
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cell. Neurosci., 12 March 2025
Sec. Cellular Neuropathology
Volume 19 - 2025 | https://doi.org/10.3389/fncel.2025.1550333
This article is part of the Research TopicRole of Microbiota in Neurocognitive Disorders: A Developmental Origin PerspectiveView all 9 articles
 Claudio Singh Solorzano1
Claudio Singh Solorzano1 Cristina Festari1
Cristina Festari1 Peppino Mirabelli2
Peppino Mirabelli2 Elisa Mombelli3
Elisa Mombelli3 Luigi Coppola4
Luigi Coppola4 Delia Luongo5
Delia Luongo5 Daniele Naviglio6
Daniele Naviglio6 Andrea Soricelli4,7
Andrea Soricelli4,7 Giulia Quattrini1
Giulia Quattrini1 Marco Salvatore4
Marco Salvatore4 Michela Pievani1
Michela Pievani1 Annamaria Cattaneo3,8
Annamaria Cattaneo3,8 Giovanni B. Frisoni9,10
Giovanni B. Frisoni9,10 Moira Marizzoni3*
Moira Marizzoni3*Introduction: A growing body of evidence recognises the role of signaling molecule of the microbiota-gut-brain axis (MGBA) in cognitive impairment (CI), but data on the link with alterations in specific cognitive domains are limited. We compared the functioning in several cognitive domains (i.e., memory, visuo-constructional, executive, and language) among cognitively unimpaired (CU) subjects, patients with CI due to Alzheimer’s disease (CI-AD) and not due to AD (CI-NAD). Then, we investigated the association of these cognitive domains with the gut microbiota (GM), MGBA mediators, and neurodegeneration-related markers.
Materials and methods: The study included 34 CI-AD, 38 CI-NAD, and 13 CU. Memory, visuo-constructional, executive, and language domains were assessed using composite measures. Faecal GM composition was inferred using 16S rRNA gene sequencing. MGBA mediators included the blood quantification of bacterial products (lipolysaccharide, LPS), cell adhesion molecules indicative of endothelial damage, vascular changes or overexpressed in response to infections, and pro- and anti-inflammatory cytokines. Neurodegeneration-related markers included plasma phosphorylated tau (p-tau181), neurofilament light chain (NfL), and glial fibrillary protein (GFAP).
Results: The CI-NAD and CI-AD groups had significantly lower scores than the CU group for all cognitive domains (p < 0.043). Associations of MGBA modulators with cognitive functioning included pro-inflammatory cytokines, markers of endothelial dysfunction or overexpressed in response to infection in both groups of patients (|ρ| > 0.33, ps < 0.042). In the CU and CI-AD pooled group, lower cognitive functioning was specifically associated with higher abundance of Dialister and Clostridia_UCG-014, higher levels of LPS and with all neurodegeneration markers (|ρ| > 0.32, p < 0.048 for all). In the CU and CI-NAD pooled group, lower cognitive performance was associated with lower abundance of Acetonema, higher abundance of Bifidobacterium, [Eubacterium]_coprostanoligenes_group and Collinsella, and higher levels of vascular changes (|ρ| > 0.30, p < 0.049).
Discussion: These results support the hypothesis that gut dysbiosis and MGBA mediators may have distinct effects on cognitive functioning and different mechanisms of action depending on the disease.
The gut microbiota (GM) includes the complex ecosystem of bacteria, fungi, archaea, and protozoa that populates the gut (Hou et al., 2022). The GM could influence brain function and behavior through the microbiota-gut-brain axis (MGBA) (Cryan et al., 2019; Morais et al., 2021) in both physiological and pathological conditions (Cryan et al., 2020; Escobar et al., 2022). A growing body of preclinical and clinical studies recognises the role of the signaling molecule of the MGBA in mediating the association between GM dysbiosis and cognitive impairment (CI) in several neurocognitive disorders, including the Alzheimer’s disease (AD). Indeed, it has been suggested that GM dysbiosis might promote the imbalance of bacteria metabolites (MahmoudianDehkordi et al., 2019) and local inflammation (Grabrucker et al., 2023) in AD patients. These conditions have been linked to increased intestinal permeability (Pei et al., 2023), increased passage of bacteria components and cytokines into the bloodstream, and systemic inflammation (Cryan et al., 2019; Kowalski and Mulak, 2019). These processes might contribute to the alteration of the blood–brain barrier, neuroinflammation, and to the accumulation of toxic proteins in the brain (Erny et al., 2015), ultimately leading to the loss of neurons and CI (Marizzoni et al., 2017; Liu et al., 2024; Yang et al., 2024). In line, recent clinical studies in AD found a relationship between alterations of MGBA mediators (e.g., increased lipopolysaccharide and inflammatory mediators), endothelial integrity reduction, brain pathology and CI (Verhaar et al., 2022; Zhang et al., 2022; Chen et al., 2023a,b; Marizzoni et al., 2023). Similarly, several studies in healthy middle-aged and elderly subjects found that microbial community composition may be associated with cognitive performance (Canipe et al., 2021; Haimov et al., 2022; Meyer et al., 2022). However, to our knowledge, no studies described the simultaneous assessment of GM and a large panel of MGBA mediators in association with specific cognitive domains in a memory clinic population. A better understanding of the impact of MGBA on cognitive performance could help to identify potential mechanisms and new therapeutic targets for the treatment and prevention of CI.
In this cross-sectional study, we explored the association between specific cognitive domains (i.e., memory, visuo-constructional, executive, and language) with faecal bacterial genera, MGBA mediators, and neurodegenerative-related markers in older adults with normal cognition (CU), patients with CI due to AD (CI-AD), and patient with CI not due to AD (CI-NAD). Our main hypothesis is that the CI-AD and CI-NAD groups showed specific MGBA mediator profiles related to the altered cognitive domains.
Several aspects of the subjects, study design and data analyses used in the present study have been described elsewhere (Marizzoni et al., 2023). Here, we added the assessment of cognitive functioning domains in relationship with GM and a wide range of MGBA mediators, and we included the quantification of plasma levels of GFAP, considered a proxy for neuroinflammation (Abdelhak et al., 2022) and recently recognized as biomarker of AD pathophysiology (Jack et al., 2024).
Participants (n = 85) were selected from a large Italian study on amyloid imaging, the Incremental Diagnostic Value of [18F]florbetapir Amyloid Imaging [INDIA-FBP] study (Boccardi et al., 2016) and underwent a multi-domain neuropsychological evaluation, a [18F]florbetapir PET, and blood and stool exams. Inclusion criteria were age between 50 and 85 years, availability of an informant (spouse, adult child, or another knowledgeable informant), and being native/fluent Italian speakers to complete the neuropsychological tests correctly. Exclusion criteria included being under antibiotic and anti-inflammatory treatment over the past 3 months or a past diagnosis of major depression or any other psychiatric disorders.
The neuropsychological evaluation covered four cognitive domains and related tests: (i) memory [Story Recall Test, total immediate and delayed recall of the Rey Auditory Verbal Learning Task (RAVLT)] (Novelli et al., 1986a; Carlesimo et al., 1996); (ii) visuo-constructional ability (Rey-Osterrieth Complex Figure) (Caffarra et al., 2002); (iii) executive functions [Trail Making Test part A (TMT-A), Raven progressive matrices] (Giovagnoli et al., 1996; Amodio et al., 2002; Caffarra et al., 2003); (iv) language (Token Test, phonemic and semantic verbal fluency test) (Novelli et al., 1986b; Spinnler and Tognoni, 1987). Participants were classified as CU (i.e., no more than one neuropsychological test was abnormal) and CI (i.e., two or more neuropsychological tests were beyond the normal range). Some participants could not complete specific neuropsychological tests due to the severity of cognitive impairment. Composite scores for each cognitive domain could not be calculated for these individuals, leading to missing data in the analysis including the cognitive domains. The [18F]florbetapir standardized uptake value ratio (SUVr) was computed as the ratio of the global cortical (frontal, parietal, temporal, anterior cingulate, posterior cingulate, and precuneus) to the cerebellar uptake. Participants were also classified as amyloid-positive (CI-AD) and amyloid-negative (CI-NAD and CU) based on an established cut-off (SUVr>1.10) (Clark et al., 2012).
Written informed consent was obtained from all participants and covered sample processing and analyses. The study was approved by the Ethics Committee of the IRCCS Fatebenefratelli (approval date November 18, 2014, number 57/2014) and was conducted according to the Declaration of Helsinki.
Stool samples were collected in sterile plastic cups from participants at their own home, stored at −20°C, and delivered within 24 h to the IRCCS Fatebenefratelli Institute in Brescia, where they were stored at −20°C until processing.
Faecal microbiota analyses were performed as reported elsewhere (Marizzoni et al., 2023). Briefly, faecal DNA was extracted using the QIAamp Fast DNA Stool Mini Kit (Qiagen Retsch GmbH, Hannover, Germany), the V3-V4 regions of the bacterial 16S rRNA gene were amplified by Illumina’s 16S Metagenomic Sequencing Library Preparation protocol and sequenced on Illumina MiSeq platform. The raw 16S data were processed using QIIME2 (Bolyen et al., 2019) and underwent the denoising process using DADA2 (Callahan et al., 2016). The SILVA reference 16S rRNA gene database 138 was used to assign taxonomies (Quast et al., 2012).
A series of markers were selected to investigate various potential metabolic, endothelial, and immune mediators of MGBA, and measured in plasma by ELISA (Pierce LAL Chromogenic Endotoxin Quantitation Kit, Thermo Fisher Scientific): (i) lipopolysaccharide (LPS), an important microbial-generated neurotoxin (Zhao et al., 2019), (ii) sVCAM-1 and sPECAM-1, as endothelial damage markers (Chen et al., 2023a,b; Sim et al., 2024), (iii) sP-Selectin, as an indicator of vascular damage (Zuliani et al., 2008), (iv) sICAM3 and sCD44, as markers of immune response to infection (Baaten et al., 2010; Guerra-Espinosa et al., 2024).
A panel of cytokines typically altered in AD (i.e., IL1β, TNFα, IL-18, IL-10) was measured using semi-quantitative real-time PCR (Brosseron et al., 2018). The total RNA isolation was performed using the PAXgene blood miRNA kit and according to the manufacturer’s protocol (PreAnalytiX, Hombrechtikon, CHE). Each target gene was normalized to the geometric mean of the expression of three reference genes (i.e., glyceraldehyde-3-phosphate dehydrogenase, beta-actin, and beta-2-microglobulin) using the TaqMan assays on a 384-well Real-Time PCR system (Biorad Laboratories, Hercules, USA). The relative target gene expression of each gene in patients compared to controls was determined using the Pfaffl method (Pfaffl, 2001).
The SUVrs were calculated as global measures (Marizzoni et al., 2020). Venous blood samples were collected from all participants using a 4 mL K3-ethylenediaminetetraacetic acid (EDTA) vacutainer, and centrifuged within two hours of collection at 3400 g for 10 min at 4°C to obtain plasma. Plasma samples were then aliquoted and stored at −80°C until testing.
Plasma concentrations of neurofilament light chain (NfL) (NF-Light immunoassay Advantage kit; Cat. No. 103400), glial fibrillary acidic protein (GFAP) (GFAP Human Discovery Kit; Cat. No. 102336), and p-tau181 (p-tau181 V2 Advantage Kit; Cat. No. 103714) were measured using the ultrasensitive Simoa SR-X instrument following the manufacturer’s recommended protocol obtained by Quanterix, Billerica, USA.
Statistical analyses were performed using Rstudio version 4.4.1. We computed composite scores for each cognitive domain (i.e., memory, visuo-constructional, executive, and language). Raw scores on each test were z-transformed according to the performance distribution of the entire sample. Then, z-scores for each test were averaged for each domain. Higher scores mean better functioning in the specific domain. For this purpose, we computed the reverse score of the TMT-A to associate higher scores with a better performance on the test.
The normal distribution of the variables was determined by the Shapiro–Wilk test. Descriptive statistics for the total sample and the three groups of interest (i.e., CU, CI-AD, CI-NAD) were reported as mean (M) and standard deviation (SD) for continuous variables and as number of participants (N) and percentage (%) for categorical variables. The ANOVA or Kruskall-Wallis test, according to data distribution with Bonferroni corrections for multiple comparisons, were used to compare continuous variables. The Pearson chi-square test was used to compare categorical variables. For graphical purposes, GM and putative MGBA mediators were reported as percentage differences of the CI groups (i.e., CI-NAD or CI-AD) versus CU.
Partial Spearman’s rank correlation was used to test the association between cognitive domains with genera, MGBA mediators, and neurodegeneration-related markers, controlling for the effect of age. Partial correlations were performed for the CU and CI-NAD pooled group and for the CU and CI-AD pooled group. The significance was set at p < 0.050 (two-tailed). For hypothesis validation analyses, associations were selected if their Spearman’s rho value was>0.4 to include only those with at least moderate association (Prion and Haerling, 2014).
Demographic and clinical characteristics were as expected for this population (Table 1 and Supplementary Table 1) Reported medications were comparable between the two patient groups. Cognitive domains.
All domains showed significant differences between groups memory domain: H (2) = 20.373, p < 0.001, n = 84; visuo-constructional domain: F(2,70) = 7.942, p < 0.001, n = 73; executive domain: H (2) = 14.75, p < 0.001, n = 84; language domain: H (2) = 15.049, p < 0.001, n = 80. In particular, the CI-NAD and CI-AD groups had significantly lower scores than the CU group for all domains (Figure 1). However, no significant differences were found when comparing CI-NAD and CI-AD (Figure 1).
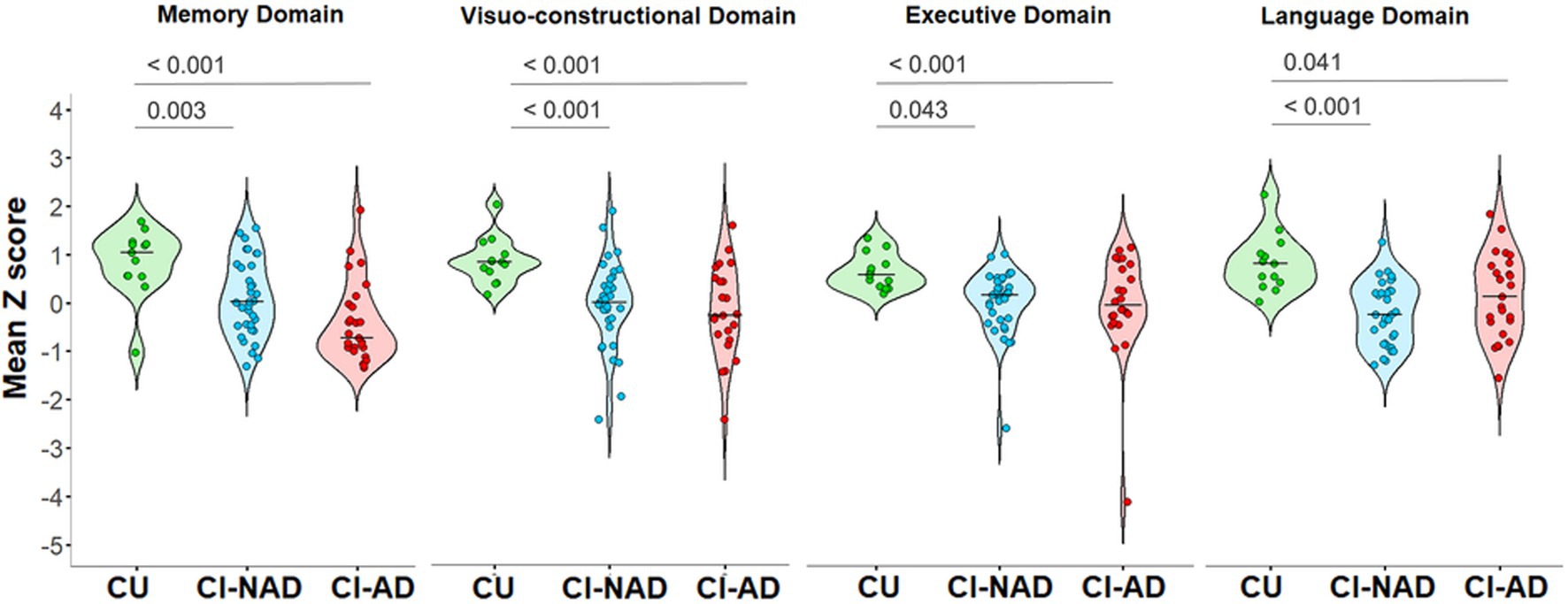
Figure 1. Violin plot of the distribution of cognitive domains’ z scores for the three groups (CU, CI-NAD, CI-AD). The plots show the median (indicated by the black horizontal band), the first through the third interquartile range (the vertical band), and an estimator of the density (thin vertical curves) of each cognitive domain functioning in each group. The reported p-values were calculated by using one-way ANOVA with Bonferroni’s correction for normal distributed variables (i.e., visuo-constructional domain) or Kruskall-Wallis test with Bonferroni’s correction for non-normal distributed variables (i.e., memory domain, executive domain, and language domain). CI-AD, patients with cognitive impairment due to AD; CI-NAD, patients with cognitive impairment not due to AD; CU, cognitively unimpaired persons.
When focusing on GM composition, both CI groups showed a lower abundance of Acetonema (−96.3% in CI-NAD, −109.5% in CI-AD; Figure 2) and a higher abundance of Bifidobacterium (+187.2% in CI-NAD, +125.3% in CI-AD) and Dialister (+187.1% in CI-NAD, +217.7% in CI-AD) compared to CU. Similarly, when considering MGBA mediators and neurodegenerative markers, higher levels of sP-Selectin (+82.2% in CI-NAD, +183.4% in CI-AD), sCD44 (+30.3% in CI-NAD, +39.8% in CI-AD), TNFα (+21.1% in CI-NAD, +18.7% in CI-AD), and NfL (+46.6% in CI-NAD, +69.1% in CI-AD) were reported in both CI groups compared to CU.
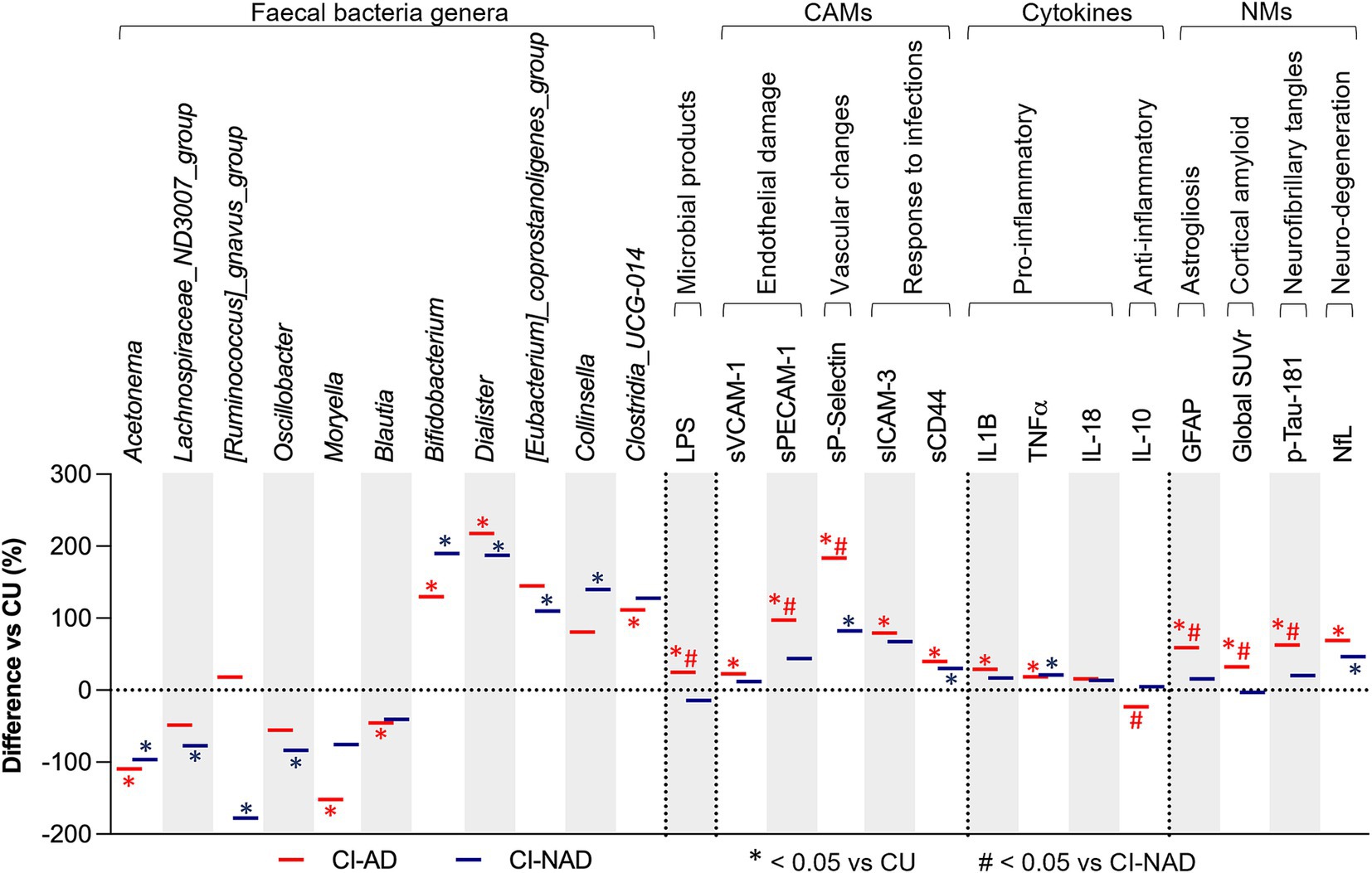
Figure 2. GM and MGBA putative mediators of study participants. Bars denote percentage difference in CI-AD and CI-NAD patients versus unimpaired control subjects (CU). The percentage difference has been calculated using control subjects as reference (represented by the threshold line at 0). p-values were calculated by using one-way ANOVA or Kruskall-Wallis test (accordingly with data distribution) with Bonferroni’s test for multiple comparison correction on raw data. Statistical significances is represented by * at p < 0.05, *** at p < 0.001 comparing CI-AD and CI-NAD versus CU and by # at p < 0.05 comparing CI-AD versus CI-NAD. CI-AD, patients with cognitive impairment due to AD; CI-NAD, patients with cognitive impairment not due to AD; CU = cognitively unimpaired persons.
The CI-AD group was characterized by a decreased abundance of Moryella (−151.3%), Blautia (−45.5%), and an increased abundance of Clostridia_UCG-014 (+111.6) compared to CU. The CI-NAD showed a lower abundance of Lachnospiraceae_ND3007_group (−77.2%), [Ruminococcus]_gnavus_group (−177.5%), and Oscillobacter (−83.5%), and a higher abundance of Collinsella (139.6%) compared to CU. On the other hand, CI-AD group was characterized by higher levels of LPS (+24.7%), sVCAM-1 (+22.7% vs CU), sPECAM1 (+97.4% vs CU), sICAM-3 (+97.2% vs CU), and the pro-inflammatory cytokine IL-1β (+28.9% vs. CU), and lower levels of the anti-inflammatory cytokine IL-10 (−22.9% vs. CI-NAD). Significantly greater levels of plasma GFAP (+59.1% vs. CU), amyloid (+32.2% vs. CU), and p-tau181 (+62.6% vs. CU) were found for the CI-AD when compared to CU.
Figure 3 shows the association of cognitive domains with microbial genera, MGBA, and neurodegenerative-related markers. In both patient groups, lower levels of pro-inflammatory cytokines, CAMs indicative of endothelial damage or upregulated immune response, and NfL were associated with better cognitive performances (|ρ| > 0.33, ps < 0.042). GM alteration was directly associated with cognitive performance, but the genera involved were different in CI-AD and CI-NAD (Supplementary Table 2). Furthermore, lower levels of LPS, amyloid, p-tau181, GFAP, and high expression of IL-10 were associated with better cognitive performance only in the CU and CI-AD pooled group (|ρ| > 0.34, ps < 0.037).
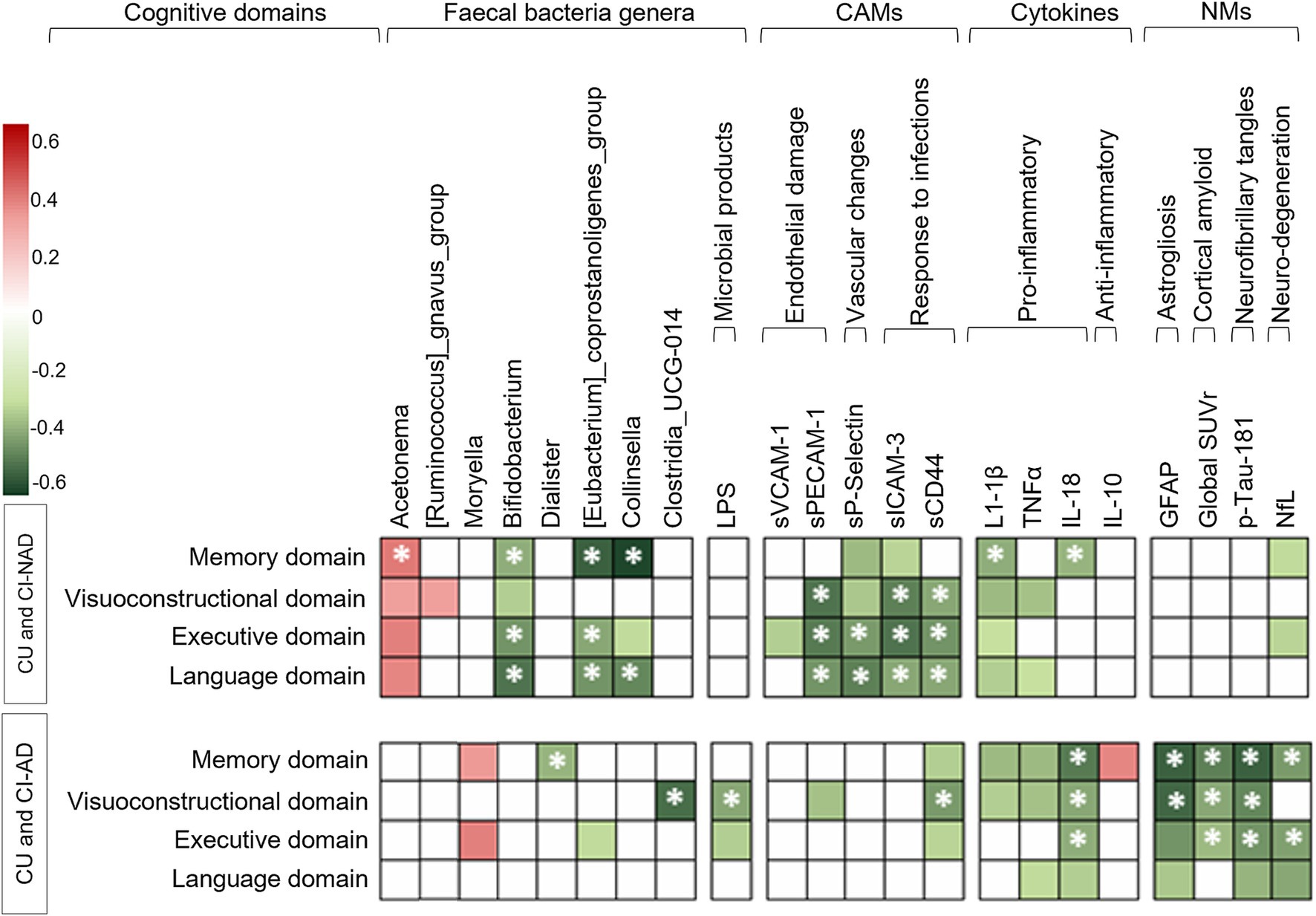
Figure 3. Heatmap of the Spearman’s rho coefficient values (red: positive; green: negative) indicating significant age-adjusted association in CU and CI-NAD or CU and CI-AD (p < 0.05, for exact p-values and confidence intervals please refer to Supplementary Table 2). Asterisks indicate moderate associations ( ρ > 0.4). For cognition domains, higher values reflected better cognitive performance. CAMs, cell adhesion molecules; CI-AD, patients with cognitive impairment due to AD; CI-NAD, patients with cognitive impairment not due to AD; CU, cognitively unimpaired persons; NMs, neurodegenerative-related markers.
Figure 4 refines the previously published model (Marizzoni et al., 2023) on the possible relationships between GM, MGBA mediators, and neurodegeneration-related markers in the CI-AD (panel A) and CI-NAD (panel B) groups. Overall, alterations in the GM composition and inflammatory profile (increased expression of cytokines and upregulation of CAMs) were associated with greater cognitive impairment in both CI-NAD and CI-AD. Interestingly, direct and group-specific associations between MGBA mediators and CI involved endothelial and vascular damage markers in CI-NAD and LPS in CI-AD. Furthermore, MGBA mediators were widely associated with neurodegeneration-related markers, and the latter were associated with CI only in CI-AD.
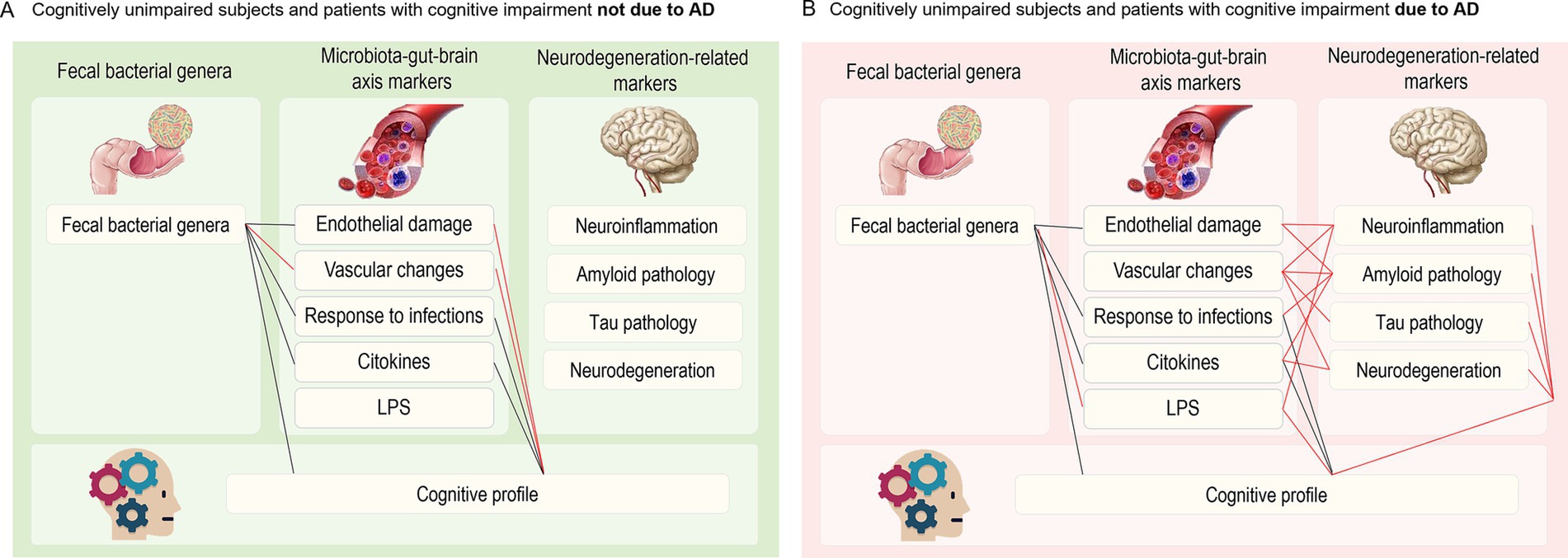
Figure 4. Closest associations (ρ > 0.4) between faecal bacterial genera, microbiota-gut-brain axis mediators, neurodegeneration-related markers and cognitive profile in CU and CI-NAD (A) and CU and CI-AD (B). Black lines indicate common paths for both groups, whereas red lines indicate paths specific to CI-NAD or CI-AD.
This cross-sectional study primarily investigated the association of cognitive domains (i.e., memory, visuo-constructional, executive, and language) with faecal bacterial genera, MGBA mediators, and neurodegenerative-related markers in a cohort of cognitively unimpaired persons, patients with cognitive impairment due to AD, and patients with cognitive impairment not due to AD. Our principal results revealed the presence of an extensive association of GM and MGBA mediators with cognitive impairment, some common to both patient groups, and some specific to CI-AD or CI-NAD.
Common associations included pro-inflammatory cytokines, soluble CAMs involved in endothelial damage or overexpressed in response to infection, and neurodegeneration markers. These results confirmed previous human findings showing that levels of pro-inflammatory cytokines (i.e., IL-1β, TNFα, and IL-18), sCAMs (i.e., sVCAM1, sPECAM1, sICAM3, sCD44), and NfL were associated with the progression of cognitive decline in dementia (Nielsen et al., 2007; Leblhuber et al., 2015; Ashton et al., 2021; Drake et al., 2021; Rasi Marzabadi et al., 2021; Hosoki et al., 2023). The increase in sCAMs is strictly related with systemic inflammation and the disruption of the blood–brain barrier’s integrity, which ultimately might lead to neuroinflammation and loss of neurons (Cryan et al., 2019; Liu et al., 2020; Loh et al., 2024). Therefore, these findings support the crucial role of systemic inflammation in influencing brain functioning and contributing to the development of cognitive impairment.
Specific associations of MGBA modulators with cognitive functioning for the CI-AD group included the abundance of Dialister and Clostridia_UCG-014, as well as the levels of LPS and IL-10 expression. Accumulating evidence supports a close connection between GM dysbiosis and AD, although studies disagree on the identity of the genera involved (Cattaneo et al., 2017; Vogt et al., 2017; Guo et al., 2021; Ling et al., 2021; Laske et al., 2022). A few studies showed that the Dialister genus and Clostridia class could be related to pathological mechanisms in AD (Vogt et al., 2017; Ling et al., 2021; Kaiyrlykyzy et al., 2022; Khedr et al., 2022). In line, we found a specific association between the higher abundance of Dialister and Clostridia_UCG-014 genera and greater impairment of memory, executive, and language cognitive domains in the CI-AD group. In our study, higher LPS levels were related to poor visuo-constructional and executive cognitive functioning, following the evidence of a strict relationship between LPS and the progressive cognitive decline associated with AD (Zhan et al., 2018; Ghosh et al., 2020). Thus, these findings support the endotoxin hypothesis of AD (Brown and Heneka, 2024) and the evidence of higher levels of LPS in AD patients compared to CU (Zhang et al., 2009; Andreadou et al., 2021). The relationship between IL-10 and the memory domain aligns with the neuroprotective role of anti-inflammatory processes in AD (Su et al., 2016; Porro et al., 2020). Some evidence reported that high expression of IL-10 was associated with low brain amyloid load in humans (D’Anna et al., 2017; Marizzoni et al., 2020) and with neurogenesis processes and enhanced cognition in animal models of AD (Kiyota et al., 2012; Guillot-Sestier et al., 2015).
Specific associations of MGBA modulators with cognitive functioning for the CI-NAD group included the abundance of Acetonema, Bifidobacterium, [Eubacterium]_coprostanoligenes_group, Collinsella, and sP-Selectin levels. These genera have been associated with cognitive decline but with conflicting results (Li et al., 2019; Rueda-Ruzafa et al., 2019; Nishiwaki et al., 2022). In particular, we found specific associations between the high abundance of Bifidobacterium, [Eubacterium]_coprostanoligenes_group, Collinsella genera and greater impairment of memory, executive, and language cognitive domains; on the other side, the high abundance of Acetonema could be a protective factor for cognitive functioning. Moreover, vascular damage and platelet activation markers (i.e., sP-Selectin) were strictly associated with cognitive impairment in all the evaluated domains, suggesting a potentially high number of vascular dementias in the CI-NAD group (Toth et al., 2017; van der Flier et al., 2018; Morgan and Mc Auley, 2024).
Concerning the neurodegeneration-related biomarkers, neuroinflammation and tau pathology play a significant role in the definition of cognitive impairment only in the CI-AD group. Beyond the expected association between ATN cascade markers and cognitive impairment (Hanseeuw et al., 2019), astrogliosis, as reflected by the increase in GFAP levels, seems to play a role in the cognitive profile of AD patients. The association between increased levels of GFAP and higher cognitive impairment in the AD group are in line with recent studies that posited specific reactive astrogliosis in AD and its correlation with the severity of cognitive impairment (Bettcher et al., 2021; Oeckl et al., 2022; Peretti et al., 2024).
These findings highlight the potential of MGBA mediators as promising biomarkers for cognitive impairment (Cryan et al., 2019; Morais et al., 2021). The possibility to integrate MGBA variables with neuroimaging and genetic markers for AD diagnosis or for differentiating AD-related and non-AD-related cognitive impairment needs further investigation. Moreover, future research should prioritize longitudinal studies to validate the association of MGBA mediators with cognitive decline. Mechanistic investigations are needed to unravel causal relationships between specific microbial genera, MGBA signaling molecules, and cognitive functions. Such studies could pave the way for targeted therapeutic interventions, such as microbiota-based therapies, dietary interventions, or pharmacological modulation of MGBA pathways, to mitigate or prevent cognitive decline (Li et al., 2023).
We are aware of several limitations of the study. Firstly, its cross-sectional design and the small sample size make it difficult to generalize the results and the causality of the conclusions. More independent, longitudinal, and large cohort studies are needed to confirm the present results. Secondly, other potential MGBA mediators that could play an important role in neurodegenerative diseases (e.g., short-chain fatty acids, neurotransmitters, estrogens, etc.) (Mayer et al., 2022) were not considered. Third, we did not include the severity and duration of dementia as covariates in the models. The progression of cognitive decline should be considered in future studies to identify better the timing and the most important players involved in the different phases of the disease. Fourth, the CI-NAD group is not characterized by a specific neurodegenerative diagnosis. However, this could represent a starting point for future works with well-characterized forms of non-AD dementias (e.g., vascular cognitive impairment, frontotemporal lobar degeneration) better to outline the cognitive impairment profile of non-AD-related neurodegenerative disorders. Finally, it should be noted that studies addressing changes in the whole GM in AD often report conflicting results due to the use of different methods for extraction, sequencing, and analyses of the microbiome profile. A harmonization procedure is needed to move the field forward.
In conclusion, these results suggest that gut microbiota and MGBA mediators in cognitive impairment may have distinct effects and mechanisms of action depending on the disease. In the CI-NAD group, MGBA mediators – particularly endothelial damage and vascular changes – are directly associated with cognitive impairment. In the CI-AD group, the effect of the MGBA mediators on cognition seemed associated with the modulation of the central neurodegeneration-related markers (i.e., GFAP, cortical amyloid, and p-tau181). MGBA variables are promising markers for cognitive impairment monitoring and treatment and deserve further investigations.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found at: https://www.ebi.ac.uk/ena, PRJEB55056.
The studies involving humans were approved by the Comitato Etico dell’IRCCS San Giovanni di Dio – Fatebenefratelli (Brescia, Italy) under registration number 57/2014. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
CS: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Visualization, Writing – original draft, Writing – review and editing. CF: Investigation, Methodology, Resources, Visualization, Writing – review & editing. PM: Investigation, Writing – review & editing. EM: Investigation, Writing – review & editing. LC: Investigation, Writing – review & editing. DL: Investigation, Writing – review & editing. DN: Investigation, Writing – review & editing. AS: Investigation, Writing – review & editing. GQ: Investigation, Writing – review & editing. MS: Investigation, Writing – review & editing. MP: Investigation, Writing – review & editing. AC: Funding acquisition, Investigation, Methodology, Project administration, Supervision, Writing – review & editing. GF: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Writing – review & editing. MM: Conceptualization, Data curation, Investigation, Methodology, Project administration, Resources, Supervision, Visualization, Writing – review & editing.
The author(s) declare that financial support was received for the research and/or publication of this article. This study was partially funded by the Alzheimer’s Association Grant (NIRG) and the Italian Ministry of Health (RF-2021-12372224 and Ricerca Corrente). Amyloid PET was collected thanks to an unrestricted grant from Avid Radiopharmaceuticals, Inc. which was unrelated to the topic of this study. The Centre de la mémoire is funded by the following private donors under the supervision of the Private Foundation of Geneva University Hospitals: APRA-Association Suisse pour la Recherche sur la Maladie d’Alzheimer, Genève; Fondation Segré, Genève; Race Against Dementia Foundation, London, UK; Fondation Child Care, Genève; Fondation Edmond J. Safra, Genève; Fondation Minkoff, Genève; Fondazione Agusta, Lugano; McCall Macbain Foundation, Canada; Nicole et René Keller, Genève; Fondation AETAS, Genève. The Clinical Research Center, University Hospital and Faculty of Medicine, Geneva, provides valuable support for regulatory submissions and data management. The Centre de la mémoire has received unrestricted grants and support for event organizations from ROCHE Pharmaceuticals; OM Pharma; EISAI Pharmaceuticals; and Biogen Pharmaceuticals. Competitive research projects have been funded by the H2020, Innovative Medicines Initiative (IMI), IMI2, Swiss National Science Foundation, and VELUX Foundation.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that no Gen AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fncel.2025.1550333/full#supplementary-material
Abdelhak, A., Foschi, M., Abu-Rumeileh, S., Yue, J. K., D’Anna, L., Huss, A., et al. (2022). Blood GFAP as an emerging biomarker in brain and spinal cord disorders. Nat. Rev. Neurol. 18, 158–172. doi: 10.1038/s41582-021-00616-3
Amodio, P., Wenin, H., del Piccolo, F., Mapelli, D., Montagnese, S., Pellegrini, A., et al. (2002). Variability of trail making test, symbol digit test and line trait test in normal people. A normative study taking into account age-dependent decline and sociobiological variables. Aging Clin. Exp. Res. 14, 117–131. doi: 10.1007/BF03324425
Andreadou, E. G., Katsipis, G., Tsolaki, M., and Pantazaki, A. A. (2021). Involvement and relationship of bacterial lipopolysaccharides and cyclooxygenases levels in Alzheimer’s disease and mild cognitive impairment patients. J. Neuroimmunol. 357:577561. doi: 10.1016/j.jneuroim.2021.577561
Ashton, N. J., Janelidze, S., al Khleifat, A., Leuzy, A., van der Ende, E. L., Karikari, T. K., et al. (2021). A multicentre validation study of the diagnostic value of plasma neurofilament light. Nat. Commun. 12:3400. doi: 10.1038/s41467-021-23620-z
Baaten, B. J. G., Li, C.-R., and Bradley, L. M. (2010). Multifaceted regulation of T cells by CD44. Commun. Integrative Biol. 3, 508–512. doi: 10.4161/cib.3.6.13495
Bettcher, B. M., Olson, K. E., Carlson, N. E., McConnell, B. V., Boyd, T., Adame, V., et al. (2021). Astrogliosis and episodic memory in late life: higher GFAP is related to worse memory and white matter microstructure in healthy aging and Alzheimer’s disease. Neurobiol. Aging 103, 68–77. doi: 10.1016/j.neurobiolaging.2021.02.012
Boccardi, M., Altomare, D., Ferrari, C., Festari, C., Guerra, U. P., Paghera, B., et al. (2016). Assessment of the incremental diagnostic value of Florbetapir F 18 imaging in patients with cognitive impairment: the incremental diagnostic value of amyloid PET with [18F]-Florbetapir (INDIA-FBP) study. JAMA Neurol. 73, 1417–1424. doi: 10.1001/JAMANEUROL.2016.3751
Bolyen, E., Rideout, J. R., Dillon, M. R., Bokulich, N. A., Abnet, C. C., al-Ghalith, G. A., et al. (2019). Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 37, 852–857. doi: 10.1038/s41587-019-0209-9
Brosseron, F., Traschütz, A., Widmann, C. N., Kummer, M. P., Tacik, P., Santarelli, F., et al. (2018). Characterization and clinical use of inflammatory cerebrospinal fluid protein markers in Alzheimer’s disease. Alzheimers Res. Ther. 10:25. doi: 10.1186/s13195-018-0353-3
Brown, G. C., and Heneka, M. T. (2024). The endotoxin hypothesis of Alzheimer’s disease. Mol. Neurodegener. 19:30. doi: 10.1186/s13024-024-00722-y
Caffarra, P., Vezzadini, G., Dieci, F., Zonato, F., and Venneri, A. (2002). Rey-Osterrieth complex figure: normative values in an Italian population sample. Neurol. Sci. 22, 443–447. doi: 10.1007/s100720200003
Caffarra, P., Vezzadini, G., Zonato, F., Copelli, S., and Venneri, A. (2003). A normative study of a shorter version of Raven’s progressive matrices 1938. Neurol. Sci. 24, 336–339. doi: 10.1007/s10072-003-0185-0
Callahan, B. J., McMurdie, P. J., Rosen, M. J., Han, A. W., Johnson, A. J. A., and Holmes, S. P. (2016). DADA2: high-resolution sample inference from Illumina amplicon data. Nat. Methods 13, 581–583. doi: 10.1038/nmeth.3869
Canipe, L. G., Sioda, M., and Cheatham, C. L. (2021). Diversity of the gut-microbiome related to cognitive behavioral outcomes in healthy older adults. Arch. Gerontol. Geriatr. 96:104464. doi: 10.1016/j.archger.2021.104464
Carlesimo, G. A., Caltagirone, C., Gainotti, G., Fadda, L., Gallassi, R., Lorusso, S., et al. (1996). The mental deterioration battery: normative data, diagnostic reliability and qualitative analyses of cognitive impairment. Eur. Neurol. 36, 378–384. doi: 10.1159/000117297
Cattaneo, A., Cattane, N., Galluzzi, S., Provasi, S., Lopizzo, N., Festari, C., et al. (2017). Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiol. Aging 49, 60–68. doi: 10.1016/j.neurobiolaging.2016.08.019
Chen, J., Dai, A.-X., Tang, H.-L., Lu, C.-H., Liu, H.-X., Hou, T., et al. (2023a). Increase of ALCAM and VCAM-1 in the plasma predicts the Alzheimer’s disease. Front. Immunol. 13:1097409. doi: 10.3389/fimmu.2022.1097409
Chen, J., Doyle, M. F., Fang, Y., Mez, J., Crane, P. K., Scollard, P., et al. (2023b). Peripheral inflammatory biomarkers are associated with cognitive function and dementia: Framingham heart study offspring cohort. Aging Cell 22:e13955. doi: 10.1111/acel.13955
Clark, C. M., Pontecorvo, M. J., Beach, T. G., Bedell, B. J., Coleman, R. E., Doraiswamy, P. M., et al. (2012). Cerebral PET with florbetapir compared with neuropathology at autopsy for detection of neuritic amyloid-β plaques: a prospective cohort study. Lancet Neurol. 11, 669–678. doi: 10.1016/S1474-4422(12)70142-4
Cryan, J. F., O'Riordan, K. J., Cowan, C. S. M., Sandhu, K. V., Bastiaanssen, T. F. S., Boehme, M., et al. (2019). The microbiota-gut-brain Axis. Physiol. Rev. 99, 1877–2013. doi: 10.1152/physrev.00018.2018
Cryan, J. F., O'Riordan, K. J., Sandhu, K., Peterson, V., and Dinan, T. G. (2020). The gut microbiome in neurological disorders. Lancet Neurol. 19, 179–194. doi: 10.1016/S1474-4422(19)30356-4
D’Anna, L., Abu-Rumeileh, S., Fabris, M., Pistis, C., Baldi, A., Sanvilli, N., et al. (2017). Serum Interleukin-10 levels correlate with cerebrospinal fluid amyloid Beta deposition in Alzheimer disease patients. Neurodegener. Dis. 17, 227–234. doi: 10.1159/000474940
Drake, J. D., Chambers, A. B., Ott, B. R., and Daiello, L. A.Alzheimer’s Disease Neuroimaging Initiative. (2021). Peripheral markers of vascular endothelial dysfunction show independent but additive relationships with brain-based biomarkers in association with functional impairment in Alzheimer’s disease. J. Alzheimers Dis. 80, 1553–1565. doi: 10.3233/JAD-200759
Erny, D., Hrabě de Angelis, A. L., Jaitin, D., Wieghofer, P., Staszewski, O., David, E., et al. (2015). Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 18, 965–977. doi: 10.1038/nn.4030
Escobar, Y.-N. H., O’Piela, D., Wold, L. E., and Mackos, A. R. (2022). Influence of the microbiota-gut-brain Axis on cognition in Alzheimer’s disease. J. Alzheimers Dis. 87, 17–31. doi: 10.3233/JAD-215290
Ghosh, S. S., Wang, J., Yannie, P. J., and Ghosh, S. (2020). Intestinal barrier dysfunction, LPS translocation, and disease development. J. Endocrine Soc 4, 1–15. doi: 10.1210/jendso/bvz039
Giovagnoli, A. R., del Pesce, M., Mascheroni, S., Simoncelli, M., Laiacona, M., and Capitani, E. (1996). Trail making test: normative values from 287 normal adult controls. Ital. J. Neurol. Sci. 17, 305–309. doi: 10.1007/BF01997792
Grabrucker, S., Marizzoni, M., Silajdžić, E., Lopizzo, N., Mombelli, E., Nicolas, S., et al. (2023). Microbiota from Alzheimer’s patients induce deficits in cognition and hippocampal neurogenesis. Brain 146, 4916–4934. doi: 10.1093/brain/awad303
Guerra-Espinosa, C., Jiménez-Fernández, M., Sánchez-Madrid, F., and Serrador, J. M. (2024, 2024). ICAMs in immunity, intercellular adhesion and communication. Cells 13:339. doi: 10.3390/CELLS13040339
Guillot-Sestier, M.-V., Doty, K. R., Gate, D., Rodriguez, J. Jr., Leung, B. P., Rezai-Zadeh, K., et al. (2015). Il10 deficiency rebalances innate immunity to mitigate Alzheimer-like pathology. Neuron 85, 534–548. doi: 10.1016/j.neuron.2014.12.068
Guo, M., Peng, J., Huang, X., Xiao, L., Huang, F., and Zuo, Z. (2021). Gut microbiome features of Chinese patients newly diagnosed with Alzheimer’s disease or mild cognitive impairment. J. Alzheimers Dis. 80, 299–310. doi: 10.3233/JAD-201040
Haimov, I., Magzal, F., Tamir, S., Lalzar, M., Asraf, K., Milman, U., et al. (2022). Variation in gut microbiota composition is associated with sleep quality and cognitive performance in older adults with insomnia. Nature Sci. Sleep 14, 1753–1767. doi: 10.2147/NSS.S377114
Hanseeuw, B. J., Betensky, R. A., Jacobs, H. I. L., Schultz, A. P., Sepulcre, J., Becker, J. A., et al. (2019). Association of Amyloid and tau with Cognition in preclinical Alzheimer disease. JAMA Neurol. 76, 915–924. doi: 10.1001/jamaneurol.2019.1424
Hosoki, S., Hansra, G. K., Jayasena, T., Poljak, A., Mather, K. A., Catts, V. S., et al. (2023). Molecular biomarkers for vascular cognitive impairment and dementia. Nat. Rev. Neurol. 19, 737–753. doi: 10.1038/s41582-023-00884-1
Hou, K., Wu, Z. X., Chen, X. Y., Wang, J. Q., Zhang, D., Xiao, C., et al. (2022). Microbiota in health and diseases. Signal Transduct. Target. Ther. 7:135. doi: 10.1038/s41392-022-00974-4
Jack, C. R.Jr, Andrews, J. S., Beach, T. G., Buracchio, T., Dunn, B., Graf, A., et al. (2024). Revised criteria for diagnosis and staging of Alzheimer’s disease: Alzheimer’s association workgroup. Alzheimer’s Dementia 20, 5143–5169. doi: 10.1002/alz.13859
Kaiyrlykyzy, A., Kozhakhmetov, S., Babenko, D., Zholdasbekova, G., Alzhanova, D., Olzhayev, F., et al. (2022). Study of gut microbiota alterations in Alzheimer’s dementia patients from Kazakhstan. Sci. Rep. 12:15115. doi: 10.1038/s41598-022-19393-0
Khedr, E. M., Omeran, N., Karam-Allah Ramadan, H., Ahmed, G. K., and Abdelwarith, A. M. (2022). Alteration of gut microbiota in Alzheimer’s disease and their relation to the cognitive impairment. J. Alzheimers Dis. 88, 1103–1114. doi: 10.3233/JAD-220176
Kiyota, T., Ingraham, K. L., Swan, R. J., Jacobsen, M. T., Andrews, S. J., and Ikezu, T. (2012). AAV serotype 2/1-mediated gene delivery of anti-inflammatory interleukin-10 enhances neurogenesis and cognitive function in APP+PS1 mice. Gene Ther. 19, 724–733. doi: 10.1038/gt.2011.126
Kowalski, K., and Mulak, A. (2019). Brain-gut-microbiota Axis in Alzheimer’s disease. J. Neurogastroenterol. Motility 25, 48–60. doi: 10.5056/jnm18087
Laske, C., Müller, S., Preische, O., Ruschil, V., Munk, M. H. J., Honold, I., et al. (2022). Signature of Alzheimer’s disease in intestinal microbiome: results from the AlzBiom study. Front. Neurosci. 16:792996. doi: 10.3389/fnins.2022.792996
Leblhuber, F., Geisler, S., Steiner, K., Fuchs, D., and Schütz, B. (2015). Elevated fecal calprotectin in patients with Alzheimer’s dementia indicates leaky gut. J. Neural Transm. 122, 1319–1322. doi: 10.1007/s00702-015-1381-9
Li, B., He, Y., Ma, J., Huang, P., du, J., Cao, L., et al. (2019). Mild cognitive impairment has similar alterations as Alzheimer’s disease in gut microbiota. Alzheimers Dement. 15, 1357–1366. doi: 10.1016/j.jalz.2019.07.002
Li, J., Zhang, F., Zhao, L., and Dong, C. (2023). Microbiota–gut–brain axis and related therapeutics in Alzheimer’s disease: prospects for multitherapy and inflammation control. Rev. Neurosci. 34, 695–718. doi: 10.1515/revneuro-2023-0006
Ling, Z., Zhu, M., Yan, X., Cheng, Y., Shao, L., Liu, X., et al. (2021). Structural and functional Dysbiosis of fecal microbiota in Chinese patients with Alzheimer’s disease. Front. Cell Developmental Biol. 8:634069. doi: 10.3389/fcell.2020.634069
Liu, S., Gao, J., Zhu, M., Liu, K., and Zhang, H. L. (2020). Gut microbiota and Dysbiosis in Alzheimer’s disease: implications for pathogenesis and treatment. Mol. Neurobiol. 57, 5026–5043. doi: 10.1007/s12035-020-02073-3
Liu, X., Liu, Y., Liu, J., Zhang, H., Shan, C., Guo, Y., et al. (2024). Correlation between the gut microbiome and neurodegenerative diseases: a review of metagenomics evidence. Neural Regen. Res. 19, 833–845. doi: 10.4103/1673-5374.382223
Loh, J. S., Mak, W. Q., Tan, L. K. S., Ng, C. X., Chan, H. H., Yeow, S. H., et al. (2024). Microbiota–gut–brain axis and its therapeutic applications in neurodegenerative diseases. Signal Transduct. Target. Ther. 9:37. doi: 10.1038/s41392-024-01743-1
MahmoudianDehkordi, S., Arnold, M., Nho, K., Ahmad, S., Jia, W., Xie, G., et al. (2019). Altered bile acid profile associates with cognitive impairment in Alzheimer’s disease—an emerging role for gut microbiome. Alzheimers Dement. 15, 76–92. doi: 10.1016/j.jalz.2018.07.217
Marizzoni, M., Cattaneo, A., Mirabelli, P., Festari, C., Lopizzo, N., Nicolosi, V., et al. (2020). ‘Short-chain fatty acids and lipopolysaccharide as mediators between gut Dysbiosis and amyloid pathology in Alzheimer’s disease. J. Alzheimer’s Disease 78, 683–697. doi: 10.3233/JAD-200306
Marizzoni, M., Mirabelli, P., Mombelli, E., Coppola, L., Festari, C., Lopizzo, N., et al. (2023). A peripheral signature of Alzheimer’s disease featuring microbiota-gut-brain axis markers. Alzheimers Res. Ther. 15:101. doi: 10.1186/s13195-023-01218-5
Marizzoni, M., Provasi, S., Cattaneo, A., and Frisoni, G. B. (2017). Microbiota and neurodegenerative diseases. Curr. Opin. Neurol. 30, 630–638. doi: 10.1097/WCO.0000000000000496
Mayer, E. A., Nance, K., and Chen, S. (2022). The gut–brain Axis. Annu. Rev. Med. 73, 439–453. doi: 10.1146/annurev-med-042320-014032
Meyer, K., Lulla, A., Debroy, K., Shikany, J. M., Yaffe, K., Meirelles, O., et al. (2022). Association of the gut Microbiota with Cognitive Function in midlife. JAMA Netw. Open 5:e2143941. doi: 10.1001/jamanetworkopen.2021.43941
Morais, L. H., Schreiber, H. L., and Mazmanian, S. K. (2021). The gut microbiota–brain axis in behaviour and brain disorders. Nat. Rev. Microbiol. 19, 241–255. doi: 10.1038/s41579-020-00460-0
Morgan, A. E., and Mc Auley, M. T. (2024). Vascular dementia: from pathobiology to emerging perspectives. Ageing Res. Rev. 96:102278. doi: 10.1016/j.arr.2024.102278
Nielsen, H. M., Londos, E., Minthon, L., and Janciauskiene, S. M. (2007). Soluble adhesion molecules and angiotensin-converting enzyme in dementia. Neurobiol. Dis. 26, 27–35. doi: 10.1016/j.nbd.2006.11.011
Nishiwaki, H., Ueyama, J., Kashihara, K., Ito, M., Hamaguchi, T., Maeda, T., et al. (2022). Gut microbiota in dementia with Lewy bodies. NPJ Parkinson’s Disease 8:169. doi: 10.1038/s41531-022-00428-2
Novelli, G., Papagno, C., Capitani, E., Laiacona, M., Cappa, S., and Vallar, G. (1986a). Tre test clinici di memoria verbale a lungo termine: Taratura su soggetti normali. Arch. Psicol. Neurol. Psichiatr. 47, 278–296. Available at: https://psycnet.apa.org/record/1988-70649-001.
Novelli, G., Papagno, C., Capitani, E., Laiacona, M., Cappa, S., and Vallar, G. (1986b). Tre test clinici di ricerca e produzione lessicale. Taratura su soggetti normali. Arch. Psicol. Neurol. Psichiatr. 47, 477–506. Available at: https://www.researchgate.net/publication/281981350_Tre_test_clinici_di_ricerca_e_produzione_lessicale_Taratura_su_soggetti_normal.
Oeckl, P., Anderl-Straub, S., von Arnim, C. A. F., Baldeiras, I., Diehl-Schmid, J., Grimmer, T., et al. (2022). Serum GFAP differentiates Alzheimer’s disease from frontotemporal dementia and predicts MCI-to-dementia conversion. J. Neurol. Neurosurg. Psychiatry 93, 659–667. doi: 10.1136/jnnp-2021-328547
Pei, Y., Lu, Y., Li, H. Z., Jiang, C. Y., and Wang, L. (2023). Gut microbiota and intestinal barrier function in subjects with cognitive impairments: a cross-sectional study. Front. Aging Neurosci. 15:1174599. doi: 10.3389/fnagi.2023.1174599
Peretti, D. E., Boccalini, C., Ribaldi, F., Scheffler, M., Marizzoni, M., Ashton, N. J., et al. (2024). Association of glial fibrillary acid protein, Alzheimer’s disease pathology and cognitive decline. Brain 147, 4094–4104. doi: 10.1093/brain/awae211
Pfaffl, M. W. (2001). A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29, 45e–445e. doi: 10.1093/nar/29.9.e45
Porro, C., Cianciulli, A., and Panaro, M. A. (2020). The regulatory role of IL-10 in neurodegenerative diseases. Biomol. Ther. 10:1017. doi: 10.3390/biom10071017
Prion, S., and Haerling, K. A. (2014). Making sense of methods and measurement: spearman-rho ranked-order correlation coefficient. Clin. Simul. Nurs. 10, 535–536. doi: 10.1016/J.ECNS.2014.07.005
Quast, C., Pruesse, E., Yilmaz, P., Gerken, J., Schweer, T., Yarza, P., et al. (2012). The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 41, D590–D596. doi: 10.1093/nar/gks1219
Rasi Marzabadi, L., Sadigh-Eteghad, S., and Talebi, M. (2021). Circulating inflammatory cytokine levels correlates with cognitive impairment. Clin. Exp. Neuroimmunol. 12, 66–71. doi: 10.1111/cen3.12613
Rueda-Ruzafa, L., Cruz, F., Roman, P., and Cardona, D. (2019). Gut microbiota and neurological effects of glyphosate. Neurotoxicology 75, 1–8. doi: 10.1016/j.neuro.2019.08.006
Sim, M. A., Tan, E. S. J., Chan, S. P., Cai, Y., Chai, Y. L., Chong, J. R., et al. (2024). Associations of circulating platelet endothelial cell adhesion Molecule-1 levels with progression of cerebral small-vessel disease, cognitive decline, and incident dementia. J. Am. Heart Assoc. 13:e035133. doi: 10.1161/JAHA.124.035133
Spinnler, H., and Tognoni, G. (1987). Standardizzazione e taratura italiana di test neuropsicologici. Ital. J. Neurol. Sci. 8, 7–120.
Su, F., Bai, F., and Zhang, Z. (2016). Inflammatory cytokines and Alzheimer’s disease: a review from the perspective of genetic polymorphisms. Neurosci. Bull. 32, 469–480. doi: 10.1007/s12264-016-0055-4
Toth, P., Tarantini, S., Csiszar, A., and Ungvari, Z. (2017). Functional vascular contributions to cognitive impairment and dementia: mechanisms and consequences of cerebral autoregulatory dysfunction, endothelial impairment, and neurovascular uncoupling in aging. Am. J. Phys. Heart Circ. Phys. 312, H1–H20. doi: 10.1152/ajpheart.00581.2016
van der Flier, W. M., Skoog, I., Schneider, J. A., Pantoni, L., Mok, V., Chen, C. L. H., et al. (2018). Vascular cognitive impairment. Nat. Rev. Dis. Prim. 4:18003. doi: 10.1038/nrdp.2018.3
Verhaar, B. J. H., Hendriksen, H. M. A., de Leeuw, F. A., Doorduijn, A. S., van Leeuwenstijn, M., Teunissen, C. E., et al. (2022). Gut microbiota composition is related to AD pathology. Front. Immunol. 12:794519. doi: 10.3389/fimmu.2021.794519
Vogt, N. M., Kerby, R. L., Dill-McFarland, K. A., Harding, S. J., Merluzzi, A. P., Johnson, S. C., et al. (2017). Gut microbiome alterations in Alzheimer’s disease. Sci. Rep. 7:13537. doi: 10.1038/s41598-017-13601-y
Yang, J., Liang, J., Hu, N., He, N., Liu, B., Liu, G., et al. (2024). The gut microbiota modulates Neuroinflammation in Alzheimer’s disease: elucidating crucial factors and mechanistic underpinnings. CNS Neurosci. Ther. 30:e70091. doi: 10.1111/cns.70091
Zhan, X., Stamova, B., and Sharp, F. R. (2018). ‘Lipopolysaccharide associates with amyloid plaques, neurons and oligodendrocytes in Alzheimer’s disease brain: a review. Front. Aging Neurosci. 10:285773. doi: 10.3389/fnagi.2018.00042
Zhang, R., Miller, R. G., Gascon, R., Champion, S., Katz, J., Lancero, M., et al. (2009). Circulating endotoxin and systemic immune activation in sporadic amyotrophic lateral sclerosis (sALS). J. Neuroimmunol. 206, 121–124. doi: 10.1016/j.jneuroim.2008.09.017
Zhang, Y.-R., Wang, J. J., Chen, S. F., Wang, H. F., Li, Y. Z., Ou, Y. N., et al. (2022). Peripheral immunity is associated with the risk of incident dementia. Mol. Psychiatry 27, 1956–1962. doi: 10.1038/s41380-022-01446-5
Zhao, J., Bi, W., Xiao, S., Lan, X., Cheng, X., Zhang, J., et al. (2019). Neuroinflammation induced by lipopolysaccharide causes cognitive impairment in mice. Sci. Rep. 9:5790. doi: 10.1038/s41598-019-42286-8
Keywords: dementia, Alzheimer’s disease, gut microbiota, microbiota-gut-brain axis, cognitive function
Citation: Singh Solorzano C, Festari C, Mirabelli P, Mombelli E, Coppola L, Luongo D, Naviglio D, Soricelli A, Quattrini G, Salvatore M, Pievani M, Cattaneo A, Frisoni GB and Marizzoni M (2025) Association between cognitive functioning and microbiota-gut-brain axis mediators in a memory clinic population. Front. Cell. Neurosci. 19:1550333. doi: 10.3389/fncel.2025.1550333
Received: 23 December 2024; Accepted: 28 February 2025;
Published: 12 March 2025.
Edited by:
Nouha Bouayed Abdelmoula, University of Sfax, TunisiaReviewed by:
Rongchen Huang, University of Colorado, United StatesCopyright © 2025 Singh Solorzano, Festari, Mirabelli, Mombelli, Coppola, Luongo, Naviglio, Soricelli, Quattrini, Salvatore, Pievani, Cattaneo, Frisoni and Marizzoni. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Moira Marizzoni, bW1hcml6em9uaUBmYXRlYmVuZWZyYXRlbGxpLmV1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.