
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Chem. Biol., 09 May 2024
Sec. Quantitative and Analytical Techniques
Volume 3 - 2024 | https://doi.org/10.3389/fchbi.2024.1400481
 Claudia Foerster1*
Claudia Foerster1* Andrea Müller-Sepúlveda2
Andrea Müller-Sepúlveda2 Marina Venturini Copetti3
Marina Venturini Copetti3 Andrea Alejandra Arrúa4
Andrea Alejandra Arrúa4 Liliam Monsalve1
Liliam Monsalve1 María Laura Ramirez5
María Laura Ramirez5 Adriana M. Torres5
Adriana M. Torres5Mycotoxins are natural metabolites produced by species of filamentous fungi belonging mainly to the genera Aspergillus, Fusarium, Penicillium, and Alternaria, which can grow in various crops and foodstuffs. The South American climate is diverse, varying from tropical, temperate, and arid to cold, ideal for the growth of different types of fungi and mycotoxin production. This mini review aimed to describe the natural occurrence of mycotoxin in food in South America from 2018 to 2023, identifying research gaps and challenges in an era of climate change. We analyzed 53 studies, 21 from Brazil. Most of the mycotoxins analyzed in South America were the traditional and regulated mycotoxins, with variable occurrences depending on the region, climatic conditions, and methodology used. Emerging and modified mycotoxins have only been studied in Argentina and Brazil, where some studies have shown high occurrences. Given this, it is essential to strengthen food safety laboratories and surveillance capabilities and establish early warning systems. It is also essential to continue working to raise awareness of mycotoxins as a public health issue and to study and prevent the impact of climate change on soil microbial population, the new prevalence of fungi, and the profile of toxigenic species. An effective connection and collaboration between disciplines and sectors in different countries is needed to meet this research challenge.
South America (14.6048°S, 59.0625°W) is a continent that has an area of 17,840,000 square kilometers (6,890,000 sq mi). Consistent with its size, it extends from a broad equatorial zone in the north to a narrow sub-Arctic zone in the south and includes twelve sovereign states: Argentina, Bolivia, Brazil, Chile, Colombia, Ecuador, Guyana, Paraguay, Peru, Suriname, Uruguay, and Venezuela. The South American climate varies from tropical to temperate, arid, and cold (Garreaud et al., 2009). This variable climate is ideal for different types of fungal growth and mycotoxin production. Mycotoxins are secondary metabolites produced by filamentous fungal species mainly belonging to Aspergillus, Fusarium, Penicillium, and Alternaria, which can grow in various crops and foodstuffs. Mycotoxins have a particular structural and chemical characteristic, which determine their biological and toxicological properties. Between 300 and 400 types are recognized, and about a dozen of them are considered important threats to human or animal health (Bennett and Klitch, 2003). Some mycotoxins stand out for their high toxicity, such as aflatoxins, which are among the most potent natural hepatocarcinogens known to date. Others, like ochratoxin A (OTA) and citrinin (CIT), can affect the kidney, the nervous system (patulin, PAT), or the reproductive system (zearalenone, ZEN). Some have multiple toxic effects in humans and animals like deoxynivalenol (DON), nivalenol (NIV), and T-2 toxin are most likely associated with the high incidence of esophageal cancer in specific populations like fumonisins B1 and B2 (FB1- FB2) (International Agency for Research on Cancer, 2012). The latest mycotoxins are regulated in many countries of South America and the rest of the world and have been widely studied (van Egmond et al., 2007). However, it is also essential to study emerging mycotoxins, which are increasing in incidence and are defined as mycotoxins that are neither routinely determined nor have legislative regulation (Vaclavikova et al., 2013). Some of the emerging mycotoxins of interest are Fusarium metabolites fusaproliferin (FP), beauvericin (BEA), enniatins (ENNs), and moniliformin (MON), fusaric acid (FA), culmorin (CUL), and butenolide (BUT), the Aspergillus metabolites sterigmatocystin (STE) and emodin (EMO), the Penicillium metabolite mycophenolic acid (MPA), and the Alternaria metabolites alternariol (AOH), alternariol monomethyl ether (AME), and tenuazonic acid (TeA) (Gruber-Dorninger et al., 2017).
South America’s economy is centered on the export of natural resources, including diverse food products prone to mycotoxins, like nuts, coffee, and cacao, and industrial crops such as corn, wheat, soybeans, rice, quinoa, and cotton (Cardenas and Orozco, 2022). Likewise, South America is experiencing the effects of climate change, including extreme weather events and changes in temperature and precipitation patterns (Fernandez-Guzman et al., 2023). Climate change is expected to increase and modify mycotoxin contamination. Research has demonstrated that only a slight elevation of CO2 levels will stimulate the growth of mycotoxin-producing fungi. There is an increased risk for mycotoxin contamination of corn, wheat, and other small grain species. In a changing climate, mycotoxins will increase and contaminate new crops and new geographical areas (Zingales et al., 2022).
This mini review aimed to describe the natural occurrence of mycotoxin in food in South America over the last 5 years (2018–2023) and identify the research gaps and challenges in a climate change era.
Bolivia, Guyana, Suriname, French Guiana, Trinidad and Tobago, and Venezuela have not reported mycotoxin data in food from 2018 to 2023. The collected data from the rest of the countries (n = 53) are summarized in Table 1. Details of the studies regarding occurrence, levels, and methodology are collected in the Supplementary Table S1.
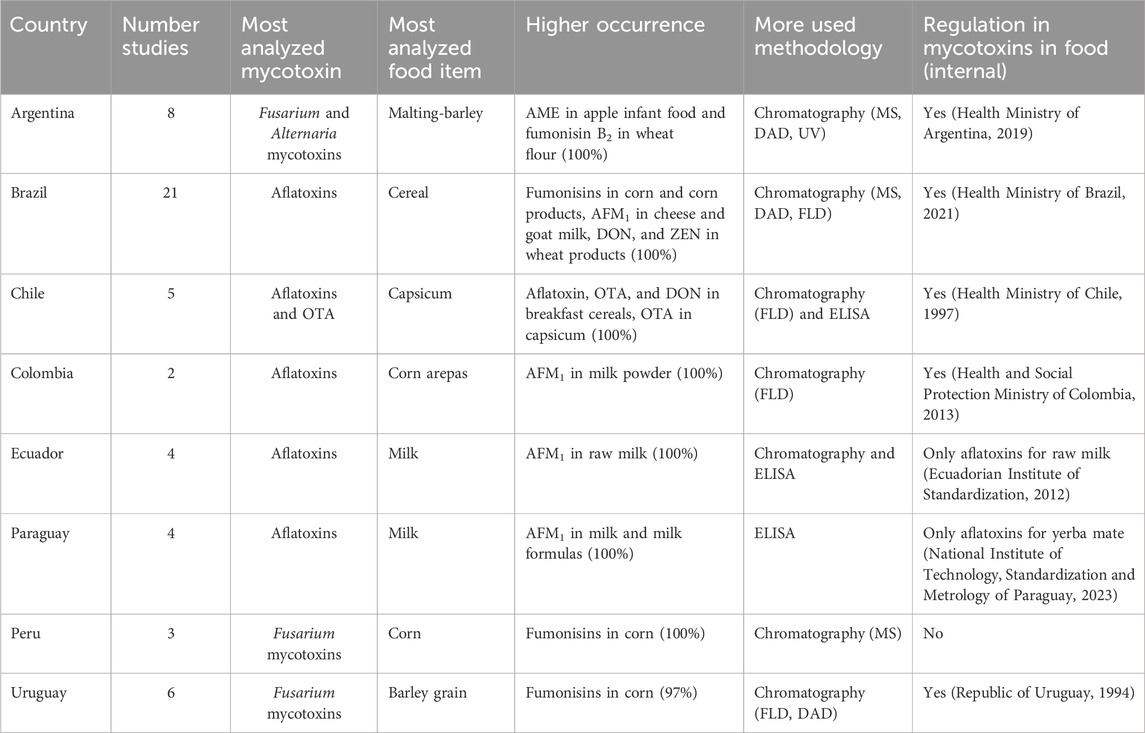
Table 1. Studies of mycotoxins natural contamination in food for human consumption, with data collected 2018–2023 in countries of South America.
This country has had eight studies in the last 5 years. Corn was analyzed for DON and their metabolites 3-ADON, 15-ADON, also FB1, FB2, NIV, and ZEN, finding DON and 3-ADON in 90% and 40% of the samples, respectively (Castañares et al., 2019). Other cereals analyzed were malting barley, which found 16% DON and 22% NIV (Nogueira et al., 2018). In wheat: AOH, AME, and TeA were found with occurrences of 19%, 38%, and 50–62%, respectively (Romero Bernal et al., 2019), and FB1 and FB2 with occurrences ranging from 50 to 100% in wheat flour (Cendoya et al., 2019). Regarding fruits, apples, tomatoes, and grapes have been analyzed. Interestingly, apple baby food (n = 20) was found to have AOH in 35%, AME in 100%, TeA in 70%, and tentoxin (TEN) in 95% of the samples, while altertoxin-I (ATX-I) and altenuene (ALT) were not found (Pavicich et al., 2023). This is important as emerging mycotoxins are not regulated in Argentina or the rest of South America. Regarding tomatoes, occurrences of AOH in 18%, AME in 8%, TeA in 21%, TEN in 13%, ATX-Iin 5%, and ALT in 8% of the samples (Maldonado Haro et al., 2023). Grapes for winemaking were found to have TeA in 16% of the cases, ranging from 77 to 133 ng/g (Prendes et al., 2018). Finally, milk samples were found (78%) with AFM1 ranging from 3 to 64 ng/mL (Costamagna et al., 2019).
Brazil is the country with the most studies made in the period (n = 21) on all types of foods. The first study analyzed aflatoxins and OTA in various spices, finding aflatoxins in fennel at 27% and 20% in rosemary (Valle Garcia et al., 2018). Also, in spices, Persson da Silva et al. (2021) found OTA in 55% of black pepper samples. Regarding nuts, Silva et al. (2018) found aflatoxins in 64% of peanut samples (n = 42) and 28% in blanched peanuts (n = 18). No aflatoxins were found in Pecan nuts (n = 52) (Valle Garcia et al., 2019), while Kluczkovski et al. (2022) found 28% of aflatoxin contamination in Brazil nut oil (n = 25). Milk and milk products were also analyzed for different mycotoxins, most frequently AFM1. This mycotoxin was found in raw milk (12.5%), pasteurized milk (36%), UHT milk (48%), Minas cheese (47%), and yogurt (25%) (Corassin et al., 2022); was also present in all samples (n = 7) of artisanal mozzarella, manufactured mozzarella, artisanal rennet and manufactured rennet (Costa da Silva et al., 2021), and goat milk (n = 108) (de Matos et al., 2021). Frey et al. (2021) analyzed AFM1, DOM-1, OTA, FB1, FB2, alpha-zearalenol, and beta-zearalenol in different types of milk, finding occurrences from 9% to 25%, 8.8% to 9.4%, 18.7% to 25%, 12.5% to 50%, 4.4% to 25%, 25% to 57.4%, and 25% to 50%, respectively. Most of the studies have been done on cereals and cereal products. Regarding breakfast and infant cereals, Mallmann et al. (2020) analyzed aflatoxins, fumonisins, ZEN, DON, T-2 toxin, HT-2, NIV, fusarone-x, 3-AcDON, 15-AcDON, Diacetoxyscirpenol, and OTA, finding 9% aflatoxins, 27% fumonisins, 15% ZEN, 13% DON and 3% OTA in breakfast cereals and 28% fumonisins (mean 196.2 ng/g), 7% ZEN (mean 47.5 ng/g), and 10% DON (mean 351.6 ng/g) in infant cereals. Another study found that 100% of the samples of breakfast cereals had fumonisins, and 10% had DON (Andrade et al., 2020). These authors also analyzed popcorn (n = 13) finding fumonisins in all samples, DON in 8% and ZEN in 15% of the samples; cornstarch and corn pasta (n = 7) finding fumonisins in all samples; corn grits-canjiquinha (n = 3), finding fumonisins in all samples, also DON and ZEN; and corn flour (n = 248) with an occurrence of 95.5% of fumonisins, 36% DON and 11% ZEN. The authors also analyzed wheat flour, pasta, crackles, and snacks, finding fumonisins, DON and ZEN in 0–97-2.5%, 13–100-66%, 0–100-100%, and 0–67-67%, respectively. The rice sampled was not contaminated (Andrade et al., 2020). Furthermore, Dos Santos et al. (2021) found DON in all wheat flour samples (n = 200), along with a 51% occurrence of ZEN and 13.5% of T-2 toxin. Iwase et al. (2023) found high occurrence and levels of DON (70%, mean 1,112 ng/g) in barley, also finding ZEN (40%, mean 256 ng/g). Also, in barley, dos Santos Caramês et al. (2022) found a 70% occurrence of enniatins. In cassava and tapioca, a low occurrence of aflatoxin was found in cassava flour (Ono et al., 2021). In oats samples, Pinheiro et al. (2021) found DON (44%), NIV (29%), 3-ADON (19%) and 15-ADON (8%). In beer, 80–100% of aflatoxins have been reported (Reboucas Rocha et al., 2023), and 21% of OTA was found in fermented coffee (Silva et al., 2023). In tilapia muscle, 19% of AFB1 was found by Seraphin de Godoy et al. (2022). Finally, Abreu et al. (2023) analyzed 135 samples of cocoa beans by a multi mycotoxin analysis, finding AFB1 (17%), AFB2 (8%), AFG1 (6%), AFG2 (1%), AFBG (14%), OTA (20%), STG (8%), roquefortine C (1%), FB2 (6%), CIT (26%), mycophenolic acid (9%), paxilline (34%), ZEN (15%), cyclopiazonic acid (27%) and TeA (21%).
Five studies have been published regarding the occurrence of mycotoxins in foods for human consumption. Two studies focused on the presence of OTA and aflatoxins in capsicum, one of the most contaminated foods observed in a previous study (Foerster et al., 2020). The first study was part of the national monitoring program and found OTA in 59%–63% of the samples of capsicum (chili, paprika, merken, i.e., chili with spices), with levels of >LOQ- 39.6 ng/g (Palma et al., 2023). The second study found OTA in all farmers’ and markets’ capsicum samples (n = 21) and 46%–75% occurrence of aflatoxin B1 (Costa et al., 2022). A current study described the findings of OTA and aflatoxins of the mycotoxin monitoring program in all food samples; spices were the most contaminated, and aflatoxins were mainly found in nutmeg, pepper, and ginger, and their levels exceeded the limits of international regulations. For OTA, the occurrence was found in spices, coffee, cocoa, and flour (Calderon et al., 2023). The last two studies were in milk and milk formulas, where AFM1 was found in 29% and 63%, respectively (Foerster et al., 2023), and in breakfast cereal, where DON, ZEN, fumonisins, OTA, and aflatoxins were found. Levels were below the Chilean regulatory limits but above the EU regulation for processed cereal-based foods and baby foods for infants and young children. Fumonisins were above 1,000 ng/g in three cornflake samples and DON >750 ng/g in one cornflake sample (Foerster et al., 2022).
In the last years, only aflatoxins have been analyzed: in corn arepas (n = 168), 27% of AFB1 was found (Blanco-Lizarazo et al., 2019), and in milk powder (n = 51), AFM1 was found in 100% of the samples, and no positives for AFB1 were found (Marimón Sibaja et al., 2019).
Four studies were conducted in Ecuador, mainly on milk. Puga-Torres et al. (2020) found AFM1 in 100% of the 209 samples of raw milk and ZEN in 99.5% (Puga-Torres et al., 2021). Also, 78 breast milk samples were analyzed for aflatoxins, finding 13% of AFM1 and 9% of AFB1 (Ortiz et al., 2018). Finally, 28 samples of corn were analyzed for aflatoxins, finding a 50% occurrence and levels of 0.42–107.7 μg/kg (Abel-Palacios et al., 2022).
Four studies have been reported in Paraguay, including wheat, beer, wine, milk, and milk formulas. The first described DON in wheat flour, bread, and crackers found high levels in most manufactured food (crackers, 0.038 ± 0.049 ng/g) (Arrua Alvarenga et al., 2019a). Furthermore, Arrua Alvarenga et al. (2019b) reported 25% OTA in wine and 24% of DON in beer. In milk formulas, AFM1 was found in all fluid formulas analyzed (median 33.6 ng/kg) and in 9.75% of the powder formulas, but with greater levels (median 1820 ng/kg) (Arrua Alvarenga et al., 2021a). Finally, in UHT and pasteurized milk (sachet and cartons), Arrua Alvarenga et al. (2021b) found AFM1 in 100% of the samples analyzed (n = 80).
Three studies were conducted in the period. The first study found aflatoxin with levels over 20 ng/g in 6/20 samples of capsicum, peanuts, and barley (Rojas Jaimes et al., 2021). Vásquez-Ocmín et al. (2023) found beauvericin in 59% of the 27 samples of quinoa, canihua, and kiwicha analyzed. Finally, Ducos et al. (2021) found fumonisins in 100% of the corn samples (n = 14) and DON (51%), NIV (6%), and ZEN (22%) in corn and wheat.
Six studies have data from 2018, five of them in grains and one of them in milk. The first publication found 67% of NIV in n = 154 barley (Garmendia et al., 2018), and the second found DON (90%) and ZEN (9%) also in barley (n = 89) (Palladino et al., 2021). The third study reported occurrences of fumonisins, DON, ZEN, and NIV in corn during 2018 and 2019, highlighting the high occurrences and levels of FB1 (96.7%, mean 4,860 ng/g) and FB2 (90.2%, mean 1,695 ng/g) in 2018 (del Palacio et al., 2023). The fourth publication found AFB1 (8%) and fumonisins (4%) in sorghum grains (n = 50) (García y Santos et al., 2022). The fifth publication found 30% of AFs in 80 samples of wheat and sorghum grains (del Palacio and Pan, 2020). Finally, Capelli et al. (2019) found AFM1 in 91.8% of cow milk samples from 18 farms in the country.
Different mycotoxins produced by toxigenic fungi were detected in foods from all over South America. Most of the contaminated products have great economic importance in the region. The type of food and climate conditions of each country influenced the occurrence of mycotoxins (Figure 1A). Fungal contamination and mycotoxin occurrence in the food and feed chains represent a high risk to human and animal health and considerable economic losses due to restrictions to the domestic and international markets. Studies on the occurrence of mycotoxins in crops and processed food are essential because they are reliable approaches to evaluating the potential exposure risk of the populations to these contaminants (Chiotta et al., 2020).
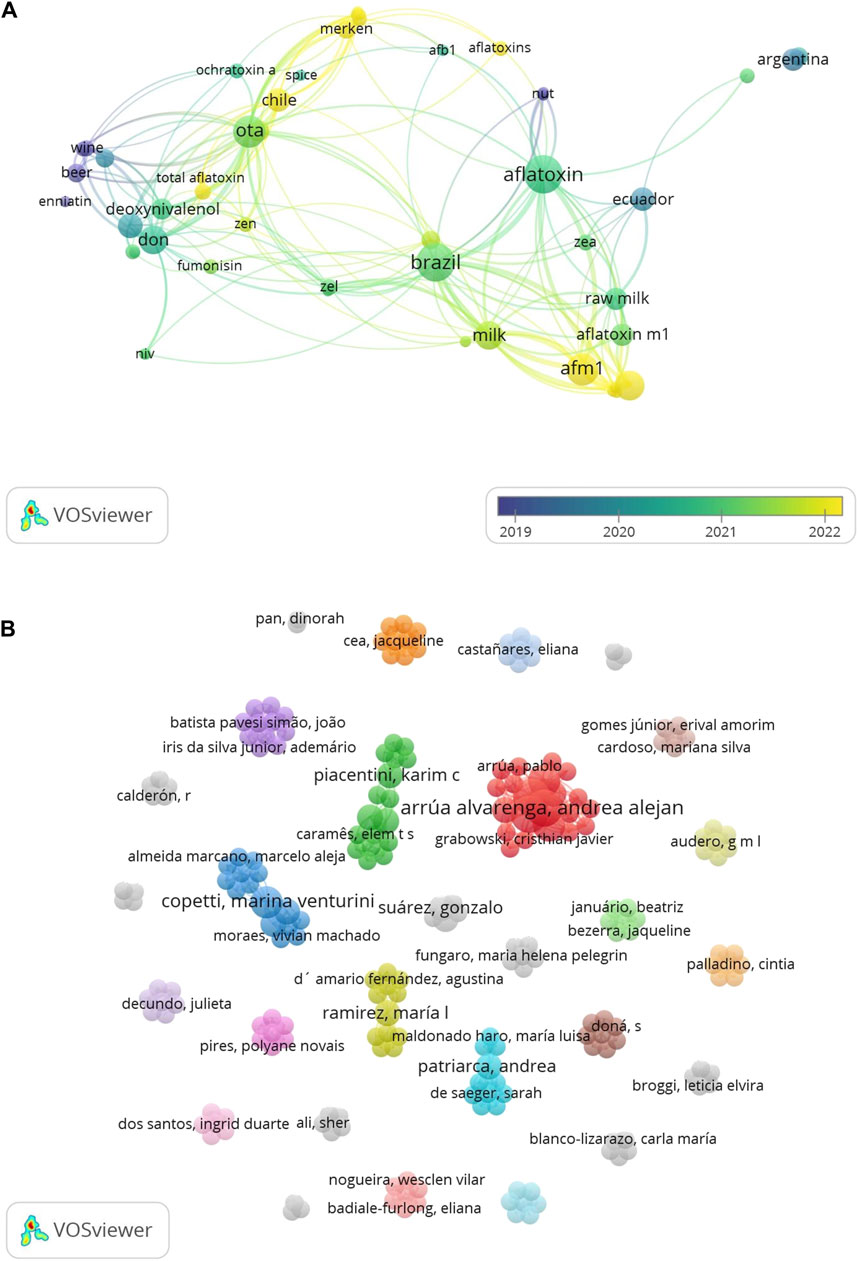
Figure 1. Scientific mapping of linked networks for different mycotoxins and foods in South America over the last 5 years. The methodology of the figure is described in the Supplementary Material. (A) Connections between the mycotoxin research in the countries of the study. (B) Collaboration map of the authors of the publications included in this mini review.
Most of the mycotoxins analyzed in South America were the “traditional and regulated mycotoxins” like aflatoxins, AFM1, OTA, fumonisins, DON, and ZEN, found with variable occurrences depending on the region, climatic conditions, and methodology used. Emerging and modified toxins like AOH, AME, TeA, TEN, ATX-I, and ALT have been studied only in Argentina and Brazil, where some studies have shown high occurrences. Also, in Brazil and Argentina, the methodology is more complex, using chromatography-mass spectrometry (LC-MS) with MS/MS detection, which can give more precise results. Robust analytical methods are crucial to ensuring food safety. LC-MS-based methods are becoming increasingly popular as they allow sensitive simultaneous determination of multiple fungal metabolites in many matrices (Gruber-Dorninger et al., 2017). ELISA methods are widely used in South America for being inexpensive and easy to use but tend to overestimate the levels because they usually detect other forms of mycotoxins (Gerding et al., 2015). Also, frequently, the limits of detection and quantification of these methods are higher than recommended. Despite these limitations, ELISA has proven to be an excellent qualitative method with high sensitivity, but additional quantification and validation must be made by chromatographic methods for reliable results (Gerding et al., 2015). In this regard, strengthening food safety laboratories and surveillance capabilities and establishing early warning systems is paramount. Also, countries must continue working and searching for efficient, cost-effective sampling and analytical methods to detect traditional and modified mycotoxins in the region.
Most studies in the region were conducted to find mycotoxins in grains and cereals, with an increasing analysis of AFM1 in milk (Figure 1A). These studies were independent of the climatic and economic conditions of feed, so it was difficult to establish causality of the occurrences. In some countries like Argentina and Paraguay, feeding has been changed from grass to grains, which could increase aflatoxin occurrence. In Brazil, milk production comes from different production systems, and frequently, there is no access to production control and history. This fact is a significant research gap in South America.
Furthermore, few countries have an estimation of exposure from the occurrences and levels of mycotoxins in food. Most of South America’s current food safety regulations were based on international risk assessments like Codex Alimentarius (e.g., Chilean Food Sanitary Regulation, 2023) and the Southern Common Market, MERCOSUR (Organization of American States, 2022). This last regulation typically applies to food exports. Although harmonized regulatory limits would be beneficial from a commercial point of view, this does not necessarily promote equal protection of human health in a homogeneous way around the world. The risks associated with mycotoxins depend on both the hazard and the exposure. The danger posed by mycotoxins to humans is probably similar worldwide, but not the exposure, because of differences in the levels of contamination and diet habits; therefore, reliable exposure assessments for mycotoxins in each country are necessary (Food and Agriculture Organization, 2004). Some South American countries have an important gap in surveillance and internal regulatory issues, having no regulations or partial ones. For example, Paraguay only has an internal regulation for aflatoxins for yerba mate (National Institute of Technology, Standardization and Metrology of Paraguay, 2023) but lacks the rest of the foodstuffs. Only Brazil and Argentina have a special regulation for mycotoxins for foods destined for little children and infants (Health Ministry of Argentina, 2019; Health Ministry of Brazil, 2021). In this sense, it is crucial to continue working to bring awareness of mycotoxins as a public health issue.
Strategies to reduce mycotoxin contamination of foodstuffs require a multifaceted approach combining pre- and post-harvest interventions. In low- and middle-income countries in South America, where technology and infrastructure are not always adequate, ensuring low mycotoxin occurrence remains a significant challenge (Ducos et al., 2021). Furthermore, changes in climate systems suggest that slightly elevated CO2 concentrations and interactions with temperature and water availability may stimulate the growth of some mycotoxigenic species, especially under water stress (Magan et al., 2011). For example, Paterson and Lima (2010) suggested that a significant risk of climate change will be developed in countries with temperate climates, like Chile and part of Argentina, which would be conducive to aflatoxin production, and that in colder climates, mycotoxins such as PAT and OTA may become more critical. The impact of climate change will also be remarkable for soil microbial populations; these will be affected and subsequently will affect the prevalence of some fungi. Due to the changes of given fungal species to colonize new environments, the profile of toxigenic species occurring in different geographical areas could be modified, leading to new mycotoxin risks in specific regions (Moretti et al., 2019). Given these potential impacts, it is crucial for research efforts to focus on monitoring the occurrence of mycotoxins in foods, evaluating population exposure, and understanding the prevalence of different toxigenic fungal species in various regions.
The following steps for South American countries are to increase food surveillance, internal mycotoxin regulation, biomonitoring analysis, and estimation of human health exposure based on contamination levels and dietary habits in each country. Epidemiological studies are urgently needed to understand the source of exposure in the population and the chronic health consequences of this exposure. Evidence on the intersection between climate change and health is limited in South America and has been generated in silos, with limited transdisciplinary research (Palmeiro-Silva et al., 2023). This information was corroborated in this study, where collaborations in research were null (Figure 1B). Effective connection and collaboration between disciplines and sectors in different countries is urgently needed to address this challenging research.
CF: Conceptualization, Formal Analysis, Methodology, Writing–original draft, Writing–review and editing. AM-S: Formal Analysis, Writing–original draft, Writing–review and editing. MVC: Formal Analysis, Writing–original draft, Writing–review and editing. AA: Formal Analysis, Writing–original draft, Writing–review and editing. LM: Formal Analysis, Writing–original draft, Writing–review and editing. MR: Formal Analysis, Writing–original draft, Writing–review and editing. AT: Supervision, Writing–original draft, Writing–review and editing.
The authors declare that financial support was received for the research, authorship, and/or publication of this article. ANID Fondecyt #11190700, CONICET PUE N° 22920200100004CO. MVC acknowledges “Conselho Nacional de Desenvolvimento Científico e Tecnológico” (CNPq) for a research grant (process 306902/2023-0).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fchbi.2024.1400481/full#supplementary-material
Abel Palacios, H., Stefanello, A., García Gavilánez, M. S., Castro Demera, D. A., Garcia, M. V., Vásquez Castillo, W. A., et al. (2022). Relationship between the fungal incidence, water activity, humidity, and aflatoxin content in maize samples from the highlands and coast of Ecuador. Toxins 14, 196. doi:10.3390/toxins14030196
Abreu, D. C. P., Vargas, E. A., Oliveira, F. A. D. S., Uetanabaro, A. P. T., Pires, P. N., Bazzana, M. J. F., et al. (2023). Study of co-occurrence of mycotoxins in cocoa beans in Brazil by liquid chromatography-tandem mass spectrometry (LC-MS/MS). Food Addit. Contam. Part A 40 (8), 1049–1058. doi:10.1080/19440049.2023.2238838
Andrade, P. D., Dias, J. V., Souza, D. M., Brito, A. P., van Donkersgoed, G., Pizzutti, I. R., et al. (2020). Mycotoxins in cereals and cereal-based products: incidence and probabilistic dietary risk assessment for the Brazilian population. Food Chem. Toxicol. 143, 111572. doi:10.1016/j.fct.2020.111572
Arrúa, A. A., Arrúa, P. D., Moura-Mendes, J., Cazal, C., Ferreira, F. P., Grabowski, C. J., et al. (2021b). Presence of aflatoxin M1 in commercial milk in Paraguay. J. Food Prot. 84 (12), 2128–2132. doi:10.4315/JFP-21-196
Arrúa, A. A., Arrua, P. D., Ulke, M. G., Quezada Viay, M. Y., Moreno Lara, J., Moura Mendes, J., et al. (2021a). Presencia de aflatoxina M1 en fórmulas lácteas infantiles comercializadas en el área metropolitana a Asunción, Paraguay. Pediatría (Asunción) 48 (1), 37–43. doi:10.31698/ped.48012021007
Arrúa, A. A., Mendes, J. M., Arrúa, P., Ferreira, F. P., Caballero, G., Cazal, C., et al. (2019b). Occurrence of Deoxynivalenol and Ochratoxin A in beers and wines commercialized in Paraguay. Toxins 11, 308. doi:10.3390/toxins11060308
Arrúa Alvarenga, A. A., Moura Mendes Arrua, J., Cazal Martínez, C. C., Arrúa Alvarenga, P. D., Fernández Ríos, D., Pérez Estigarribia, P. E., et al. (2019a). Deoxynivalenol screening in wheat-derived products in Gran Asunción, Paraguay. J. Food Saf. 39, 12580. doi:10.1111/jfs.12580
Bennett, J. W., and Klich, M. (2003). Mycotoxins. Clin. Microbiol. Rev. 16 (3), 497–516. doi:10.1128/CMR.16.3.497-516.2003
Blanco-Lizarazo, C. M., Gamboa-Marín, A., Vega, S., Jiménez-Rodríguez, L. P., and Sánchez B, I. C. (2019). Assessment of dietary exposure to aflatoxin B1 from corn arepas in Colombia. Food Addit. Contam. Part A 36 (7), 1109–1117. doi:10.1080/19440049.2019.1615643
Calderón, R., Palma, P., Godoy, M., Vidal, M., and Rivera, A. (2023). Co-occurrence and estimation of the risk of total aflatoxins (B1, B2, G1, and G2) and ochratoxin A in agri-food products consumed in Chile. Food control. 146, 109493. doi:10.1016/j.foodcont.2022.109493
Capelli, A., Suárez, G., and García y Santos, C. (2019). Aflatoxinas en alimentos y leche de vacas de 18 establecimientos comerciales de las regiones centro-sur y este de Uruguay. [in Spanish]. Veterinaria (Montevideo) 55 (212), 52–56. doi:10.29155/VET.55.212.2
Caramês, E. T. d. S., Piacentini, K. C., Aparecida Almeida, N., Lopes Pereira, V., Azevedo Lima Pallone, J., and de Oliveira Rocha, L. (2022). Rapid assessment of enniatins in barley grains using near infrared spectroscopy and chemometric tools. Food Res. Int. 161, 111759. doi:10.1016/j.foodres.2022.111759
Cárdenas, M., and Orozco, S. (2022). The challenges of climate mitigation in Latin America and the Caribbean: some proposals for action. UNDP Lat. Am. Caribb. policy documents Ser. UNDP LAC PDS Nº. 40.
Castañares, E., Martínez, M., Cristos, D., Rojas, D., Lara, B., Stenglein, S., et al. (2019). Fusarium species and mycotoxin contamination in maize in Buenos Aires province, Argentina. Eur. J. Plant Pathol. 155, 1265–1275. doi:10.1007/s10658-019-01853-5
Cendoya, E., Nichea, M. J., Monge, M. P., Sulyok, M., Chiacchiera, S. M., and Ramirez, M. L. (2019). Fumonisin occurrence in wheat-based products from Argentina. Food Addit. Contam. Part B 12 (1), 31–37. doi:10.1080/19393210.2018.1520308
Chiotta, M. L., Fumero, M. V., Cendoya, E., Palazzini, J. M., Alaniz-Zanon, M. S., Ramirez, M. L., et al. (2020). Toxigenic fungal species and natural occurrence of mycotoxins in crops harvested in Argentina. Rev. Argent. Microbiol. 52 (4), 339–347. doi:10.1016/j.ram.2020.06.002
Corassin, C. H., Borowsky, A., Ali, S., Rosim, R. E., and de Oliveira, C. A. F. (2022). Occurrence of aflatoxin M1 in milk and dairy products traded in são paulo, Brazil: an update. Dairy 3, 842–848. doi:10.3390/dairy3040057
Costa, J., Santos, C., Soares, C., Rodríguez, R., Lima, N., and Santos, C. (2022). Occurrence of aflatoxins and ochratoxin A during merkén pepper powder production in Chile. Foods 11, 3843. doi:10.3390/foods11233843
Costa da Silva, M., da Silva G de Castro, E., do N Barreto, J., Vitor de Oliveira Martins, P., Lopes da Silva, G., Ferreira da Silva, R., et al. (2021). Ochratoxin A levels in fermented specialty coffees from Caparaó, Brazil: is it a cause of concern for coffee drinkers? Food Addit. Contam. Part A 38 (11), 1948–1957. doi:10.1080/19440049.2021.1943542
Costamagna, D., Gaggiotti, M., Chiericatti, C. A., Costabel, L., Audero, G. M. L., Taverna, M., et al. (2019). Quantification of aflatoxin M1 carry-over rate from feed to soft cheese. Toxicol. Rep. 6, 782–787. doi:10.1016/j.toxrep.2019.07.004
de Godoy, S. H. S., Gomes, A. L., Burbarelli, M. F. d. C., Bedoya-Serna, C. M., Vasquez-Garcia, A., Chaguri, M. P., et al. (2022). Aflatoxins in fish feed and Tilapia (Oreochromis niloticus) tissues in Brazil. J. Aquatic Food Prod. Technol. 31 (7), 726–734. doi:10.1080/10498850.2022.2095879
Del Palacio, A., Corallo, B., Simoens, M., Cea, J., de Aurrecoechea, I., Martinez, I., et al. (2023). Major Fusarium species and mycotoxins associated with freshly harvested maize grain in Uruguay. Mycotoxin Res. 39 (4), 379–391. doi:10.1007/s12550-023-00498-y
Del Palacio, A., and Pan, D. (2020). Occurrence and toxigenic potential of Aspergillus section Flavi on wheat and sorghum silages in Uruguay. Mycology 11 (2), 147–157. doi:10.1080/21501203.2020.1752321
De Matos, C. J., Schabo, D. C., do Nascimento, Y. M., Tavares, J. F., Lima, E. d. O., da Cruz, P. O., et al. (2021). Aflatoxin M1 in Brazilian goat milk and health risk assessment. J. Environ. Sci. Health, Part B 56 (4), 415–422. doi:10.1080/03601234.2021.1892434
Dos Santos, I. D., Pizzutti, I. R., Días, J. V., Fontana, M. E. Z., Souza, D. M., and Cardoso, C. D. (2021). Mycotoxins in wheat flour: occurrence and co-occurrence assessment in samples from Southern Brazil. Food Addit. Contam. Part B 14 (2), 151–161. doi:10.1080/19393210.2021.1920053
Ducos, C., Pinson-Gadais, L., Chereau, S., Richard-Forget, F., Vásquez-Ocmín, P., Cerapio, J. P., et al. (2021). Natural occurrence of mycotoxin-producing fusaria in market-bought Peruvian cereals: a food safety threat for andean populations. Toxins 13 (2), 172. doi:10.3390/toxins13020172
Ecuadorian Institute of Standardization (2012). Instituto Ecuatoriano de Estandarización NTE INEN 9 Leche cruda Requisitos. Available at: https://www.gob.ec/regulaciones/nte-inen-9-leche-cruda-requisitos (Accessed March 8, 2024).
Fernandez-Guzman, D., Lavarello, R., Yglesias-González, M., Hartinger, S. M., and Rojas-Rueda, D. (2023). A scoping review of the health co-benefits of climate mitigation strategies in South America. Lancet Regional Health – Am. 26, 100602. doi:10.1016/j.lana.2023.100602
Foerster, C., Monsalve, L., and Ríos-Gajardo, G. (2022). Mycotoxin exposure in children through breakfast cereal consumption in Chile. Toxins 14, 324. doi:10.3390/toxins14050324
Foerster, C., Monsalve, L., and Ríos-Gajardo, G. (2023). Occurrence of aflatoxin M1 in milk and exposure estimation for its consumption in the Chilean population. Food control. 148, 109677. doi:10.1016/j.foodcont.2023.109677
Foerster, C., Muñoz, K., Delgado-Rivera, L., Rivera, A., Cortés, S., Müller, A., et al. (2020). Occurrence of relevant mycotoxins in food commodities consumed in Chile. Mycotoxin Res. 36 (1), 63–72. doi:10.1007/s12550-019-00369-5
Food and Agriculture Organization (2004). FAO Technical Papers: worldwide regulations for mycotoxins in food and feed in 2003. FAO Food Nutr. Pap. 81.
Frey, M., Rosim, R., and Oliveira, C. (2021). Mycotoxin Co-occurrence in milks and exposure estimation: a pilot study in são paulo, Brazil. Toxins 13, 507. doi:10.3390/toxins13080507
Garcia, M. V., Mallmann, C. A., and Copetti, M. V. (2018). Aflatoxigenic and ochratoxigenic fungi and their mycotoxins in spices marketed in Brazil. Food Res. Int. 106, 136–140. doi:10.1016/j.foodres.2017.12.061
Garcia, M. V., Moraes, V. M., Bernardi, A. O., Oliveira, M. S., Mallmann, C. A., Boscardin, J., et al. (2019). Mycological quality of pecan nuts from Brazil: absence of aflatoxigenic fungi and aflatoxins. Cienc. Rural. 49, 6. doi:10.1590/0103-8478cr20190076
García y Santos, C., Cajarville, C., Suárez, G., and Bettucci, L. (2022). How do time, tannin, and moisture content influence toxicogenic fungal populations during the storage of sorghum grains? J. Food Prot. 85 (5), 778–785. doi:10.4315/JFP-21-239
Garmendia, G., Pattarino, L., Negrín, C., Martínez-Silveira, A., Pereyra, S., Ward, T. J., et al. (2018). Species composition, toxigenic potential and aggressiveness of Fusarium isolates causing Head Blight of barley in Uruguay. Food Microbiol. 76, 426–433. doi:10.1016/j.fm.2018.07.005
Garreaud, R. D., Vuille, M., Compagnucci, R., and Marengo, J. (2009). Present-day South American climate. Palaeogeogr. Palaeoclimatol. Palaeoecol. 281 (3–4), 180–195. doi:10.1016/j.palaeo.2007.10.032
Gerding, J., Ali, N., Schwartzbord, J., Cramer, B., Brown, D. L., Degen, G. H., et al. (2015). A comparative study of the human urinary mycotoxin excretion patterns in Bangladesh, Germany, and Haiti using a rapid and sensitive LC-MS/MS approach. Mycotoxin Res. 31, 127–136. doi:10.1007/s12550-015-0223-9
Gruber-Dorninger, C., Novak, B., Nagl, V., and Berthiller, F. (2017). Emerging mycotoxins: beyond traditionally determined food contaminants. J. Agric. Food Chem. 65 (33), 7052–7070. doi:10.1021/acs.jafc.6b03413
Health and Social Protection Ministry of Colombia (2013). Ministerio de Salud y Protección Social Resolución 4506 de 2013. Available at: https://www.minsalud.gov.co/Normatividad_Nuevo/Resoluci%C3%B3n%204506%20de%202013.pdf (Accessed March 8, 2024).
Health Ministry of Argentina (2019). Codigo alimentario argentino. Article 156: limits for mycotoxins (incorporated in 2019). Available at: https://www.argentina.gob.ar/anmat/codigoalimentario (Accessed February 9, 2024).
Health Ministry of Brazil (2021). Ministério da Saúde – MS. Agência Nacional de Vigilância Sanitária – ANVISA. Instrução normativa - N° 88, de 26 de março de, 2021.
Health Ministry of Chile (1997). Chilean Food Sanitary Regulation [Reglamento Sanitario de los alimentos] DTO. N° 977/96 (D.OF. 13.05.97) República de Chile, Ministerio de Salud. Available at: https://dinta.cl/reglamento-sanitario-de-los-alimentos-actualizacion-enero-2023/ (Accessed February 8, 2024).
International Agency for Research on Cancer (2012) Improving public health through mycotoxin control. Lyon, France: IARC Scientific Publication No. 158.
Iwase, C. H. T., Piacentini, K. C., Silva, N. C. C., Rebellato, A. P., and Rocha, L. O. (2023). Deoxynivalenol and zearalenone in Brazilian barley destined for brewing. Food Addit. Contam. Part B 16 (2), 86–92. doi:10.1080/19393210.2022.2151046
Kluczkovski, A., Bezerra, L., Januário, B., Lima, E., Campelo, P., Machado, M., et al. (2022). Nuclear magnetic resonance approach in Brazil nut oil and the occurrence of aflatoxins. J. Oleo Sci. 71 (10), 1439–1444. doi:10.5650/jos.ess22067
Magan, N., Medina, A., and Aldred, D. (2011). Possible climate-change effects on mycotoxin contamination of food crops pre- and postharvest. Plant Pathol. 60, 150–163. doi:10.1111/j.1365-3059.2010.02412.x
Maldonado Haro, M. L., Cabrera, G., Fernandez Pinto, V., and Patriarca, A. (2023). Alternaria toxins in tomato products from the Argentinean market. Food control. 147, 109607. doi:10.1016/j.foodcont.2023.109607
Mallmann, C. A., Tyska, D., Almeida, C. A. A., Oliveira, M. S., and Gressler, L. T. (2020). Mycotoxicological monitoring of breakfast and infant cereals marketed in Brazil. Int. J. Food Microbiol. 331, 108628. doi:10.1016/j.ijfoodmicro.2020.108628
Marimón Sibaja, K. V., Gonçalves, K. D. M., Garcia, S. D. O., Feltrin, A. C. P., Nogueira, W. V., Badiale-Furlong, E., et al. (2019). Aflatoxin M1 and B1 in Colombian milk powder and estimated risk exposure. Food Addit. Contam. Part B 12 (2), 97–104. doi:10.1080/19393210.2019.1567611
Moretti, A., Pascale, M., and Logrieco, A. F. (2019). Mycotoxin risks under a climate change scenario in Europe. Trends food Sci. Technol. 84, 38–40. doi:10.1016/j.tifs.2018.03.008
National Institute of Technology, Standardization and Metrology of Paraguay (2023). Norma paraguaya NP 35 001 93 yerba mate. Available at: https://portal.intn.gov.py/index.php/organismos/organismo-nacional-de-normalizacion/citn/normas-obligatorias-y-reglamentarias (Accessed February 8, 2024).
Nogueira, M. S., Decundo, J., Martinez, M., Dieguez, S. N., Moreyra, F., Moreno, M. V., et al. (2018). Natural contamination with mycotoxins produced by Fusarium graminearum and Fusarium poae in malting barley in Argentina. Toxins (Basel) 10 (2), 78. doi:10.3390/toxins10020078
Ono, L. T., Silva, J. J., Doná, S., Martins, L. M., Iamanaka, B. T., Fungaro, M. H. P., et al. (2021). Aspergillus section Flavi and aflatoxins in Brazilian cassava (Manihot esculenta Crantz) and products. Mycotoxin Res. 37, 221–228. doi:10.1007/s12550-021-00430-2
Organization of American States (2022). Reglamento Técnico sobre Límites Máximos de Aflatoxinas. Available at: http://www.sice.oas.org/trade/mrcsrs/resolutions/indice.asp (Accessed February 8, 2024).
Ortiz, J., Jacxsens, L., Astudillo, G., Ballesteros, A., Donoso, S., Huybregts, L., et al. (2018). Multiple mycotoxin exposure of infants and young children via breastfeeding and complementary/weaning foods consumption in Ecuadorian highlands. Food Chem. Toxicol. 118, 541–548. doi:10.1016/j.fct.2018.06.008
Palladino, C., Puigvert, F., Muela, A., Taborda, B., Perez, C. A., Perez-Parada, A., et al. (2021). Evaluation of Fusarium mycotoxins and fungicide residues in barley grain produced in Uruguay. J. Agric. Food Res. 3, 100092. doi:10.1016/j.jafr.2020.100092
Palma, P., Calderón, R., Godoy, M., Vidal, M., and Rivera, A. (2023). Human exposure to ochratoxin A and its natural occurrence in spices marketed in Chile (2016–2020): a case study of merkén. J. Food Compos. Analysis 115, 104885. doi:10.1016/j.jfca.2022.104885
Palmeiro-Silva, Y. K., Lescano, A. G., Flores, E. C., Astorga E, Y., Rojas, L., Chavez, M. G., et al. (2023). Identifying gaps on health impacts, exposures, and vulnerabilities to climate change on human health and wellbeing in South America: a scoping review. Lancet Regional Health – Am. 26, 100580. doi:10.1016/j.lana.2023.100580
Paterson, R. R. M., and Lima, N. (2010). How will climate change affect mycotoxins in food? Food Res. Int. 43, 1902–1914. doi:10.1016/j.foodres.2009.07.010
Pavicich, M. A., De Boevre, M., Vidal, A., Mikula, H., Warth, B., Marko, D., et al. (2023). Natural occurrence, exposure assessment and risk characterization of Alternaria mycotoxins in apple by-products in Argentina. Expo. Health 16, 149–158. doi:10.1007/s12403-023-00544-1
Pinheiro, M., Iwase, C. H. T., Bertozzi, B. G., Caramês, E. T. S., Carnielli-Queiroz, L., Langaro, N. C., et al. (2021). Survey of freshly harvested oat grains from southern Brazil reveals high incidence of type B trichothecenes and associated Fusarium species. Toxins 13, 855. doi:10.3390/toxins13120855
Prendes, L. P., Fontana, A. R., Merín, M. G., D´ Amario Fernández, A., Bottini, R. A., Ramirez, M. L., et al. (2018). Natural occurrence and production of tenuazonic acid in wine grapes in Argentina. Food Sci. Nutr. 6, 523–531. doi:10.1002/fsn3.577
Puga-Torres, B., Cáceres-Chicó, M., Alarcón-Vásconez, D., and Gómez, C. (2021). Determination of zearalenone in raw milk from different provinces of Ecuador. Vet. World 14 (8), 2048–2054. doi:10.14202/vetworld.2021.2048-2054
Puga-Torres, B., Salazar, D., Cachiguango, M., Cisneros, G., and Gómez-Bravo, C. (2020). Determination of Aflatoxin M1 in raw milk from different provinces of Ecuador. Toxins (Basel). 12 (8), 498. doi:10.3390/toxins12080498
Republic of Uruguay (1994). Reglamento Bromatologico Nacional Decreto Nº 315/994 de 05/07/1994. Available at: https://www2.sag.gob.cl/pecuaria/establecimientos_habilitados_exportar/normativa/uruguay/2_reglamento_bromatologico_decreto_315-994-1994.pdf (Accessed March 8, 2024).
Rocha, A. R., Cardoso, M. S., Souza Júnior, J. A., Gomes Júnior, E. A., Maciel, L. F., and Menezes-Filho, J. A. (2023). Occurrence of aflatoxins B1, B2, G1, and G2 in beers produced in Brazil and their carcinogenic risk evaluation. Food control. 145, 109348. doi:10.1016/j.foodcont.2022.109348
Rojas Jaimes, J., Chacón-Cruzado, M., Castañeda-Peláez, L., and Díaz-Tello, A. (2021). Quantification of carcinogenic aflatoxins in unprocessed foods and their implication for consumption in Lima, Peru. Nutr. Hosp. 38 (1), 146–151. doi:10.20960/nh.03240
Romero Bernal, Á. R., Reynoso, C. M., García Londoño, V. A., Broggi, L. E., and Resnik, S. L. (2019). Alternaria toxins in Argentinean wheat, bran, and flour. Food Addit. Contam. Part B 12 (1), 24–30. doi:10.1080/19393210.2018.1509900
Silva, A. R. da ., Zanin, L. M. M., Ishikawa, A. T., Yamashita, C. R. T., Fracalossi, F. P., Amorim, T. M., et al. (2018). Development of ic-Elisa for the screening of aflatoxin contamination in the peanut production chain. Pesqui. Agropecuária Bras. 53 (3), 361–370. doi:10.1590/S0100-204X2018000300011
Silva, A. R. P. d., Fungaro, M. H. P., Silva, J. J., Martins, L. M., Taniwaki, M. H., and Iamanaka, B. T. (2021). Ochratoxin A and related fungi in Brazilian black pepper (Piper nigrum L.). Food Res. Int. 142, 110207. doi:10.1016/j.foodres.2021.110207
Silva, I. M. d.M., da Cruz, A. G., Ali, S., Freire, L. G. D., Fonseca, L. M., Rosim, R. E., et al. (2023). Incidence and levels of aflatoxin M1 in artisanal and manufactured cheese in pernambuco state, Brazil. Toxins 15, 182. doi:10.3390/toxins15030182
Vaclavikova, M., Malachova, A., Veprikova, Z., Dzuman, Z., Zachariasova, M., and Hajslova, J. (2013). Emerging’ mycotoxins in cereals processing chains: changes of enniatins during beer and bread making. Food Chem. 136, 750–757. doi:10.1016/j.foodchem.2012.08.031
Van Egmond, H. P., Schothorst, R. C., and Jonker, M. A. (2007). Regulations relating to mycotoxins in food: perspectives in a global and European context. Anal. Bioanal. Chem. 389, 147–157. doi:10.1007/s00216-007-1317-9
Vásquez-Ocmín, P. G., Marti, G., Gadea, A., Cabanac, G., Vásquez-Briones, J. A., Casavilca-Zambrano, S., et al. (2023). Metabotyping of Andean pseudocereals and characterization of emerging mycotoxins. Food Chem. 407, 135134. doi:10.1016/j.foodchem.2022.135134
Keywords: mycotoxins, occurrence, natural contamination, foodstuffs, emerging mycotoxins, human consumption, South America
Citation: Foerster C, Müller-Sepúlveda A, Copetti MV, Arrúa AA, Monsalve L, Ramirez ML and Torres AM (2024) A mini review of mycotoxin’s occurrence in food in South America in the last 5 years: research gaps and challenges in a climate change era. Front. Chem. Biol 3:1400481. doi: 10.3389/fchbi.2024.1400481
Received: 13 March 2024; Accepted: 26 April 2024;
Published: 09 May 2024.
Edited by:
Rohit Mahar, Hemwati Nandan Bahuguna Garhwal University, IndiaReviewed by:
Florin Oancea, National Institute for Research & Development in Chemistry and Petrochemistry (ICECHIM), RomaniaCopyright © 2024 Foerster, Müller-Sepúlveda, Copetti, Arrúa, Monsalve, Ramirez and Torres. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Claudia Foerster, Y2xhdWRpYS5mb2Vyc3RlckB1b2guY2w=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.