, United States) were connected to the UV detector. Throughout all experiments performed in this study, the detectors were arranged in the following sequence: SEC-UV-MALS-RI.
Analysis of CPS by 1H NMR spectroscopy (Bruker, Avance III 400 and 600) was conducted at Beijing Center for Physical and Chemical Analysis, Beijing, China. Prior to analysis, the CPS samples were freeze-dried and reconstituted to final concentration of 2–4 mg/mL in deuterium oxide. In 1H NMR data, splitting patterns are indicated as follows: s for singlet, d for doublet, t for triplet, q for quartet, m for multiplet, and br for broad singlet. NMR chemical shifts (δ) are reported in parts per million (ppm), and coupling constants (J) are reported in Hertz (Hz).
GraphPad Prism was employed to conduct a one-way analysis of variance (ANOVA) to assess the influence of soy peptones on the molecular size and molecular weight of the refined CPSs for serotypes 6A, 6B, 11A, and 33F.
For S. pneumoniae serotype 6A, A3X soy peptone and Casein Tryptone showed similar growth curves, while Difco soy peptone and HY-SOY 4D soy peptone demonstrated consistent growth trends with higher maximum bacterial densities. Notably, HY-SOY 4D soy peptone supported slightly slower growth (Figure 1). Corresponding CPS concentrations were highest with A3X soy peptone (727 mg/L), exceeding Difco soy peptone (655 mg/L), Casein Tryptone (639 mg/L) and HY-SOY 4D soy peptone (625 mg/L) (Table 2).
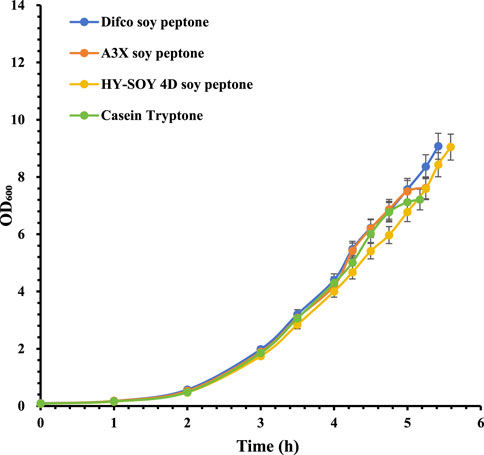
Figure 1. Growth profiles of S. pneumoniae serotype 6A fermentation with various peptones. Difco soy peptone is marked with blue circles; A3X soy peptone is marked with orange circles; HY-SOY 4D soy peptone is marked with yellow circles; Casein Tryptone is marked with green circles. The error bars indicate standard deviation of three replicates.
S. pneumoniae serotype 6B exhibited similar growth curves with A3X soy peptone and Difco soy peptone, reaching stationary phase faster than the other peptones. Casein Tryptone and HY-SOY 4D soy peptone showed similar growth trends with slightly slower growth observed with HY-SOY 4D (Figure 2). Final CPS yields were highest with A3X soy peptone (793 mg/L), followed by HY-SOY 4D soy peptone (742 mg/L), Difco soy peptone (681 mg/L), and Casein Tryptone (695 mg/L) (Table 2).
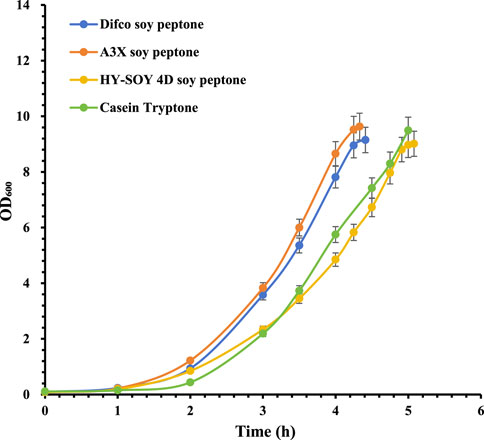
Figure 2. Growth profiles of S. pneumoniae serotype 6B fermentation with various peptones. Difco soy peptone is marked with blue circles; A3X soy peptone is marked with orange circles; HY-SOY 4D soy peptone is marked with yellow circles; Casein Tryptone is marked with green circles. The error bars indicate standard deviation of three replicates.
In serotype 11A, A3X soy peptone showed a slightly higher maximum bacterial density, with HY-SOY 4D soy peptone again showing slower growth (Figure 3). CPS yields were highest with A3X soy peptone (377 mg/L), followed by Difco soy peptone (359 mg/L), Casein Tryptone (328 mg/L), and HY-SOY 4D soy peptone (319 mg/L) (Table 2).
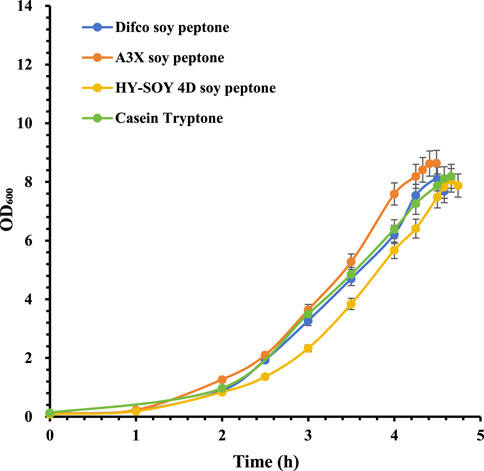
Figure 3. Growth profiles of S. pneumoniae serotype 11A fermentation with various peptones. Difco soy peptone is marked with blue circles; A3X soy peptone is marked with orange circles; HY-SOY 4D soy peptone is marked with yellow circles; Casein Tryptone is marked with green circles. The error bars indicate standard deviation of three replicates.
Serotype 33F showed significant differences among nitrogen sources, with A3X soy peptone providing the highest bacterial density, followed by Difco soy peptone (*P < 0.05), HY-SOY 4D soy peptone (**p < 0.01) and Casein Tryptone (***p < 0.001) (Figure 4). CPS productivity mirrored these trends, with A3X soy peptone yielding 1,565 mg/L, higher than Difco soy peptone (1,335 mg/L), HY-SOY 4D soy peptone (1,215 mg/L), and Casein Tryptone (1,242 mg/L) (Table 2).
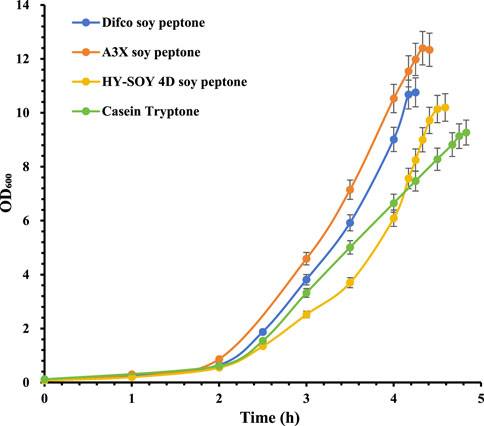
Figure 4. Growth profiles of S. pneumoniae serotype 33F fermentation with various peptones. Difco soy peptone is marked with blue circles; A3X soy peptone is marked with orange circles; HY-SOY 4D soy peptone is marked with yellow circles; Casein Tryptone is marked with green circles. The error bars indicate standard deviation of three replicates.
These results indicate that different soy peptones can moderately affect growth profiles and CPS productivity in S. pneumoniae fermentations. A3X soy peptone from ORGANOTECHINE consistently supported the fast pneumococcal growth and high CPS productivity across the four serotypes, followed by Difco soy peptone, HY-SOY 4D soy peptone and Casein Tryptone. On the contrary, the HY-SOY 4D soy peptone exhibited reduced pneumococcal density and a decelerated growth rate across the majority of serotypes. This characteristic could potentially facilitate a more extended period for the elongation of pneumococcal CPSs.
To determine the effect of nitrogen sources on the quality attributes of pneumococcal CPSs, we conducted three consecutive batches of a uniform fermentation and purification process. The purification process comprised ultrafiltration, acid precipitation, alcohol precipitation, final ultrafiltration and diafiltration, excluding traditional CTAB precipitation, phenol extraction, and costly chromatography steps. This streamlined process significantly reduced costs and time compared to conventional methods. The purified CPSs from each serotype were analyzed to determine the quality attributes of CPSs, including percentage content of components, molecular size, molecular weight, etc.
Analysis of purified CPSs from S. pneumoniae serotypes 6A, 6B, 11A, and 33F revealed that all CPSs met European Pharmacopeia standards regardless of nitrogen source. However, the type of nitrogen source significantly influenced the molecular size, molecular weight and content of functional chemical groups of the pure CPSs (Figures 5, 6). The results from Table 2 indicate that nitrogen source selection significantly influences the molecular size, molecular weight, and methyl pentose content of pneumococcal CPSs for serotypes 6A and 6B, without affecting residual protein and nucleic acid, nitrogen, or phosphorus content. HY-SOY 4D soy peptone supports the synthesis of higher molecular weight CPSs with higher content of methyl pentose, suggesting better immunogenicity. For serotype 11A, nitrogen sources moderately affect molecular size, molecular weight, and O-acetyl group content, with HY-SOY 4D soy peptone promoting higher molecular weight CPSs and higher O-acetyl content. In serotype 33F, nitrogen sources greatly impact molecular size and weight, with HY-SOY 4D soy peptone and Casein tryptone leading to higher molecular weight CPSs.

Figure 5. The comparison of molecular size levels of refined CPS with four different nitrogen sources (Difco soy peptone, A3X soy peptone, HY-SOY 4D soy peptone and casein tryptone) for serotype 6A, 6B, 11A, and 33F (ns: not significant, *p < 0.05, **p < 0.01).
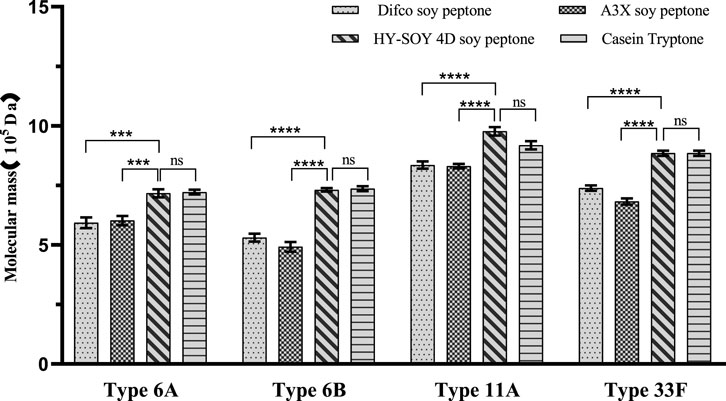
Figure 6. The comparison of molecular mass levels of refined CPS with four different nitrogen sources (Difco soy peptone, A3X soy peptone, HY-SOY 4D soy peptone and casein tryptone) for serotype 6A, 6B, 11A, and 33F (ns: not significant, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001).
Overall, HY-SOY 4D soy peptone can support the synthesis of high molecular weight CPSs, with a higher content of functional chemical groups, which is associated with better immunogenicity (Howard et al., 1971; Loetscher, Mottlau, and Hochuli, 1992; Balázs et al., 2002; MacLennan and Vinuesa, 2002; Craxton et al., 2003; Xiao et al., 2006; Zhao, Zhao, and Xie, 2012; Soubal et al., 2013; Qiong et al., 2022). Despite this, HY-SOY 4D soy peptone is preferred over Casein tryptone due to its plant origin, lower risk of contamination (Calix et al., 2011a; Calix et al. 2011b; Calix et al. 2012; Camilli et al., 2014).
Peptones are derived from the partial hydrolysis of proteins from plant, yeast, or animal sources. The protein hydrolysis process can be achieved by using strong acids, bases and proteolytic enzymes, the final complex, nutritionally enriched product can be easily consumed by microbes and cells. The generic composition of a typical peptone includes 25% polypeptides, 30% free amino acids, 20% carbohydrates, 15% salts and trace metals, and 10% of other components (vitamins, organic acids). While peptones generally share similar components, the specific hydrolysis method and protein source can lead to distinct product profiles. Microorganisms require accessible carbon, nitrogen, phosphate, sulfur, trace metals, and vitamins for growth, which are readily provided by peptones. The choice of peptone must match the microorganism’s needs, with some preferring short-chain polypeptides and others long-chain ones.
HY-SOY 4D soy peptone, Casein tryptone, Difco soy peptone, and A3X soy peptone exhibit unique profiles in total nitrogen (TN), amino nitrogen (AN) Table 3. Their molecular weight distributions also vary significantly. For example, HY-SOY 4D has a molecular weight distribution with 48.6% below 500 Da and 20.4% between 500 and 1,000 Da, while A3X has 77.0% below 500 Da and 21.1% between 500 and 1,000 Da. This difference in small polypeptide percentages may affect S. pneumoniae growth and polysaccharide biosynthesis. Additionally, these peptones differ in their total and free amino acid compositions, which can influence microbial uptake and utilization. HY-SOY 4D contains notably higher levels of glutamic acid (11.5 mg/g) and tyrosine (2.1 mg/g) compared to other peptones. A3X stands out with a higher percentage of free amino acids, which can be directly absorbed by microorganisms, potentially impacting CPS synthesis.
The purified CPSs derived from fermentation with HY-SOY 4D were characterized using 1H NMR spectroscopy, as depicted in Figure 7, and were compared with the structures reported in the literature (Gisch et al., 2013; Geno et al., 2015). The analysis revealed that our prepared CPSs possess a complete structure with a notably low impurity content; specifically, the content of CWPS in all samples is less than 6%. The impurity content within the CPS was determined by integrating the phosphocholine structure on LTA in the 1H NMR spectrum. The acetylation sites of type 11A CPS are known to be complex (Zartler et al., 2009), and the degree of acetylation varies at each site. In conjunction with the literature, it was established that there are four acetylation sites present. Our capsules exhibit 100% acetylation at the sites on glycerol and galactose, and a partial acetylation on glucose. However, the acetyl resonances could not be definitively assigned to specific sites due to their complexity.
As antimicrobial resistance in S. pneumoniae grows and complicates clinical treatment, vaccines become increasingly vital for infection prevention and control (Geno et al., 2015; Ganaie et al., 2020). S. pneumoniae CPSs, as major vaccine antigens, stimulate the production of protective antibodies. However, CPSs must meet stringent quality standards for high immunogenicity and safety (Zhao, Zhao, and Xie, 2012; Jones, 2015; Qiong et al., 2022). Immunogenicity is influenced by factors such as chain length and the content of functional chemical groups like methyl pentoses, phosphate, acetate, and pyruvate (Szu et al., 1991; McNeely et al., 1998; Werz and Seeberger, 2005; Bentley et al., 2006; Fusco et al., 2007; Berti, De Ricco, and Rappuoli, 2018; Anish et al., 2014). These critical quality attributes of CPSs are influenced by the S. pneumoniae genome and production processes (Anish, Beurret, and Poolman, 2021; Hegerle et al., 2018; Kalynych et al., 2011; Stefanetti et al., 2019). Given the complexity of S. pneumoniae fermentation medium and the regulation of CPS synthesis through complex metabolic pathways, few studies have explored the effect of peptone source on the quality attributes of purified CPSs.
Our study evaluated the effects of nitrogen source on the yield and characteristics of CPSs for S. pneumoniae serotype 6A, 6B, 11A, and 33F. We found that all purified CPSs met European Pharmacopeia standards. Different nitrogen sources led to moderate differences in bacterial growth density and CPS productivity but significant differences in molecular size, molecular weight, and content of functional chemical groups of the CPSs. While A3X soy peptone showed higher growth and productivity, HY-SOY 4D soy peptone was superior in synthesizing higher molecular weight CPSs with higher content of functional chemical groups, indicating better immunogenicity. Considering the risks associated with animal-derived Casein tryptone, plant-derived HY-SOY 4D soy peptone is the preferred nitrogen source for fermentation medium. This study identified the selection of peptones as a key fermentation factor affecting S. pneumoniae CPS critical quality attributes, offering insights for optimizing the polysaccharide preparation process and enhancing the immunogenicity of purified polysaccharides.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
YuL: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Software, Validation, Writing–original draft, Writing–review and editing. YaL: Methodology, Writing–review and editing. HY: Methodology, Writing–original draft. YW: Writing–review and editing. YZ: Writing–review and editing. HZ: Conceptualization, Visualization, Writing–review and editing. YnL: Methodology, Writing–review and editing. XY: Writing–review and editing. XC: Methodology, Writing–review and editing. JL: Funding acquisition, Resources, Writing–review and editing.
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by a grant of Beijing Postdoctoral Research Foundation (2023-ZZ-32), Beijing, China.
Authors YuL, YaL, YW, YZ, YnL, XY, XC, and JL were employed by Beijing Minhai Biotechnology Co. Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Aanensen, D. M., Mavroidi, A., Bentley, S. D., Reeves, P. R., and Spratt, B. G. (2007). Predicted functions and linkage specificities of the products of the Streptococcus pneumoniae capsular biosynthetic loci. J. Bacteriol. 189, 7856–7876. doi:10.1128/jb.00837-07
Adamo, R., Nilo, A., Castagner, B., Boutureira, O., Berti, F., and Bernardes, G. J. (2013). Synthetically defined glycoprotein vaccines: current status and future directions. Chem. Sci. 4, 2995–3008. doi:10.1039/c3sc50862e
Al-Jumaili, A., Dawood, H. N., Ikram, D., and Al-Jabban, A. (2023). 'Pneumococcal disease: global disease prevention strategies with a focus on the challenges in Iraq. Int. J. Gen. Med. 16, 2095–2110. doi:10.2147/ijgm.s409476
Anish, C., Beurret, M., and Jan, P. (2021). Combined effects of glycan chain length and linkage type on the immunogenicity of glycoconjugate vaccines. NPJ Vaccines 6, 150. doi:10.1038/s41541-021-00409-1
Anish, C., Schumann, B., Pereira, C. L., and Seeberger, P. H. (2014). Chemical biology approaches to designing defined carbohydrate vaccines. Chem. Biol. 21, 38–50. doi:10.1016/j.chembiol.2014.01.002
Avery, O. T., and Heidelberger, M. (1925). 'Immunological relationships of cell constituents of pneumococcus: second paper. J. Exp. Med. 42, 367–376. doi:10.1084/jem.42.3.367
Avery, O. T., Heidelberger, M., and Goebel, W. F. (1925). The soluble specific substance of friedlander's bacillus: paper II. chemical and immunological relationships of pneumococcus type II and of a strain of friedlander's bacillus. J. Exp. Med. 42, 709–725. doi:10.1084/jem.42.5.709
Balázs, M., Martin, F., Zhou, T., and Kearney, J. (2002). 'Blood dendritic cells interact with splenic marginal zone B cells to initiate T-independent immune responses. Immunity 17, 341–352. doi:10.1016/s1074-7613(02)00389-8
Bentley, S. D., Aanensen, D. M., Mavroidi, A., Saunders, D., Rabbinowitsch, E., Collins, M., et al. (2006). Genetic analysis of the capsular biosynthetic locus from all 90 pneumococcal serotypes. PLoS Genet. 2, e31. doi:10.1371/journal.pgen.0020031
Berti, F., De Ricco, R., and Rappuoli, R. (2018). Role of O-acetylation in the immunogenicity of bacterial polysaccharide vaccines. Molecules 23, 1340. doi:10.3390/molecules23061340
Bröker, M., Berti, F., and Costantino, P. (2016). Factors contributing to the immunogenicity of meningococcal conjugate vaccines. Hum. Vaccin Immunother. 12, 1808–1824. doi:10.1080/21645515.2016.1153206
Calix, J. J., Nahm, M. H., and Zartler, E. R. (2011a). 'Elucidation of structural and antigenic properties of pneumococcal serotype 11A, 11B, 11C, and 11F polysaccharide capsules. J. Bacteriol. 193, 5271–5278. doi:10.1128/jb.05034-11
Calix, J. J., Oliver, M. B., Sherwood, L. K., Beall, B. W., Hollingshead, S. K., and Nahm, M. H. (2011b). Streptococcus pneumoniae serotype 9A isolates contain diverse mutations to wcjE that result in variable expression of serotype 9V-specific epitope. J. Infect. Dis. 204, 1585–1595. doi:10.1093/infdis/jir593
Calix, J. J., Saad, J. S., Brady, A. M., and Nahm, M. H. (2012). Structural characterization of Streptococcus pneumoniae serotype 9A capsule polysaccharide reveals role of glycosyl 6-O-acetyltransferase wcjE in serotype 9V capsule biosynthesis and immunogenicity. J. Biol. Chem. 287, 13996–14003. doi:10.1074/jbc.m112.346924
Camilli, R., Spencer, B. L., Moschioni, M., Pinto, V., Berti, F., Nahm, M. H., et al. (2014). 'Identification of Streptococcus pneumoniae serotype 11E, serovariant 11Av and mixed populations by high-resolution magic angle spinning nuclear magnetic resonance (HR-MAS NMR) spectroscopy and flow cytometric serotyping assay (FCSA). PLoS One 9, e100722. doi:10.1371/journal.pone.0100722
Craxton, A., Magaletti, D., Ryan, E. J., and Clark, E. A. (2003). 'Macrophage- and dendritic cell-dependent regulation of human B-cell proliferation requires the TNF family ligand BAFF. Blood 101, 4464–4471. doi:10.1182/blood-2002-10-3123
Defrance, T., Taillardet, M., and Genestier, L. (2011). 'T cell-independent B cell memory. Curr. Opin. Immunol. 23, 330–336. doi:10.1016/j.coi.2011.03.004
Degeest, B., and De Vuyst, L. (1999). Indication that the nitrogen source influences both amount and size of exopolysaccharides produced by Streptococcus thermophilus LY03 and modelling of the bacterial growth and exopolysaccharide production in a complex medium. Appl. Environ. Microbiol. 65, 2863–2870. doi:10.1128/aem.65.7.2863-2870.1999
Fitzgerald, D., and Waterer, G. W. (2019). 'Invasive pneumococcal and meningococcal disease. Infect. Dis. Clin. North Am. 33, 1125–1141. doi:10.1016/j.idc.2019.08.007
Fusco, P. C., Farley, E. K., Huang, C. H., Moore, S., and Michon, F. (2007). 'Protective meningococcal capsular polysaccharide epitopes and the role of O acetylation. Clin. Vaccine Immunol. 14, 577–584. doi:10.1128/cvi.00009-07
Ganaie, F., Saad, J. S., McGee, L., van Tonder, A. J., Bentley, S. D., Lo, S. W., et al. (2020). A new pneumococcal capsule type, 10D, is the 100th serotype and has a large cps fragment from an oral Streptococcus. mBio 11, 009377-20–e1020. doi:10.1128/mbio.00937-20
Geno, K. A., Gilbert, G. L., Song, J. Y., Skovsted, I. C., Klugman, K. P., Jones, C., et al. (2015). Pneumococcal capsules and their types: past, present, and future. Clin. Microbiol. Rev. 28, 871–899. doi:10.1128/cmr.00024-15
Gisch, N., Kohler, T., Ulmer, A. J., Müthing, J., Pribyl, T., Fischer, K., et al. (2013). Structural reevaluation of Streptococcus pneumoniae Lipoteichoic acid and new insights into its immunostimulatory potency. J. Biol. Chem. 288, 15654–15667. doi:10.1074/jbc.m112.446963
Gonçalves, V. M., Dias, W. O., Campos, I. B., Liberman, C., Sbrogio-Almeida, M. E., Silva, E. P., et al. (2014). 'Development of a whole cell pneumococcal vaccine: BPL inactivation, cGMP production, and stability. Vaccine 32, 1113–1120. doi:10.1016/j.vaccine.2013.10.091
Gonçalves, V. M., Zangirolami, T. C., Giordano, R. L., Raw, I., Tanizaki, M. M., and Giordano, R. C. (2002). 'Optimization of medium and cultivation conditions for capsular polysaccharide production by Streptococcus pneumoniae serotype 23F. Appl. Microbiol. Biotechnol. 59, 713–717. doi:10.1007/s00253-002-1075-8
Grobben, G. J., van Casteren, W. H. M., Arie Schols, H., Oosterveld, A., Sala, G., Smith, M. R., et al. (1997). “Analysis of the exopolysaccharides produced by Lactobacillus delbrueckii subsp. bulgaricus NCFB 2772 grown in continuous culture on glucose and fructose.” Appl. Microbiol. Biotechnol. 48, 516–521. doi:10.1007/s002530051089
Guo, Y., and Qiao, L.-Na (2021). Clinical features and antibiotic sensitivity of invasive pneumococcal disease versus noninvasive pneumococcal disease in children. Zhongguo dang dai er ke za zhi = Chin. J. Contemp. Pediatr. 23, 466–470. doi:10.7499/j.issn.1008-8830.2011125
Gururao, D. S., Allen, H. M., Patrick, K. J., Lasko, D. R., Lomberk, S. E., Jason Arnold, L., et al. (2018). “Media and fermentation methods for producing polysaccharides in bacterial cell culture.” In, edited by China national intellectual property administration, 41. CN.
Hamaguchi, S., Zafar, M. A., Cammer, M., and Weiser, J. N. (2018). Capsule prolongs survival of Streptococcus pneumoniae during starvation. Infect. Immun. 86. doi:10.1128/iai.00802-17
Hefti, M. H., Milder, F. J., Boeren, S., Vervoort, J., and van Berkel, W. J. H. (2003). A His-tag based immobilization method for the preparation and reconstitution of apoflavoproteins. Biochimica biophysica acta 1619, 139–143. doi:10.1016/s0304-4165(02)00474-9
Hegerle, N., Bose, J., Ramachandran, G., Galen, J. E., Levine, M. M., Simon, R., et al. (2018). Overexpression of O-polysaccharide chain length regulators in Gram-negative bacteria using the Wzx-/Wzy-dependent pathway enhances production of defined modal length O-polysaccharide polymers for use as haptens in glycoconjugate vaccines. J. Appl. Microbiol. 125, 575–585. doi:10.1111/jam.13772
Howard, J. G., Zola, H., Christie, G. H., and Courtenay, B. M. (1971). Studies on immunological paralysis. V. The influence of molecular weight on the immunogenicity, tolerogenicity and antibody-neutralizing activity of the 3 pneumococcal polysaccharide. Immunology 21, 535–546.
Hyams, C., Camberlein, E., Cohen, J. M., Bax, K., and Brown, J. S. (2010). The Streptococcus pneumoniae capsule inhibits complement activity and neutrophil phagocytosis by multiple mechanisms. Infect. Immun. 78, 704–715. doi:10.1128/iai.00881-09
Jha, V., and Janoff, E. N. (2019). Complementary role of CD4+ T cells in response to pneumococcal polysaccharide vaccines in humans. Complementary role CD4+ T cells response pneumococcal polysaccharide vaccines humans', Vaccines 7, 18. doi:10.3390/vaccines7010018
Jones, C. (2015). Glycoconjugate vaccines: the regulatory framework. Methods Mol. Biol. Clift. N.J. 1331, 229–251. doi:10.1007/978-1-4939-2874-3_14
Jones, C., and Currie, F. (1991). Control of components of bacterial polysaccharide vaccines by physical methods. Biologicals 19, 41–47. doi:10.1016/1045-1056(91)90023-d
Kabat, E. A., and Bezer, A. E. (1958). The effect of variation in molecular weight on the antigenicity of dextran in man. Arch. Biochem. Biophys. 78, 306–318. doi:10.1016/0003-9861(58)90354-0
Kadioglu, A., Weiser, J. N., Paton, J. C., and Andrew, P. W. (2008). The role of Streptococcus pneumoniae virulence factors in host respiratory colonization and disease. Nat. Rev. Microbiol. 6, 288–301. doi:10.1038/nrmicro1871
Kalynych, S., Ruan, X., Valvano, M. A., and Cygler, M. (2011). Structure-guided investigation of lipopolysaccharide O-antigen chain length regulators reveals regions critical for modal length control. J. Bacteriol. 193, 3710–3721. doi:10.1128/jb.00059-11
Kolkman, M. A., van der Zeijst, B. A., and Nuijten, P. J. (1998). 'Diversity of capsular polysaccharide synthesis gene clusters in Streptococcus pneumoniae. J. Biochem. 123, 937–945. doi:10.1093/oxfordjournals.jbchem.a022028
Lees, J. A., Croucher, N. J., Goldblatt, D., Nosten, F., Parkhill, J., Turner, C., et al. (2017). 'Genome-wide identification of lineage and locus specific variation associated with pneumococcal carriage duration. eLife 6, e26255. doi:10.7554/elife.26255
Li, Y., Xu, Y., Cao, X., Wang, Y., Wang, J., Zhao, Yi, et al. (2024). Optimization of manufacturing process for serotype 14 pneumococcal capsular polysaccharide. Front. Chem. Eng. 6. doi:10.3389/fceng.2024.1481257
Liberman, C., Takagi, M., Cabrera-Crespo, J., Sbrogio-Almeida, M. E., Dias, W. O., Leite, L. C. C., et al. (2008). 'Pneumococcal whole-cell vaccine: optimization of cell growth of unencapsulated Streptococcus pneumoniae in bioreactor using animal-free medium. J. Ind. Microbiol. Biotechnol. 35, 1441–1445. doi:10.1007/s10295-008-0445-3
Loetscher, P., Mottlau, L., and Hochuli, E. (1992). Immobilization of monoclonal antibodies for affinity chromatography using a chelating peptide. J. Chromatogr. 595, 113–119. doi:10.1016/0021-9673(92)85151-i
MacLennan, I., and Vinuesa, C. (2002). 'Dendritic cells, BAFF, and APRIL: innate players in adaptive antibody responses. Immunity 17, 235–238. doi:10.1016/s1074-7613(02)00398-9
McNeely, T. B., Staub, J. M., Rusk, C. M., Blum, M. J., and Donnelly, J. J. (1998). 'Antibody responses to capsular polysaccharide backbone and O-acetate side groups of Streptococcus pneumoniae type 9V in humans and rhesus macaques. Infect. Immun. 66, 3705–3710. doi:10.1128/iai.66.8.3705-3710.1998
Micoli, F., Stefanetti, G., and MacLennan, C. A. (2023). Exploring the variables influencing the immune response of traditional and innovative glycoconjugate vaccines. Front. Mol. Biosci. 10, 1201693. doi:10.3389/fmolb.2023.1201693
Mond, J. J., Lees, A., and Snapper, C. M. (1995b). T cell-independent antigens type 2. Annu. Rev. Immunol. 13, 655–692. doi:10.1146/annurev.immunol.13.1.655
Mond, J. J., Vos, Q., Lees, A., and Snapper, C. M. (1995a). 'T-cell independent antigens. Curr. Opin. Immunol. 7, 349–354. doi:10.1016/0952-7915(95)80109-x
Morais, V., Dee, V., and Suárez, N. (2018). Purification of capsular polysaccharides of Streptococcus pneumoniae: traditional and new methods. Front. Bioeng. Biotechnol. 6, 145. doi:10.3389/fbioe.2018.00145
Morais, V., Texeira, E., and Suarez, N. (2019). 'Next-Generation whole-cell pneumococcal vaccine. Vaccines (Basel) 7, 151. doi:10.3390/vaccines7040151
Paton, J. C., and Trappetti, C. (2019). 'Streptococcus pneumoniae capsular polysaccharide. Microbiol. Spectr. 7. doi:10.1128/microbiolspec.gpp3-0019-2018
Qiong, C., Ji-Chun, S., Wang, C.-E., Shan-Shan, W., and Qiang, Ye (2022). Current status and prospect of quality control of 23-valent pneumococcal polysaccharide vaccine. Drug Stand. China 023. doi:10.19778/j.chp.2022.02.010
Restrepo, A. V., Salazar, B. E., Agudelo, M., Rodriguez, C. A., Zuluaga, A. F., and Omar, V. (2005). 'Optimization of culture conditions to obtain maximal growth of penicillin-resistant Streptococcus pneumoniae. BMC Microbiol. 5, 34. doi:10.1186/1471-2180-5-34
Soubal, J. P., Peña, L., Santana, D., Valdés, Y., García, D., Pedroso, J., et al. (2013). Procedure for the conjugation of the Streptococcus pneumoniae serotype 6B capsular polysaccharide to the tetanus toxoid. Biotecnol. Apl. 30, 208–215.
Stefanetti, G., Okan, N., Fink, A., Gardner, E., and Kasper, D. L. (2019). Glycoconjugate vaccine using a genetically modified O antigen induces protective antibodies to Francisella tularensis. Proc. Natl. Acad. Sci. U. S. A. 116, 7062–7070. doi:10.1073/pnas.1900144116
Szu, S. C., Li, X. R., Stone, A. L., and Robbins, J. B. (1991). Relation between structure and immunologic properties of the Vi capsular polysaccharide. Infect. Immun. 59, 4555–4561. doi:10.1128/iai.59.12.4555-4561.1991
Talaga, P., Vialle, S., and Moreau, M. (2002). 'Development of a high-performance anion-exchange chromatography with pulsed-amperometric detection based quantification assay for pneumococcal polysaccharides and conjugates. Vaccine 20, 2474–2484. doi:10.1016/s0264-410x(02)00183-4
Texeira, E., Checa, J., Rial, A., Chabalgoity, J. A., and Suarez, N. (2015). A new chemically defined medium for cultivation of Streptococcus pneumoniae serotype 1. J. Biotech Res., 54–62.
Vos, Q., Lees, A., Wu, Z. Q., Snapper, C. M., and Mond, J. J. (2000). 'B-cell activation by T-cell-independent type 2 antigens as an integral part of the humoral immune response to pathogenic microorganisms. Immunol. Rev. 176, 154–170. doi:10.1034/j.1600-065x.2000.00607.x
Weiser, J. N., Ferreira, D. M., and Paton, J. C. (2018). Streptococcus pneumoniae: transmission, colonization and invasion. Nat. Rev. Microbiol. 16, 355–367. doi:10.1038/s41579-018-0001-8
Werz, D. B., and Seeberger, P. H. (2005). Total synthesis of antigen bacillus anthracis tetrasaccharide-creation of an anthrax vaccine candidate. Angew. Chem. Int. Ed. Engl. 44, 6315–6318. doi:10.1002/anie.200502615
Xiao, L., Fang, Y., Cheng, L., and Kong, J. (2006). Advances in the development of meningococcal vaccines. Prog Microbiol Immunol 34, 3. doi:10.13309/j.cnki.pmi.2006.01.014
Zartler, E. R., Porambo, R. J., Anderson, C. L., Chen, L. H., Yu, J., and Nahm, M. H. (2009). Structure of the capsular polysaccharide of pneumococcal serotype 11A reveals a novel acetylglycerol that is the structural basis for 11A subtypes. J. Biol. Chem. 284, 7318–7329. doi:10.1074/jbc.m807952200
Zeidan, A. A., Poulsen, V. K., Janzen, T., Buldo, P., Derkx, P. M. F., Øregaard, G., et al. (2017). 'Polysaccharide production by lactic acid bacteria: from genes to industrial applications. FEMS Microbiol. Rev. 41, S168–s200. doi:10.1093/femsre/fux017
Keywords: Streptococcus pneumoniae, capsular polysaccharide, nitrogen source, yield, chain length, functional chemical group
Citation: Li Y, Liu Y, Yao H, Wang Y, Zhou Y, Zheng H, Liu Y, You X, Cao X and Liu J (2025) Effect of nitrogen sources on the yield and quality attributes of capsular polysaccharides in Streptococcus pneumoniae. Front. Chem. Eng. 7:1518165. doi: 10.3389/fceng.2025.1518165
Received: 28 October 2024; Accepted: 26 February 2025;
Published: 12 March 2025.
Edited by:
Muhammad Rizwan Javed, Government College University, PakistanReviewed by:
Lance Edward Keller, University of Mississippi Medical Center, United StatesCopyright © 2025 Li, Liu, Yao, Wang, Zhou, Zheng, Liu, You, Cao and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xin Cao, Y2FveGluQGJpb21pbmhhaS5jb20=; Jiankai Liu, bGl1amlhbmthaUBiaW9taW5oYWkuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.