
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Chem. Biol. , 30 May 2024
Sec. Structure, Spectroscopy & Imaging
Volume 3 - 2024 | https://doi.org/10.3389/fchbi.2024.1400642
This article is part of the Research Topic Bridging Chemistry with Biology through Speciation Chemistry in Important Processes Involving Essential and Non-essential Metal Ions: Developed from the ICBIC 2023 View all 3 articles
The presence of isomers is a huge challenge in the development of new medicinal or pharmaceutical agents because the main goal is to obtain the most active compound with high purity and yield. Tautomerism is a phenomenon quite common in biomolecules that also appears in many drugs, and strategies to control the corresponding desired species and related equilibrium conditions leading to efficient chemical speciation are frequently required. There are many reports in the literature about the presence of tautomers, although some articles do not properly emphasize their occurrence or their importance for the differences verified in biological activity. Herein, a discussion about tautomers observed in both metalated and non-metalated compounds and their importance in the biological properties of promising drugs is revisited. Mainly, keto-enol equilibria among imines, hydrazones, and oxindole derivatives are showcased, based on significant examples, and strategies to improve their speciation or to better elucidate their modes of action are suggested.
Speciation of an element refers to its distribution among defined chemical species formed or present in a system, according to the IUPAC definition (Templeton et al., 2000). Elemental speciation is commonly described by considering isotopic composition, oxidation state, inorganic, organic, and organometallic structures, and complexes with macromolecules (Templeton and Fujishiro, 2017). In many areas, particularly in catalysis, synthesis, separation of intermediate products, and pharmacology, the precise characterization of formed chemical species is crucial to achieving the best results in developing more efficient catalysts or more effective medicinal agents.
Isomers always constitute an important challenge in the synthesis and isolation of desired products. Selective formation of one of the isomers is sometimes possible in high purity and yield by choosing adequate conditions to shift the equilibrium to the more efficient isomeric form when performing the reactions. This is mostly important when a selected biological target is uniquely bound by only one of the isomeric species, and a more effective interaction with inhibitors is crucial.
Tautomers occur in many simple molecules and biomolecules, differing from each other frequently by protonation of the molecules in solution and, therefore, in a dependent correlation with the pH (Martin, 2018). Nucleobases can present tautomers that differ in the position of protons, and such structural differences play crucial roles in their hydrogen bonding interactions, leading to altered base pairing and consequently to serious effects on biochemical processes where nucleic acids are involved. For instance, minor tautomers that are transiently formed during replication could generate undesirable mutations (Fedeles et al., 2022).
Tautomeric equilibria are also intensely dependent on the dielectric constant of the medium and the ability of solvents to form hydrogen bonds with each tautomer. More polar solvents typically favor the more polar tautomer. Different techniques, mainly nuclear magnetic resonance (NMR), infrared (IR), ultraviolet (UV), Raman, X-ray diffraction, and terahertz spectroscopies are useful and have been used to monitor tautomers (Bharatam et al., 2023). A comprehensive tautomer database included more than 2,800 tautomeric structures, mainly prototropic tautomerism (79%) but also ring-chain and valence tautomerism (Dhaked et al., 2020).
Diverse classes of compounds with biological activity display tautomerism, such as oximes, oxindoles, diketones, and hydrazones, and are potentially valuable as building blocks in medicinal chemistry. Some of them have entered clinical tests, and a few of these have been approved as therapeutics (Bharatam et al., 2023), such as piroxicam, chlorthalidone, favipiravir, and topotecan (Sanna et al., 2005), discussed in Section 2. In keto-enol tautomerization, a carbonyl double bond (keto form) is interconverted to an alkene double bond (enol form), simultaneously with the shift of the alpha proton in the keto form to the hydroxyl group in the enol form. A few examples of keto-enol tautomers with some pharmacological or medicinal interests are shown in Scheme 1. Frequently, more than two tautomers can be formed.
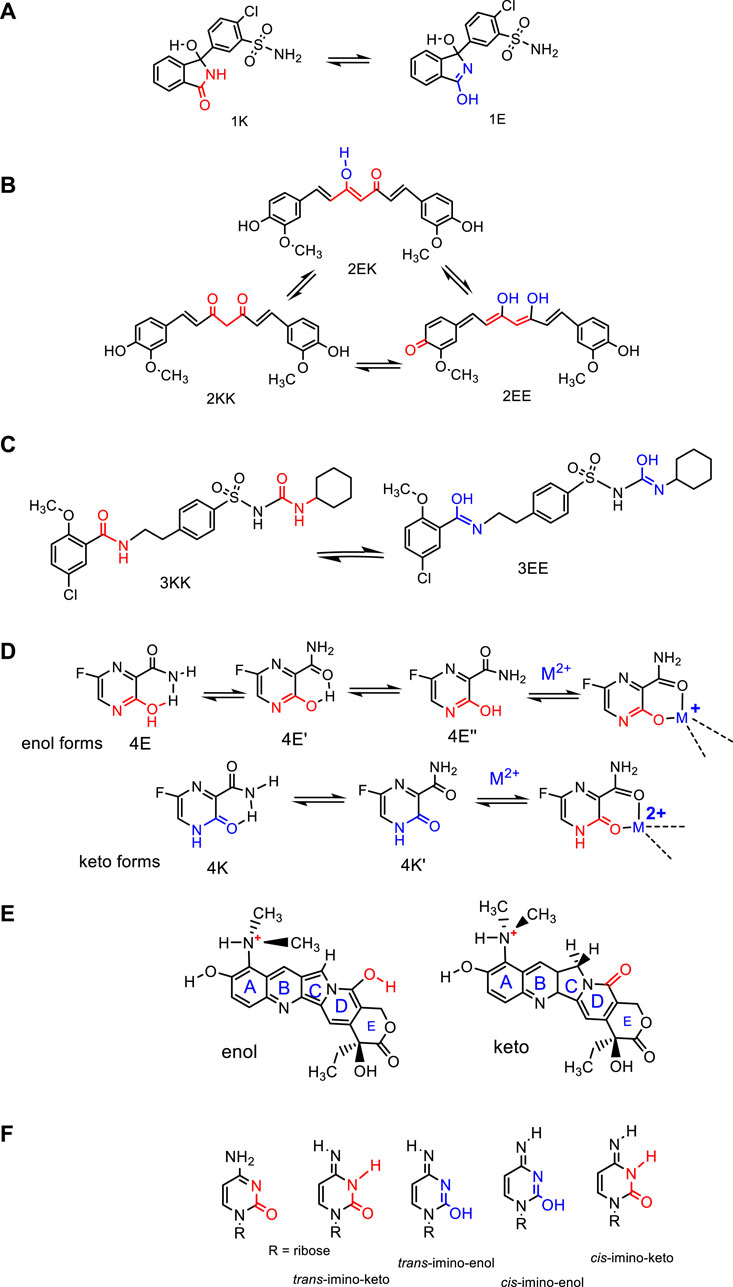
Scheme 1. Keto-enol tautomers occurring in molecules with some pharmacological or medicinal interest: (A) Chlorthalidone, a drug to treat heart failure and renal impairment (Temperini et al., 2009); (B) curcumin (Manolova et al., 2014); (C) glibenclamide, an antidiabetic agent (Wolnica et al., 2019); (D) favipiravir, an anti-influenza drug (Deneva et al., 2023), and (E) topotecan, the most commonly used agent for the treatment of ovarian carcinoma (Sanna et al., 2005). (F) Possible amino-imino and keto-enol tautomers of nucleobase cytosine.
In solution, tautomerism involves dynamic equilibria between two or more structurally different molecular forms in which a proton transfer (prototropy) usually occurs. Therefore, tautomers coexist as a mixture of possible compounds in concentrations proportional to their relative stability in a particular solvent. They usually show different properties such as water solubility, acid-base character, partition coefficient, or drug effectiveness. Tautomerization usually converts a hydrogen bond donor into an acceptor while simultaneously changing a hydrogen bond acceptor into a donor (Martin, 2018).
β-diketones constitute an early typical example of ligands capable of exhibiting keto-enol tautomerism, and because enolates can be easily formed, they give rise to metal complexes with several divalent metal ions (as Mn, Fe, Co, Ni, Zn, Cd, and Mg) acting as efficient Lewis acid catalysts, in addition to showing interesting structural features, and spectroscopic and magnetic properties (Graddon, 1969). These complexes commonly form octahedral, tetragonal, square planar, or tetrahedral structures, depending on the metal ion and on the steric effects caused by bulky ligands. With Lewis base molecules as water or amines, β-diketones can also form square-pyramidal adducts. In anhydrous conditions, they can even form polymeric species where the ligand act as a bridge between metal centers through their oxygen atoms (Hansen, 2021).
Recently, a novel strategy was developed to probe the G-protein coupled receptor domain rotation by introducing a tri-fluorinated group in a diketone compound and exploring the 19F NMR signal variability and high sensitivity to changes in its surrounding environment based on keto-enol equilibrium (Wang H. et al., 2021). In this case, three tautomers are possible (enol-RCOCHCOHCF3, enol-RCOHCHCOCF3, and diketo-RCOCH2COCF3), and the fluorinated derivative marker showed very distinct, not overlapping signals that were extremely sensitive to the microenvironment of the protein. Therefore, the opposite movements of different receptor domains could be monitored spectroscopically relative to the protein hydrophobic core as a result of an existent tautomeric equilibrium.
In many reported cases, the desirable most active tautomer was not appropriately detected and characterized, or its importance in explaining the mode of action is not evident. Sometimes, the biological target is found to interact with a less stable tautomer instead of the expected predominant one in solution.
Herein, a selected series of molecules with potential application as pharmacological or medicinal agents that show possible tautomeric species are discussed, with the aim of better understanding their influence in interactions with main biological targets in their mechanisms of action and pointing out some strategies to achieve the most effective or desired tautomer. These molecules display tautomerism even when metalated, although the coordination to metal ions frequently leads to the preferential stabilization of one of the tautomers.
Over the last decades, the influence of tautomerism in drug discovery has elicited a huge interest, discussed in terms of thermodynamic and kinetic aspects, where different methodologies, mainly NMR but also infrared, were used to monitor tautomeric equilibria in solution (Claramunt et al., 2006; Alkorta et al., 2020), and often including support by extensive simulation studies (Katritzky et al., 2010; Göller, 2022). An extensive open-source database reporting measured and estimated tautomer ratios, mainly in water, is available (Wahl and Sander, 2020). A hybrid quantum-classical workflow was proposed to predict the relative stability of tautomers to identify the dominant species (Shee et al., 2023). Recently, complex data mining on tautomeric polymorphism in crystal structure databases was reported (Woods-Ryan et al., 2023), extending its interest beyond aqueous solutions or solvents to the solid state.
There are some interesting examples of drugs in which the presence of tautomers is crucial for their medicinal properties. Sulfonamides such as the therapeutic chlorthalidone (shown in Scheme 1A), a widely prescribed drug in the United States, used to treat high blood pressure, swelling, diabetes insipidus, and renal impairment, show an irregular pattern of inhibition toward carbonic anhydrase, an enzyme that presents several isoforms (Temperini et al., 2009). X-ray crystal structure analysis revealed the effect of tautomerization on the selectivity of inhibition, with the enol form being the most effective through the formation of strong hydrogen bonds with residues Thr200 and Asn67, besides three water molecules at W142, W146, and W161 (2.54–3.16 Å) in the active site of the enzyme.
Curcumin (shown in Scheme 1B), found in an Asian Curcuma longa plant, shows diverse therapeutic properties, being investigated as an anti-inflammatory, antiviral, antibacterial, antifungal, and anticancer agent. It also shows tautomeric equilibria (see Scheme 1) involving three tautomers (Manolova et al., 2014). The effectiveness of the pharmacological activity of curcumin is limited by its poor solubility, fast metabolism, fast systemic elimination, and, therefore, low bioavailability. Therefore, different techniques and analytical methods for improving curcumin formulations in diverse matrices have been developed, trying to improve its biological activity (Urošević et al., 2022). A strong predominance of the KE tautomer in solid state and in organic solvents was reported in many studies. However, when inserted in a calix[4]arene host system, results indicated that the binding of the KK tautomer is more favorable (by 1.7 kcal/mol in water) than the binding of the preferred tautomeric KE form (Angelova and Antonov, 2017).
Topotecan (Hycamtin; GlaxoSmithKline), an antitumor agent from the class of camptothecins, is commonly used against ovarian carcinoma and was also approved for the treatment of small-cell lung cancer, which is a lethal malignancy (shown in Scheme 1E). It shows three different types of enol and keto tautomers and has topoisomerase I, an essential enzyme in DNA replication, transcription, and recombination, as the main target (Sanna et al., 2005).
Piroxicam, a non-steroidal anti-inflammatory drug described as very effective against various inflammatory conditions, can exist in seven different tautomeric forms, and it seems that the enol-amide form is the most stable, present in ethanol or DMSO solution as a monomer-dimer equilibrium. However, in addition to its therapeutic uses, there are some reports of undesirable effects correlated to its structural characteristics (Ivanova et al., 2015). Only its neutral species was suggested to be biologically active, and by the addition of water, the equilibrium shifted to the zwitterionic tautomer. Therefore, it is crucial to understand the conversion between tautomers to define the desired drug action and its better mode of delivery.
Favipiravir, an anti-influenza drug (see Scheme 1D) that was also tested against the SARS-CoV-2 virus, had its tautomers studied by molecular spectroscopy (UV/Vis absorption, fluorescence, and NMR), as well as by DFT quantum chemical calculations (Deneva et al., 2023). The enol form is the most stable in many organic solvents, although the equilibrium is shifted in the presence of water, and a keto form appears to be favored due to drug–solvent interactions. With the addition of Mg2+ or Ca2+ ions, complexes [ML2] are formed at a 2:1 ligand:metal ratio, both in the enol or keto tautomeric state, with coordination by an amide -C=O(NH2) and a keto group, or by an amide -C=O(NH2) and an enol group. It seems that a simultaneous tautomeric transition and complexation occur, preceded by a change from the most stable enol form to the keto form or by a deprotonation followed by complexation in the case of the enol tautomer, as shown in Scheme 1D (Deneva et al., 2023).
In the development of new promising drugs, a wide range of different ligands and corresponding metal complexes have been investigated regarding the occurrence of tautomerism.
Oximes exhibit diverse biological and pharmacological applications, including antibacterial, antifungal, anti-inflammatory, antioxidant, and anticancer activities, and usually are found in the form of interconvertible E and Z stereoisomers, showing different physical and pharmacological properties (Dhuguru et al., 2022). Studies usually indicated that Z-isomers are more stable and predominant than E-isomers. Oximes can also show three main tautomeric forms: oximes, nitrones, and nitroso compounds. Particularly, some derivatives of indirubin, an indole-based oxime (Wang X. et al., 2021), have been broadly investigated for their anticancer activity, probably due to their strong affinity for cyclin-dependent kinases (CDKs), proteins with an essential role in controlling cell cycle and proliferation (Knockaert et al., 2002). Different pharmacological inhibitors of CDKs that contain an oxindole moiety in their structure show possible tautomers and are being tested for diverse diseases (cancer, neurodegenerative, cardiovascular, parasitic, or viral infections). Indirubin itself has 26 possible E/Z isomers and tautomers, some of which are more stable than others (Alkorta et al., 2020). A recent publication reports a very rapid keto-enol tautomerization (<1 ps) when an oxime group is introduced in an indirubin derivative, promoting an excited-state proton transfer process and additionally improving the photostability of the compound compared to the precursor (Nobre et al., 2024).
Hydrazones constitute another versatile class of compounds that show tautomers and are used in a wide range of biological applications as antibacterials, antiprotozoals, antifungals, antituberculars, anticonvulsants, anti-inflammatories, cytotoxic agents, antimalarials, antivirals, and vasodilator agents (Sztanke et al., 2013). Aroylhydrazones exhibiting keto-enol tautomerism formed a series of deprotonated enolate species with marked antitumor activity. This activity was significantly improved when coordinated with copper(II) ions and having a pyridine as a co-ligand (Gou et al., 2017). In methanol solution of free ligands, the keto form is more stable, with tautomerization energy being approximately a few kcal/mol, according to quantum chemical calculations. In contrast, the inverse conversion of enol to keto forms was predicted to require more than 60 kcal/mol (Gou et al., 2017). Similar results were observed with tridentate hydrazone-zinc(II) and manganese(II) complexes; [ML2] in distorted octahedral species was isolated in enolate forms where a strong intermolecular H-bonding interaction between two ligand units occurs (Ray et al., 2008).
In our studies of oxindole derivatives, tautomers of corresponding imines and hydrazones (shown in Scheme 2) were found to have a crucial role in their biological activities. Oxindole groups show intrinsic keto-enol tautomerism, and this characteristic remains in its derivatives, such as imines or hydrazones, as well as in corresponding metalated species (da Silveira et al., 2008; da Silveira et al., 2009). Particularly when coordinated with essential metal ions such as copper or zinc, the influence of their structural features on the reactivity exhibited was critical. This influence took the form of improving its biological activities, mainly by improving its ability to make hydrogen bonds with targeted biomolecules.
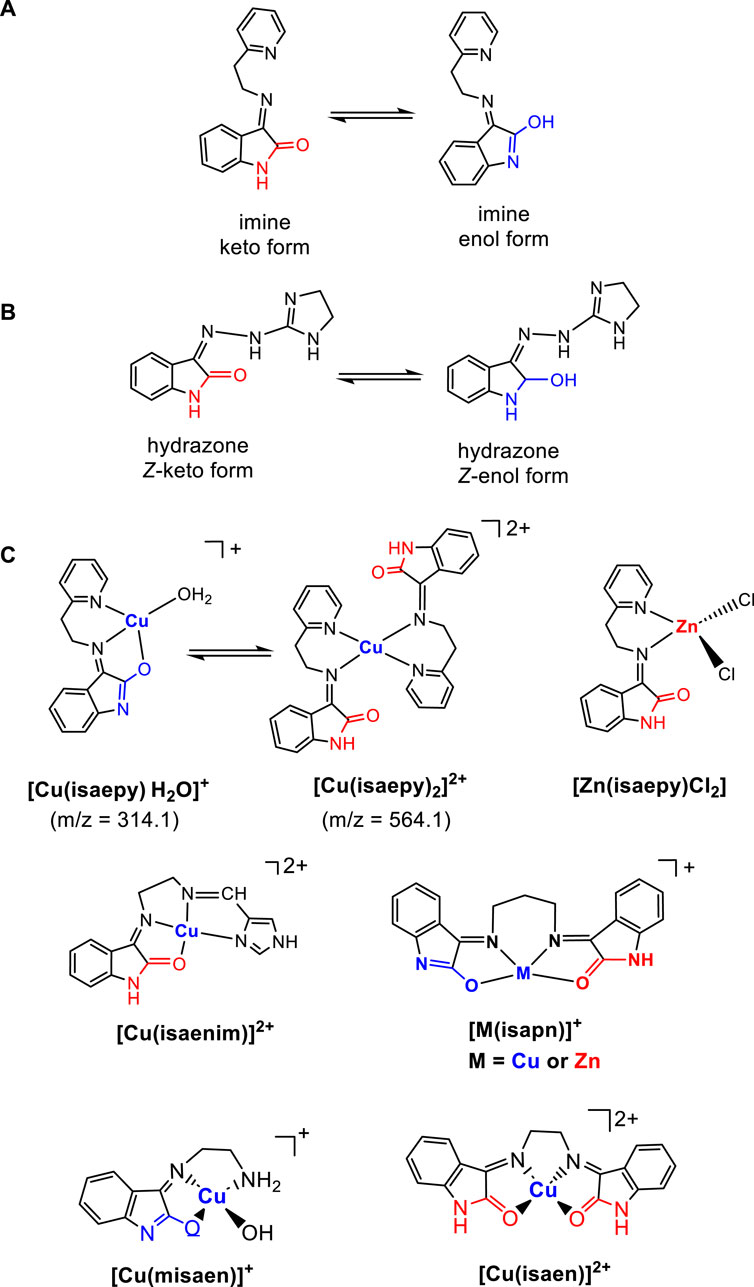
Scheme 2. Some oxindolimine (A) and hydrazone (B) ligands and corresponding metal complexes (C) showing keto-enol tautomerism. Both copper(II) complexes with ligand isaepy were isolated, monitored, and characterized by ESI-MS measurements, showing both signals due to tautomeric equilibrium (M:L 1:1, enol form, and M:L 1:2, keto form). Other metal complexes were isolated in one of the tautomeric forms.
In aqueous solution, the keto forms of free oxindolimine ligands are favored, isolated preferentially at pH 5 (Cerchiaro et al., 2005). The proportion of the enol form increases at pH ≥ 8. In contrast, the coordination of such ligands to metal ions stabilizes mostly the corresponding enol forms. Particularly, complexes with enol tautomers of tridentate ligands, as ligand isaepy (see Scheme 2), seem to be the most active due to their more planar structure and the presence of a labile water molecule in the coordination sphere. At physiological pH, those tautomers show stronger donor–acceptor interactions with biomolecules such as DNA or proteins through hydrogen bond formation.
A recent study on the tautomerism observed in glibenclamide (see Scheme 1C), an important pharmaceutical for diabetes mellitus (type 2), indicated that the amide-to-imide ratio was different if the active pharmaceutical was prepared in the glass state (bulk system) or in the form of spin-coated homogeneously distributed thin films with various thickness (49–220 nm) (Wolnica et al., 2019). Kinetic curves obtained from infrared data for the bulk system follow the sigmoidal shape, characteristic of the autocatalytic reaction, while results obtained for the confined samples provide exponential character, indicating first-order transformation. It seems that the autocatalytic nature of tautomerization in bulk samples arises from the initial formation of the amide form that further catalyzes the amide-imide transformation.
In our studies on supported oxindolimine-copper(II) complexes, we verified that the tautomeric equilibria can be shifted not only in solution by pH variation but also by interactions of the metal complexes with different inorganic matrices.
In solution, oxindolimine-metal complexes have their properties as antitumor agents modulated by both the nature of the metal ion and by the structural features of the coordinated ligand in a synergistic way. When inserted or supported in inorganic matrices, such as beidellite clay or functionalized silica (MCM- and MCM-atzac) materials, the tautomeric equilibria suffered some remarkable shifts, as monitored by IR and Raman spectroscopies (Vieira et al., 2019). The hybrid materials MCM-[Zn(isapn)] and MCM-atzac-[Zn(isapn)] show high discrepancy when compared to the free [Zn(isapn)]+ spectral profile due to H-bond interactions with the matrix, probably indicating a preferential stabilization of one of the tautomers, probably the keto-enol form. In contrast, a remarkable similarity of results with MCM-[Cu(isapn)] and MCM-atzac-[Cu(isapn)] compared to those of free copper complex attested to the absence of such interaction factors. When supported in beidellite, the asymmetric keto-enol form of ligand isapn is favored (Couto et al., 2023) for both copper(II) and zinc(II) compounds.
Studies of the influence of analogous hydrazones derived from oxindoles in the aggregation inhibition of β−amyloid peptides, enhanced in the presence of metal ions such as copper, zinc, or iron, indicated the possibility of six isomers, detected by NMR spectroscopy among the E- and Z-forms, and corresponding keto-enol tautomers (Wegermann et al., 2022). DFT simulations supported the experimental results, attesting that the most stable isomers in solution are the keto isomers in corresponding Z-forms. However, these studies also attested that there is a proton transfer from the hydrazone ligand to the amyloid peptide (Aβ1-16), interfering in the tautomeric equilibrium, favoring the enol form (Wegermann et al., 2023). A pioneering work on curcumin binding to amyloid aggregates reports the predominant presence of the enol form and that the enolization is crucial for this interaction, making it a powerful tool for the detection of and therapy for Alzheimer’s disease (Yanagisawa et al., 2010).
Tautomeric equilibria continue to elicit great interest in both chemical (structural and spectroscopic features) and biological studies (interactions and activity). Different classes of ligands (imines, hydrazones, polyphenols, oximes, and oxindoles) exhibit tautomers that show different reactivity toward biomolecules and, thus, significantly influence their properties and applications. More in-depth studies could provide better conditions for developing new and more efficient compounds regarding each of the possible tautomeric forms in order to optimize their syntheses and explore their potential medicinal uses more accurately.
AF: conceptualization, formal analysis, funding acquisition, investigation, supervision, writing–original draft, and writing–review and editing. AA: formal analysis, investigation, writing–original draft, and writing–review and editing. CW: formal analysis, investigation, writing–original draft, and writing–review and editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. The authors are thankful to the Sao Paulo State Research Foundation (FAPESP, project CEPID Redoxoma, grant 2013/07937-8) for financial support. They are also grateful to Portal CAPES for providing access to the literature.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author AF declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Alkorta, I., Elguero, J., Dardonville, C., Reviriego, F., Santa María, D., Claramunt, R. M., et al. (2020). A theoretical and spectroscopic (NMR and IR) study of indirubin in solution and in the solid state. J. Phys. Org. Chem. 33, 4043. doi:10.1002/poc.4043
Angelova, S., and Antonov, L. (2017). Molecular insight into inclusion complex formation of curcumin and calix[4]arene. Chem. Sel. 2, 9658–9662. doi:10.1002/slct.201701865
Bharatam, P. V., Valanju, O. R., Wani, A. A., and Dhaked, D. K. (2023). Importance of tautomerism in drugs. Drug Discov. Today 28, 103494. doi:10.1016/j.drudis.2023.103494
Cerchiaro, G., Aquilano, K., Filomeni, G., Rotilio, G., Ciriolo, M. R., and Da Costa Ferreira, A. M. (2005). Isatin-Schiff base copper(II) complexes and their influence on cellular viability. J. Inorg. Biochem. 99, 1433–1440. doi:10.1016/j.jinorgbio.2005.03.013
Claramunt, R. M., López, C., Santa María, M. D., Sanz, D., and Elguero, J. (2007). The use of NMR spectroscopy to study tautomerism. ChemInform 38, 169–206. doi:10.1002/chin.200711279
Couto, R. A. A., Miguel, R. B., Vieira, E. G., Brendlé, J., Limousy, L., Constantino, V. R. L., et al. (2023). Synthetic beidellite clay as nanocarrier for delivery of antitumor oxindolimine-metal complexes. J. Inorg. Biochem. 240, 112099. doi:10.1016/j.jinorgbio.2022.112099
Deneva, V., Slavova, S., Kumanova, A., Vassilev, N., Nedeltcheva-Antonova, D., and Antonov, L. (2022). Favipiravir—tautomeric and complexation properties in solution. Pharmaceuticals 16, 45. doi:10.3390/ph16010045
da Silveira, V., Silva, J. P., Oliveira, C. C., Graziani, I., Ciriolo, M. R., and Da Costa Ferreira, A. M. (2008). Double-strand DNA cleavage induced by Oxindole-Schiff base copper(II) complexes with potential antitumor activity. J. Inorg. Biochem. 102, 1090–1103. doi:10.1016/j.jinorgbio.2007.12.033
da Silveira, V., Caramori, G. F., Abbott, M. P., Gonçalves, M. B., Petrilli, H. M., and Da Costa Ferreira, A. M. (2009). Oxindole-Schiff base copper(II) complexes interactions with human serum albumin: spectroscopic, oxidative damage, and computational studies. J. Inorg. Biochem. 103, 1331–1341. doi:10.1016/j.jinorgbio.2009.05.015
Dhaked, D. K., Guasch, L., and Nicklaus, M. C. (2020). Tautomer database: a comprehensive resource for tautomerism analyses. J. Chem. Inf. Model. 60, 1090–1100. doi:10.1021/acs.jcim.9b01156
Dhuguru, J., Zviagin, E., and Skouta, R. (2022). FDA-approved oximes and their significance in medicinal chemistry. Pharmaceuticals 15, 66. doi:10.3390/ph15010066
Fedeles, B. I., Li, D., and Singh, V. (2021). Structural insights into tautomeric dynamics in nucleic acids and in antiviral nucleoside analogs. Front. Mol. Biosci. 8, 823253. doi:10.3389/fmolb.2021.823253
Göller, A. H. (2022). Reliable gas-phase tautomer equilibria of drug like molecule scaffolds and the issue of continuum solvation. J. Comput. Aided Mol. Des. 36, 805–824. doi:10.1007/s10822-022-00480-3
Gou, Y., Li, J., Fan, B., Xu, B., Zhou, M., and Yang, F. (2017). Structure and biological properties of mixed-ligand Cu(II) Schiff base complexes as potential anticancer agents. Eur. J. Med. Chem. 134, 207–217. doi:10.1016/j.ejmech.2017.04.026
Graddon, D. P. (1969). Divalent transition metal β-keto-enolate complexes as lewis acids. Coord. Chem. Rev. 4, 1–28. doi:10.1016/S0010-8545(00)80090-9
Guimarães Vieira, E., Miguel, R. B., Rodrigues da Silva, D., Boni Fazzi, R., de Couto, R. A. A., Marin, J. H., et al. (2019). Functionalized nanoparticles as adjuvant to increase the cytotoxicity of metallodrugs toward tumor cells. New J. Chem. 43, 386–398. doi:10.1039/c8nj04654a
Hansen, P. E. (2021). Structural studies of β-diketones and their implications on biological effects. Pharmaceuticals 14, 1189. doi:10.3390/ph14111189
Ivanova, D., Deneva, V., Nedeltcheva, D., Kamounah, F. S., Gergov, G., Hansen, P. E., et al. (2015). Tautomeric transformations of piroxicam in solution: a combined experimental and theoretical study. RSC Adv. 5, 31852–31860. doi:10.1039/c5ra03653d
Katritzky, A. R., Hall, C. D., El-Gendy, B. E. D. M., and Draghici, B. (2010). Tautomerism in drug discovery. J. Comput. Aided Mol. Des. 24, 475–484. doi:10.1007/s10822-010-9359-z
Knockaert, M., Greengard, P., and Meijer, L. (2002). Pharmacological inhibitors of cyclin-dependent kinases. TRENDS Pharmacol. Sci. 23, 417–425. doi:10.1016/S0165-6147(02)02071-0
Manolova, Y., Deneva, V., Antonov, L., Drakalska, E., Momekova, D., and Lambov, N. (2014). The effect of the water on the curcumin tautomerism: a quantitative approach. Spectrochimica Acta Part A Mol. Biomol. Spectrosc. 132, 815–820. doi:10.1016/j.saa.2014.05.096
Martin, Y. C. (2018). Experimental and pK prediction aspects of tautomerism of drug-like molecules. Drug Discov. Today Technol. 27, 59–64. doi:10.1016/j.ddtec.2018.06.006
Nobre, D. C., Delgado-Pinar, E., Cunha, C., and Sérgio Seixas de Melo, J. (2024). The role of the oxime group in the excited state deactivation processes of indirubin. Phys. Chem. Chem. Phys. 26, 7416–7423. doi:10.1039/d3cp05260e
Ray, A., Banerjee, S., Sen, S., Butcher, R. J., Rosair, G. M., Garland, M. T., et al. (2008). Two Zn(II) and one Mn(II) complexes using two different hydrazone ligands: spectroscopic studies and structural aspects. Struct. Chem. 19, 209–217. doi:10.1007/s11224-007-9274-7
Sanna, N., Chillemi, G., Grandi, A., Castelli, S., Desideri, A., and Barone, V. (2005). New hints on the pH-driven tautomeric equilibria of the topotecan anticancer drug in aqueous solutions from an integrated spectroscopic and quantum-mechanical approach. J. Am. Chem. Soc. 127, 15429–15436. doi:10.1021/ja052637u
Shee, Y., Yeh, T.-L., Hsiao, J.-Y., Yang, A., Lin, Y. C., and Hsieh, M. H. (2023). Quantum simulation of preferred tautomeric state prediction. NPJ Quantum Inf. 9, 102. doi:10.1038/s41534-023-00767-9
Sztanke, K., Maziarka, A., Osinka, A., and Sztanke, M. (2013). An insight into synthetic Schiff bases revealing antiproliferative activities in vitro. Bioorg. Med. Chem. 21, 3648–3666. doi:10.1016/j.bmc.2013.04.037
Temperini, C., Cecchi, A., Scozzafava, A., and Supuran, C. T. (2009). Carbonic anhydrase inhibitors. Comparison of chlorthalidone and indapamide X-ray crystal structures in adducts with isozyme II: when three water molecules and the Keto−Enol tautomerism make the difference. J. Med. Chem. 52, 322–328. doi:10.1021/jm801386n
Templeton, D. M., Ariese, F., Cornelis, R., Danielsson, L. G., Muntau, H., Van Leeuwen, H. P., et al. (2000). Guidelines for terms related to chemical speciation and fractionation of elements. Definitions, structural aspects, and methodological approaches (IUPAC recommendations 2000). Pure Appl. Chem. 72, 1453–1470. doi:10.1351/pac200072081453
Templeton, D. M., and Fujishiro, H. (2017). Terminology of elemental speciation – an IUPAC perspective. Coord. Chem. Rev. 352, 424–431. doi:10.1016/j.ccr.2017.02.002
Urošević, M., Nikolić, L., Gajić, I., Nikolić, V., Dinić, A. ; V., and Miljković, V. (2022). Curcumin: biological activities and modern pharmaceutical forms. Antibiot. (Basel) 11, 135. doi:10.3390/antibiotics11020135
Wahl, O., and Sander, T. (2020). Tautobase: an open tautomer database. J. Chem. Inf. Model. 60, 1085–1089. doi:10.1021/acs.jcim.0c00035
Wang, H., Wang, Z., Wei, C., Wang, J., Xu, Y., Bai, G., et al. (2021a). Anticancer potential of indirubins in medicinal chemistry: biological activity, structural modification, and structure-activity relationship. Eur. J. Med. Chem. 223, 113652. doi:10.1016/j.ejmech.2021.113652
Wang, X., Zhao, W., Al-Abdul-Wahid, S., Lu, Y., Cheng, T., Madsen, J. J., et al. (2021b). Trifluorinated keto-enol tautomeric switch in probing domain rotation of a G protein-coupled receptor. Bioconjugate Chem. 32, 99–105. doi:10.1021/acs.bioconjchem.0c00670
Wegermann, C. A., Monzani, E., Casella, L., Ribeiro, M. A., Bruzeguini, C. E. T., Vilcachagua, J. D., et al. (2022). Unveiling geometrical isomers and tautomers of isatin-hydrazones by NMR spectroscopy. J. Mol. Struct. 1250, 131633. doi:10.1016/j.molstruc.2021.131633
Wegermann, C. A., Pirota, V., Monzani, E., Casella, L., Costa, L. A. S., Novato, W. T. G., et al. (2023). Interaction studies of oxindole-derivatives with β-amyloid peptides inhibiting its aggregation induced by metal ions. J. Inorg. Biochem. 245, 112227. doi:10.1016/j.jinorgbio.2023.112227
Wolnica, K., Szklarz, G., Dulski, M., Wojtyniak, M., Tarnacka, M., Kaminska, E., et al. (2019). Studying tautomerism in an important pharmaceutical glibenclamide confined in the thin nanometric layers. Colloids Surfaces B Biointerfaces 182, 110319. doi:10.1016/j.colsurfb.2019.06.049
Woods-Ryan, A., Doherty, C. L., and Cruz-Cabeza, A. J. (2023). A to Z of polymorphs related by proton transfer. Cryst. Eng. Comm. 25, 2845–2858. doi:10.1039/D3CE00216K
Yanagisawa, D., Shirai, N., Amatsubo, T., Taguchi, H., Hirao, K., Urushitani, M., et al. (2010). Relationship between the tautomeric structures of curcumin derivatives and their Aβ-binding activities in the context of therapies for Alzheimer’s disease. Biomaterials 31, 4179–4185. doi:10.1016/j.biomaterials.2010.01.142
Keywords: chemical speciation, tautomerism, keto-enol equilibria, medicinal agents, promising scaffolds
Citation: Araujo de Oliveira AP, Wegermann CA and Ferreira AMDC (2024) Keto-enol tautomerism in the development of new drugs. Front. Chem. Biol 3:1400642. doi: 10.3389/fchbi.2024.1400642
Received: 13 March 2024; Accepted: 29 April 2024;
Published: 30 May 2024.
Edited by:
Michal Jan Gajda, Migamake Pte Ltd, SingaporeReviewed by:
Hector Alfredo Calderon, National Polytechnic Institute (IPN), MexicoCopyright © 2024 Araujo de Oliveira, Wegermann and Ferreira. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ana Maria Da Costa Ferreira, YW1kY2ZlcnJAaXEudXNwLmJy
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.